Introduction
Werner Syndrome (WS) is an
autosomal recessive segmental aging disorder associated with a marked
predisposition to cancer and vascular disease [1,2]. The
first signs of this disorder appear after puberty and the disease is usually
diagnosed in individuals 20 to 30 years of age. WS patients show increased
predisposition to diseases observed during normal aging such as
arteriosclerosis, osteoporosis, type II diabetes mellitus and a variety of
tumors, primarily of mesenchymal origin [1,3].
Myocardial infarction (MI) and cancer are the most common causes of death among
WS patients, with a median age of death of approximately 47 years.
WS
is caused by mutations in the gene that encodes for the Werner syndrome protein
(WRN), a protein that belongs to the class of enzymes termed RecQ helicases [4,5]. In
contrast to other members of this family of helicases, WRN has an exonuclease
domain, which is highly homologous to the nuclease domain of E. coli DNA
polymerase I and ribonuclease D (RNase D) [6]. Helicase
and exonuclease activities with a 3' to 5' directionality have been
demonstrated in vitro using recombinant WRN [7-14]. WRN can
unwind and/or hydrolyze a number of different DNA structures, from linear
duplex DNA to single stranded regions of flap DNA substrates to synthetic
replication forks and Holliday junctions [15,16]. WRN
exonuclease is highly processive on substrates with a recessed 3' end
structure, an activity that is independent of 5' overhang length and nucleotide
sequence. In addition, WRN displays weak length-dependent degradation of 3'
overhang DNA substrates, which is independent of nucleotide sequence [17].
The
presence of helicase and exonuclease activities have suggested that WRN may
function in the processing of pathological DNA ends during DNA repair and/or
recombination [18]. Moreover,
since a subpopulation of WRN is localized at telomeres, it has also been
proposed that natural DNA ends are genuine substrates of this enzyme [19,20]. Indeed,
WRN has been shown to bind TRF2 and POT1 [21-23], two
telomere-specific proteins, and in vitro studies have indicated that
both TRF2 and POT1 stimulate WRN helicase activity on substrates that mimic
telomere ends [20,22].
However, while TRF2 has been reported to either stimulate or repress WRN
exonuclease activity [20,21], POT1
does not appear to influence this activity, at least in the context of the
substrates tested in these studies. Significantly, recent studies have
indicated that WRN is important for maintaining the G-rich lagging strand of
telomeric DNA [19,20] and our
own work has demonstrated that WRN is required for proper telomere homeostasis
by preventing the formation of extrachromosomal telomeric circles [24].
Human
telomeres are composed of several kilobases of the repetitive hexamer TTAGGG
and contain a 3' single-stranded DNA extension that is thought to loop back and
invade the proximal complementary strand thereby leading to the establishment
of a protective structure termed telomeric-loop (t-loop)[25-27]. The 3'
overhang is generated either by removal of the RNA primer from the newly
replicated lagging strand or by nucleolytic attach in the 5' to 3' direction
after replication of the leading strand. In telomerase-positive cells, the 3'
overhang is utilized as template by the RNA subunit of telomerase to extend
telomeres.
Telomere homeostasis is maintained by a
multiprotein complex that includes telomere-specific proteins including
telomere repeat factors 1 and 2 (TRF1 and TRF2), which binds telomeres through
a myb-like DNA binding domain located at the carboxyl-terminal end [28-30], and
protection of telomeres (POT1), a G-strand specific single-stranded DNA binding
protein [31]. These
proteins are though to protect telomeres from end-to-end fusion and intra- or
inter-telomeric recombination events [32,33].
Disruption of this protective structure activates a DNA damage response pathway
and leads to cell cycle arrest, cell senescence or apoptosis [34-37].
To
gain mechanistic insights on the function of WRN at telomeres, we have carried
out exonuclease assays utilizing telomeric templates bearing a 3' G-rich
overhang. Interestingly, we found that the 3' overhang of telomeric but not
non-telomeric DNA substrates is specifically hydrolyzed by WRN exonuclease in
vitro. 3' to 5' processing of the telomeric DNA substrates by WRN
exonuclease activity is limited to nucleotides within the single-stranded
region of the substrate, does not depends on the length of the single-stranded
region and does not require ATPase or helicase activities. Notably, resection
of the 3' overhang is precisely dependent on the presence of bona-fide
telomeric repeat sequences in both the double and single-stranded regions of
the DNA molecule, as any modification within the substrate that alters the
TTAGGG repeat unit results in the complete inhibition of DNA processing.
Importantly, sequence-specific processing of the telomeric substrate is
inhibited by the single-stranded telomeric DNA binding protein POT1, suggesting
a functional interplay between this protein and WRN in the homeostasis of the
telomeric 3' overhang.
Results
Limited processing of
telomeric DNA substrates with a 3' G-rich overhang by WRN exonuclease in vitro
In
previous studies, we and others have demonstrated that standard double-stranded
DNA substrates with a 3' overhang are not significantly processed by WRN
exonuclease in vitro [12,13,38,39].
Yet, the presence of WRN at telomeres prompted us to test whether telomeric
sequences with a 3' single-stranded overhang are particularly susceptible to
WRN-mediated processing. For this purpose, non-telomeric or telomeric DNA
substrates with a 15 nucleotides 3' overhang were incubated with increasing
amounts of purified WRN in cell-free exonuclease assays. DNA products from
these reactions were separated by denaturing polyacrylamide gel electrophoresis
and visualized by autoradiography. In agreement with our prior data [38], a
non-telomeric double-stranded DNA substrate with a 3' overhang is not degraded
by WRN (Figure 1). In contrast, we observed that WRN exonuclease removes
several nucleotides from most of the 3' overhangs of the telomeric substrate.
Notably, 3' processing of the telomeric substrate is not highly processive, as
exonuclease activity slows down as it moves across the GGG trinucleotide
repeats and in proximity of the junction between single-stranded and double-stranded DNA,
and produces weaker degradation further inward (Figure 1 and Supplementary Figure 1). Thus, the majority
of the processing occurs within the single-stranded region of the DNA substrate
leading to the generation of products with a shorter
3' overhang. This striking profile of DNA proces- sing
is strictly dependent on a functional WRN, since degradation of the 3' overhang
from the telomeric substrate is abolished when DNA is incubated with a mutant
WRN lacking exonuclease activity (WRN-D82A).
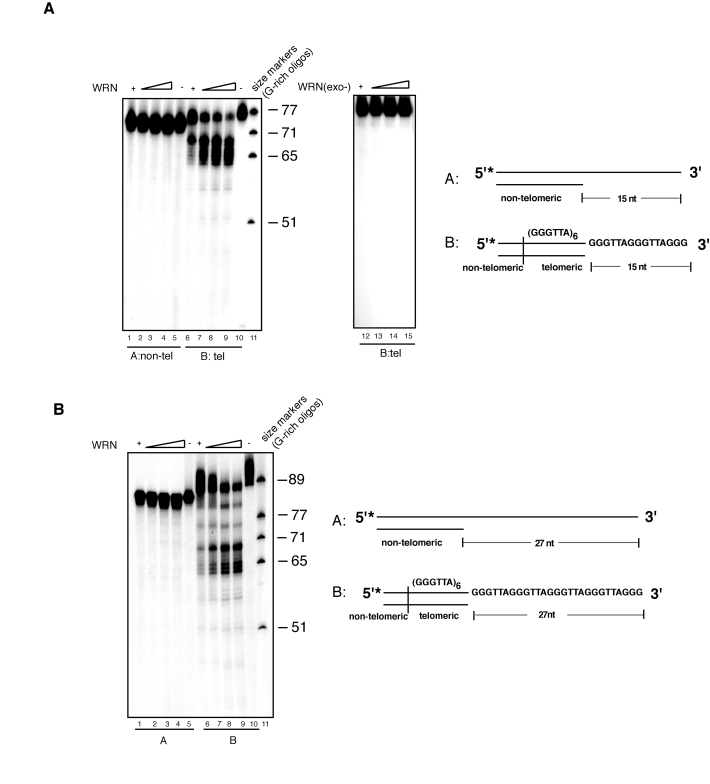
Figure 1. WRN exonuclease resects the 3' single-stranded overhang of telomeric DNA substrates. (A) 100 to 400 fmol of purified
recombinant wild-type WRN or exonuclease mutant WRN (WRN D82A) were
incubated with 5'-32P-labeled, 15 nt 3'-overhang DNA substrates
containing non-telomeric sequences (lanes 1-5) or telomeric (TTAGGG)
repeats (lanes 6-10) at 37°C for 10 min. The reaction products were
analyzed by 12% polyacrylamide-urea denaturing gel and autoradiography (lane
1 to 4, 100, 200, 300, and 400 fmol of WRN; lane 5,
non-telomeric DNA substrate; lane 6 to 9, 100, 200, 300, and 400
fmol of WRN; lane 10, telomeric DNA substrate; lane 11, (TTAGGG)
repeats molecular size markers, lane 12 to 15, 100, 200, 300, and
400 fmol of exonuclease mutant WRN(D82A).
(B) 100 to 400 fmol of purified recombinant WRN were incubated with
5'-32P-labeled, non-telomeric (lanes 1-5) or telomeric (lanes
6-10) DNA substrates with 27 nt 3'-overhang at 37°C for 10 min. The
reaction products were analyzed by 12% polyacrylamide-urea denaturing gel
and autoradiography (lane 1 to 4; 100, 200, 300, and 400 fmol of
WRN; lane 5, non-telomeric DNA substrate; lane 6 to 9, 100,
200, 300, and 400 fmol of WRN; lane 10, telomeric DNA substrate,
lane 11, (TTAGGG) repeats molecular size markers.
To
determine whether exonucleolytic processing was influenced by overhang length,
we performed exonuclease assays using a telomeric substrate with a 27 nucleotides
3' overhang. The result of this experiment shows that this substrate is
processed by WRN exonuclease to generate a periodic pattern of DNA products
with shorter 3' overhangs (Figure 1B), while under the same experimental
conditions incubation of WRN with a non-telomeric DNA substrate results in the
removal of mostly one to three nucleotides from some of the substrate molecules
without any significant furtherinward degradation (Figure 1B). This result is
in agreement with a previous study, which reported limited degradation of
substrates bearing a 3' overhang longer than 25 nucleotides by WRN [17]; (see also
Supplementary Figure 1). Telomeric substrates with shorter overhangs but not
substrates with non-telomeric sequences were also processed by WRN exonuclease
(data not shown). Collectively, these results demonstrate that the exonuclease
activity of WRN specifically processes telomeric DNA substrates with 3'
overhang independently of the length of the single-stranded overhang.
Concentration and time
dependency of exonuclease activity on telomeric DNA substrate
To examine in more details
the kinetics of WRN-mediated processing of telomeric substrates with a 3'
overhang, we titrated the amount of WRN added to the exonuclease reactions. As
shown in Figure 2A, at low concentrations, WRN hydrolyzes a few nucleotides
from the 3' overhang of a small percentage of telomeric substrate molecules,
while increasing concentrations of enzyme results in the step-wise processing
of increasing amounts of telomeric DNA substrate into a number of products with
shorter 3' overhangs (Figure 2A). In a complementary experiment, the telomeric
DNA substrate was incubated with WRN for 1 to 10 minutes and the products of
the reactions were analyzed by denaturing polyacrylamide gel electrophoresis.
The results of this experiment show that removal of a few nucleotides from the
3' overhang occurs within one minute of WRN addition and then continues inward
to produce time-dependent accumulation of progressively smaller products
differing by 1 to 6 nucleotides (Figure 2A).
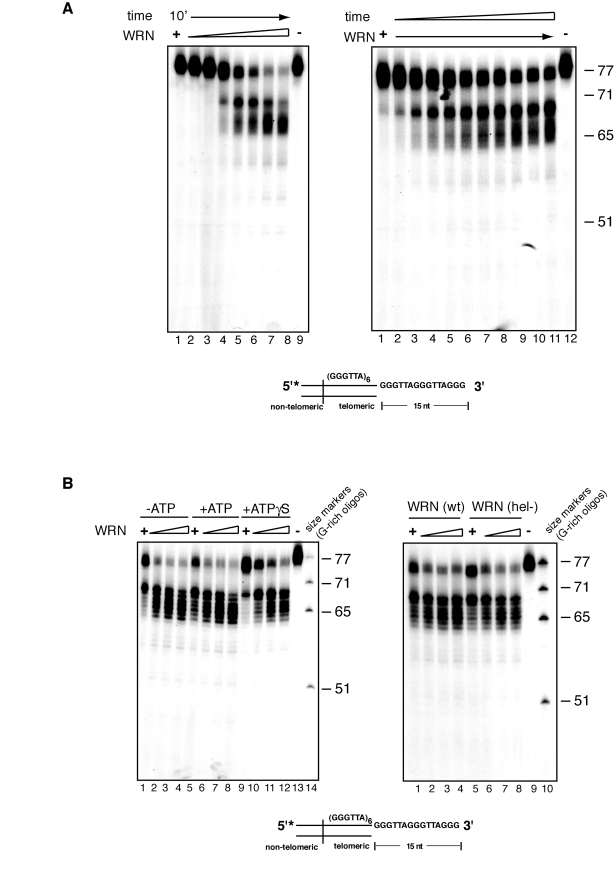
Figure 2. Concentration and time dependency of WRN exonuclease activity on telomeric substrates. (A) (left) 25 to 500 fmol of purified WRN were
incubated with 5'-32P-labeled, 3'-overhang telomeric DNA
substrate at 37°C for 10 min. The reaction products were analyzed by 12%
polyacrylamide-urea denaturing gel and autoradiography (lane 1 to 10, 25,
50, 100, 150, 200, 250, 300, 350, 400, 500 fmol of purified WRN; lane 11, DNA
substrate. (Right) 200 fmol of purified WRN was incubated with 5'-32P-labeled,
3'-overhang telomeric DNA substrate at 37°C from 0 to 10 min. The reaction
products were analyzed by 12% polyacrylamide-urea denaturing gel and
autoradiography (lane 1 to 11, 0, 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 min;
lane 12, DNA substrate. (B) (left) 100 to 400 fmol of
purified WRN were incubated with 5' 32P-labeled, 3'-overhang
telomeric DNA substrate in the absence or presence of 1.0 mM ATP or 1.0 mM
Adenosine 5'-[γ-thio]triphosphate
(ATPγS) at 37°C for 10 min. The
reaction products were resolved by 12% polyacrylamide-urea denaturing gel
and visualized by autoradiography (lane 1 to 4, 100, 200, 300, and 400 fmol
of WRN without ATP; lane 5 to 8, 100, 200, 300, and 400 fmol of WRN in the
presence of ATP, lane 9 to 12, 100, 200, 300, and 400 fmol of WRN in the
presence of 1.0 mM ATPγS; lane 13, DNA substrate; lane
14, (TTAGGG) repeats molecular size markers. (Right) 100 to 400 fmol
of purified WRN or WRN helicase mutant (K577M) were incubated with
telomeric DNA substrates at 37°C for 10 min. The reaction products were
analyzed by 12% polyacrylamide-urea denaturing gel and autoradiography
(lane 1 to 4, 100, 200, 300, and 400 fmol of WRN; lane 5 to 8, 100, 200,
300, and 400 fmol of helicase mutant WRN; lane 9, DNA substrate; lane 10,
(TTAGGG) repeats molecular size markers.
ATPase and helicase
activities are not required for 3' processing of telomeric substrates
In addition to a 3' to 5' exonuclease
activity, WRN possesses intrinsic ATPase and ATP-dependent helicase activities.
To determine if these activities assist in the nucleolytic processing of the
G-rich overhang of telomeric substrates, exonuclease reactions were carried out
in the presence or absence of ATP, or the non-hydrolysable ATP analogue ATPγS. A similar pattern of DNA products is observed in the presence or
absence of ATP, demonstrating that 3' end processing of telomeric substrates is
independent of ATP hydrolysis (Figure 2C). Reactions carried out in the
presence of ATPγS display a modest decrease in the amount of processed
molecules, although the pattern of products is unaffected.
To confirm that ATPase and
helicase activities do not influence the rate of extent of nucleolytic
processing of the 3' overhang, a telomeric DNA substrate was incubated with a
WRN variant lacking both activities (WRN K577M) and the products of the
reactions were analyzed by denaturing polyacrylamide gel electro-phoresis. As
shown on the right panel of Figure 2B, the pattern and intensity of DNA
products generated by WRN K577M, does not significantly differ from that of
wild-type WRN. Identical results were obtained when WRN K577M was incubated
with the telomeric substrate in the presence or absence of ATP (data not
shown).
Degradation of telomeric
substrate by WRN is dependent on the presence of telomeric sequences in both
the single and double stranded region of the DNA
Our data indicates that the
exonuclease activity of WRN on substrates with 3' overhangs is
telomere-specific. To confirm the specificity of this reaction for 5'-TTAGGG-3'
repeated sequences we carried out exonuclease assays with modified substrates.
First, to determine whether the presence of a repeated motif was sufficient for
3' end processing by WRN, we tested a DNA substrate composed of several
5'-AATCCC-3' repeats with a 15 nucleotides C-rich 3' overhang (Figure 3A;
compl-telomere). Incubation of this substrate with WRN does not yield any
detectable processing product, even at the highest WRN concentration tested.
Next, to determine if limited processing of the 3'overhang requires the
presence of telomeric repeats in both the duplex portion of the DNA and 3'
single-stranded overhang, WRN was incubated with substrates bearing altered
sequences in either the double-stranded or single-stranded DNA region of the
telomeric substrate. Changing the double-stranded portion of the telomeric
substrate to non-telomeric sequences results in the complete inhibition of WRN
exonuclease activity (Figure 3B). Similarly, replacing the single-stranded 3'
overhang from a telomeric repeat sequence to a non-telomeric sequence is
sufficient to prevent DNA degradation by WRN (Figure 3C). To corroborate the strict requirement for the
presence of precise telomeric 5'-TTAGGG-3' repeat units, we introduced three
nucleotides substitutions at two distinct positions within the sequence of the
G-rich 3' overhang. We measured the extent of WRN exonuclease activity on
telomeric substrates in which the GGG triplet at nucleotides 69 to 71 or the
TTA triplet at nucleotides 69 to 71 were replaced with a CCC triplet. The
results of these experiments show that each of
these changes within the 3' G-rich overhang is sufficient to completely inhibit
WRN exonuclease activity on telomeric DNA substrates (Figure 4A). Collectively,
these data demonstrate that WRN exonuclease activity on 3' overhangs of
telomeric DNA substrates is strictly dependent on the presence of perfect
telomeric TTAGGG motifs in both double-stranded and single-stranded regions of
the substrate.
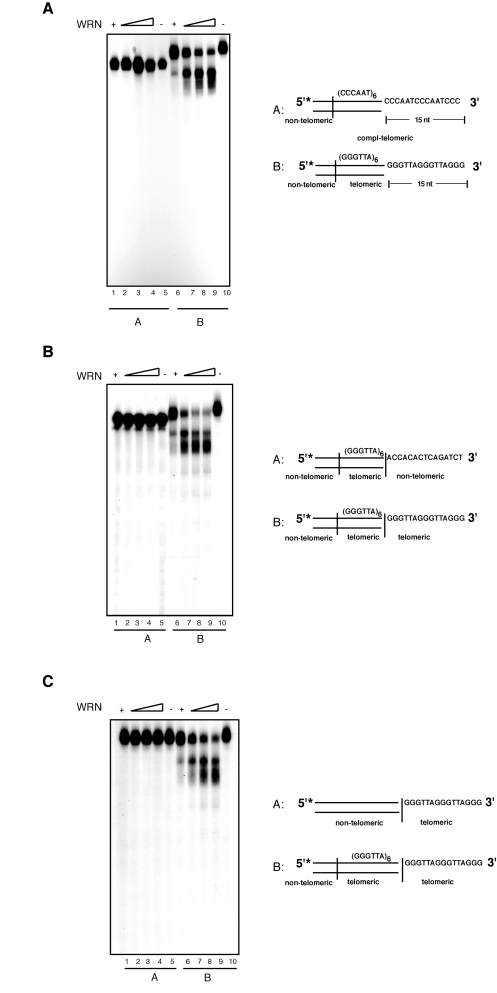
Figure 3. Both single- and double-stranded telomeric DNA sequences are required for the processing of the 3' overhang by WRN exonuclease. (A) 100 to
400 fmol of purified WRN were incubated with 5'-32P-labeled,
3'-overhang DNA substrate with (CCCAAT) repeats sequence (lanes 1-5)
and telomeric DNA substrate (lanes 6-10) at 37°C for 10 min. The
reaction products were analyzed by 12% polyacrylamide-urea denaturing gel
and autoradiography (lane 1 to 4, 100, 200, 300, and 400 fmol of
WRN; lane 5, (CCCAAT) repeat DNA substrate; lane 6 to 9, 100,
200, 300, and 400 fmol of WRN; lane 10, telomeric DNA substrate. (B)
100 to 400 fmol of purified WRN were incubated with 5'-32P-labeled
DNA substrate with 3' of non-telomeric overhang (lanes 1-5) and
telomeric DNA substrate (lanes 6-10) at 37°C for 10 min. The
reaction products were analyzed by 12% polyacrylamide-urea denaturing gel
and autoradiography (lane 1 to 4, 100, 200, 300, and 400 fmol of
WRN; lane 5, telomeric DNA substrate with non-telomeric overnhang; lane
6 to 9, 100, 200, 300, and 400 fmol of WRN; lane 10, telomeric
DNA substrate. (C) 100 to 400 fmol of purified WRN were
incubated with 5'-32P-labeled, 3'-overhang DNA substrate with
double-stranded non-telomeric sequence (lanes 1-5) and telomeric DNA
substrate (lanes 6-10) at 37°C for 10 min. The reaction products
were analyzed by 12% polyacrylamide-urea denaturing gel and autoradiography
(lane 1 to 4, 100, 200, 300, and 400 fmol of WRN; lane 5, DNA
substrate with double-stranded non-telomeric DNA sequence; lane 6 to 9,
100, 200, 300, and 400 fmol of WRN; lane 10, telomeric DNA
substrate.
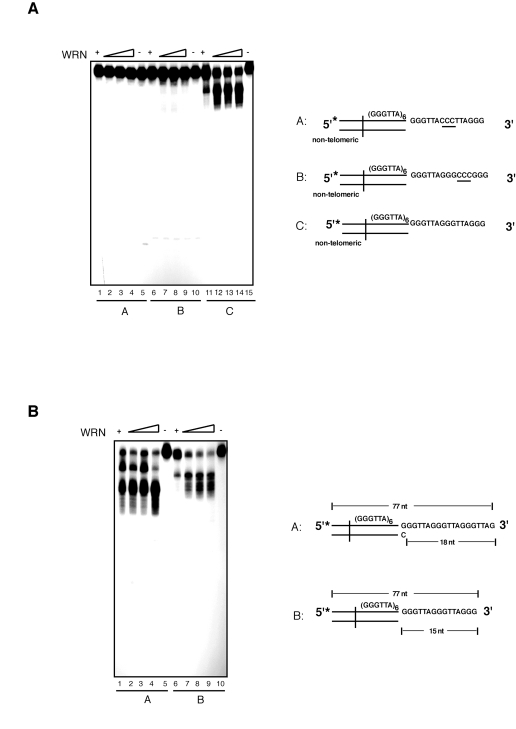
Figure 4. WRN exonuclease does not process telomeric DNA substrates with nucleotide substitutions within the 3' overhang sequence that alter the telomeric repeat unit. (A) 100 to 400 fmol of purified WRN were incubated
with 5'-32P-labeled telomeric DNA substrates with nucleotide
substitutions within the 3' overhang at 37°C for 10 min. The reaction
products were analyzed by 12% polyacrylamide-urea denaturing gel and
autoradiography (lane 1 to 4, 100, 200, 300, and 400 fmol of WRN; lane
5, telomeric DNA substrate with 3' overhang CCC (69 to 71) substitution
(substrate A); lane 6 to 9, 100, 200, 200, and 400 fmol of WRN; lane
10, telomeric DNA substrate with 3'overhang CCC (72 to 74) substitution
(substrate B); lane 11 to 14, 100, 200, 300, and 400 fmol of WRN; lane
15, telomeric DNA substrate C. (B) 100 to 400 fmol of
purified WRN were incubated with 5'-32P-labeled telomeric DNA
substrates with either 18 nt (substrate A) or 15 nt (substrate B) 3'
overhangs at 37°C for 10 min. The reaction products were analyzed by 12%
polyacrylamide-urea denaturing gel and autoradiography (lane 1 to 4,
100, 200, 300, and 400 fmol of WRN; lane 5, DNA substrate A; lane
6 to 9, 100, 200, 300, and 400 fmol of WRN; lane 10, DNA
substrate B.
Next, to determine whether
the identity of the 3' terminal nucleotide influences resection of the 3' end,
we measured the extent of WRN exonuclease activity on a telomeric DNA substrate
with a 5'-GGTTAG-3' terminal sequence. This is the preferred 3' terminal
sequence of human telomeres in vivo [40]. As shown
in Figure 4B, incubation of WRN with the telomeric DNA substrate terminating
with the GGTTAG-3' sequence leads to a distinct pattern of products than the
telomeric substrate terminating with the 5'-TTAGGG-3' sequence. Significantly,
comparison of the digestion patterns indicates that resection of the 3'
overhang of each substrate follows a sequence-specific pattern that stalls
within the GGG trinucleotide unit (see also Supplementary Figure 1). These
results indicate that the terminal nucleotide of the 3' overhang influences the
pattern of products but does not alter exonuclease activity, further stressing
the strict telomere sequence-specificity of the processing reaction.
POT1 but not TRF2
inhibits 3' end processing of telomeric DNA substrates by WRN exonuclease
TRF2 and POT1 are two
components of the shelterin complex that function in the protection of
telomeric ends [41]. TRF2 binds
at the junction of the double/single stranded telomeric DNA sequence and
interacts with WRN through its basic amino terminal domain [24]. To assess
whether TRF2 modulates WRN exonuclease activity on telomeric DNA substrates,
TRF2 or TRF2ΔB, a TRF2
mutant that does not bind WRN, were first preincubated with the telomeric DNA
substrate at room temperature for 20 minutes, then WRN was added to the
reaction and the incubation was continued at 370C for an additional
15 minutes. The results of this experiment, which are shown in Figure 5A,
indicate that neither TRF2 nor TRF2ΔB influence the pattern of digested products,
demonstrating that TRF2 does not alter the pattern of DNA processing of
telomeric overhangs by WRN.
Next we tested whether POT1 affects WRN
exonuclease activity. The single-stranded telomeric DNA binding protein POT1
plays a key role in telomere end protection and telomere length regulation [41]. POT1 has
been shown to stimulate WRN helicase activity on forked telomeric substrates,
however no effect on WRN exonuclease was reported, at least in the context of
the substrates used in this study [22]. To
determine whether POT1 influences the processing of the 3' G-rich
single-stranded overhang by WRN, we preincubated POT1 or a mutant form of POT1
(POT1Δ1-140),
which lacks the single-stranded DNA binding domain, with a telomeric DNA
substrate at room temperature for 20 minutes before the addition of WRN and
further incubation at 370C for 15 minutes. Gel shift assays with a
radiolabeled (GGGATT)5 single-stranded telomeric oligonucleotide
confirmed that POT1 but not POT1Δ1-140 binds to single-stranded telomeric DNA (data not
shown). The results of the exonuclease assays indicate that addition of
increasing amounts of POT1 inhibits the processing of substrates with either a
15 or 27 nucleotides 3' single-stranded telomeric overhang by WRN exonuclease (Figure 5B and C). Importantly,
the DNA binding activity of POT1 is required to prevent the limited processing
of the 3' telomeric overhang by WRN, since POT1Δ1-140 is unable to repress WRN
exonuclease activity on both substrates (Figure 5B and C).
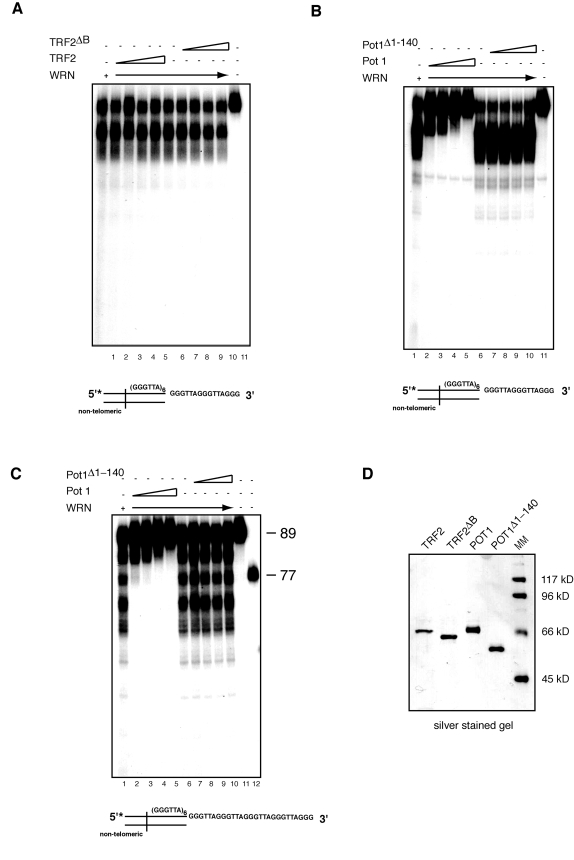
Figure 5. POT1 but not TRF2 inhibits processing of the 3' overhang of telomeric DNA substrates by WRN exonuclease. (A) 200 to 800 fmol of purified TRF2 or TRF2ΔB were incubated
with telomeric DNA substrates at room temperature for 20 min, then 200 fmol
of WRN was added into the reaction to incubate at 37ºC for 15 min. The
products were analyzed by 12% polyacrylamide-urea denaturing gel and
autoradiography (lane 1, 200 fmol of WRN; lane 2 to 5, 200,
400, 600, 800 fmol of TRF2 and 200 fmol of WRN; lane 6, 200 fmol of
WRN; lane 7 to 10, 200, 400, 600, 800 fmol of TRF2ΔB and 200fmol of
WRN; lane 11, DNA substrate. (B)200 to 800 fmol of purified
POT1 or POT1Δ1-140 were incubated with telomeric DNA
substrates at room temperature for 20 min, then 200 fmol of WRN was added
into the reaction to incubate at 37ºC for 15 min. The products were
analyzed by 12% polyacrylamide-urea denaturing gel and autoradiography (lane
1, 200 fmol of WRN; lane 2 to 5, 200, 400, 600, 800 fmol POT1
and 200 fmol of WRN; lane 6, 200 fmol of WRN; lane 7 to 10,
200, 400, 600, 800 fmol POT1Δ1-140 and 200 fmol of
WRN; lane 11, DNA substrate). (C) 200 to 800 fmol of purified
POT1 or POT1Δ1-140 were incubated with telomeric substrates
with 27 nt 3' overhang at room temperature for 20 min, then 200 fmol of WRN
was added into the reaction to incubate at 37ºC for 15 min. The
products were analyzed by 12% polyacrylamide-urea denaturing gel and
autoradiography (lane 1, 200 fmol of WRN; lane 2 to 5, 200,
400, 600, 800 fmol of POT1 and 200 fmol of WRN; lane 6, 200 fmol of
WRN;lane 7 to 10, 200, 400, 600, 800 fmol of POT1Δ140
and 200 fmol of WRN; lane 11, 89-mer telomeric oligo, lane 12 77-mer
telomeric oligo. (D) Silver stained SDS-PAGE gel showing
the purity of purified recombinant TRF2, TRF2ΔB, Pot1,
and POT1Δ1-140 used in
the exonuclease assays.
Discussion
In
this study we investigated the biochemical properties of the WRN protein at
telomeres and describe in vitro studies that expose a unique property of
WRN exonuclease on 3' single-stranded overhang of telomeric DNA substrates
mimicking natural telomere termini. Here, we demonstrate that WRN exhibits
sequence-specific exonuclease activity that specifically removes, in a
restricted fashion, several nucleotides inward from the G-rich 3'
single-stranded overhang of telomeric substrates. Close inspection and
comparison of the digestion pattern among the different substrates used in this
study (see Supplementary Figure 1) reveals that processing of each telomeric substrate
occurs readily up to the first GGG repeat unit where it stalls; it then
proceeds further inward until the next GGG repeat where it slows down again,
and this pattern continues until WRN approaches the double-stranded
single-stranded junction where further digestion is dramatically reduced. This
activity requires the presence of telomeric sequences in both the duplex and 3'
G-rich overhang DNA segments, as replacements in either the double-stranded
portion or the single-stranded DNA extension that alter the normal GGGATT
telomeric repeat unit result in the complete inhibition of WRN exonuclease.
Several
groups have characterized the activity of WRN exonuclease on 3' recessed duplex
DNA substrates or blunt duplexes with an internal bubble or fork on the opposite
end [12,14,16,42,43]. However, only one group has previously reported that
WRN exonuclease can digest 3' overhangs of sufficient length [17]. This study
reported that WRN degrades non-telomeric DNA substrates with more than 20
nucleotides overhangs to generate products that are generally one nucleotides
shorter [17].
Interestingly, while WRN ATPase and helicase activities do not influence
processing of telomeric substrates (Figure 2B), WRN exonuclease on
non-telomeric 3' overhang substrates is stimulated by ATP hydrolysis [17]. These
results demonstrate that the ATPase and helicase activities play a role in the
processing of 3' overhangs of non-telomeric but not telomeric substrates, and
suggest that WRN can resect 3' overhangs through two distinct mechanisms, one
of which is telomeric repeats sequence specific. As we observed a minor but
reproducible inhibition of DNA processing by ATPγS, it is
possible that nucleotide binding without hydrolysis may induce a conformational
change in WRN that limits the attack of telomeric substrates.
WRN
is a nuclear protein that has been implicated in several nuclear processes
ranging from DNA repair, transcription, recombination and telomere metabolism [18]. This
multiplicity of cellular functions is reflected in the variable pattern of
localization of this protein in the nucleus [44,45].
Significantly, immuno-histochemical studies and chromatin immuno-precipitation
assays have revealed that a subpopulation of WRN is associated with chromosome
ends, primarily during the S-phase of the cell cycle [19], and lack of
WRN or expression of an helicase-negative WRN protein in normal cells causes
telomere loss [19,46]. In
vitro studies have further indicated that WRN cooperates with telomeric
repeat binding factors TRF1 and
TRF2 to unwind the telomeric displacement loop (D-loop), a structure formed by
invasion of the 3' single-stranded telomeric overhang of internal homologous
double-stranded DNA region [20]. Although
these studies have exposed an important function for the WRN helicase activity
in the maintenance of telomere homeostasis, our understanding of how WRN
regulates telomere length and stability is far from being well understood.
Moreover, the role that the exonuclease activity plays in this and possibly
other processes remains unknown. Unraveling the cellular function of WRN
exonuclease activity is of critical importance, since this is the domain that
radically differentiates WRN from other human RecQ helicases such as BLM and
RecQ4 [47].
Nucleolytic activities at telomeres must
be properly controlled, since failure to restrain it would cause telomere
attrition and chromosome instability, which may affect cell viability by
inducing cell senescence or apoptosis, or promote tumorigenesis [37,41,48]. To
prevent unwanted degradation, telomeres are bound by telomere-specific factors
known as the shelterin complex that collectively contribute to the protection
of telomere termini from excessive processing or fusion with other telomere
ends. Significantly, we found that the telomeric protein POT1 but not TRF2
inhibits WRN processing of the 3' overhang, suggesting that POT1, in addition
to its role in protecting the 5' telomeric end from nucleolytic resection, is
important for regulating WRN-mediated processing of the 3' telomeric overhang.
A previous study has reported that POT1 influence WRN helicase activity,
however no alterations in exonuclease activity by POT1 were observed [22]. It is
likely that lack of inhibition of WRN exonuclease activity by POT1 was due to
the nature of the telomeric substrate used in this study, which was a forked
blunt-ended DNA duplex.
Our
demonstration that WRN exhibits sequence-specific exonuclease activity that
specifically removes several nucleotide inward, in a controlled manner, from
the G-rich 3' overhang of telomeric substrates, suggests that WRN exonuclease
activity may play a role in telomere homeostasis. We envision that WRN
exonuclease activity at chromosome ends may have an important role in
configuring the telomere 3' termini prior to the formation of the protective
t-loop structure and shaping the ends of telomeres for proper replication,
telomerase elongation, or protection. Future studies testing the function of
WRN exonuclease activity at chromosome ends in vivo will be critical for
defining the role of WRN and its activities in telomere homeostasis.
Methods
DNA substrates used in this study:
1. 77-mer (G77/C62) telomeric substrate with 15 nucleotides 3' overhang
5'AGCTGAGCATGTCCAGACATGTCCTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGG3'
5'TCGACTCCTACAGGTCTGTACAGGATCCCAATCCCAATCCCAATCCCAATCCCAATCCCAAT3'
2. 77-mer (77/62) non-telomeric substrate with 15 nucleotides 3'overhang
5'AGCTGAGCATGTCCAGACATGTCCTACCAGAGCCATATGAGTCAAACCGTCATCGAGCTCCGTGTGAACTAGCTCATTCGAC3'
5'TCGACTCGTACAGGTCTGTACAGGATGGTCTCGGTATACTCAGTTTGGCAGTAGCTCGAGGC3'
3. 77-mer non-telomeric substrate with telomeric 3' overhang sequence
5'AGCTGAGCATGTCCAGACATGTCCTACCAGAAGCCATATGAGTCAAACCGTCATCGAGCTCCGGGGTTAGGGTTAGGG3'
5'TCGACTCGTACAGGTCTGTACAGGATGGTCTTCGGTATACTCAGTTTGGCAGTAGCTCGAGGC3'
4. 77-mer telomeric substrate with non-telomeric 3'overhang sequence
5'AGCTGAGCATGTCCAGACATGTCCTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAACCACACTCAGATCT3'
5'TCGACTCGTACAGGTCTGTACAGGATCCCAATCCCAATCCCAATCCCAATCCCAATCCCAAT3'
5. 89-mer (G89/C62) telomeric substrate with 27 nucleotides 3'overhang
5'GGGTTAGGGTTAAGCTGAGCATGTCCAGACATGTCCTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTT AGGGTTAGGGTTAGGG3'
5'TCGACTCCTACAGGTCTGTACAGGATCCCAATCCCAATCCCAATCCCAATCCCAATCCCAAT3'
6. 89-mer (89/62) non-telomeric substrate with 27 nucleotides 3'overhang
5'ACCTGCAACTAGAGCTGAGCATGTCCAGACATGTCCTACCAGAGCCATATGAGTCAAACCGTCATCGAGCTCCG TGTGAACTAGCTCAT3'
5'TCGACTCGTACAGGTCTGTACAGGATGGTCTCGGTATACTCAGTTTGGCAGTAGCTCGAGGC3'
7. 77-mer telomeric substrate with mutant (69-CCC) 3' overhang
5'AGCTGAGCATGTCCAGACATGTCCTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTACCCTTAGGG3'
5'TCGACTCCTACAGGTCTGTACAGGATCCCAATCCCAATCCCAATCCCAATCCCAATCCCAAT3'
8. 77-mer telomeric substrate with mutant (72-CCC) 3' overhang
5'AGCTGAGCATGTCCAGACATGTCCTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGCCCGGG3'
5'TCGACTCCTACAGGTCTGTACAGGATCCCAATCCCAATCCCAATCCCAATCCCAATCCCAAT3'
9. 77-mer (CCCTAA) repeat
sequence substrate with 3'overhang (compl-telomeric)
5'AGCTGAGCATGTCCAGACATGTCCTACCCAATCCCAATCCCAATCCCAATCCCAATCCCAATCCCAATCCCAATCCC3'
5'TCGACTCCTACAGGTCTGTACAGGATGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTA3'
10. 77-mer Telomeric
substrate with 18 nucleotides 3' overhang and terminal GTTAG sequence
5'AGCTGAGCATGTCCAGACATGTCGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTA3'
5'TCGACTCCTACAGGTCTGTACAGCCCAATCCCAATCCCAATCCCAATCCCAATCCCAAT3'
11. 70mer non-telomeric
substrate with 25 nucleotides 3' overhang
5'GCTGATCAACCCTACATGTGTAGGTAACCCTAACCCTAACCCTAAGGACAACCCTAGTGAAGCTTGTAAC3'
5'CGACTAGTTGGGATGTACACATCCATTGGGATTGGGATTGGGATT3'
All telomeric DNA substrates
have a 26 nucleotides non-telomeric 5' end to allow proper annealing of the
oligonucleotides.
Protein expression and purification.
Recombinant Flag-tagged wild-type
WRN, exonuclease mutant WRN (D82A), helicase mutant WRN (K577M), TRF2, TRF2ΔB, POT1, and POT1(Δ1-140) cDNAs were cloned into baculovirus expression
vectors to generate recombinant viruses used to infect Sf9 cells. 48 hours
after infection, cells were collected and lysed in lysis buffer (10 mM
Hepes pH 7.5, 100 mM NaCl, 1.5 mM MgCl2, 0.5% Nonidet P-40).
Recombinant proteins were purified by DEAE-cellulose and affinity
chromatography on anti-Flag resin [38-39].
Exonuclease assay.
DNA exonuclease activity of was measured as described
in [39]. Briefly,
oligonucleotides were labeled at the 5' end with [32P] ATP and T4
polynucleotide kinase. The appropriate oligonucleotides were then annealed by
boiling followed by slowly cooling to room temperature. Reaction mixtures
containing 50 fmol of DNA substrate (100,000 cpm) and increasing
amounts of WRN in 40 mM Tris-HCl pH 7.5, 4 mM MgCl2, 5 mM
dithiothreitol, 0.1mg/ml bovine serum albumin, in the absence or presence of
1 mM ATP or 1 mM ATPγS, in a final volume of 10 μl were incubated at
37ºC for 10 minutes. Reactions were terminated by the addition of
2.0 μl of a 95% formamide solution and after incubation at 95°C for
3 minutes, DNA products were resolved by 12% polyacrylamide-urea gel electrophoresis
and visualized by autoradiography.
To assess WRN exonuclease activity in the presence of TRF2 and
POT1 variants, telomeric DNA substrates (50 fmol, 100,000 cpm) were
incubated with increasing amounts (200-800 fmol) of either POT1, POT1(Δ1-140), TRF2 or TRF2ΔB in 10 μl of Buffer A (10 mM Tris-HCl pH
7.5, 80 mM NaCl, 4 mM KCl, 4 mM MgCl2, 1mM ATP, and 5%
glycerol) at room temperature for 20 minutes. Then, 200 fmol of WRN was
added into the reaction mixture and incubate at 37ºC for an additional 15
minutes. Each reaction was then terminated by the addition of 2.0 μl of a
95% formamide solution. After incubation at 95 °C for 3 minutes, DNA
products were resolved by 12% polyacrylamide-urea gel electrophoresis and
visualized by autoradiography.
Supplementary Materials
Processing of telomeric substrates with 3' overhangs by WRN exonuclease. (A)
100 to 400 fmol of purified WRN were
incubated with 5'-radiolabeled non-telomeric DNA substrate with 25 nt
3' overhang or telomeric substrate with 15 nt 3' overhang at 37°C for 10
min. The reaction products were analyzed by 12% polyacrylamide-urea
denaturing gel and autoradiography (lane 1 to 4, 100, 200, 300, and
400 fmol of WRN; lane 5, nont-telomeric DNA substrate; lane 6 to
9, 100, 200, 300, and 400 fmol of WRN; lane 10, telomeric DNA
substrate; lane 11, G-rich molecular size markers.
(B) (left) 400 fmol of purified
recombinant wild-type WRN was incubated with 5'-32P-labeled
3'-overhang telomeric DNA substrates at 37°C for 10 min. The reaction
products were resolved on a long 12% polyacrylamide-urea denaturing gel to
improve bands resolution and visualized by autoradiography. M= G-rich
telomeric oligonucleotides were used as molecular size markers. (right)
Schematic representation of substrates used in the exonuclease assay.
Arrows and brackets denote major processing products identified in this
study.
Acknowledgments
We thank members of the Comai and Reddy labs for valuable suggestions
during the course of this study. This investigation was supported by NIH grant
R01AG023873 from the National Institute on Aging awarded to L.C., and was
conducted in a facility constructed with support from Research Facilities
Improvement Program Grant Number C06 RR014514-01, C06 RR10600-01 and C06
CA62528 from the National Center for Research Resources, National Institutes of
Health.
Conflicts of Interest
The
authors have declared that no competing interests exist.
References
-
1.
Epstein
CJ
, Martin
CM
, Schultz
AL
and Motulsky
AG.
Werner's syndrome. A review of its symptomatology, natural history, phatological features, genetics and relationship to the natural aging process.
Medicine.
1966;
45:
177
-221.
[PubMed]
.
-
2.
Dyer
C
and Sinclair
A.
The premature ageing syndromes: insights into the ageing process.
Age and Ageing.
1998;
27:
73
-80.
[PubMed]
.
-
3.
Goto
M
Hierarchical deterioration of body systems in Werner's syndrome: implications for normal aging.
Mech Aging Dev.
1997;
98:
239
-254.
[PubMed]
.
-
4.
Yu
CE
, Oshima
J
, Fu
YH
, Wijsman
EM
, Hisama
F
, Alisch
R
, Matthews
S
, Nakura
J
, Miki
T
, Ouais
S
, Martin
GM
, Mulligan
J
and Schellenberg
GD.
Positional Cloning of the Werner's Syndrome Gene.
Science.
1996;
272:
258
-262.
[PubMed]
.
-
5.
Karow
JK
, Wu
L
and Hickson
ID.
RecQ family helicases: roles in cancer and aging.
Curr Opin Genet Dev.
2000;
10:
32
-38.
[PubMed]
.
-
6.
Moser
MJ
, Holley
WR
, Chatterjee
A
and Mian
IS.
The proofreading domain of Escherichia coli DNA polymerase I and other DNA and/or RNA exonuclease domains.
Nucl Acids Res.
1997;
25:
5110
-5118.
[PubMed]
.
-
7.
Gray
M
, Shen
JC
, Kamath-Loeb
A
, Blank
A
, Sopher
B
, Martin
G
, Oshima
J
and Loeb
L.
The Werner syndrome protein is a DNA helicase.
Nature Genet.
1997;
17:
100
-103.
[PubMed]
.
-
8.
Shen
JC
, Gray
M
, Oshima
J
and Loeb
L.
Characterization of Werner syndrome protein DNA helicase activity: directionality, substrate dependence and stimulation by replication protein A.
Nucl Acids Res.
1998;
26:
2879
-2885.
[PubMed]
.
-
9.
Shen
JC
, Gray
M
, Oshima
J
, Kamath-Loeb
A
, Fry
M
and Loeb
L.
Werner Syndrome Protein: I. DNA Helicase and DNA Exonuclease Reside On The Same Polypeptide.
J Biol Chem.
1998;
273:
34139
-34144.
[PubMed]
.
-
10.
Suzuki
N
, Shimamoto
A
, Imamura
O
, Kuromitsu
J
, Kitao
S
, Goto
M
and Furuichi
Y.
DNA helicase activity in Werner's syndrome gene product synthesized in a baculovirus system.
Nucleic Acids Res.
1997;
25:
2973
-2978.
[PubMed]
.
-
11.
Suzuki
N
, Shiratori
M
, Goto
M
and Furuichi
Y.
Werner syndrome helicase contains a 5'--3' exonuclease activity that digests DNA and RNA strands in DNA/DNA and RNA/DNA duplexes dependent on unwinding.
Nucleic Acids Res.
1999;
27:
2361
-2368.
[PubMed]
.
-
12.
Kamath-Loeb
A
, Shen
JC
, Loeb
L
and Fry
M.
Werner Syndrome Protein: II. Characterization of the integral 3' --> 5' DNA exonuclease.
J Biol Chem.
1998;
273:
34145
-34150.
[PubMed]
.
-
13.
Huang
S
, Li
B
, Gray
M
, Oshima
J
, Mian
LS
and Campisi
J.
The premature ageing syndrome protein, WRN, is a 3' --> 5' exonuclease.
Nature Genet.
1998;
20:
114
-116.
[PubMed]
.
-
14.
Huang
S
, Beresten
S
, Li
B
, Oshima
J
, Ellis
NA
and Campisi
J.
Characterization of the human and mouse WRN 3'-5' exonuclease.
Nucleic Acid Res.
2000;
12:
2396
-2405.
[PubMed]
.
-
15.
Mohaghegh
P
, Karow
JK
, Brosh
Jr RM
, Bohr
VA
and Hickson
ID.
The Bloom's and Werner's syndrome proteins are DNA structure-specific helicases.
Nucleic Acids Res.
2001;
29:
2843
-2849.
[PubMed]
.
-
16.
Shen
JC
and Loeb
LA.
Werner syndrome exonuclease catalyzes structure-dependent degradation of DNA.
Nucleic Acids Res.
2000;
28:
3260
-3268.
[PubMed]
.
-
17.
Machwe
A
, Xiao
L
and Orren
DK.
Length-dependent degradation of single-stranded 3' ends by the Werner syndrome protein (WRN): implications for spatial orientation and coordinated 3' to 5' movement of its ATPase/helicase and exonuclease domains.
BMC Mol Biol.
2006;
7:
6
[PubMed]
.
-
18.
Opresko
PL
Telomere ResQue and preservation - roles for the Werner syndrome protein and other RecQ helicases.
Mech Ageing Dev.
2008;
129:
79
-90.
[PubMed]
.
-
19.
Crabbe
L
, Verdun
RE
, Haggblom
CI
and Karlseder
J.
Defective telomere lagging strand synthesis in cells lacking WRN helicase activity.
Science.
2004;
306:
1951
-1953.
[PubMed]
.
-
20.
Opresko
PL
, Otterlei
M
, Graakjaer
J
, Brheim
P
, Dawut
L
, Kolvraa
S
, May
A
, Seidman
MM
and Bohr
VA.
The Werner syndrome helicase and exonuclease cooperate to resolve telomeric D loops in a manner regulated by TRF1 and TRF2.
Mol Cell.
2004;
14:
763
-774.
[PubMed]
.
-
21.
Machwe
A
, Xiao
L
and Orren
DK.
TRF2 recruits the Werner syndrome (WRN) exonuclease for processing of telomeric DNA.
Oncogene.
2004;
23:
149
-56.
[PubMed]
.
-
22.
Opresko
PL
, Mason
PA
, Podell
ER
, Lei
M
, Hickson
ID
, Cech
TR
and Bohr
VA.
POT1 stimulates RecQ helicases WRN and BLM to unwind telomeric DNA substrates.
J Biol Chem.
2005;
280:
32069
-32080.
[PubMed]
.
-
23.
Opresko
PL
, von
Kobbe C
, Laine
JP
, Harrigan
J
, Hickson
ID
and Bohr
VA.
Telomere-binding protein TRF2 binds to and stimulates the Werner and Bloom syndrome helicases.
J Biol Chem.
2002;
277:
41110
-41119.
[PubMed]
.
-
24.
Li
B
, Jog
S
, Reddy
S
and Comai
L.
WRN controls formation of extrachromosomal telomeric circles and is required for TRF2DeltaB-mediated telomere shortening.
Mol Cell Biol.
2008;
28:
1892
-1904.
[PubMed]
.
-
25.
Griffith
JD
, Comeau
L
, Rosenfield
S
, Stansel
RM
, Bianchi
A
, Moss
H
and de Lange
T.
Mammalian telomeres end in a large duplex loop.
Cell.
1999;
97:
503
-514.
[PubMed]
.
-
26.
Nikitina
T
and Woodcock
CL.
Closed chromatin loops at the ends of chromosomes.
J Cell Biol.
2004;
166:
161
-165.
[PubMed]
.
-
27.
Murti
KG
and Prescott
DM.
Telomeres of polytene chromosomes in a ciliated protozoan terminate in duplex DNA loops.
Proc Natl Acad Sci U S A.
1999;
96:
14436
-14439.
[PubMed]
.
-
28.
Bilaud
T
, Brun
C
, Ancelin
K
, Koering
CE
, Laroche
T
and Gilson
E.
Telomeric localization of TRF2, a novel human telobox protein.
Nat Genet.
1997;
17:
236
-239.
[PubMed]
.
-
29.
Bilaud
T
, Koering
CE
, Binet-Brasselet
E
, Ancelin
K
, Pollice
A
, Gasser
SM
and Gilson
E.
The telobox, a Myb-related telomeric DNA binding motif found in proteins from yeast, plants and human.
Nucleic Acids Res.
1996;
24:
1294
-1303.
[PubMed]
.
-
30.
Broccoli
D
, Smogorzewska
A
, Chong
L
and de Lange
T.
Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2.
Nat Genet.
1997;
17:
231
-235.
[PubMed]
.
-
31.
Baumann
P
and Cech
TR.
Pot1, the putative telomere end-binding protein in fission yeast and humans.
Science.
2001;
292:
1171
-1175.
[PubMed]
.
-
32.
de Lange
T
Protection of mammalian telomeres.
Oncogene.
2002;
21:
532
-540.
[PubMed]
.
-
33.
de Lange
T
T-loops and the origin of telomeres.
Nat Rev Mol Cell Biol.
2004;
5:
323
-239.
[PubMed]
.
-
34.
Karlseder
J
, Broccoli
D
, Dai
Y
, Hardy
S
and de Lange
T.
p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2.
Science.
1999;
283:
1321
-1325.
[PubMed]
.
-
35.
Karlseder
J
, Smogorzewska
A
and de Lange
T.
Senescence induced by altered telomere state, not telomere loss.
Science.
2002;
295:
2446
-2449.
[PubMed]
.
-
36.
d'Adda
di Fagagna F
, Teo
SH
and Jackson
SP.
Functional links between telomeres and proteins of the DNA-damage response.
Genes Dev.
2004;
18:
1781
-1799.
[PubMed]
.
-
37.
Ferreira
MG
, Miller
KM
and Cooper
JP.
Indecent exposure: when telomeres become uncapped.
Mol Cell.
2004;
13:
7
-18.
[PubMed]
.
-
38.
Li
B
and Comai
L.
Functional interaction between Ku and the werner syndrome protein in DNA end processing.
J Biol Chem.
2000;
275:
28349
-28352.
[PubMed]
.
-
39.
Li
B
and Comai
L.
Requirements for the nucleolytic processing of DNA ends by the Werner syndrome protein-Ku70/80 complex.
J Biol Chem.
2001;
276:
9896
-9902.
[PubMed]
.
-
40.
Sfeir
AJ
, Chai
W
, Shay
JW
and Wright
WE.
Telomere-end processing the terminal nucleotides of human chromosomes.
Mol Cell.
2005;
18:
131
-138.
[PubMed]
.
-
41.
de Lange
T
Shelterin: the protein complex that shapes and safeguards human telomeres.
Genes Dev.
2005;
19:
2100
-2110.
[PubMed]
.
-
42.
Machwe
A
, Ganunis
R
, Bohr
VA
and Orren
DK.
Selective blockage of the 3'-- 5' exonuclease activity of WRN protein by certain oxidative modifications and bulky lesions in DNA.
Nucleic Acids Res.
2000;
28:
2762
-2770.
[PubMed]
.
-
43.
Orren
DK
, Theodore
S
and Machwe
A.
The Werner syndrome helicase/exonuclease (WRN) disrupts and degrades D-loops in vitro.
Biochemistry.
2002;
41:
13483
-13488.
[PubMed]
.
-
44.
Marciniak
RA
, Lombard
DB
, Johnson
FB
and Guarente
L.
Nucleolar localization of the Werner syndrome protein in human cells.
Proc Natl Acad Sci U S A.
1998;
95:
6887
-6892.
[PubMed]
.
-
45.
Sakamoto
S
, Nishikawa
K
, Heo
SJ
, Goto
M
, Furuichi
Y
and Shimamoto
A.
Werner helicase relocates into nuclear foci in response to DNA damaging agents and co-localizes with RPA and Rad51.
Genes Cells.
2001;
6:
421
-430.
[PubMed]
.
-
46.
Bai
Y
and Murnane
JP.
Telomere instability in a human tumor cell line expressing a dominant-negative WRN protein.
Hum Genet.
2003;
113:
337
-347.
[PubMed]
.
-
47.
Brosh
RM
and Bohr
VA.
Human premature aging, DNA repair and Rec Q helicases.
Nucleic Acid Res.
2007;
35:
7527
-7544.
[PubMed]
.
-
48.
Blasco
MA
Telomeres and human disease: ageing, cancer and beyond.
Nat Rev Genet.
2005;
6:
611
-622.
[PubMed]
.