Introduction
The
complexity of mammalian development is intrinsic to the zygote with genes
encoding the information necessary for every tissue and cellular sub-type.
Development initiates with totipotent embryonic stem cells (ESCs), which give
rise to the three germ layers, the ectoderm, the mesoderm and the endoderm,
eventually constructing the tissues of the body. ESCs possess characteristics
such as asymmetric cellular division, the ability to differentiate into all
three germ layers, telomerase activity, and a cell cycle that has
significantly diminished gap phases. In adulthood, multipotent tissue specific
stem cells regulate homeostatic tissue regeneration. These adult stem cells
(ASCs) while lacking the capacity to differentiate into the three germ layers
are capable of regenerating a cellular popula-tion of a specific tissue type
and maintain asymmetric cellular division. ASCs are characterized as being in
a state of relative proliferative quiescence, which they can exit from under
the proper conditions, to obtain the proliferative potential necessary for
tissue regeneration.
ASCs
are primarily responsible for maintaining tissue structure; they accomplish this
through their functional duality between self-renewal and commitment. The
tissue specific ASC populations are vital to survival and therefore must be
maintained through self-renewal. However, the necessity for self-renewal must
be transient, as the cells are also required to differentiate and commit to a
specific lineage. The balance between self-renewal and commitment is
critical. If the ASC population leans towards the self-renewal pathway, it
risks the loss of differentiation capacity and could malignantly transform into
a highly proliferative nondifferentiating cellular population. On the other
hand, if the balance shifts towards differentiation, there is a risk that the
stem cell population would be lost accompanied by an increased potential for
degenerative disease occurrence, a mechanism that is believed to be a component
of the aging process.
Organismal
aging and age-related diseases are often associated with senescence. Hayflick
originally described senescence as a permanent cell cycle arrest due to the
limited replicative potential of cultured human fibroblasts [1]. Telomere
shortening, oncogene activation or DNA damaging events can trigger the
senescence pathway. Senescence plays a critical role in maintaining properly
functioning ASC populations. Under normal conditions ASCs divide to replace
aging tissue. During lifetime, extrinsic sources (such as ionizing radiation,
genotoxic drugs, chemicals, etc.) and intrinsic factors (DNA replication
errors, spontaneous chemical changes to DNA, programmed DNA recombination) can
lead to mutations, which could accumulate over time in the progenitor
population.
Regeneration
can also be triggered due to tissue damaging events, which could directly
expose the ASC population to mutations and/or alter the regulatory tissue
microenvironment. Stem cells possess inherent damage repair mechanisms that
can respond to DNA-damage, reactive oxygen species (ROS) damage and mutations
that de-regulate the cell-cycle and other cellular functions. When these repair
mechanisms fail the cell will accrue increased levels of damage, initiating
cell-death pathways such as senescence or apoptosis (programmed cell death).
The depletion of the progenitor cell population results in an inability for
tissue renewal, aging and possible development of degenerative diseases. On
the other side, failing to properly repair DNA damage along with escape from
apoptosis and/or senescence could trigger neoplastic transformation of stem
cells.
Stem cells encapsulate such an immense clinical
therapeutic potential that understanding their intricate biological role is
paramount. Unfortunately, the definition of stem cells remains to be nebulous
and data can be contradictory. In this review we will attempt to describe stem
cell properties in both embryonic and adult stem cells and the intriguing
regulation of the cell cycle in these systems. We then discuss the role of the
senescence process in ASCs and its relation to aging and age-related diseases.
Concluding, we will examine how the de-regulation of the mechanisms discussed
may lead to carcinogenesis and what stem cell research may hold for future
therapeutic prospects.
Embryonic
stem cells and their origins
Thomson
and Gearhart are attributed with initial isolation and characterization of
human ESCs (hESCs) from the inner cell mass of the blastocyst, noting the
differentiation and self-renewing capacity of the cells in vitro [2,3].
Further characterization demonstrated that the cells expressed cell surface
markers typical of undifferentiated nonhuman primate ESCs (pESCs) and human
embryonic carcinoma cells as was originally described [2,3,4,5]. These
specific markers included stage-specific embryonic antigen (SSEA)-1, SSEA-3,
SSEA-4, TRA-1-60, TRA-1-81, alkaline phosphatase activity and high levels of
telomerase activity [2,3]. Telomerase is a ribonucleoprotein enzyme that
preserves the telomeric regions at the ends of chromosomes by de novo
oligonucleotide synthesis [6]. Telomerase activity is not present in normal
diploid somatic cells, which incur shorted telomeres with age leading to
replicative senescence after a finite number of replications [1,7,8,9]. It
has been shown that TRA-1-60 and TRA-1-81 are specific epitopes of a larger
membrane-bound protein podocalyxin, which under-goes retinoic acid modification
when ESCs differentiate losing its reactivity with the TRA-1-60 and TRA-1-81
antibodies [10]. These characterizations remain to be used to identify stem
cells today, along with the expression of the intrinsic transcription factor
Oct-4 and in mouse ESCs (mESCs) the constitutive ability to receive extrinsic
signals from the cytokine leukemia inhibitory factor (LIF) [11,12,13].
Soon
after the initial isolations and characterizations of hESCs, interest shifted towards
understanding the factors involved in their differentiation. For example, if
all cells are derived from initial progenitor cells, what directs
differentiation towards glial cells versus adipocytes? Brüstle et al were
among the first to demonstrate in vitro controlled differentiation of
hESCs using a series of growth factor combinations, which successfully elicited
a reactivity to a monoclonal antibody specific for a membrane epitope typically
found on the membranes of glial precursors [14]. They initially grew ES cells
in a media that favored the growth of neural precursors. They then exposed
cells to the following series of growth factors: i) basic fibroblast growth
factor (FGF2), ii) FGF2 and epidermal growth factor (EGF) and iii) FGF2 and
platelet-derived growth factor (PDGF) [14]. The cells maintained in the final
growth factor-supplemented media were able to be stored and kept in culture
without further differentiation for many passages. However, as growth factors
were removed cells further differentiated into more specific neural cell types
such as oligodendrocytes and astrocytes [14]. The cells that were
preferentially differentiated were injected into a rat model of a human
hereditary myelin disorder, Pelizaeus-Marzbacher disease, and effectively
remyelinated the axons of the brain and spinal chord [14]. These results as
well as others [15,16] demonstrated the potential to manipulate the
differentiation of isolated hESCs in vitro for therapeutic treatment of
human disease.
Embryonic
stem cells and their regulation of the cell cycle
A
major difference between stem cells and somatic cells is found in the basic
regulation of the cell cycle. In somatic cells the cell cycle is controlled
mainly by Rb-E2F family complexes, cyclin-cyclin dependent kinases (Cdks), and
Cdk inhibitors through the INK4a/ARF pathway. Undulations in expression and
post-translational modifications of the proteins involved in these pathways
result in the control and regulation of the cell cycle. Likewise, mutations or
de-regulation of these proteins can lead to uncontrolled cell proliferation,
aneuploidy, and genomic instability [17,18].
The
cell cycle regulatory mechanisms, which differ between somatic cells and ESCs
have been determined using the mESC model in combination with mESCs
representing a pluripotent lineage (mEPLC) [19]. mESCs of late
pre-implantation and early post-implantation embryos proliferate at an
unusually rapid rate [20]. Between 4.5 and 6.0 dpc (days post coitum), the
epiblast expands with a generation time of approximately 10 hours [21]. This
increases between 6.5 and 7.0 dpc, where mean generation times are found to be
approximately 4.4 hours [21,22]. The cell cycle in mESCs and mEPLCs has been
found to curtail G1 and G2 phases with an increased proportion of the cycle,
approximately 50-60%, spent in S phase [23,24].
Under normal somatic cell cycle
conditons, Rb/p105, in the hypophosphorylated state, interacts with E2F
transcription factors inhibiting the transcription of genes necessary for the
progression of the cell cycle through the restriction point (R point). The
phosphorylation levels of Rb/p105 are dependent upon the CDK activity present
in the cell. Mitogen signaling through the Ras/Raf/mitogen activated protein
kinase (MAPK) pathway activates the cyclin D - CDK4/6 complexes, which are
believed to initially activate Rb/p105 activity by hypophosphorylating the
unphosphorylated protein. To pass the R point of the cell cycle cyclin E/CDK2
hyperphosphorylates Rb/p105 inhibiting the protein from binding to E2F
transcription factors thus initiating the transcription of genes required in
the S phase of the cell cycle. To obtain a cell cycle that is less influenced
by mitogen variations, stem cells appear to adopt a different regulation
mechanism as depicted in Figure 1 [25,26].
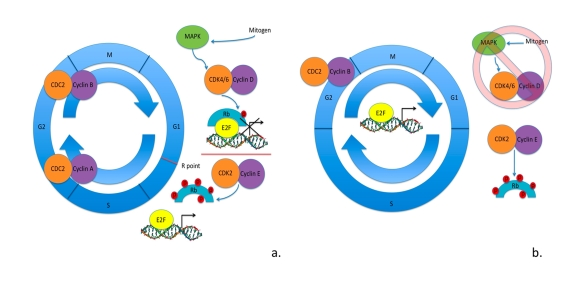
Figure 1. Cell cycle in somatic cells vs. ESCs. (a) Cell cycle regulation
in somatic cells: mitogen signaling through MAPK pathway activates cyclin D
- CDK4/6 kinase activity hypophosphorylating Rb family member proteins.
Hypophosphorylated Rb family member proteins bind to E2F transcription
factors blocking the transcription of E2F-regulated genes. To surpass the
R point cyclin E - CDK2 kinase activity is activated hyperphosphorylating
Rb family member proteins. Hyperphosphorylated Rb family member proteins
are unable to interact with E2F factors, allowing them to activate
transcription of genes necessary in the progression of cell cycle. (b)
Cell cycle regulation in ESCs as is currently understood. Mitogen
signaling through MAPK pathways seems to be irrelevant in the progression
of cell cycle. There is cell cycle-independent expression of cyclin E -
CDK2 maintaining the hyperphosphorylated levels of Rb family member
proteins. This results in cell cycle-independent expression of
E2F-regulated genes. Cyclin B - CDC2 is the only CDK activity that appears
to be regulated by the cell cycle. ESCs have shortened gap phases and an
elongated S phase of the cell cycle, with an apparent lack in the R point
for G1-S transition.
Along
with shortened gap phases in the ESC cell cycle, the R point does not seem to
regulate the G1 - S transition. Stead and collegues found that in both mESCs
and mEPLCs there was a precocious cell cycle-independent expression of CDK2,
cyclin A and cyclin E kinase activity [24]. Furthermore, when CDK2 was
suppressed they found a significant decrease in cell proliferation rate.
Instead CDC2 - cyclin B, essential to G2 - M transition, was the only CDK activity
that was found to be cell cycle-dependent and E2F target genes were
constitutively expressed throughout the cell cycle [24]. Evidence has also
shown a lack in hypophosphorylated Rb/p105, instead findings support the
presence of hyperphosphorylated Rb/p105 in mESCs and mEPLCs [23,27]. Given
the cell-cycle independent expression of cyclin E and CDK2, it would be
logical that Rb/p105 would be found in the hyperphosphorylated state, further
supporting the absence of the R point in ESC cell cycle progression (Figure 1).
Mitogen
signaling through the MAPK pathway normally stimulates cell division in somatic
cells, however, this signaling when prolonged is a potent inducer of
differentiation. mESCs appear to avoid this stimulation by maintaining low
levels of cyclin D expression and almost no detectable CDK4 kinase activity
[28]. This corresponds to the lack in hypophosphorylated Rb/p105 levels
previously detected in mESCs. These findings support the absence of early G1
in mESCs, allowing them to avoid the differentiation-inducing effects of MAPK
signaling as is found in other cell types.
Although
the majority of studies thus far have been performed in mESCs, hESCs similarly
show a truncation of the G1 phase of the cell cycle, however not much else is
known about cell cycle regulation in hESCs. Interestingly, primate ESCs behave
similarly to mESCs in having cell cycle-independent expression of cyclin E,
constitutive hyperphosphorylation of Rb/p105 and serum and MAPK-independent
cell cycle progression [28,29]. Therefore, it could be inferred that through
conserved evolution hESCs may regulate the cell cycle in a similar fashion.
Taken together these data lead to the hypothesis that the ESC cell cycle is
rate-dependent upon high levels of CDK activity, is not regulated by Rb/p105 or
E2F gene expression, lacks the G1 check point and the traditional periodicity
found during the somatic cell cycle.
Adult
stem cell characteristics
The
first evidences of adult stem cells were described as lympho-haematopoietic
stem cells, which were capable of giving rise to both erythroid and lymphoid progeny [30]. The previous medical studies, showing the
capability of bone marrow to regenerate a transplanted patient's bone marrow
attributed credibility to these finding [31]. Presently, adult stem cell
tissue regeneration is not a foreign concept and it is well accepted as the
regenerative mechanism in tissues such as the intestinal epithelium, bone
marrow, and skin. The almost constant regeneration of these tissues has been
linked to tissue specific adult stem cell populations, which when deregulated
have been associated with various diseases and cancers [32,33,34,35]. While
these were the most physiologically obvious tissues in which stem cell
regeneration could occur, adult stem cell populations have also been identified
and characterized in the retina [36], the pancreas [37], the liver [38], the
central nervous system [39], and in skeletal muscle [40].
The
most heavily studied populations of ASCs are the haematopoietic and mesenchymal
stem cells (HSCs and MSCs). HSCs are the progenitor lineage that produces all
of the mature blood cells throughout an organism's life. It was originally
noted that the HSC population contained two populations of stem cells, which
responded differently to radiation [41]. The cycling population was unable to
repair DNA-damage and produced acute marrow failure, whereas the more primitive
quiescent population appeared to repair radiation induced damage [41]. These
findings have been further supported and outline the classification of HSCs
into the two following groups: the long-term repopulating HSCs (LTR-HSCs),
primarily found in a quiescent state, and the short-term repopulating HSCs
(STR-HSCs), which undergo haematopoiesis supplying the daily replenishment of
mature blood cells. This mechanism that HSCs have adopted has allowed the
progenitor population of LTR-HSCs to maintain genomic integrity by reduced
replication events [42]. MSCs are a cellular population found in the bone
marrow alongside the HSCs, which differentiate into cells of the mesenchymal
lineage including bone, cartilage, fat, connective tissue, muscle and marrow
stroma [43,44]. The MSC population is quite heterogeneous and more recently
multiple pre-MSC lineages have been described: MAPC, hBMSC, USSC, FSSC, AFS,
MIAMI cells, hFLMPC, and MASC [45,46,47,48,49,50,51,52]. Pre-MSCs have
been shown to differentiate and form the three germinal layers, furthermore
multiple lineages have been shown to proliferate without telomere shortening
[42]. These lineages speak to the complexity of the regenerative mechanism
that still has yet to be well defined.
Aside
from haematopoietic and mesenchymal stem cells (HSCs and MSCs) the identified
adult stem cell populations have been onerous to study, due to the difficulty
in isolating and culturing the cells in vitro [35]. Since their
discovery, it has been understood that adult stem cells reside in niches that
supply the cells with necessary growth factors and stimulation to undergo
self-renewal and proliferation [53,54]. When these growth factors are applied
to in vitro culture conditions, viable adult stem cell culture has been
achieved [55,56,57,58,59,60]. However, there is still restricted
understanding of these adult stem cell populations and their properties.
Genomic studies, utilizing microarray
technology, have identified molecular signatures for specific and across
diverse populations of stem cells [61,62,63]. Particular genes were found to
span both ESCs as well as diverse adult stem cell lineages. These studies
concluded that while many of these genes were ubiquitously expressed in other
tissues, a subset of this grouping could represent genes involved in general
stem cell growth and maintenance [35]. More recently, Rossi et al. using an
oligonucleotide microarray, identified 907 out of 34,000 genes that were
significantly differentially expressed between HSCs from young and old mice,
sixteen of the genes more highly expressed in older animals have been
implicated in human leukemia. [64,65,66]. A similar study found that genes
of the functional categories DNA repair, chromatin remodeling, and silencing
genes were expressed less in HSCs from aged animals. These findings may
suggest genetic and epigenetic alterations that are responsible for the
differences observed between young and old HSCs.
Adult
stem cells and their regulation of the cell cycle
Adult
stem cells, differing from ES cells, maintain a quiescent state in vivo
unless they are stimulated by tissue damage or regenerative signals to
differentiate. In normally dividing cells entering into the quiescent state
there is an upregulation of CDK inhibitors, which act to block the kinase
activity of CDKs effectively blocking cell proliferation [67,68,69,70,71].
Further-more, CDK inhibitor expression is independently sufficient to inhibit
proliferation [67]. CDK inhibitor expression is found in quiescent ASCs and
when downregulated can initiate proliferation and differentiation in HSCs [72,73,74].
Unfortunately,
due to the limitations in the data that are currently available, it is not
possible to construct a detailed ASC cell cycle model. From what has been
gleaned of the ESC model, it is believed that the G1 phase and the R point are
critical in the decision between self-renewal and differentiation, as well as
the directionality of differentiation. Much of the data seems to suggest that
the mechanisms regulating the cell cycle are extrinsically supplied from the
cellular microenvironment, the niche. However, more studies will be needed
before we can truly understand the roles of the regulatory protein mechanisms
recognized for so long as the classical cell cycle model.
Aging
and maintenance of adult stem cells
Self-renewal
of stem cells is critical for their persistence through life, however the
capacity to maintain this characteristic declines with age [75,76]. The
decline in the maintenance of the self-renewal pathway is considered one of the
major mechanisms attributing to aging. p16Ink4a, a cyclin-dependent
kinase inhibitor, promotes Rb/p105 activation and is associated with the
triggering of the senescence pathway [77]. It has more recently been ascribed
to stem cell aging and loss in self-renewing properties [75,78]. In fetal
stem cell populations there is no detectable expression of p16Ink4a,
however increasing levels of p16Ink4a expression have been detected
in stem cells of aging tissues [76,79].
TheINK4a/ARF tumor suppressor locus encodes for p16Ink4a and p19Arf,
which act respectively through the Rb and p53 cell death pathways [80]. The INK4a/ARF
locus is activated in tissues under oncogenic stresses, such as DNA damage and
telomere shortening. p16Ink4a then acts to inhibit the kinase
activities of cyclin D1 - CDK4, cyclin D2 - CDK4, and cyclin D3 - CDK6. D-type
cyclin and CDK complexes phosphorylate Rb/p105, when in the hypophosphorylated
form Rb/p105 binds to E2F-1, 2, 3, and 4 blocking their activity as
transcriptional activators. E2F target genes are required for progression of
the cell cycle and their transcriptional repression results in G1 cell cycle
arrest and eventual replicative senescence. p19Arf interacts with
p53 initiating p53-dependent cell death, or apoptosis. p19Arf can
also slow the cell cycle and lead to senescence. Similar to p16Ink4a,
p19Arf is not expressed in fetal stem cells but is found to increase
in aging stem cells [76,79].
To
maintain their replicative and self-renewing potential stem cells have in place
mechanisms to repress activation of cell death pathways. Bmi-1 has been shown
to promote self-renewal in stem cells by repressing the expression of p16Ink4a
and p19Arf through negative regulation of the INK4a/ARF locus
(Figure 2). In Bmi-1-/- neural stem cells, Molofsky and
collegues found overexpression of p16Ink4a and reduced rates of
proliferation [81,82]. Park and collegues determined that Bmi-1 is essential
for the self-renewal of HSCs. They utilized a Bmi-1-/- mouse
model and showed that there was an increase in both p16Ink4a and p19Arf
expression in HSCs leading to proliferation arrest and p53-dependent cell
death. The subsequent loss of p16Ink4a expression in Bmi-1-/-cells was able to partially rescue the self-renewal capacity of the stem
cells [82,83].
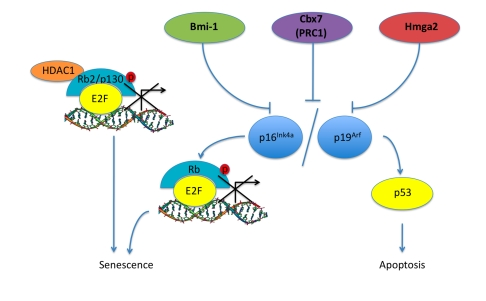
Figure 2. Pathways associated with aging in ASCs. Bmi-1, Cbx7
(PRC1), Hmga2 are proteins that have been shown to increase in expression
levels in aging ASCs along with corresponding inhibition of the INK4a/ARF
locus leading to a progression into senescence and apoptosis. Rb2/p130
also shows an increase in senescent MSCs, this could be a result of HDAC1 -
Rb2/p130 complex repressing E2F target gene transcription and initiating
the senescence pathway.
Bmi-1 is a Polycomb group (PcG) RING
finger protein found to associate with the multiprotein PcG complex PRC1. PRC1
is a complex that maintains the repressive state of heterochromatin by
modifying histone protein complexes. It includes at least one paralogue of the
Pcgf, Ring1, Phc and Cbx components as has been described [84]. PRC2 is the
second complex that can associate with heterochromatin. It is hypothesized
that both PRC1 and PRC2 act in maintaining the heterochromatic structure
necessary for stem cell self-renewal and with age it is believed that these
mechanisms become inadequate and can lead to neoplastic transformation of stem
cell populations. In ESCs mutant for PRC2, there is a loss in the ability to
maintain an un-differentiated state of self-renewal [85,86]. Futhermore, both
PRC1 and PRC2 have been shown to be inter-dependent in their effects on stem
cell self-renewal and cancer development [87,88].
In
similar studies, other PcG proteins have been found to affect the INK4a/ARF
locus in aging stem cells. Jacobs and collegues show that Mel-18 and Cbx7 were
found to regulate the INK4a/ARF locus [81]. Cbx7 is a PcG protein that
helps form PRC1, this protein was independently found to extend the lifespan of
primary human cell lines (Figure 2) [89]. When introduced into mouse
fibroblasts, Cbx7 can result in immortalization of the cell line through the
downregulation of the INK4a/ARF locus and interference with the p16Ink4a/Rb
and p19Arf/p53 tumor suppressor pathways [89].
The
age-dependent decline in ASC self-renewing capacity has been associated with
various transcriptional regulators other than the PcG proteins discussed above.
Nishino
and colleagues have recently discovered the involvement of Hmga2 in stem cell
self-renewal [90]. Hmga2 is a transcriptional regulator and was found to be
highly expressed in young neural stem cell populations in mice. Its expression
declines with age and is believed to be regulated by the microRNA let-7b.
Induced expression of let-7b in stem cells with high levels of Hmga2, showed a
decrease in Hmga2 levels in concordance with a decreased ability for
self-renewal. This corresponded to increased expression of p16Ink4a
and p19Arf (Figure 2). Furthermore, in mice deficient for Hmga2
there were reduced stem cell numbers and self-renewal throughout the central
and peripheral nervous system of fetal and young-adult mice [90].
Owing
to the fact that the regulation of the ASC cell cycle is still not completely
understood and because of the importance that the INK4a/ARF locus seems
to play in aging ASCs, we performed a study on the senescence of rat MSCs in
vitro looking specifically at the expression levels of Rb family proteins
[91,92]. We observed that the induction of senescence was associated with a
decrease in expression of genes involved in stem cell self-renewal, DNA-damage
repair genes, p107 and Rb/p105. However, Rb2/p130 expres-sion surprisingly
increased during senescence in MSCs [92]. This suggested that Rb2/p130 plays a
prominent role in either MSC specific aging and/or senescence. It has
previously been shown that Rb2/p130 can bind to HDAC1 repressing E2F-dependent gene expression, such as cyclin A,
ultimately resulting in G0 growth arrest, supporting the possibility
that Rb2/p130 has a more global role in cellular senescence (Figure 2) [92,93].
Over the past decade evidence has mounted in favor of
the hypothesis that stem cell self-renewal is regulated through heterochromatin
conformation under the control of PcG protein complexes. This regulation
appears to repress the INK4a/ARF locus, thus inhibiting the progression of tumor suppressive mechanisms such
as senescence and apoptosis. However, other regulatory mechanisms are present
as seen with Hmga2, let-7b, and Rb2/p130, therefore the story is far from
complete. The necessity for stem cells to maintain self-renewability appears to
be in balance with the risk to enter into un-controlled proliferation and
possibly cancer. Further studies are necessary to clarify how these mechanisms
play a role in the self-renewal, main- tenance,
and senescence of stem cells.
Concluding
remarks
The
area of stem cell research is vastly and rapidly expanding with the hope of its
potential in therapeutic applications. In this review we have discussed the
current characterizations and understandings of ESCs and ASCs. ESCs have been
utilized not only to understand development, but to obtain a manipulative
system that could be applied towards regenerative and disease based therapies.
The first stem cell trial of this kind has just recently been approved by the
Food and Drug Administration for Phase I clinical trial and is based upon the
pre-clinical studies published in 2005 on hES cell-derived oligodendrocytes and
their ability to remyelinate and restore function of the spinal chord in mice
after injury [94]. This is the first example of a therapeutic potential that we
have yet to reap and that will surely be expanded upon in the years to come.
Utilizing
and understanding ESC differentiation in vitro may help elucidate the
ASC populations, which until now have been tedious to isolate and lack accurate
and universal cell markers. Here we have outlined the current understanding of
ESC populations and their regulation of the cell cycle. More importantly we
highlight the significance of maintaining a self-renewable population of ASCs
and the regulation mechanisms that have been associated with this maintenance.
The pathway that appears to be involved across the board is the INK4a/ARF
CDK-inhibitor pathway, which regulates the two major mechanisms of cell death,
senescence and apoptosis. Both p16Ink4a and p19Arf have
been found to be un-detectable in young stem cell populations but increase in
aging populations, lending to the importance of these pathways in stem cell
maintenance. As we have discussed, there are multiple genetic and epigenetic
factors that appear to be associated with the INK4a/ARF pathway
regulation in ASCs, speaking to the complexity and the profundity of what we
have yet to ascertain.
There
is a cogent belief that organismal aging is linked to the aging and the loss of
functional ASC populations. The data discussed here support the role of
senescence and apoptosis as self-regulative mechanisms in aging ASCs.
Clarification and in-depth comprehension of these pathways may unveil needed
therapeutic potentialities that could be applicative to both aging and
age-related diseases.
This
work was supported by the Sbarro Health Research Organization (www.shro.org)
and Human Health Foundation (www.hhfonlus.com).
The
authors declare no conflict of interests.