Positive association of common variants in CD36 with neovascular age-related macular degeneration
Abstract
Age-related macular degeneration (AMD) is a leading cause of legal blindness among older individuals of industrialized countries. In neovascular AMD, which is an advanced stage of AMD, choroidal neovascularization develops underneath the macula and destroys central vision. Oxidative stress is a hypothesized pathway for the pathophysiology of AMD. CD36 was chosen as a candidate gene for neovascular AMD because the protein plays an important role in this pathway as well as in angiogenesis and in maintaining chorioretinal homeostasis. We tested 19 tag single nucleotide polymorphisms (SNPs) across CD36 for their association with the disease in a Japanese population comprising 109 neovascular AMD subjects and 182 unrelated controls. Five of the 19 SNPs demonstrated a nominally significant association with neovascular AMD (P < 0.05), of which two (rs3173798 and rs3211883) withstood Bonferroni correction for multiple testing (rs3173798, nominal P = 9.96 × 10−4, allele-specific odds ratio = 0.55; rs3211883, nominal P = 2.09 × 10−4, allele-specific odds ratio = 0.50). Population structure analyses excluded stratification artifacts in our study cohort. This study supports the candidacy of CD36 as a novel susceptibility gene for neovascular AMD. Replication of our results in other populations will provide further convincing evidence for the genetic association.
Introduction
Age-related macular degeneration (AMD) is
a leading cause of legal blindness among older individuals of industrialized
countries [1]. The advanced stage of AMD is
classified into atrophic (dry) or neovascular (wet) types. The atrophic type
features a geographic atrophy of the retinal pigment epithelium (RPE) and
photoreceptors of the macula, whereas the neovascular type is characterized by
choroidal neovascularization (CNV) and its sequela. Although the growing
prevalence of AMD could be attributed to an aging population, the precise
etiology remains elusive. Many investigations have established that genetics
plays a role in the pathogenesis of AMD. To date, genetic variants in the
complement factor H (CFH) gene on chromosome 1q32 [2-7] and in two
tightly linked genes — age-related maculopathy susceptibility 2 (ARMS2),
also known as LOC387715, and high-temperature requirement factor A1 (HTRA1)
on 10q26 [8-13] — have demonstrated the strongest replicable
associations with AMD across multiple ethnic groups. Variants in two adjacent
genes complement factor B, complement component 2 on 6p21 [14,15], and
complement component 3 gene on 19p13 [16-18]
have also demonstrated replicable associations with AMD among Caucasians.
CD36 is involved in diverse physiological and
pathological processes, including scavenger receptor functions (e.g., uptake of
oxidized lipids and advanced glycation end products), transforming growth
factor-β activation, lipid metabolism, angiogenesis, atherogenesis, and
inflammation [19-21]. These wide variety
functions are a result of the diverse ligands with which CD36 can interact [19-21].
In particular, CD36 is known as a critical receptor for thrombospondin-1
(TSP-1). The CD36/TSP-1 signal is essential for the inhibition of
neovascularization, thereby maintaining the quiescence of the normal
vasculature [19,20]. A recent in vivo study demonstrated that down-regulation
of CD36 in capillary sprout endothelial cells facilitated angiogenesis
and results indicated that the cells were becoming insensitive to
antiangiogenic TSP-1 signaling [22]. In the eye, CD36 was reported to play a
major role in the inhibition and regression of corneal neovascularization [23].
CD36 also seems to play an important role in maintaining chorioretinal
homeostasis. Notably, rats carrying a specific genetic variant of CD36
have been found to be more susceptible to light-induced retinal damage [24],
and are more likely to develop age-related retinal degeneration and chorio-capillary
rarefaction [25].
Oxidative stress is widely recognized as
an important component in the pathogenesis of AMD [26,27]. The susceptibility of RPE cells to oxidative stress
progressively increases with age, and the cumulative oxidative damage causes
RPE dysfunction and apoptosis, either directly or through inflammatory
processes [26,27]. CD36 could be regarded as a
link between oxidative stress and oxidative RPE damage, given that CD36 is
involved in the uptake of oxidized lipids by RPE cells [28], which can initiate
many of the cellular events relevant to AMD pathogenesis. A recent in vitro
study reported that the uptake of oxidized low-density lipoprotein (oxLDL)
induces the expression of several genes related to oxidative stress,
inflammation, and apoptosis for RPE cells [29]. An immuno-histochemical study
reported the presence of oxLDL in surgically excised CNV membranes [30].
Furthermore, CD36 is involved in the phagocytosis of photoreceptor outer
segments (OSs), where light-induced oxidation of retinal OS phospholipids
enhances CD36-mediated phagocytosis [31]. In vitro evidence indicates that an
exposure of RPE cells to oxLDL compromises the phagocytic ability of RPE cells [32].
This dysfunction can give rise to the accumulation of lipofuscin in RPE cells,
which further precipitates oxidative conditions and RPE damage [26,27].
Taken together, CD36 can have specific and important
functions in the pathological events involved in AMD and neovascularization.
With the hypothesis that genetic variants in CD36 could be associated
with neovascular AMD, we examined the presence of an association of CD36
variants with the disease.
Results
Single-marker
associations
The demographic details of the study population are
listed in Table 1. Marker information, allelic frequencies, and summary
statistics for all evaluated single nucleotide polymorphisms (SNPs) are shown
in Table 2. Five of the 19 SNPs showed nominally significant associations with
neovascular AMD (P < 0.05), of which two (rs3173798 and rs3211883)
withstood Bonferroni correction for multiple testing (Bonferroni-corrected P= 0.0189 and 0.00397, respectively; Table 2). Applying a permutation
procedure for multiple testcorrection
also yielded significant P values only for thetwo SNPs, rs3173798 and rs3211883 (correctedempirical P = 0.0155 and 0.0043,
respectively). Theminor allele C at
rs3173798 was associated withprotection
against neovascular AMD, with a frequency of 0.307 in cases and 0.445 in
controls (nominal P = 9.96 × 10−4; empirical
pointwise P = 0.0018; per allele odds ratio = 0.55 [95% confidence
interval: 0.39-0.79]). The minor allele A at rs3211883 was also protective
against the disease, with a frequency of 0.248 in cases and 0.398 in controls
(nominal P = 2.09 × 10−4; empirical pointwise P
= 5.0 × 10−4; per allele odds ratio = 0.50 [95%
confidence interval: 0.34-0.72]). Inclusion of age and sex as covariates in
logistic regression models did not substantially change the significance of the
observed associations (rs3173798, age- and sex-adjusted odds ratio = 0.59 [95%
confidence interval = 0.41-0.84], P = 3.10 × 10−3,
additive model; rs3211883, age- and sex-adjusted odds ratio = 0.5 [95%
confidence interval = 0.36 - 0.77], P
= 7.0 × 10−4, additive the model). The two SNPs, rs3173798 and rs3211883, were
highly correlated with each other (r2 = 0.80); thus, their effects
could not be separated statistically (fitting one in conditional logistic
regression model rendered the other redundant). When either SNP rs3173798 or
rs3211883 was fitted in the logistic regression, all other SNPs showing
nominally significant association (rs10499862, rs3173800, and rs17154232) were
redundant.
Table 1. Characteristics of the study population.
| Neovascular AMD | Controls |
|
Number
of subjects
|
109
|
182
|
|
Gender
(male/female)
|
87/22
|
110/72
|
|
Mean
age ± SD (years)
|
76 ±
7.3
|
72 ±
5.8
|
|
Age
range (years)
|
57-91
|
56-95
|
Table 2. Results of single-marker association test.
| | |
Minor
Allele Frequency
|
Association
Results
|
|
SNP
|
Location
|
Minor Allele
|
Cases
|
Controls
|
Allelic
P-value (Empirical Pointwise P-value)*
|
Allelic OR
(95% CI)
|
Corrected
Empirical P-value†
|
Bonferroni
Corrected P-value‡
|
|
rs12531609
|
Intron
1
|
T
|
0.165
|
0.223
|
0.0945
(0.138)
|
0.69
(0.45-1.07)
|
0.608
|
1
|
|
rs3211816
|
Intron
3
|
A
|
0.509
|
0.475
|
0.428
(0.451)
|
1.15
(0.82-1.60)
|
0.995
|
1
|
|
rs10499862
|
Intron
3
|
C
|
0.106
|
0.187
|
0.00895
(0.0126)
|
0.51
(0.31-0.85)
|
0.113
|
0.17
|
|
rs3211849
|
Intron
3
|
A
|
0.289
|
0.269
|
0.606
(0.628)
|
1.10
(0.76-1.60)
|
1
|
1
|
|
rs3211851
|
Intron
3
|
C
|
0.202
|
0.253
|
0.160
(0.20)
|
0.75
(0.50-1.12)
|
0.799
|
1
|
|
rs1054516
|
Intron
3
|
C
|
0.395
|
0.459
|
0.130
(0.136)
|
0.77
(0.55-1.08)
|
0.726
|
1
|
|
rs3173798
|
Intron
3
|
C
|
0.307
|
0.445
|
9.96 ×
10−4 (0.0018)
|
0.55
(0.39-0.79)
|
0.0155
|
0.0189
|
|
rs3211870
|
Intron
4
|
C
|
0.454
|
0.511
|
0.184
(0.181)
|
0.80
(0.57-1.12)
|
0.839
|
1
|
|
rs1358337
|
Intron
4
|
G
|
0.349
|
0.319
|
0.457
(0.454)
|
1.14
(0.80-1.63)
|
0.996
|
1
|
|
rs3211883
|
Intron
4
|
A
|
0.248
|
0.398
|
2.09 ×
10−4 (5.0 × 10−4)
|
0.50
(0.34-0.72)
|
0.0043
|
0.00397
|
|
rs3173800
|
Intron
4
|
T
|
0.404
|
0.289
|
0.00427
(0.00570)
|
1.67
(1.17-2.38)
|
0.0538
|
0.0812
|
|
rs1924
|
Intron
5
|
A
|
0.161
|
0.220
|
0.0824
(0.0877)
|
0.68
(0.44-1.05)
|
0.570
|
1
|
|
rs17154232
|
Intron
6
|
C
|
0.087
|
0.151
|
0.0250
(0.0411)
|
0.54
(0.31-0.93)
|
0.256
|
0.475
|
|
rs17154233
|
Intron
6
|
C
|
0.266
|
0.203
|
0.0801
(0.0776)
|
1.42
(0.96-2.11)
|
0.555
|
1
|
|
rs3211908
|
Intron
7
|
T
|
0.142
|
0.146
|
0.91
(1)
|
0.97
(0.60-1.57)
|
1
|
1
|
|
rs17154258
|
Intron
8
|
G
|
0.142
|
0.184
|
0.191
(0.218)
|
0.73
(0.46-1.17)
|
0.860
|
1
|
|
rs1527483
|
Intron
11
|
A
|
0.179
|
0.176
|
0.925
(1)
|
1.02
(0.66-1.58)
|
1
|
1
|
|
rs3211958
|
Intron
14
|
G
|
0.367
|
0.396
|
s0.492
(0.531)
|
0.89
(0.63-1.25)
|
0.998
|
1
|
|
rs7755
|
3′UTR
|
G
|
0.491
|
0.420
|
0.0978
(0.124)
|
1.33
(0.95-1.86)
|
0.631
|
1
|
Haplotype analysis
The pairwise linkage disequilibrium (LD) structure was
constructed with all SNPs evaluated (Figure 1a). Using the criteria described
by Gabriel et al. [33], three haplotype blocks were defined (Figure 1a).
Haplotype analyses from all blocks revealed that the association with
neovascular AMD was restricted to block 1 and 2, as demonstrated by the
significant omnibus results (omnibus P = 0.00482 and 0.00181,
respectively; Table 3). As shown in Table 3, one haplotype in block 1 and two
haplotypes in block 2 were found to be significantly associated with the
disease after correction for multiple testing (permutation P < 0.05).
A risk haplotype (underlined in Table 3) showed a solid spine of LD across
blocks 1 and 2, with haplotype frequencies of 0.404 in affected individuals and
0.288 in controls (P = 0.0043; odds ratio = 1.67 [95% confidence
interval = 1.17-2.38]; Figure 1b). This haplotype was completely described by
the allele T at rs3173800. The protective allele A at rs3211883 was split into
two different haplotypes, one of which showed statistical significance for
protection against neovascular AMD (P = 0.0067; odds ratio = 0.48 [95%
confidence interval = 0.28-0.83]; Table 3).
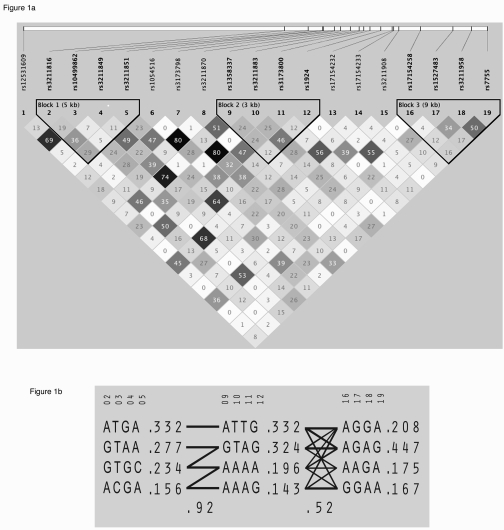
Figure 1. Linkage disequilibrium structure and haplo-typic architecture in CD36.. (A) Haploview plot
defining haplotype block structure of the CD36 region. Linkage disequilibrium
(LD) was measured using data from all subjects in the present study. The
haplotype blocks were determined using the criteria described by Gabriel et al. [33]. The physical
position of each SNP is presented in the upper diagram. Each box provides
estimated statistics of the coefficient of determination (r2),
with darker shades representing stronger LD. (B) Haplotypes in the
haplotype blocks across the CD36 region. There are three haplotype
blocks across the region. The haplotype frequencies are shown to the right
of each haplotype. Only haplotypes having a frequency greater than 1% are
shown. The SNP numbers across the top of the haplotypes correspond to those
in the Haploview plot. A multiallelic D′ statistic, which indicates the level of recombination between two
blocks, is shown in the crossing area. Connections from one block to the
next were shown for haplotypes of greater than 10% frequency with thick
lines and greater than 1% frequency with thin lines.
Table 3. Association of CD36 haplotype blocks with neovascular AMD.
Associations of 3 haplotypes,
ATGA in block 1 and ATTG and AAAG in block 2, remained statistically
significant after correction for multiple testing (permutation P =
0.0325, 0.0325, and 0.0453, respectively). The evidence for association of
haplotype ACGA in block 1 disappeared after correction for multiple testing
(permutation P = 0.0622). The risk haplotype showing a solid spine
of LD across blocks 1 and 2 was underlined.
| |
Frequency
| | | |
|
Haplotype*
|
Cases
|
Controls
| P-value†
|
OR
(95% CI)
|
Omnibus P-value‡
|
|
Block
1
| ATGA
|
0.404
|
0.288
|
0.0043
|
1.67
(1.17-2.38)
|
0.00482
|
|
GTAA
|
0.289
|
0.269
|
0.606
|
1.10
(0.76-1.60)
| |
|
GTGC
|
0.202
|
0.253
|
0.160
|
0.75
(0.50-1.12)
| |
|
ACGA
|
0.106
|
0.187
|
0.0089
|
0.51
(0.31-0.85)
| |
|
Block
2
| | | | | | |
| ATTG
|
0.404
|
0.288
|
0.0043
|
1.67
(1.17-2.38)
|
0.00181
|
|
GTAG
|
0.344
|
0.313
|
0.443
|
1.15
(0.80-1.64)
| |
|
AAAA
|
0.156
|
0.219
|
0.06
|
0.65
(0.42-1.02)
| |
|
AAAG
|
0.092
|
0.173
|
0.0067
|
0.48
(0.28-0.83)
| |
|
Block
3
| | | | | | |
|
AGAG
|
0.491
|
0.420
|
0.0978
|
1.33
(0.95-1.86)
|
0.328
|
|
AGGA
|
0.188
|
0.220
|
0.362
|
0.82
(0.54-1.25)
| |
|
AAGA
|
0.179
|
0.173
|
0.858
|
1.04
(0.67-1.62)
| |
|
GGAA
|
0.142
|
0.181
|
0.220
|
0.75
(0.47-1.19)
| |
Assessment of population stratification
Hidden population stratification between
cases and controls can generate a false positive association. The population
stratification was examined by STRUCTURE [34] using 26 unlinked
genome-wide SNPs. We found no evidence of significant stratification in our study cohort [Pr (K = 1 >
0.99)], indicating that population stratification did not account for
association signals detected in the present study.
Discussion
We tested biological candidate gene CD36 and
found that common variants in this gene are associated with neovascular AMD in
a Japanese population. We confirmed the lack of population stratification
between case and control subjects in the present study. Our results implicate CD36
as a previously unknown genetic risk factor for neovascular AMD.
We identified two protective variants satisfying
stringent statistical thresholds for significance; rs3211883 was the most
significant SNP (nominal allelic P = 2.09 × 10−4),
followed by rs3173798 (nominal allelic P = 9.96 × 10−4).
The two SNPs were highly correlated with each other (r2 = 0.80);
therefore, their effects could not be separated statistically in our dataset.
The biological basis of the associations is currently unknown because the two
SNPs do not reside in the coding sequence of CD36. Using the FASTSNP
program [35], we predicted binding of the CDX1 intronic enhancer to the
sequence containing rs3211883 and rs3173798 to be located in a potential splice
site. Thus, these two SNPs could have non-coding effects on gene function;
however, exhaustive resequencing of the locus is required to search potentially
undiscovered and more important causative variants.
CD36 is
located on chromosome 7q11.2, a region that has not been previously implicated
in AMD. We examined SNPs across a 366 kb region harboring CD36 and two flanking genes (GNAT3 and SEMA3C) in an available database, the
NEI/NCBI dbGAP database (http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?id=phs000001).
This database provides results of a
genome-wide association (GWA) analysis between 395 individuals with AMD and 198
controls from the National Eye Institute Age-Related Eye Disease Study (AREDS).
This analysis did not include the two most significant SNPs (rs3173798 and
rs3211883) or any of the three SNPs (rs10499862, rs3173800, and rs17154232)
that showed nominally significant associations in our study. The GWA study
looked at five CD36 SNPs (rs1194182, rs3211822, rs3211885, rs1405747,
and rs7755) and found no significant association (all nominal P >
0.05). Of the five GWA SNPs, rs7755 was also typed in our study and all of the
remaining GWA SNPs were captured by our tag SNPs; rs1194182 was highly correlated with rs3211849 (r2
= 0.95) and rs3211822, rs3211885, and rs1405747 were a perfect proxy (r2 =1) for rs3211816,
rs3211870, and rs7755, respectively, according to the HapMap JPT data.
Consistent with the GWA data from AREDS, none of the four proxy SNPs
(rs3211849, rs3211816, rs3211870, and rs7755) showed a significant association
with neovascular AMD in our study (all nominal P > 0.05), indicating
that the GWA study in the AREDS cohort was unable to capture the genetic
effects detected in the present study.
Cumulative oxidative stress is an important component
of AMD pathogenesis because of its contribution to RPE damage and subsequent
pathology such as the activation of inflammatory responses in the Bruch
membrane and choroids [26,27]. It is possible
that altered CD36 biologic behavior, which is defined by common variations in
this gene, could be a contributing factor for this pathogenic sequence of events given its
ability to scavenge oxidized lipids [28,29] and phagocytose OSs under conditions of increased oxidative
stress [31]. The ability of CD36 to mediate the antiangiogenic activity of
TSP-1 would also predict a proangiogenic consequence of CD36 dysfunction [19,20]. Further support for the involvement of CD36 in AMD pathogenesis can
be found in studies involving CD36-deficient animals. Rats carrying a specific
genetic variant of CD36 have been shown to be more susceptible to
light-induced retinal damage [24], and are more likely to develop an
age-related retinal degeneration and choriocapillary rarefaction [25]. This
observation could serve as a link between the genetic association observed in our study and prior evidence
that a markedly decreased choroidal circulation
precedes the appearance of CNV in neovascular AMD [36].
In conclusion, we report a novel association between
common variants in CD36 and neovascular AMD in a Japanese population.
Although the underlying causative biological perturbation related to these
variants is not yet clear, this study supports the candidacy of CD36 as
a novel susceptibility gene for neovascular AMD. Replication of our results in
other populations will provide further convincing evidence for the association
of CD36 variants with neovascular AMD.
Methods
Study participants.
This study
was approved by the Institutional Review Board at Kobe University Graduate
School of Medicine and was conducted in accordance with the Declaration of
Helsinki. Written informed consent was obtained from all subjects. All cases
and controls included in this study were Japanese individuals recruited from
the Department of Ophthalmology at Kobe University Hospital in Kobe, Japan.
All patients with neovascular AMD received ophthalmic
examinations, including visual acuity measurement, slit-lamp biomicroscopy of
the fundi, color fundus photographs, optical coherence tomography, fluorescein
angiography, and indocyanine green angiography. All of our study subjects with
neovascular AMD had CNV and associated manifestations such as nondrusenoid
pigment epithelial detachment, serous or hemorrhagic retinal detachments,
subretinal or sub-RPE hemorrhages, and fibrosis, and thus, were categorized as
having clinical age-related maculopathy staging system (CARMS) stage 5 [37].
Patients with polypoidal choroidal vasculopathy and secondary choroidal
neovascular diseases such as degenerative myopia, ocular trauma, angioid streaks,
idiopathic CNV, and presumed ocular histoplasmosis were excluded from our
study. The control subjects were 56 years of age or older and were defined as
individuals without macular degeneration and macular changes such as drusen or
pigment abnormalities. Thus, controls were categorized as having CARMS stage 1 [37]
on the basis of comprehensive ophthalmic examinations.
SNP selection.
To comprehensively yet efficiently screen CD36 sequences for genetic
variations in a Japanese population, we ran Tagger tool [38] from HapMap
Project database for the Japanese in Tokyo (JPT) population [39] (minor allele
frequency cutoff was set at 0.1; r2 cutoff was set at 0.8; and the
Tagger Pairwise mode was used). Nineteen tag SNPs across a 74.5 kb region
encompassing CD36 were selected for genotyping. Based on the HapMap JPT
data, these 19 SNPs captured 121 of 123 SNPs in CD36 exhibiting a minor
allele frequency greater than 10% with a mean r2 value of 0.97.
Thus, our set of 19 SNPs is highly representative of the common genetic
variation in CD36 because it acts as a proxy marker for other untyped
SNPs in this region.
Genotyping.
Genomic DNA was extracted from the peripheral blood
using a standard methodology. Genotyping was performed using TaqMan®
SNP Genotyping Assays (Applied Biosystems, Foster City, CA) on a StepOnePlus™ Real-Time PCR
System (Applied Biosystems) in accordance with the supplier's
recommendations.
Statistical analysis.
Each marker was tested for association using a
software package, PLINK v1.00 (http://pngu.mgh.harvard.edu/purcell/plink/)
[40]. In addition to obtaining nominal P-values, empirical P-values
were generated by 10,000 permutation tests using Max (T) permutation procedure
implemented in PLINK [40]. In this procedure, two sets of empirical
significance values were calculated: pointwise estimates of an individual SNP's
significance (empirical pointwise P-values) and corrected values for
multiple testing (corrected empirical P-values). We also applied a
Bonferroni correction [41], which is the most conservative correction for
multiple testing, where nominal P-values were multiplied by 19 (the
number of SNPs tested for association). To adjust for age and sex differences
between the case and control subjects, logistic regression analyses were
performed using SNPStats (http://bioinfo.iconcologia.net/SNPstats),
with age and sex controlled as covariates. Age and sex were included in this
model as a continuous covariate measured in years and a categorical covariate,
respectively. Deviations from Hardy-Weinberg equilibrium were tested using the chi-square test
(1 degree of freedom), and all of the 19 SNPs passed the Hardy-Weinberg
equilibrium tests in both the case and control subjects (P > 0.001) [41].
To dissect multiple association signals due to LD patterns, we conducted
conditional logistic regression analysesusing
the logistic and condition options in PLINK. The FASTSNP program (http://fastsnp.ibms.sinica.edu.tw/pages/input_CandidateGeneSearch.jsp) was used to investigate the
potential function of SNP [35].
A software package, Haploview, was used for assessing
LD patterns and haplotype association statistics [42]. Haplotype blocks were
determined using the algorithm of Gabriel et al [33]. To correct for multiple
testing in the haplotype analysis, 10,000
permutations were run using Haploview. Odds ratios and 95% confidence
intervals for haplotype-specific risks were calculated using VassarStats (http://faculty.vassar.edu/lowry/VassarStats.html). An omnibus (or global) test of the
haplotype association was conducted with PLINK.
Population stratification errors are a major
problem in case-control studies because they can generate spurious positive
associations [41]. The population stratification should be minimized in
our study cohort given the genetic homogeneity of the Japanese population.
However, to exclude a potential stratification in our study cohort, we examined
the population stratification by a software package, STRUCTURE [34], as
performed in previous genetic association studies on Japanese populations [43-45].
The following 26 polymorphic SNPs, which were randomly distributed along the
genome and are not in LD with each other (r2 < 0.035), were used
for this analysis: rs3818729 (1p13.2), rs13388696 (2p23.1), rs2305619
(3q25.32), rs6876885 (5p15.1), rs6459193 (6p11.2), rs3779109 (7p22.1),
rs6468284 (8p12), rs955220 (9p24.3), rs4838590 (10q11.22), rs12806 (10q24.2),
rs2019938 (11p15.5), rs609017 (11q24.3), rs3912640 (12p13.2), rs2283299
(12p13.33), rs715948 (12q13.3), rs7328193 (13q12.11), rs1048990 (14q13.2),
rs16948719 (15q22.31), rs11076720 (16q24.3), rs1051009 (17p13.2), rs1292033
(17q23.1), rs7239116 (18q11.2), rs892115 (19p13.2), rs844906 (20p11.21),
rs2825761 (21q21.1), and rs3884935 (22q13.1). The log likelihood of each
analysis at varying number of K (the number of populations) was
estimated from three independent runs (20,000 burn in and 30,000 iterations).
The best estimate of K was identified by computing posterior
probabilities Pr (K = 1, 2, 3, 4, or 5) based on the log
likelihood as described by Pritchard et al. [46].
Acknowledgments
The authors thank all who participated in this study.
This study was supported by a Grant-in Aid for (C) 20592042 from the Ministry
of Education, Science, and Culture, Tokyo, Japan.
Conflicts of Interest
None of the authors has a conflict of interest.
References
-
1.
Friedman
DS
, O'Colmain
BJ
, Munoz
B
, Tomany
SC
, McCarty
C
, de Jong
PT
, Nemesure
B
, Mitchell
P
and Kempen
J.
Prevalence of age-related macular degeneration in the United States.
Arch Ophthalmol.
2004;
122:
564
-572.
[PubMed]
.
-
2.
Klein
RJ
, Zeiss
C
, Chew
EY
, Tsai
JY
, Sackler
RS
, Haynes
C
, Henning
AK
, SanGiovanni
JP
, Mane
SM
, Mayne
ST
, Bracken
MB
, Ferris
FL
and Ott
J.
Complement factor H polymorphism in age-related macular degeneration.
Science.
2005;
308:
385
-389.
[PubMed]
.
-
3.
Edwards
AO
, Ritter
R 3rd
, Abel
KJ
, Manning
A
, Panhuysen
C
and Farrer
LA.
Complement factor H polymorphism and age-related macular degeneration.
Science.
2005;
308:
421
-424.
[PubMed]
.
-
4.
Haines
JL
, Hauser
MA
, Schmidt
S
, Scott
WK
, Olson
LM
, Gallins
P
, Spencer
KL
, Kwan
SY
, Noureddine
M
, Gilbert
JR
, Schnetz-Boutaud
N
, Agarwal
A
and Postel
EA.
Complement factor H variant increases the risk of age-related macular degeneration.
Science.
2005;
308:
419
-421.
[PubMed]
.
-
5.
Hughes
AE
, Orr
N
, Esfandiary
H
, Diaz-Torres
M
, Goodship
T
and Chakravarthy
U.
A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration.
Nat Genet.
2006;
38:
1173
-1177.
[PubMed]
.
-
6.
Li
M
, Atmaca-Sonmez
P
, Othman
M
, Branham
KE
, Khanna
R
, Wade
MS
, Li
Y
, Liang
L
, Zareparsi
S
, Swaroop
A
and Abecasis
GR.
CFH haplotypes without the Y402H coding variant show strong association with susceptibility to age-related macular degeneration.
Nat Genet.
2006;
38:
1049
-1054.
[PubMed]
.
-
7.
Maller
J
, George
S
, Purcell
S
, Fagerness
J
, Altshuler
D
, Daly
MJ
and Seddon
JM.
Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration.
Nat Genet.
2006;
38:
1055
-1059.
[PubMed]
.
-
8.
Yang
Z
, Camp
NJ
, Sun
H
, Tong
Z
, Gibbs
D
, Cameron
DJ
, Chen
H
, Zhao
Y
, Pearson
E
, Li
X
, Chien
J
, Dewan
A
and Harmon
J.
A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration.
Science.
2006;
314:
992
-993.
[PubMed]
.
-
9.
Dewan
A
, Liu
M
, Hartman
S
, Zhang
SS
, Liu
DT
, Zhao
C
, Tam
PO
, Chan
WM
, Lam
DS
, Snyder
M
, Barnstable
C
, Pang
CP
and Hoh
J.
HTRA1 promoter polymorphism in wet age-related macular degeneration.
Science.
2006;
314:
989
-992.
[PubMed]
.
-
10.
Kondo
N
, Honda
S
, Ishibashi
K
, Tsukahara
Y
and Negi
A.
LOC387715/HTRA1 variants in polypoidal choroidal vasculopathy and age-related macular degeneration in a Japanese population.
Am J Ophthalmol.
2007;
144:
608
-612.
[PubMed]
.
-
11.
Kanda
A
, Chen
W
, Othman
M
, Branham
KE
, Brooks
M
, Khanna
R
, He
S
, Lyons
R
, Abecasis
GR
and Swaroop
A.
A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration.
Proc Natl Acad Sci USA.
2007;
104:
16227
-16232.
[PubMed]
.
-
12.
Leveziel
N
, Souied
EH
, Richard
F
, Barbu
V
, Zourdani
A
, Morineau
G
, Zerbib
J
, Coscas
G
, Soubrane
G
and Benlian
P.
PLEKHA1-LOC387715-HTRA1 polymorphisms and exudative age-related macular degeneration in the French population.
Mol Vis.
2007;
13:
2153
-2159.
[PubMed]
.
-
13.
Fritsche
LG
, Loenhardt
T
, Janssen
A
, Fisher
SA
, Rivera
A
, Keilhauer
CN
and Weber
BH.
Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA.
Nat Genet.
2008;
40:
892
-896.
[PubMed]
.
-
14.
Gold
B
, Merriam
JE
, Zernant
J
, Hancox
LS
, Taiber
AJ
, Gehrs
K
, Cramer
K
, Neel
J
, Bergeron
J
, Barile
GR
, Smith
RT; AMD Genetics Clinical Study Group
and Hageman
GS.
Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration.
Nat Genet.
2006;
38:
458
-462.
[PubMed]
.
-
15.
Spencer
KL
, Hauser
MA
, Olson
LM
, Schmidt
S
, Scott
WK
, Gallins
P
, Agarwal
A
, Postel
EA
, Pericak-Vance
MA
and Haines
JL.
Protective effect of complement factor B and complement component 2 variants in age-related macular degeneration.
Hum Mol Genet.
2007;
16:
1986
-1992.
[PubMed]
.
-
16.
Yates
JR
, Sepp
T
, Matharu
BK
, Khan
JC
, Thurlby
DA
, Shahid
H
, Clayton
DG
, Hayward
C
, Morgan
J
, Wright
AF
, Armbrecht
AM
, Dhillon
B
and Deary
IJ.
Complement C3 variant and the risk of age-related macular degeneration.
N Engl J Med.
2007;
357:
553
-561.
[PubMed]
.
-
17.
Maller
JB
, Fagerness
JA
, Reynolds
RC
, Neale
BM
, Daly
MJ
and Seddon
JM.
Variation in complement factor 3 is associated with risk of age-related macular degeneration.
Nat Genet.
2007;
39:
1200
-1201.
[PubMed]
.
-
18.
Spencer
KL
, Olson
LM
, Anderson
BM
, Schnetz-Boutaud
N
, Scott
WK
, Gallins
P
, Agarwal
A
, Postel
EA
, Pericak-Vance
MA
and Haines
JL.
C3 R102G polymorphism increases risk of age-related macular degeneration.
Hum Mol Genet.
2008;
17:
1821
-1824.
[PubMed]
.
-
19.
Silverstein
RL
and Febbraio
M.
CD36 and atherosclerosis.
Curr Opin Lipidol.
2000;
11:
483
-491.
[PubMed]
.
-
20.
Febbraio
M
, Hajjar
DP
and Silverstein
RL.
CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism.
J Clin Invest.
2001;
108:
785
-791.
[PubMed]
.
-
21.
Kuniyasu
A
, Ohgami
N
, Hayashi
S
, Miyazaki
A
, Horiuchi
S
and Nakayama
H.
CD36-mediated endocytic uptake of advanced glycation end products (AGE) in mouse 3T3-L1 and human subcutaneous adipocytes.
FEBS Lett.
2003;
537:
85
-90.
[PubMed]
.
-
22.
Anderson
CR
, Hastings
NE
, Blackman
BR
and Price
RJ.
Capillary sprout endothelial cells exhibit a CD36low phenotype. Regulation by shear stress and vascular endothelial growth factor-induced mechanism for attenuating anti-proliferative thrombospondin-1 signaling.
Am J Pathol.
2008;
In press
.
-
23.
Mwaikambo
BR
, Sennlaub
F
, Ong
H
, Chemtob
S
and Hardy
P.
Activation of CD36 inhibits and induces regression of inflammatory corneal neovascularization.
Invest Ophthalmol Vis Sci.
2006;
47:
4356
-4364.
[PubMed]
.
-
24.
Li
S
, Lam
TT
, Fu
J
and Tso
MO.
Systemic hypertension exaggerates retinal photic injury.
Arch Ophthalmol.
1995;
113:
521
-526.
[PubMed]
.
-
25.
Houssier
M
, Raoul
W
, Lavalette
S
, Keller
N
, Guillonneau
X
, Baragatti
B
, Jonet
L
, Jeanny
JC
, Behar-Cohen
F
, Coceani
F
, Scherman
D
, Lachapelle
P
and Ong
H.
CD36 deficiency leads to choroidal involution via COX2 down-regulation in rodents.
PLoS Med.
2008;
5:
e39
[PubMed]
.
-
26.
Zarbin
MA
Current concepts in the pathogenesis of age-related macular degeneration.
Arch Ophthalmol.
2004;
122:
598
-614.
[PubMed]
.
-
27.
Beatty
S
, Koh
H
, Phil
M
, Henson
D
and Boulton
M.
The role of oxidative stress in the pathogenesis of age-related macular degeneration.
Surv Ophthalmol.
2000;
45:
115
-134.
[PubMed]
.
-
28.
Gordiyenko
N
, Campos
M
, Lee
JW
, Fariss
RN
, Sztein
J
and Rodriguez
IR.
RPE cells internalize low-density lipoprotein (LDL) and oxidized LDL (oxLDL) in large quantities in vitro and in vivo.
Invest Ophthalmol Vis Sci.
2004;
45:
2822
-2829.
[PubMed]
.
-
29.
Yamada
Y
, Tian
J
, Yang
Y
, Cutler
RG
, Wu
T
, Telljohann
RS
, Mattson
MP
and Handa
JT.
Oxidized low density lipoproteins induce a pathologic response by retinal pigmented epithelial cells.
J Neurochem.
2008;
105:
1187
-1197.
[PubMed]
.
-
30.
Kamei
M
, Yoneda
K
, Kume
N
, Suzuki
M
, Itabe
H
, Matsuda
K
, Shimaoka
T
, Minami
M
, Yonehara
S
, Kita
T
and Kinoshita
S.
Scavenger receptors for oxidized lipoprotein in age-related macular degeneration.
Invest Ophthalmol Vis Sci.
2007;
48:
1801
-1807.
[PubMed]
.
-
31.
Sun
M
, Finnemann
SC
, Febbraio
M
, Shan
L
, Annangudi
SP
, Podrez
EA
, Hoppe
G
, Darrow
R
, Organisciak
DT
, Salomon
RG
, Silverstein
RL
and Hazen
SL.
Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: a potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions.
J Biol Chem.
2006;
281:
4222
-4230.
[PubMed]
.
-
32.
Hoppe
G
, Marmorstein
AD
, Pennock
EA
and Hoff
HF.
Oxidized low density lipoprotein-induced inhibition of processing of photoreceptor outer segments by RPE.
Invest Ophthalmol Vis Sci.
2001;
42:
2714
-2720.
[PubMed]
.
-
33.
Gabriel
SB
, Schaffner
SF
, Nguyen
H
, Moore
JM
, Roy
J
, Blumenstiel
B
, Higgins
J
, DeFelice
M
, Lochner
A
, Faggart
M
, Liu-Cordero
SN
, Rotimi
C
and Adeyemo
A.
The structure of haplotype blocks in the human genome.
Science.
2002;
296:
2225
-2229.
[PubMed]
.
-
34.
Falush
D
, Stephens
M
and Pritchard
JK.
Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies.
Genetics.
2003;
164:
1567
-1587.
[PubMed]
.
-
35.
Yuan
HY
, Chiou
JJ
, Tseng
WH
, Liu
CH
, Liu
CK
, Lin
YJ
, Wang
HH
, Yao
A
, Chen
YT
and Hsu
CN.
FASTSNP: an always up-to-date and extendable service for SNP function analysis and prioritization.
Nucleic Acids Res.
2006;
34:
W635
-641.
[PubMed]
.
-
36.
Metelitsina
TI
, Grunwald
JE
, DuPont
JC
, Ying
GS
, Brucker
AJ
and Dunaief
JL.
Foveolar choroidal circulation and choroidal neovascularization in age-related macular degeneration.
Invest Ophthalmol Vis Sci.
2008;
49:
358
-363.
[PubMed]
.
-
37.
Seddon
JM
, Sharma
S
and Adelman
RA.
Evaluation of the clinical age-related maculopathy staging system.
Ophthalmology.
2006;
113:
260
-266.
[PubMed]
.
-
38.
de Bakker
PI
, Yelensky
R
, Pe'er
I
, Gabriel
SB
, Daly
MJ
and Altshuler
D.
Efficiency and power in genetic association studies.
Nat Genet.
2005;
37:
1217
-1223.
[PubMed]
.
-
39.
The
International HapMap Consortium
The International HapMap Project.
Nature.
2003;
426:
789
-796.
[PubMed]
.
-
40.
Purcell
S
, Neale
B
, Todd-Brown
K
, Thomas
L
, Ferreira
MA
, Bender
D
, Maller
J
, Sklar
P
, de Bakker
PI
, Daly
MJ
and Sham
PC.
PLINK: a tool set for whole-genome association and population-based linkage analyses.
Am J Hum Genet.
2007;
81:
559
-575.
[PubMed]
.
-
41.
Balding
DJ
A tutorial on statistical methods for population association studies.
Nat Rev Genet.
2006;
7:
781
-791.
[PubMed]
.
-
42.
Barrett
JC
, Fry
B
, Maller
J
and Daly
MJ.
Haploview: analysis and visualization of LD and haplotype maps.
Bioinformatics.
2005;
21:
263
-265.
[PubMed]
.
-
43.
Kubo
M
, Hata
J
, Ninomiya
T
, Matsuda
K
, Yonemoto
K
, Nakano
T
, Matsushita
T
, Yamazaki
K
, Ohnishi
Y
, Saito
S
, Kitazono
T
, Ibayashi
S
and Sueishi
K.
A nonsynonymous SNP in PRKCH (protein kinase C eta) increases the risk of cerebral infarction.
Nat Genet.
2007;
39:
212
-217.
[PubMed]
.
-
44.
Yamada
K
, Gerber
DJ
, Iwayama
Y
, Ohnishi
T
, Ohba
H
, Toyota
T
, Aruga
J
, Minabe
Y
, Tonegawa
S
and Yoshikawa
T.
Genetic analysis of the calcineurin pathway identifies members of the EGR gene family, specifically EGR3, as potential susceptibility candidates in schizophrenia.
Proc Natl Acad Sci USA.
2007;
104:
2815
-2820.
[PubMed]
.
-
45.
Yamada
K
, Nakamura
K
, Minabe
Y
, Iwayama-Shigeno
Y
, Takao
H
, Toyota
T
, Hattori
E
, Takei
N
, Sekine
Y
, Suzuki
K
, Iwata
Y
, Miyoshi
K
and Honda
A.
Association analysis of FEZ1 variants with schizophrenia in Japanese cohorts.
Biol Psychiatry.
2004;
56:
683
-690.
[PubMed]
.
-
46.
Pritchard
JK
, Stephens
M
and Donnelly
P.
Inference of population structure using multilocus genotype data.
Genetics.
2000;
155:
945
-959.
[PubMed]
.