Abstract
Werner syndrome (WS) is a premature aging disorder characterized by genomic instability. The WRN gene defective in WS encodes a protein with both helicase and exonuclease activities that interacts with proteins implicated in DNA metabolism. To understand its genetic functions, we examined the ability of human WRN to rescue phenotypes associated with sgs1, the sole RecQ helicase in Saccharomyces cerevisiae. WRN failed to rescue sgs1 sensitivity to the DNA damaging agent methylmethane sulfonate or replication inhibitor hydroxyurea, suggesting divergent functions of human and yeast RecQ helicases. However, physiological expression of WRN in sgs1 top3 restored top3 slow growth phenotype, whereas no effect on growth was observed with wild-type or sgs1 strains. Slow growth of WRN-transformed sgs1 top3 correlated with an elevated population of large-budded cells with undivided nuclei, indicating restoration of cell cycle delay in late S/G2 characteristic of top3. WRN helicase but not exonuclease activity was genetically required for restoration of top3 growth phenotype, demonstrating separation of function of WRN catalytic activities. A naturally occurring missense polymorphism in WRN that interferes with helicase activity abolished its ability to restore top3 slow growth phenotype. Proposed roles of WRN in genetic pathways important for the suppression of genomic instability are discussed.
Introduction
Understanding the genetic pathways of the
WRN helicase-exonuclease has posed a complex challenge to researchers. Studies
of WRN-deficient cell lines have provided evidence for a role of WRN in the response
to replicational stress through a recombinational repair pathway [1,2]; however,
the precise molecular functions and protein interactions required for WRN to
help cells proliferate, maintain genomic stability, and deal with endogenous or
exogenously induced DNA damage are not well understood. Moreover, although the
clinical and cellular phenotypes of Werner Syndrome (WS) appear to be distinct
from that of the other
human RecQ helicase disease, it is not clear if WRN has entirely unique or at
least partially overlapping roles with the other RecQ helicases to maintain
genomic stability (for review, see [3,4]).
To
investigate the genetic functions of WRN in a defined setting, we have
developed a yeast-based model system to study the functional requirements of
WRN in pathways that are conserved between yeast and human. Unlike human cells
which have five RecQ helicases (WRN, BLM, RECQ1, RECQ5, and RECQ4), Saccharomyces
cerevisiae has only a single RecQ homolog, Sgs1 [4]. Although sgs1
mutants exhibit sensitivity to DNA damaging agents or replication inhibitors
and display a shortened lifespan, the best known genetic function of sgs1
is to suppress the slow growth phenotype of a top3 mutant. It is
believed that Top3 decatenates intertwined DNA molecules generated by Sgs1
helicase during replication [5,6];
therefore, in the absence of Top3, torsional stress is not relieved resulting
in slow growth and hyper-recombination. Based on the genetic and physical
interaction of Sgs1 with Top3 [5,7,8], a
model has been proposed that together they suppress the formation of cross-over
products that arise from the resolution of Holliday Junction (HJ) recombination
intermediates [9,10].
Conserved interactions between RecQ helicases and Top3 exist in other organisms
as well [11-15].
In human cells, BLM physically interacts with Top3α, and the two proteins together have the ability to catalyse double HJ
dissolution on model DNA substrates in a reaction that requires BLM-mediated
ATP hydrolysis and the active-site tyrosine residue of Top3α [16]. This
reaction gave rise exclusively to non-cross-over products, as predicted from
the hemicatenane model, and supports a proposed role of BLM with Top3α as a suppressor of sister chromatid exchanges (SCEs). RMI1 (BLAP75)
promotes this BLM-dependent dissolution of the homologous recombination (HR)
intermediate by recruiting Top3α to the double HJ [17,18].
Interestingly, BLM appears to be unique in the double HJ dissolution reaction
since WRN, RECQ1 and RECQ5 all failed to substitute for BLM [17,19].
Moreover, association of Top3α and BLAP75 with BLM stimulates
its HJ unwinding activity; however, neither WRN nor E. coli RecQ HJ unwinding
was stimulated by Top3α BLAP75 [20]. Very recently, a new component of the BLM-Top3α complex, designated RMI2, was identified that is important for the
stability of the BLM protein complex [21,22]. RMI2 deficiency in vertebrate cells
results in chromosomal instability [21,22], suggesting its function as a tumor
suppressor. RMI2 enhanced the double HJ dissolvase activity of the
BLM-Top3α complex [21], indicating that additional proteins are likely to be involved. In fact, other proteins were isolated with the RMI2
complex, including the mismatch repair complex MSH2/6, RPA, and the Fanconi
Anemia proteins FANCM and FAAP24 [21].
The suppression of recombinant cross-over
products that are detected as sister chromatid exchanges is thought to be
specific to the coordinate functions of yeast Sgs1 and Top3, and its human
counterparts, BLM and Top3α However, RECQ5 and RECQ1 also
interact with Top3α[23,24], and
elevated SCE is also found in fibroblasts from RECQ5 [25] or RECQ1 [26] knockout
mice as well as human cells depleted of RECQ1 by RNA interference [27]. These
studies suggest that RecQ helicases participate in non-redundant pathways to
suppress cross-overs during mitosis [28].
WS
cells have a unique form of genomic instability known as variegated
translocation mosaicism, characterized by extensive deletions and
rearrangements [29]. WS cells,
like other RecQ mutants, are also defective in recombination and sensitive to
DNA damaging agents [3,30]. To
begin to understand the basis for these defects in cellular DNA metabolism, we
tested the ability of WRN in defined genetic backgrounds using yeast as a model
system. Using this approach, we discovered that WRN restores the top3
slow growth phenotype in the sgs1 top3 background, but does not
complement the DNA damage sensitivity or growth of a single sgs1
mutant. WRN helicase activity is required for genetic restoration of the top3
growth phenotype, suggesting that WRN unwinding activity creates a DNA
substrate in vivo that in the absence of top3 prevents normal
cell growth. These results have implications for the potentially overlapping
pathways between RecQ helicases, particularly WRN and the human homolog of
Sgs1, BLM. Moreover, depending on the genetic background, recombinational
events initiated or mediated by WRN helicase activity may have consequences for
cell cycle progression and cell growth.
Results
Expression
of the human Werner syndrome protein (WRN) in yeast fails to rescue the
methylmethanesulfonate or hydroxyurea hypersensitivity of an sgs1 mutant
The sgs1 strain is characterized by sensitivity to
the compound methylmethanesulfonate (MMS), which introduces alkylation base
damage or to hydroxyurea (HU), a replication inhibitor. To assess the effect
of WRN expression on the MMS or HU-sensitivity phenotype of the sgs1
mutant, sgs1 was transformed with a multi-copy TRP1 selectable plasmid
which is either empty or encoding full-length WRN protein (1-1432). Quantitative Western blot analyses using purified
recombinant WRN protein as standard indicated that 8.1 x 104WRNmolecules/cell
were present at 2% galactose (gal) concentration (data not shown). In comparison,
the level of endogenous WRN in HeLa cells was determined to be 8.9 x 104 molecules/cell
[31], in
agreement with published values for WRN copy number in other human cells [32,33].
Therefore, the level of WRN protein expression in yeast is comparable to that
in human cells and considered physiological. The sgs1 strain
transformed with empty vector (sgs1/ /vector) did not expressprotein
specifically recognized by WRN antibody (data not shown). Transformed sgs1
cells were serially diluted and spotted on to a synthetic complete (SC) 2% gal
media lacking tryptophan (Trp) and containing the indicated concentration of
either MMS or HU. As shown in Figure 1, WRN failed to rescue the sensitivity
of the sgs1 mutant to MMS or HU, and was observed to enhance the sensitivity
of sgs1 to either drug. The vector-transformed wild-type parent strain
(W3031A) was included as a control, showing resistance to MMS or HU (Figure 1). The wild-type parent strain transformed with the YEp112SpGAL-WRN plasmid
(Wild type/WRN) showed MMS and HU resistance comparable to Wild type/Vector (Figure 1), indicating that the drug sensitivity was dependent on the sgs1
mutation. Similar results were obtained at lower WRN protein levels induced by
0.5% gal (Supp. Data Figure 1).
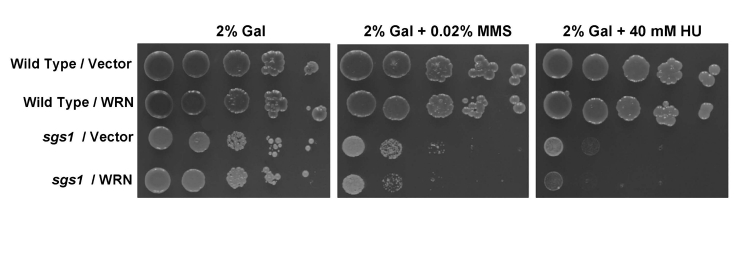
Figure 1. WRN fails to rescue the MMS and HU sensitivity of sgs1. Cultures of wild-type
parental strain (W303-1A) or sgs1 strain transformed with
YEp112SpGAL or YEp112SpGAL-WRN were grown to early log phase (OD
600
of ~0.6 to 0.8). Ten-fold serial dilutions of these cultures were spotted
onto SC-Trp plates containing 2% gal and either MMS or HU at the indicated
concentrations. Plates were incubated at 30°C for 3 days (control plates)
and 5 days (MMS or HU plates) and then photographed.
WRN
and TOP3 genetically interact
Strains
mutant for top3 have pleiotropic phenotypes including severe growth
defect, hyper-recombination at multiple loci, and sensitivity to the DNA
damaging agents MMS and HU. However, a mutation in the sgs1 gene
suppresses the slow growth rate of top3 [5]. Since it
has been proposed that Top3 may be an evolutionarily conserved partner of RecQ
helicases, we examined if the expression of WRN could substitute for Sgs1 and hence
confer slow growth in the sgs1top3
background.
sgs1
top3 double mutant cells transformed
with YEp112SpGAL or YEp112SpGAL-WRN
were streaked onto plates containing SC minus Trp media with 2% gal. As shown
in Figure 2A, the WRN transformed sgs1 top3 strain grew significantly
slowly compared to the vector transformed sgs1 top3 strain. sgs1
top3 transformed with the YEp112SpGAL-SGS1 plasmid was included as a
positive control (Figure 2A), demonstrating that wild-type Sgs1 expressed in
the sgs1 top3 mutant was able to genetically complement the growth
phenotype. The transformed sgs1 top3 strains grew similarly in the
absence of gal as shown for 2 days (Figure 2A) or earlier time points (data not
shown). Expression of WRN had no effect on the growth of parental wild-type
(W3031A) or sgs1 strains (Figure 2B), indicating that the effect of WRN
on cell growth was specific to that observed in the sgs1 top3 mutant
background.
To
further evaluate the effect of WRN expression on growth of the sgs1 top3
double mutant, liquid culture experiments were performed. After an initial lag
phase for all cultures, sgs1 top3/WRN exhibited decreased growth
compared to sgs1 top3/vector, evident at 12, 16, and 20 hr (Figure 2C).
The decrease in cell growth for the sgs1 top3/WRN cells was not quite as
great as that observed for sgs1 top3/SGS1, suggesting a partial restoration of the top3-associated growth
phenotype by WRN.
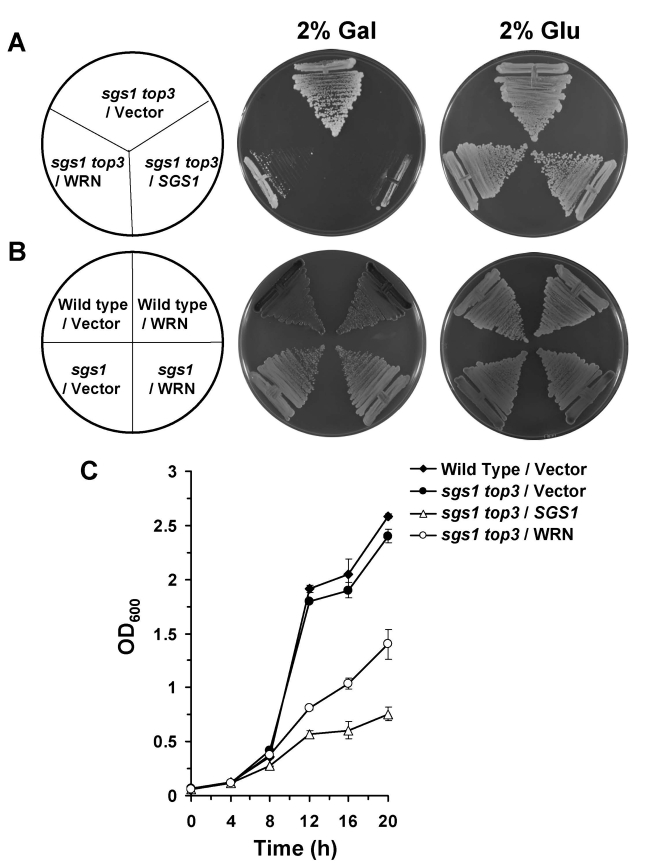
Figure 2. WRN expression in sgs1 top3 restores the slow growth phenotype of top3. Panel A, sgs1 top3 strain transformed with YEp112SpGAL or YEp112SpGAL-WRN were
streaked on an SC-Trp plate containing either 2% glu or 2% gal. As a
control sgs1 top3 strain transformed with YEp112SpGAL-SGS1
was streaked on both the plates. Plates were incubated at 30°C for 2 days
and then photographed. Panel B, Wild type parental strain
W303-1A or sgs1 strain transformed with YEp112SpGAL or
YEp112SpGAL-WRN were streaked on an SC-Trp plate either containing 2% glu
or 2% gal. Plates were incubated at 30°C for 4 days and then
photographed. Composition of the plates was as in Panel A
and Panel B respectively. Panel C, Comparison
of growth of sgs1 top3 strain transformed with YEp112SpGAL,
YEp112SpGAL-WRN or YEp112SpGAL-SGS1. Logarithmically growing
cultures of above mentioned strains were reinoculated at OD0.05
in SC-Trp medium containing 2% gal and were incubated at 30°C. Growth of
the cultures was followed by their absorbance at OD600. The
experiment was repeated twice in duplicate with similar results. Data
represent the mean with standard deviations indicated by error bars.
WRN
expression alters the cell cycle distribution of sgs1 top3
It
was previously shown that top3 mutant strains are delayed in the late
S/G2 phase of the cell cycle [5], a
characteristic that may account for their slow growth. Mutation of the SGS1
gene in the top3 background suppresses the delay in the S/G2 phase of
the cell cycle; consequently, the characteristic dumbbell shaped morphology of
the top3 mutant yeast cells that have not completed cell division is
suppressed by the sgs1 mutation.
We examined if WRN expression could restore the delay in the S/G2 phase of the
cell cycle in sgs1 top3. An elevated population of large budded cells
with undivided nuclei was observed for the sgs1 top3 mutant cells expressing
WRN (Figure 3). The percentage of sgs1 top3/WRN
cells with dumbbell morphology was approximately 2.6-fold greater than that ofsgs1 top3/vector cells, suggesting that WRN expression in the sgs1
top3 mutant restored the characteristic S/G2 delay of top3 and this
may contribute to the suppression of growth by WRN in the sgs1 top3
cells.
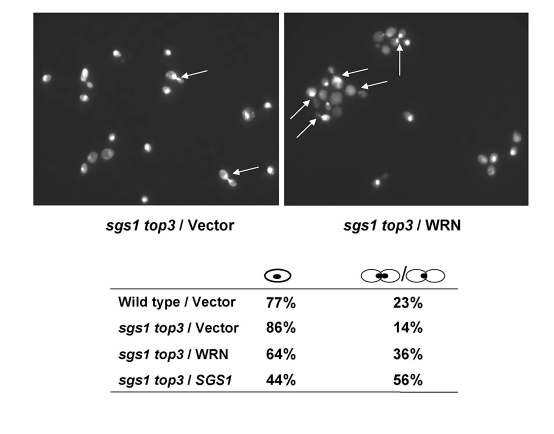
Figure 3. WRN expression induces S/G2 arrest in sgs1 top3 cells. Logarithmically growing
cultures of sgs1 top3 strain transformed with YEp112SpGAL, YEp112SpGAL-WRN,
or YEp112SpGAL-SGS1, and the vector-transformed wild-type parental strain
were induced at 2% gal concentration for 6 h. Cultures were harvested,
processed for DAPI staining as described in "Materials and Methods" and
were observed using Axiovert 200 M microscope(Zeiss; 100x lens). Shown is the DAPI staining of the sgs1
top3 transformed with YEp112SpGAL (upper left) and with YEp112SpGAL-WRN
(upper right). Arrows show cells with undivided nuclei. Distribution of
the cells in G1 (single cells) and S/G2 (budded cells) is shown in lower
panel.
WRN ATPase/ helicase but not exonuclease activity is
required to restore the top3 slow growth phenotype
To
assess the functional requirements of WRN to restore the slow growth phenotype
in the sgs1 top3 background, we assessed the importance of WRN catalytic
activities (helicase, exonuclease) for slow growth phenotype restoration. To do
this, we constructed WRN expression plasmids containing missense point mutations
in the active site of the conserved exonuclease domain (E84A) or the
ATPase/helicase domain (K577M) that were previously shown to inactivate the
respective catalytic activities of the purified recombinant proteins [34,35] (Figure 4A). Plasmids encoding the WRN mutant alleles were transformed into thesgs1 top3 cells and expression of the mutant WRN proteins was confirmed
by Western blot
analyses (Figure 4B). The effects of mutant WRN protein expression on growth
were assessed by streaking the transformed yeast strains onto SC plates media
with 2% gal but lacking Trp. Streak analysis revealed that the WRN-E84A
exonuclease dead mutant restored the top3-associated slow growth
phenotype in sgs1 top3 comparable to wild-type WRN, whereas the
WRN-K577M ATPase/ helicase dead mutant failed to restore the slow growth in thesgs1 top3 as shown by its similar level of growth to that of the sgs1
top3/vector cells (Figure 4D). However, the sgs1 top3/WRN-E84A and sgs1
top3/WRN-K577M cells grew equally well in the absence of gal induction (Figure 4E). From these results, we conclude that WRN helicase but not exonuclease
activity was required for restoration of slow growth phenotype characteristic
of top3 in the sgs1 top3 background.
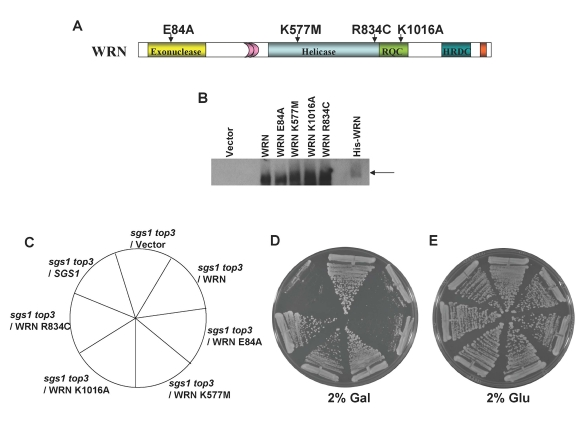
Figure 4. WRN ATPase/helicase, but not exonuclease activity, is required to restore the slow growth phenotype of top3 in sgs1 top3 background. Panel A, WRN
protein with conserved domains and positions of site-directed mutations. PanelB, Expression of WRN and WRN variants in transformed sgs1
top3 was induced at 2% gal concentration and cells were harvested after 6
h. Equal amount of total cell lysate was loaded on to 8-16% polyacrylamide
SDS gels, followed by Western blotting using anti-WRN antibody. sgs1 top3 strain
transformed with ATPase/helicase-dead (YEp195SpGAL-WRN K577M),
exonuclease-dead (YEp195SpGAL-WRN E84A), RQC mutant (YEp195SpGAL-WRN
K1016A), or polymorphic mutant (YEp195SpGAL-WRN R834C) was streaked on
SC-Trp plates containing either 2% glu (Panel E) or 2% gal (Panel
D). Plates were incubated at 30°C for 2 days and then
photographed. Composition of the plates was as in Panel C.
Genetic
analysis of a naturally occurring WRN missense polymorphism or engineered RQC
domain WRN missense mutant in the sgs1 top3 background
In
addition to the helicase domain, many RecQ helicases share another conserved
sequence C-terminal to the ATPase/helicase core domain designated the RecQ
C-terminal (RQC) region that has been implicated in DNA binding and protein
interactions [4]. To address
the potential importance of the WRN RQC domain for its biological function, we
examined the ability of a specific WRN RQC missense mutant, WRN-K1016A (Figure 4A), to restore the slow growth phenotype of top3 in sgs1 top3.
The WRN-K1016A mutant was previously characterized and shown to have
significantly reduced DNA binding and helicase activity compared to the
wild-type recombinant WRN protein [36]. sgs1
top3 expressing WRN-K1016A (Figure 4B) grew similarly to the sgs1 top3/vector
cells (Figure 4D), indicating that expression of the WRN-K1016A mutant failed
to restore the slow growth phenotype characteristic of the top3 mutant.
Although
no missense mutations have been identified that are genetically linked to
Werner syndrome, a number of WRN polymorphic missense variants exist whose
biological significance is not well understood. The lack of genetic data on
WRN polymorphisms suggested to us that the yeast-based WRN system might be
useful in their analysis. The WRN-834C polymorphism residing in the core
helicase domain wasidentified in DNA from the Polymorphism
Discovery Resource database (egp.gs.washington.edu)
(Figure 4A). Previously, it was reported by the Loeb lab that the purified
recombinant WRN-R834C protein has dramatically reduced WRN ATPase, helicase,
and helicase-coupled exonuclease activity [37]. To assess
the biological effect of the WRN R834C polymorphic change, sgs1 top3/WRN-R834C
was streaked onto a SC plate lacking Trp and containing 2% gal. Expression of
WRN-R834C (Figure 4B) demonstrated comparable growth to that of sgs1 top3/vector
(Figure 4D). These results demonstrate that the WRN-R834C polymorphism,
similar to the K577M and K1016A mutations that interfere with WRN helicase
activity, interfere with WRN function in the sgs1top3
background.
Previous studieshave shown that gene
expression levels from the GAL1/10 promotorcan be regulated
by altering the concentration of gal in thegrowth medium. We
therefore examined the level of WRN protein expression in cells
grown at lower gal concentrations (0-2%) in the presence of 2%
raffinose (raf). Western blot analyses demonstrated that WRN
expression was dependent on the concentration of gal in the media (Figure 5A).
Quantitative Western blot analyses using purified recombinant WRN
protein as standard indicated that 2.3 x 104WRN molecules/cell were present at 2% gal. As WRN expression was
regulated by the level of gal, we wanted to compare the ability of
WRN and its associated variants to
restore the slow growth phenotype of top3 in sgs1 top3 background
at lower levels of protein expression. WRN restored the slow growth phenotype of sgs1
top3 throughout the gal concentration range, including the lower gal
concentrations (Figure 5C-F). Similarly, WRN-E84A mutant restored the slow
growth top3 phenotype, whereas the ATPase/ helicase dead WRN-K577M
mutant failed to do so (Figure 5C-F). Likewise, neither WRN-R834C nor
WRN-K1016A affected the growth of the sgs1 top3 mutant (Figure 5C-F).
No effect on the growth rate of wild-type parental strain (W3031B) as well as sgs1
strain was observed with WRN or its associated variants (Supp. Data Figures 2 and
3). These results demonstrate that wild-type WRN or the WRN exonuclease dead
proteins are similarly able to restore the top3 growth phenotype in the sgs1
top3 background, whereas the WRN mutant proteins that have defective
ATPase/helicase activity did not affect the growth of the sgs1 top3
mutant at either low or high levels of gal-induced protein expression.
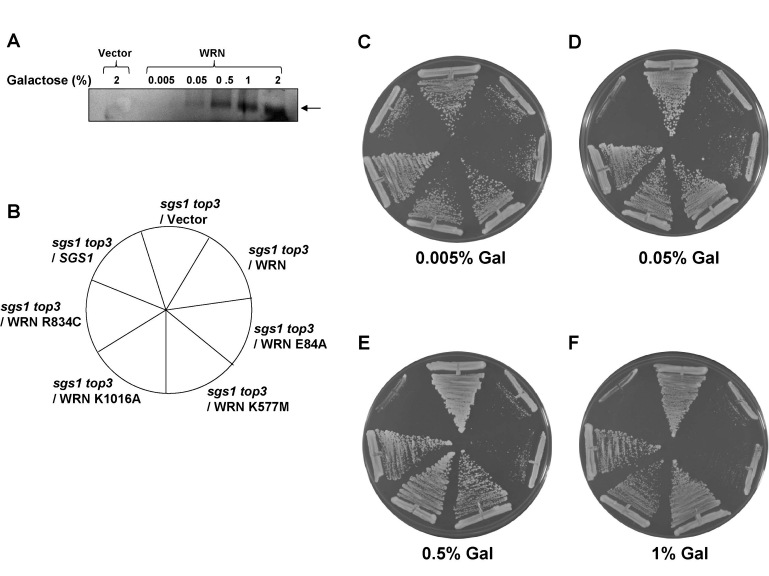
Figure 5. WRN mediated restoration of slow growth phenotype in sgs1 top3 background is independent of its expression level. WRN expression in the transformed sgs1 top3
cells was induced with the indicated gal concentrations and cells were
harvested 6 h after induction. As a control, sgs1 top3/YEp112SpGAL
was included. Equal amounts of total cell lysate were loaded on 8-16% polyacrylamide SDS gels followed by Western
blot detection using anti-WRN antibody as shown in PanelA.
sgs1 top3 strain transformed with YEp112SpGAL, YEp112SpGAL-WRN, exonuclease-dead
(YEp195SpGAL-WRN E84A), ATPase/helicase-dead (YEp195SpGAL-WRN K577M), RQC mutant (YEp195SpGAL-WRN K1016A),
polymorphic mutant(YEp195SpGAL-WRN R834C) and YEp112SpGAL-SGS1 was streaked on SC-Trp plate containing
gal at varying concentrations asindicated. Plates were incubated at 30°C for 2 days and then photographed.
Panels C-F show the effect of WRN expression onthe growth rate of sgs1 top3 transformed strains
at different gal concentrations. Composition of the plates was as in PanelB.
Effect
of WRN expression on the MMS and HU sensitivity of the sgs1 top3 mutant
Previously,
it was reported that the sgs1 top3 double mutant is less sensitive to
MMS or HU than the top3 single mutant [6]. Since WRN
affected cell growth of sgs1 top3, we next examined its effect on drug
sensitivity. As shown in Figure 6, sgs1 top3/WRN displayed sensitivity
to both MMS and HU that was comparable to sgs1 top3/SGS1, whereas sgs1
top3/vector was more resistant to either drug. Genetic analysis of the WRN
variants in the sgs1 top3 background
revealed that strains transformed with WRN-K577M, WRN-K1016A, or WRN-R834C displayed sensitivity
comparable to that of vector, whereas the sgs1 top3/WRN-E84A strain
showed HU and MMS resistance similar to sgs1 top/WRN and sgs1
top3/SGS1 (Figure 6). These results demonstrate that in addition to its
effect on growth in the sgs1 top3 background, WRN also affects
sensitivity of the sgs1 top3 mutant to HU or MMS. Furthermore, WRN
ATPase/helicase but not exonuclease activity is genetically required for its
effect on HU or MMS sensitivity in the sgs1 top3 background. Expression
of WRN in the wild-type parent strain had no effect on the strain's sensitivity
to the tested concentration of HU or MMS (Supp. Data Figure 4), indicating that
the effect of WRN expression was dependent on the sgs1 top3 genetic
background.
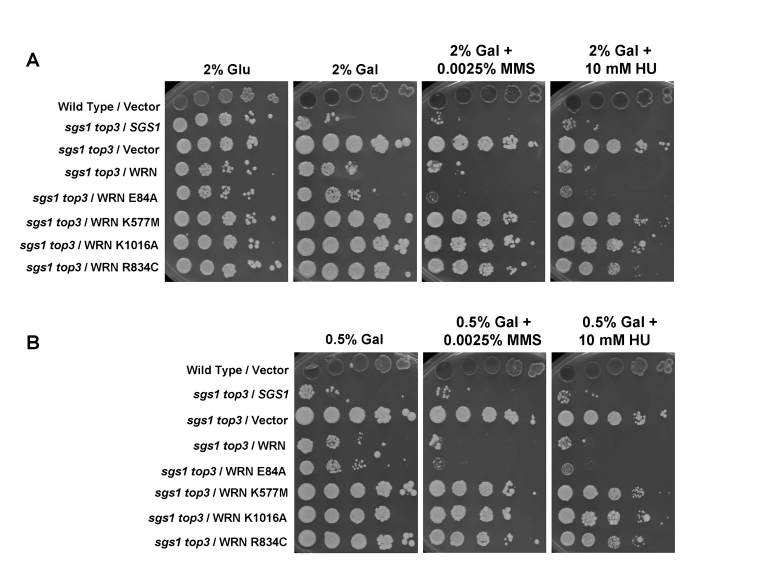
Figure 6. Effect of WRN expression on the MMS and HU sensitivity of sgs1 top3 strain. Logarithmically growing
cultures of sgs1 top3 strain transformed with YEp112SpGAL,
YEp112SpGAL-WRN, exonuclease-dead (YEp195SpGAL-WRN E84A),
ATPase/helicase-dead (YEp195SpGAL-WRN K577M), RQC mutant (YEp195SpGAL-WRN
K1016A), polymorphic mutant (YEp195SpGAL-WRN R834C), YEp112SpGAL-SGS1 and
vector transformed wild type parental strains were spotted in a ten-fold
serial dilutions onto SC-Trp plates containing glu or gal and either MMS or
HU at the indicated concentrations. Plates were incubated at 30°C for 2
days (control plates) and 4 days (MMS and HU plates) and then photographed.
Discussion
From
our studies, we conclude that WRN genetically interacts with Top3 through its
ability to restore the top3 slow growth phenotype in the sgs1 top3
background. WRN helicase activity is required for genetic restoration of slow
growth, similar to previous studies which demonstrate that sgs1 helicase
defective alleles fail to suppress top3 slow growth [38,39].
Although WRN suppressed the growth in the sgs1 top3 double mutant, WRN
did not rescue the sensitivity of sgs1 to the DNA damaging agent MMS or
the replication inhibitor HU. Thus, WRN cannot directly replace the Sgs1
helicase in the cellular response to replicational stress. However, in a
pathway defined by genetic background, human WRN expressed in yeast can clearly
exert a phenotype. The ability of WRN to affect the growth phenotype in the sgs1 top3 background is supported by our observation that the morphological
appearance of large budded cells with undivided nuclei characteristic of S/G2
arrest is restored in the WRN-transformed sgs1 top3 mutant. This work
is the first demonstration that WRN can function in a genetic pathway that
affects top3-related phenotypes, and this role is dependent on
WRN helicase but not exonuclease activity.
Previous studies demonstrated that
expression of WRN or BLM in sgs1 does not affect growth rate, but can
partially suppress illegitimate recombination or homologous recombination [40]. However,
a distinction in the biological functions between the two human RecQ helicases
was suggested based on the observation that expression of BLM, but not WRN, can
restore the resistance of the sgs1 mutant to the replication inhibitor
HU [40] and extend
the shortened lifespan caused by the sgs1 mutation [41]. It was
also reported that human BLM, but not WRN, can suppress the growth in the sgs1
top3 double mutant [40], suggesting
that yeast Sgs1 helicase has functions similar to those of human BLM helicase.
Although we also observed that WRN expression failed to correct the HU
sensitivity of the sgs1 mutant, we found that WRN expression can restore
the top3 slow growth phenotype in the sgs1 top3 background. The
difference between our study and the earlier one may reflect differences in WRN
protein expression (since the earlier study did not report a quantitative level
of WRN protein), strains, or yeast culture conditions. Our quantitative
Western blot analyses demonstrate that WRN expressed in yeast at levels
comparable to that previously reported in several human cell lines was
sufficient to restore growth in the sgs1 top3 mutant as detected by
plate streak studies or liquid culture growth analysis. From these results, we conclude that WRN can function in the sgs1
top3 background, indicating some genetic overlap between the WRN and Sgs1
pathways. By inference, WRN may also have overlapping genetic functions with
BLM. Although WRN was not observed in vitro to substitute in the BLM-Top3α complex double HJ dissolution reaction (see Introduction), it
is plausible that WRN interacts with Top3α in a related
protein complex with additional factors and can perform a function important
for genomic stability. The results from our sgs1 top3 WRN
complementation studies in yeast prompt further investigation of the
possibility that WRN functionally interacts with Top3α in human cells during cellular DNA replication or recombination.
Conceivably, in a BLM-impaired condition, WRN may partially substitute for BLM
through its protein partnership with a topoisomerase.
In
a previous study, we found that human WRN expressed in a yeast dna2-1 mutant
background can rescue the associated replication and repair phenotypes;
however, a non-catalytic C-terminal domain of WRN was sufficient for genetic
rescue, demonstrating that WRN helicase activity is not required to rescue the
mutant cellular phenotypes associated with the dna2-1 mutant [31]. Based on
genetic and biochemical results, the explanation for this finding was that WRN
rescues the dna2-1 mutant by interacting with endogenous yeast Flap
Endonuclease-1 (FEN-1) and stimulating its nuclease activity, thereby bypassing
the requirement for wild-type DNA2 nuclease to process DNA replication and
repair intermediates [31,42]. In
contrast, we show in the current study that WRN helicase activity is required
to suppress growth in the sgs1 top3 mutant. The distinct genetic
requirements for WRN to rescue different mutant backgrounds suggest that WRN
can operate in separate pathways using different catalytic or protein
interaction domains. We propose that WRN is a modular pleiotropic protein with
unique catalytic or protein interaction domains that are necessary to fulfill
its functions in different biological settings dictated by mutant genetic
background.
In
addition to the yeast studies which have examined the genetic interactions
between sgs1 and top3, the potential functional overlap of Sgs1
with other topo-isomerases has been investigated. For example, sgs1 top1 mutants
are severely growth impaired, suggesting that the synergistic defect can be
attributed to the two genes operating in separate but overlapping pathways.
This is distinct from the epistatic relationship of sgs1 and top3.
Based on their genetic findings, Weinstein and Rothstein proposed that a subset
of DNA structures which arise at stalled or collapsed replication forks
normally processed by the Sgs1-Top3 pathway can be alternatively channeled into
a Top1-dependent pathway in the absence of active Sgs1 helicase activity [39]. It is
conceivable that WRN, which interacts physically and functionally with human
TOP1 [43], may
collaborate with Top1 (or another topoisomerase) in yeast to generate a viable
but poorly growing cell in a pathway parallel to Sgs1. Alternatively, WRN may
directly substitute for Sgs1 only in a specialized situation when Top3 is
absent. It is conceivable that the poor growth of the top3 mutant is
attributed to unresolved recombinogenic DNA intermediates created by WRN
helicase activity. This last idea underscores a growing notion in the field
that genetic recombination must be properly regulated; otherwise, deleterious
recombination events acting on aberrant DNA structures prevail [44]. Thus,
RecQ helicase-mediated recombination pathways are like a double-edged sword.
In the appropriate genetic background, these pathways secure a normal growth
rate and genomic stability; however, in certain mutant backgrounds (e.g., top3),
DNA unwinding by a RecQ helicase is counter-productive to cell growth and
genome homeostasis.
Materials and Methods
Plasmid DNA constructs.
Site-directed
mutations of WRN (E84A, K577M, R834C, and K1016A) in the plasmid
YEp195SpGAL-WRN [31] were constructed using mutagenic primers
(Supplementary Table 1) and a standard protocol from Quickchange II XL site-directed
mutagenesis kit (Stratagene) by Lofstrand labs (Gaithersburg, MD). DNA fragments encoding WRN and its associated
variants were gel purified from the respective YEp195SpGAL-WRN constructs after
double digestion with SalI and MluI. Gel purified fragments were
then cloned into the SalI-MluI sites of vector
YEp112SpGAL [45], a
2 μm multi-copyplasmid containing a TRP1 selectable
marker to construct YEp112SpGAL-WRNor WRN variants under the
control ofa gal-inducible promotor. The SGS1 expression
plasmid pSGS1f2 was kindly provided by Dr. Brad Johnson (University
of Pennsylvania School of Medicine, Philadelphia, Pennsylvania). SGS1
was PCR amplified using 5'-ACGCGTCGACATGGTGACGAA GC-3' and 5'-GTCTCCTTCACTACGCGTCGAAT-3' as the forward
and the reverse primers, respectively, using pSGS1f2 as a template. PCR was
carried out with PCR super mix HiFi (Invitrogen) for 30 cycles (denaturation at
94°C for 30 s per cycle, annealing at 57°C for 50 s per cycle, and elongation
at 72°C for 5 min per cycle). The amplified product was cloned into the SalI-MluI
sites of vector YEp112SpGAL.
Yeast media and strains.
Strains
with wild-type SGS1 TOP3 (WT; W303-1A, genotype, MATa ade2-1 canl- 100 his3-11,15 leu2-3,112 trpl-l ura3-1) [5], a sgs1 mutant
(W1292-3C; genotype MATa SUP4-o::URA3 sgs1-25
ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 rad5-535) and a sgs1
top3 mutant ( W1058-11C, genotype, MATa SUP4-o::URA3
sgs1-25 top3-2::HIS3 ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1
rad5-535) have been characterized [6] and were
kindly provided by Dr. Rodney Rothstein (Columbia University). Yeast cultures
were grown using standard protocol and transformations were performed using a
Lithium Acetate-based protocol [46].
Transformed yeast strains were grown in SC media minus Trp and containing
either glucose (glu) or gal as required.
Genetic analysis of hydroxyurea or methylmethane-sulfonate
sensitivity in WRN transformed yeast strains.
To determine the effect of WRN expression on the MMS and
HU sensitivity of sgs1 or sgs1 top3 strains, strains transformed
with YEp112SpGAL or YEp112SpGAL-WRN were grown in SC raf minus Trp at 30oC.
Cultures were reinoculated in SC raf minus Trp and grown at 30°C to early log
phase (OD600 of ~0.6 to 0.8). Ten-fold serial dilutions of these
strains were spotted onto SC gal minus Trp plates containing the indicated
concentrations of HU or MMS. Plates were incubated at 30°C. As a control,
wild-type parental strain (W303-1A) transformed with YEp112SpGAL or
YEp112SpGAL-WRN, or sgs1 top3/ YEp112SpGAL-SGS1 was treated as
described above.
Genetic analysis of the slow growth phenotype in WRN transformed sgs1 top3 strain.
To examine the effect of WRN expression on
the growth of wild-type parental (W303-1A), sgs1, or sgs1 top3
strains, the corresponding strains transformed with YEp112SpGAL or
YEp112SpGAL-WRN were streaked onto SC minus Trp plates containing either glu or
gal as indicated. As a control, sgs1 top3/YEp112SpGAL-SGS1 was
included. Plates were incubated at 30°C. To determine the ability of WRN to
affect growth rate of sgs1 top3 strain at lower WRN protein expression
levels, the sgs1 top3 strain transformed with YEp112SpGAL,
YEp112SpGAL-WRN, or YEp112SpGAL-SGS1 was streaked onto SC minus Trp
plates containing varying concentrations of gal as indicated.
To assess the functional requirements of WRN
to restore the slow growth phenotype in the sgs1 top3 background, sgs1
top3 transformed with YEp112SpGAL-WRN (E84A, K577M, R834C, and K1016A) were
streaked onto SC minus Trp plates containing either glu or gal as indicated.
For controls, sgs1 top3 strain transformed with YEp112SpGAL, YEp112SpGAL-WRN
or YEp112Sp GAL-SGS1 was included.
To evaluate the growth of transformed sgs1 top3 strains in
liquid culture, yeast cells were grown in SC raf minus Trp at 30oC.
Cultures were reinoculated in SC raf minus Trp and grown at 30°C to early log
phase (A
600 of ~0.5). Cultures were then reinnoculated at
OD0.05 in media containing 2% gal and their growth was followed by
measuring absorbance at the indicated time intervals.
Cell
cycle distribution of WRN transformed sgs1 top3 strain.
Logarithmically growing cultures of sgs1 top3
transformed with YEp112SpGAL, YEp112SpGAL-WRN, or YEp112SpGAL-SGS1, and the
vector-transformed wild-type parental strain were induced at 2% gal
concentration for 6 h. For DAPI (4', 6-diamidino-2-phenylindole)
staining, 70% ethanolfixed cells were washed with phosphate-buffered
saline (PBS), stained and mounted in Vectashield mounting medium
with DAPI (1 μg/ml) (Vector,USA). Cells were examined with an
Axiovert 200 M microscope(Zeiss; 100x lens) and
composite differential interference contrast(DIC) and fluorescence
images were analyzed using AxioVision,version 3.0 program (Zeiss).
Western
blot analyses.
Transformed sgs1 or sgs1 top3 strains
were grown in SC raf minus Trp at 30oC to an OD600 of
0.5. Cultures were then induced at the indicated gal concentration for 6 h.
Cells (3 ml) were collected by centrifugation, washed with PBS, lysed in
alkaline lysis buffer [50 mM NaOH, pH 10.5, 2 mM EDTA, 1 mM
phenylmethylsulfonyl fluoride (PMSF), 2% SDS, 10% glycerol, 5%
2-mercaptoethanol and protease inhibitors (Roche Molecular Biochemicals)],
boiled for 5 min, clarified by centrifugation, and neutralized with 1 M HCl.
Proteins from equivalent amounts of cell lysate were resolved on 8-16%
polyacrylamide SDS gels. Expression of WRN or WRN mutant proteins was determined
by Western blot using a WRN mouse monoclonal antibody directed against an
epitope in a purified C-terminal fragment of WRN [47] (1:1000,
Spring Valley Labs). For quantitative Western blot analysis, increasing
concentrations of purified recombinant His-tagged full-length WRN protein were
included on gels with yeast lysate samples.
Supplementary Materials
WRN fails to rescue the MMS and HU sensitivity of sgs1. Cultures of wild-type parental
strain (W303-1A) or sgs1 strain transformed with YEp112SpGAL or
YEp112SpGAL-WRN were grown to early log phase (OD
600 of ~0.6 to 0.8). Ten-fold
serial dilutions of these cultures were spotted onto SC-Trp plates
containing 0.5% gal and either MMS or HU at the indicated concentrations.
Plates were incubated at 30°C for 3 days (control plates) and 5 days (MMS
or HU plates) and then photographed.
WRN expression has no effect on the growth rate of wild type parental strain W303-1A. Wild type parental strain
W303-1A transformed with YEp112SpGAL, YEp112SpGAL-WRN, exonuclease-dead
(YEp195SpGAL-WRN E84A), ATPase/helicase-dead (YEp195SpGAL-WRN K577M), RQC
mutant (YEp195SpGAL-WRN K1016A) and polymorphic mutant (YEp195SpGAL-WRN
R834C) was streaked on SC-Trp plate containg either 2% glu (Panel B)
or galactose at varying concentrations as indicated (Panel C-G).
Plates were incubated at 30°C for 2 days and then photographed.
Composition of the plates was as in Panel A.
WRN expression has no effect on the growth rate of sgs1 strain. sgs1 strain transformed
with YEp112SpGAL, YEp112SpGAL-WRN, exonuclease-dead (YEp195SpGAL-WRN E84A),
ATPase/helicase-dead (YEp195SpGAL-WRN K577M), RQC mutant (YEp195SpGAL-WRN
K1016A) and polymorphic mutant (YEp195SpGAL-WRN R834C) was streaked on SC-Trp
plate containing either 2% glu (Panel B) or gal at varying
concentrations as indicated (Panel C-G). Plates were
incubated at 30°C for 4 days and then photographed. Composition of the
plates was as in Panel A.
Effect of WRN expression on the MMS and HU sensitivity of wild-type parental strain W303-1A. Logarithmically growing
cultures wild type parental strain transformed with YEp112SpGAL or
YEp112SpGAL-WRN was spotted in a ten-fold serial dilutions onto SC-Trp
plates containing glu or gal and either MMS or HU at the indicated
concentrations. Plates were incubated at 30°C for 2 days.
Oligonucleotides used for WRN site-directed mutagenesis.
Acknowledgments
This work was supported in full by the
Intramural Research program of the NIH, National Institute on Aging. We thank Dr.
Rodney Rothstein (Columbia University) for the yeast strains and Dr. Brad
Johnson (University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania) for the SGS1 expression plasmid. We thank Dr. Jian Lu
(Laboratory of Molecular Gerontology, NIA-NIH) for helpful comments on the
manuscript.
Conflicts of Interest
The
authors in this manuscript have no conflict of interests to declare.
References
-
1.
Prince
PR
, Emond
MJ
and Monnat
RJ Jr.
Loss of Werner syndrome protein function promotes aberrant mitotic recombination.
Genes Dev.
2001;
15:
933
-938.
[PubMed]
.
-
2.
Saintigny
Y
, Makienko
K
, Swanson
C
, Emond
MJ
and Monnat
RJ Jr.
Homologous recombination resolution defect in Werner syndrome.
Mol Cell Biol.
2002;
22:
6971
-6978.
[PubMed]
.
-
3.
Brosh
RM Jr
and Bohr
VA.
Human premature aging, DNA repair and RecQ helicases.
Nucleic Acids Res.
2007;
35:
7527
-7544.
[PubMed]
.
-
4.
Sharma
S
, Doherty
KM
and Brosh
RM Jr.
Mechanisms of RecQ helicases in pathways of DNA metabolism and maintenance of genomic stability.
Biochem J.
2006;
398:
319
-337.
[PubMed]
.
-
5.
Gangloff
S
, McDonald
JP
, Bendixen
C
, Arthur
L
and Rothstein
R.
The yeast type I topoisomerase Top3 interacts with Sgs1, a DNA helicase homolog: a potential eukaryotic reverse gyrase.
Mol Cell Biol.
1994;
14:
8391
-8398.
[PubMed]
.
-
6.
Shor
E
, Gangloff
S
, Wagner
M
, Weinstein
J
, Price
G
and Rothstein
R.
Mutations in homologous recombination genes rescue top3 slow growth in Saccharomyces cerevisiae.
Genetics.
2002;
162:
647
-662.
[PubMed]
.
-
7.
Bennett
RJ
and Wang
JC.
Association of yeast DNA topoisomerase III and Sgs1 DNA helicase: studies of fusion proteins.
Proc Natl Acad Sci U S A.
2001;
98:
11108
-11113.
[PubMed]
.
-
8.
Fricke
WM
, Kaliraman
V
and Brill
SJ.
Mapping the DNA topoisomerase III binding domain of the Sgs1 DNA helicase.
J Biol Chem.
2001;
276:
8848
-8855.
[PubMed]
.
-
9.
Ira
G
, Malkova
A
, Liberi
G
, Foiani
M
and Haber
JE.
Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast.
Cell.
2003;
115:
401
-411.
[PubMed]
.
-
10.
Mankouri
HW
and Hickson
ID.
Understanding the roles of RecQ helicases in the maintenance of genome integrity and suppression of tumorigenesis.
Biochem Soc Trans.
2004;
32:
957
-958.
[PubMed]
.
-
11.
Ahn
JS
, Osman
F
and Whitby
MC.
Replication fork blockage by RTS1 at an ectopic site promotes recombination in fission yeast.
EMBO J.
2005;
24:
2011
-2023.
[PubMed]
.
-
12.
Harmon
FG
, DiGate
RJ
and Kowalczykowski
SC.
RecQ helicase and topoisomerase III comprise a novel DNA strand passage function: a conserved mechanism for control of DNA recombination.
Mol Cell.
1999;
3:
611
-620.
[PubMed]
.
-
13.
Harmon
FG
, Brockman
JP
and Kowalczykowski
SC.
RecQ helicase stimulates both DNA catenation and changes in DNA topology by topoisomerase III.
J Biol Chem.
2003;
278:
42668
-42678.
[PubMed]
.
-
14.
Hartung
F
, Suer
S
, Knoll
A
, Wurz-Wildersinn
R
and Puchta
H.
Topoisomerase 3alpha and RMI1 suppress somatic crossovers and are essential for resolution of meiotic recombination intermediates in Arabidopsis thaliana.
PLoS Genet.
2008;
4:
e1000285
[PubMed]
.
-
15.
Win
TZ
, Mankouri
HW
, Hickson
ID
and Wang
SW.
A role for the fission yeast Rqh1 helicase in chromosome segregation.
J Cell Sci.
2005;
118:
5777
-5784.
[PubMed]
.
-
16.
Wu
L
and Hickson
ID.
The Bloom's syndrome helicase suppresses crossing over during homologous recombination.
Nature.
2003;
426:
870
-874.
[PubMed]
.
-
17.
Raynard
S
, Bussen
W
and Sung
P.
A double Holliday Junction dissolvasome comprising BLM, Topoisomerase IIIa and BLAP75.
J Biol Chem.
2006;
281:
13861
-13864.
[PubMed]
.
-
18.
Wu
L
, Bachrati
CZ
, Ou
J
, Xu
C
, Yin
J
, Chang
M
, Wang
W
, Li
L
, Brown
GW
and Hickson
ID.
BLAP75/RMI1 promotes the BLM-dependent dissolution of homologous recombination intermediates.
Proc Natl Acad Sci USA.
2006;
103:
4068
-4073.
[PubMed]
.
-
19.
Wu
L
, Chan
KL
, Ralf
C
, Bernstein
DA
, Garcia
PL
, Bohr
VA
, Vindigni
A
, Janscak
P
, Keck
JL
and Hickson
ID.
The HRDC domain of BLM is required for the dissolution of double Holliday junctions.
EMBO J.
2005;
24:
2679
-2687.
[PubMed]
.
-
20.
Bussen
W
, Raynard
S
, Busygina
V
, Singh
AK
and Sung
P.
Holliday junction processing activity of the BLM-Topo IIIalpha-BLAP75 complex.
J Biol Chem.
2007;
282:
31484
-31492.
[PubMed]
.
-
21.
Singh
TR
, Ali
AM
, Busygina
V
, Raynard
S
, Fan
Q
, Du
CH
, Andreassen
PR
, Sung
P
and Meetei
AR.
BLAP18/RMI2, a novel OB-fold-containing protein, is an essential component of the Bloom helicase-double Holliday junction dissolvasome.
Genes Dev.
2008;
22:
2856
-2868.
[PubMed]
.
-
22.
Xu
D
, Guo
R
, Sobeck
A
, Bachrati
CZ
, Yang
J
, Enomoto
T
, Brown
GW
, Hoatlin
ME
, Hickson
ID
and Wang
W.
RMI, a new OB-fold complex essential for Bloom syndrome protein to maintain genome stability.
Genes Dev.
2008;
22:
2843
-2855.
[PubMed]
.
-
23.
Johnson
FB
, Lombard
DB
, Neff
NF
, Mastrangelo
MA
, Dewolf
W
, Ellis
NA
, Marciniak
RA
, Yin
Y
, Jaenisch
R
and Guarente
L.
Association of the Bloom syndrome protein with topoisomerase IIIa in somatic and meiotic cells.
Cancer Res.
2000;
60:
1162
-1167.
[PubMed]
.
-
24.
Shimamoto
A
, Nishikawa
K
, Kitao
S
and Furuichi
Y.
Human RecQ5b, a large isomer of RecQ5 DNA helicase, localizes in the nucleoplasm and interacts with topoisomerases 3a and 3b.
Nucleic Acids Res.
2000;
28:
1647
-1655.
[PubMed]
.
-
25.
Hu
Y
, Lu
X
, Barnes
E
, Yan
M
, Lou
H
and Luo
G.
Recql5 and Blm RecQ DNA helicases have nonredundant roles in suppressing crossovers.
Mol Cell Biol.
2005;
25:
3431
-3442.
[PubMed]
.
-
26.
Sharma
S
, Stumpo
DJ
, Balajee
AS
, Bock
CB
, Lansdorp
PM
and Brosh
RM Jr.
, Blackshear PJ. RECQL, a member of the RecQ family of DNA helicases, suppresses chromosomal instability.
Mol Cell Biol.
2007;
27:
1784
-1794.
[PubMed]
.
-
27.
Sharma
S
and Brosh
RM Jr.
Human RECQ1 is a DNA damage responsive protein required for genotoxic stress resistance and suppression of sister chromatid exchanges.
PLoS ONE.
2007;
2:
e1297
[PubMed]
.
-
28.
Sharma
S
and Brosh
RM Jr.
Unique and important consequences of RECQ1 deficiency in mammalian cells.
Cell Cycle.
2008;
7:
989
-1000.
[PubMed]
.
-
29.
Fukuchi
K
, Martin
GM
and Monnat
RJ Jr.
Mutator phenotype of Werner syndrome is characterized by extensive deletions.
Proc Natl Acad Sci U S A.
1989;
86:
5893
-5897.
[PubMed]
.
-
30.
Kudlow
BA
, Kennedy
BK
and Monnat
RJ Jr.
Werner and Hutchinson-Gilford progeria syndromes: mechanistic basis of human progeroid diseases.
Nat Rev Mol Cell Biol.
2007;
8:
394
-404.
[PubMed]
.
-
31.
Sharma
S
, Sommers
JA
and Brosh
RM Jr.
In vivo function of the conserved non-catalytic domain of Werner syndrome helicase in DNA replication.
Hum Mol Genet.
2004;
13:
2247
-2261.
[PubMed]
.
-
32.
Kawabe
T
, Tsuyama
N
, Kitao
S
, Nishikawa
K
, Shimamoto
A
, Shiratori
M
, Matsumoto
T
, Anno
K
, Sato
T
, Mitsui
Y
, Seki
M
, Enomoto
T
, Goto
M
, Ellis
NA
, Ide
T
, Furuichi
Y
and Sugimoto
M.
Differential regulation of human RecQ family helicases in cell transformation and cell cycle.
Oncogene.
2000;
19:
4764
-4772.
[PubMed]
.
-
33.
Moser
MJ
, Kamath-Loeb
AS
, Jacob
JE
, Bennett
SE
, Oshima
J
and Monnat
RJ Jr.
WRN helicase expression in Werner syndrome cell lines.
Nucleic Acids Res.
2000;
28:
648
-654.
[PubMed]
.
-
34.
Brosh
RM Jr
, Orren DK, Nehlin JO, Ravn PH, Kenny MK, Machwe A, Bohr VA. Functional and physical interaction between WRN helicase and human Replication Protein A.
J Biol Chem.
1999;
274:
18341
-18350.
[PubMed]
.
-
35.
Huang
S
, Li
B
, Gray
MD
, Oshima
J
, Mian
IS
and Campisi
J.
The premature ageing syndrome protein, WRN, is a 3'-->5' exonuclease.
Nat Genet.
1998;
20:
114
-116.
[PubMed]
.
-
36.
Lee
JW
, Kusumoto
R
, Doherty
KM
, Lin
GX
, Zeng
W
, Cheng
WH
, von
Kobbe C
, Brosh
RM Jr
, Hu
JS
and Bohr
VA.
Modulation of Werner syndrome protein function by a single mutation in the conserved RecQ domain.
J Biol Chem.
2005;
280:
39627
-39636.
[PubMed]
.
-
37.
Kamath-Loeb
AS
, Welcsh
P
, Waite
M
, Adman
ET
and Loeb
LA.
The enzymatic activities of the Werner syndrome protein are disabled by the amino acid polymorphism R834C.
J Biol Chem.
2004;
279:
55499
-55505.
[PubMed]
.
-
38.
Mullen
JR
, Kaliraman
V
and Brill
SJ.
Bipartite structure of the SGS1 DNA helicase in Saccharomyces cerevisiae.
Genetics.
2000;
154:
1101
-1114.
[PubMed]
.
-
39.
Weinstein
J
and Rothstein
R.
The genetic consequences of ablating helicase activity and the Top3 interaction domain of Sgs1.
DNA Repair (Amst).
2008;
7:
558
-571.
[PubMed]
.
-
40.
Yamagata
K
, Kato
J
, Shimamoto
A
, Goto
M
, Furuichi
Y
and Ikeda
H.
Bloom's and Werner's syndrome genes suppress hyperrecombination in yeast sgs1 mutant: implication for genomic instability in human diseases.
Proc Natl Acad Sci USA.
1998;
95:
8733
-8738.
[PubMed]
.
-
41.
Heo
SJ
, Tatebayashi
K
, Ohsugi
I
, Shimamoto
A
, Furuichi
Y
and Ikeda
H.
Bloom's syndrome gene suppresses premature ageing caused by Sgs1 deficiency in yeast.
Genes Cells.
1999;
4:
619
-625.
[PubMed]
.
-
42.
Sharma
S
, Sommers
JA
and Brosh
RM Jr.
Processing of DNA replication and repair intermediates by the concerted action of RecQ helicases and Rad2 structure-specific nucleases.
Protein Pept Lett.
2008;
15:
89
-102.
[PubMed]
.
-
43.
Laine
JP
, Opresko
PL
, Indig
FE
, Harrigan
JA
, von
Kobbe C
and Bohr
VA.
Werner protein stimulates topoisomerase I DNA relaxation activity.
Cancer Res.
2003;
63:
7136
-7146.
[PubMed]
.
-
44.
Sung
P
and Klein
H.
Mechanism of homologous recombination: mediators and helicases take on regulatory functions.
Nat Rev Mol Cell Biol.
2006;
7:
739
-750.
[PubMed]
.
-
45.
Clark
AB
, Cook
ME
, Tran
HT
, Gordenin
DA
, Resnick
MA
and Kunkel
TA.
Functional analysis of human MutSa and MutSb complexes in yeast.
Nucleic Acids Res.
1999;
27:
736
-742.
[PubMed]
.
-
46.
Gietz
RD
, Schiestl
RH
, Willems
AR
and Woods
RA.
Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure.
Yeast.
1995;
11:
355
-360.
[PubMed]
.
-
47.
von
Kobbe C
, Thoma
NH
, Czyzewski
BK
, Pavletich
NP
and Bohr
VA.
Werner syndrome pretein contains three structure specific DNA binding domains.
J Biol Chem.
2003;
52997
-53006.
[PubMed]
.