Introduction
There is a growing interest in the role
of macroautophagy, herein simply termed autophagy, in both normal homeostasis
and in a variety of pathological conditions [1,2]. This
interest has been sparked in part by observations suggesting that in lower
organisms, autophagy is an important determinant of lifespan [3]. For instance,
in C elegans, the life extending effects of mutation in the daf-2
pathway requires an intact autophagy program [4,5]. Similarly, in the worm, the increase in lifespan seen with
dietary restriction is not evident when autophagy is impaired [6]. Consistent
with these observations, genetic manipulations that can increase autophagy in Drosophila
result in flies with an extended lifespan and an increase in overall stress
resistance [7].
Less is known regarding the role of autophagy in
mammalian systems. Prior to the establishment of the molecular and biochemical
basis for autophagy, it was well appreciated that aging tissues were often
characterized by the accumulation of damaged cellular components. In addition,
consistent with a defect in autophagy, it was also evident that animal tissues
exhibited an age-dependent decline in the turnover rates of long lived proteins
[8]. These and
other studies have suggested that autophagic flux declines with age and that
the magnitude and timing of this decline is in general concordance with the
age-dependent accumulation of damaged proteins and organelles seen within aging
tissues. Recent genetic mouse models have strengthened this association. While
complete knockouts of essential autophagy genes appear to be lethal in the
neonatal stage, various conditional knockout models have been recently
described. Among these recent results are the description of tissue specific
deletions of essential autophagy genes in liver, brain, pancreas and heart [9-14]. While
significant differences exist in these various model systems, most were
characterized by the rapid appearance of various pathologies and physiological
impairments that can also be observed as a consequence of normal aging.
Relatively little is known about the downstream
mediators of the often profound physiological alterations observed following
the disruption of autophagic flux. Most likely there is no single pathway
across all tissues and organs, and even within a single tissue type, multiple
mediators may exist. For instance, while the accumulation of misfolded and
aggregated proteins normally cleared in part by autophagy are likely to play a
prominent role in models of neurodegeneration, in other tissues, the role of
accumulation of damaged protein aggregates is less clear. This tissue
specificity was reinforced by recent observations demonstrating that deletion
of p62, a ubiquitin and LC3/Atg8 binding protein, rescues the pathological
changes observed in autophagy deficient liver but does not appear to alter the
phenotypic changes seen following deletion of Atg7 in the brain [15].
One important function of autophagy is
the turnover of organelles including mitochondria. While several reports have
described the structural changes in mitochondrial appearance evident in
electron micrographs taken of autophagy deficient mammalian tissues [9,12-14], the
functional alterations, if any, of these mitochondria have not been reported.
Here, using a variety of cellular and in vivo models of Atg7 deficiency,
we have assessed the magnitude of mitochondrial dysfunction and the
contribution of the corresponding oxidative stress in mediating the
physiological impairment observed following disruption of autophagic flux.
Results
Mitochondrial dysfunction in Atg7 deficient skeletal
muscle
In an effort to more fully characterize the role of
autophagy in the maintenance of normal mitochondrial function, we created a
conditional knockout model in which Atg7, an essential gene required for
autophagosome formation, was deleted from mouse skeletal muscle. We initially
chose this model because skeletal muscle is an abundant tissue that is also a
rich source of mitochondria. As expected, muscle protein lysate from animals
expressing the Cre recombinase under the control of the muscle creatine kinase
(MCK) promoter demonstrated reduced Atg7 expression when compared to skeletal
muscle tissue obtained from control mice (Figure 1A). Coincident with a
reduction in Atg7 expression, we noted a marked increase in the level of p62, a
protein cleared in large part through autophagy and whose levels are routinely
used as a marker of overall autophagic flux [16]. Although
these results suggest that autophagy in skeletal muscle was largely impaired in
these conditionally ablated animals, when compared to control animals, we
observed no obvious differences in terms of viability, overall size and
activity, serum parameters or generalized appearance of the Atg7F/F:MCK-Cre
mice throughout the first year of life (unpublished observations). In addition,
histological analysis revealed no discernable skeletal muscle structural
abnormalities between knockout and control mice (Figure 1B). In contrast,
electron micrographs demonstrated that Atg7F/F:MCK-Cre mice accumulated
markedly abnormal mitochondria that were especially evident in the
sub-sarcolemma region of muscle fibers. These abnormal mitochondria appeared
less electron dense and were often swollen, lacking in cristae, or dysmorphic
in appearance (Figure 1C). Despite these profound differences in mitochondrial
appearance, we observed no obvious alterations in the composition of the
various cytochrome complexes (Figure 1D), nor were there obvious differences in
the assembly of individual electron transfer components using Blue Native Gel
analysis (Figure 1E).
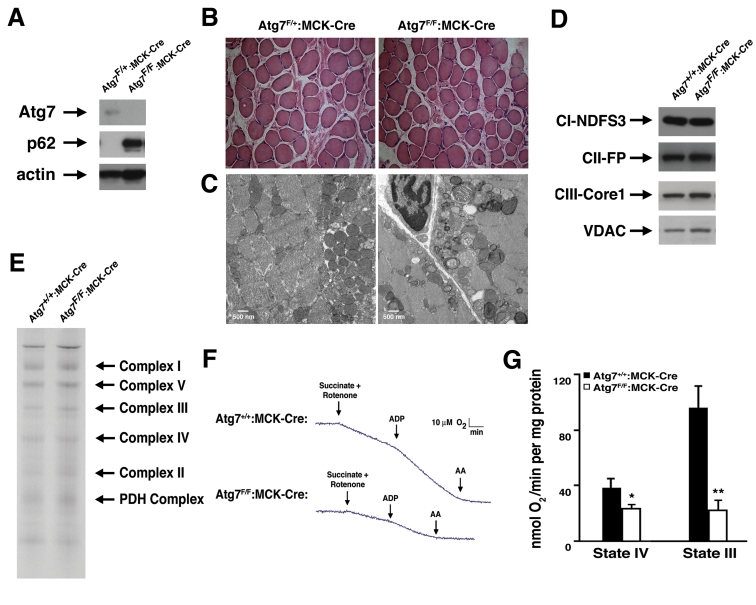
Figure 1. Impaired mitochondrial function in Atg7 deficient skeletal muscle. (A)
Western blot analysis of protein lysate obtained from mice with a
conditional deletion of Atg7 within skeletal muscle (Atg7F/F:MCK-Cre)
or control animals (Atg7F/+:MCK-Cre). Relative levels of Atg7,
p62 and actin (loading control) were assessed. (B) Representative
stained histological sections from soleus muscle. (C) Electron
micrographs of skeletal muscle from 8 week old Atg7F/+:MCK-Cre
or Atg7F/F:MCK-Cre mice. Electron micrographs demonstrate the
accumulation of dysmorphic mitochondria within the Atg7F/F:MCK-Cre
muscle. (D) Western blot analysis of purified mitochondria obtained
from Atg7F/F:MCK-Cre or control animals Atg7+/+:MCK-Cre
demonstrating an apparent similar level of electron transfer subunit
composition. Mitochondrial protein lysates were probed for the Complex I
component protein NDFS3 (NADH-ubiquinone oxidoreductase 30 kDa subunit),
the Complex II component FP (flavoprotein subunit of complex II ), the
Complex III protein Core 1 (Ubiquinol-cytochrome-c reductase complex core
protein 1) and the mitochondrial membrane protein VDAC. (E) Blue
Native gel electrophoresis using mitochondrial extracts isolated from
control and Atg7-/- tissues demonstrating similar stoichiometry
and assembly of electron transfer complex components. (F)
Representative oxygen consumption tracings of mitochondria isolated from
the skeletal muscle of 12 month old Atg7+/+:MCK-Cre or Atg7F/F:MCK-Cre
mice. Atg7-deficient skeletal muscle demonstrated a pronounced reduction in
respiration when assessed in the presence of the Complex II dependent
substrate succinate. Rotenone was routinely added to prevent reverse
electron transport. Measurements were made in the absence (State IV) and
presence (State III) of ADP and following the Complex III inhibitor
antimycin A (AA). (G) Composite determinations of mitochondrial
respiration in the presence of succinate and rotenone. Graph represents the
mean +/- SEM from Atg7+/+:MCK-Cre (n=3) or Atg7F/F:MCK-Cre
mice (n=3). * p≤0.05; ** p≤0.01.
In order to test whether the observed alterations in
mitochondrial appearance were also accompanied by corresponding functional
changes, we measured the basal and stimulated respiration of mitochondria
isolated from Atg7F/F:MCK-Cre and control animals. Preliminary
experiments suggested that while Complex I dependent respiration was reduced,
Complex II dependent respiration was even more impaired in Atg7 deficient
muscle. Using succinate as a substrate, mitochondrial function was noted to be markedly reduced when we tested mitochondria
isolated from Atg7 deficient animals (Figure 1F). These defects were evident
under basal conditions (State IV) and even more so, under conditions of maximal
stimulated respiration (State III) induced by the addition of ADP (Figure 1G).
Atg7-/- MEFs demonstrate impaired cellular
respiration and increased ROS levels
In order to further characterize the defect in
mitochondrial function within the context of intact cells, we next isolated
mouse embryonic fibroblasts (MEFs) from wild type or Atg7-/- embryos
(Figure 2A). Compared to WT MEFs, Atg7-/- MEFs exhibited a reduction
in basal oxygen consumption (Figure 2B). In addition, we noted that Atg7-/-
MEFs demonstrated a marked reduction in maximal mitochondrial oxidative
capacity, as assessed by the levels of FCCP-stimulated
respiration. These differences were not a result of any apparent differences
in overall mitochondrial numbers between the two cell types (Figure 2C).
Coincident with this decrease in mitochondrial oxygen consumption, we noted
that Atg7-/- MEFs generated more lactic acid, consistent with an
increase reliance on glycolysis (Figure 2D). This shift away from aerobic
respiration and towards cytosolic glycolysis in Atg7-/- MEFs
presumably represents a compensatory mechanism to maintain intracellular
energetic homeostasis in the setting of dysfunctional mitochondria.
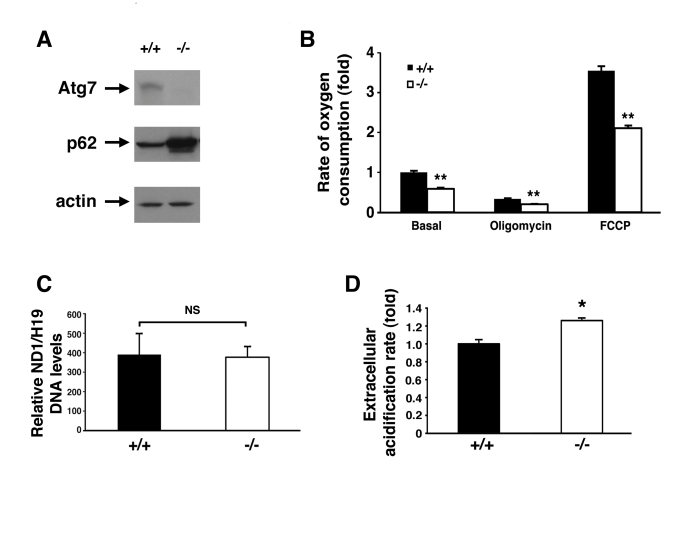
Figure 2. Alterations in the energetics of Atg7 -/- MEFs.
(A) Western blot
analysis of wild type (+/+) or Atg7-/- MEFs for the expression
of Atg7, p62 and actin (loading control). (B) Measurement of oxygen
consumption for WT and Atg7-/- MEFs under basal conditions,
following the addition of the mitochondrial electron chain inhibitor
oligomycin (0.5 μM), or in the presence of the mitochondrial uncoupler FCCP
(1 μM), to determine maximal oxidative capacity. Shown is the average fold
change +/- SEM in oxygen consumption (WT MEFs basal respiration=1) obtained
from 5 experiments each performed in triplicate. (C) Assessment of
mitochondrial number in WT or Atg7-/- MEFs. DNA was isolated
from WT (n=3 independent WT MEF cell isolates) and Atg7-/- MEFs (n=3
independent Atg7-/- MEF cell isolates) and quantitative PCR
analysis performed for the mitochondrial-encoded gene ND1 and the
nuclear-encoded gene H19. (D) Relative extracellular acidification
rates indicating lactic acid production and hence glyolytic rates in WT or
Atg7-/- MEFs. Shown is the average +/- SEM fold change in
lactic acid production from 8 experiments each performed in triplicate. *
p≤0.05; ** p≤0.01.
Damaged mitochondria often produce increased levels of
reactive oxygen species (ROS). This increase in ROS can further increase
mitochondrial damage leading in turn to more oxidant release and additional
mitochondrial damage, in a process termed the ‘vicious cycle'[17]. In some
circumstances, this perpetuating cycle of mitochondrial damage and oxidative
stress is thought to contribute to normal aging as well as many age-related
diseases [18]. Given the
above observations, we next sought to assess whether continuous oxidative
stress was evident in autophagy deficient cells. As noted in Figure 3A, Atg7-/-
MEFs had increased levels of intracellular ROS. Culturing these cells in the
presence of the antioxidant N-acetylcysteine (NAC) resulted in a reduction in
ROS levels (Figure 3B).
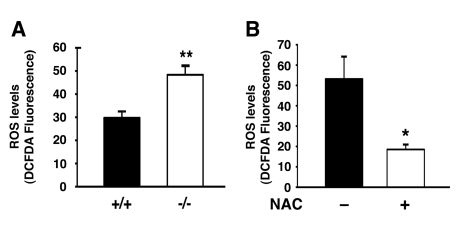
Figure 3. Atg7 deficient cells exhibit increased levels of ROS. (A)
Intracellular ROS levels as assessed by DCFDA fluorescence intensity
(arbitrary units) in WT and Atg7-/- MEFs. ROS measurements were
made from three independent WT or Atg7-/- MEF primary cell
isolates and the fluorescent intensity of more than 250 cells of each
genotype were assessed. (B) NAC treatment reduces the levels of ROS
in MEFs lacking Atg7. Levels of ROS were assessed by DCFDA fluorescence in
Atg7-/- MEFs untreated or treated with NAC (500 μM) for 4 days
prior to imaging. Values represent the normalized fluorescent intensity
(arbitrary units) of approximately 300 cells per condition. Graphs
represent the mean +/- SEM.
Antioxidant treatment did not appear to alter the
level of autophagic flux in Atg7-/- MEFs as the level of p62 was
unaltered in NAC treated cell (Figure 4A). However, chronic NAC treatment did
partially ameliorate the observed metabolic defect seen in these cells (Figure 4 B, C). These results suggest that the continuous oxidative stress observed
in Atg7-/- MEFs contributes to the decline in mitochondrial
function.
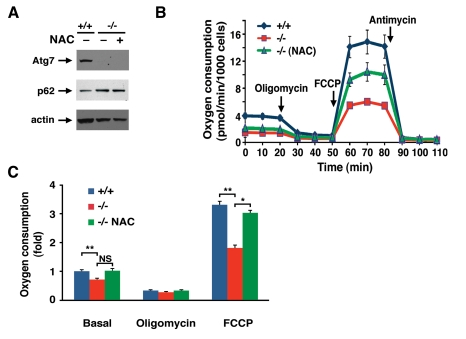
Figure 4. NAC treatment partially corrects the metabolic defect observed in Atg7 -/- MEFs.
(A) Western blot analysis of wild type (+/+) or Atg7-/-
MEFs for the expression of Atg7, p62 and actin (loading control) cultured
in the presence or absence on the antioxidant NAC (500 μM) for ten days. (B)
Primary wild-type and Atg7-/- MEFs that were cultured in the
absence or presence of 500 μM NAC for 10
days prior to cellular respiration measurement. Shown is a representative
tracing of oxygen consumption performed in triplicate under basal
conditions, following the addition of oligomycin (0.5 μM), the
pharmacological uncoupler FCCP (1 μM) or the Complex III inhibitor
antimycin A (0.25 μM). (C) Averaged metabolic profile from 4
separate experiments employing 3 independent primary isolates of WT and
Atg7-/- MEFs. Shown is the fold change +/- SEM in oxygen
consumption (WT MEF basal respiration =1) for WT MEFs and for Atg7-/-
MEFs that were cultured in the absence or presence of 500 μM NAC for 10
days prior to metabolic assessment.* p≤0.05; ** p≤0.01; NS= not
significant.
Oxidative stress and glucose intolerance in pancreatic
Atg7-/- mice
Given the profound alterations observed in isolated
mitochondria derived from Atg7 deficient skeletal muscle and the observation that
NAC treatment could at least partially reverse the metabolic defects observed
in Atg7-/- MEFs, we next sought to assess whether these principles
could be applied to the physiological defects seen in an in vivo model
of Atg7 deficiency. Since our skeletal muscle conditional Atg7-/-
mice did not exhibit an overt phenotype, we created an additional model in
which Atg7 was deleted within pancreatic β cells by crossing the
Atg7-floxed mice with RIP2-Cre animals. Western blot analysis from purified
pancreatic islets demonstrated that conditional knockout animals (Atg7F/F:RIP2-Cre)
had reduced or absent Atg7 expression and a corresponding increase in p62
levels (Figure 5A). In young mice, deletion of Atg7 within β cells did not
result in significant alterations in pancreatic insulin expression (Figure 5B).
Similarly, the cellular composition of individual pancreatic islets was
largely unperturbed in 8 week old mice (Figure 5 C, D). In contrast, electron
micrographs of control or knockout tissues revealed the early accumulation of
markedly abnormal mitochondria within the β cells of Atg7 deficient mice
(Figure 5E). Analysis of basal and FCCP-stimulated respiration from isolated
pancreatic islets revealed a significant decrease in basal mitochondrial
respiration and a marked decrease inmitochondrial oxidative capacity in Atg7
deficient islets (Figure 5F). Consistent with previous reports describing
animals with alterations in β cell mitochondria [12,13,19],
mice lacking Atg7 within their β cells also exhibited marked abnormalities
in glucose tolerance (Figure 5G).
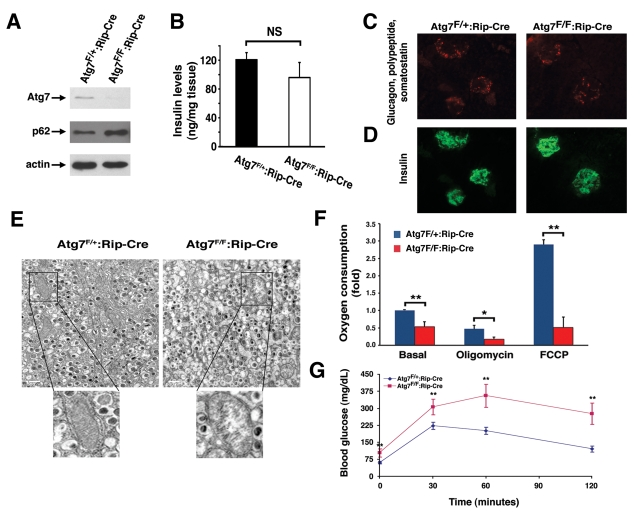
Figure 5. Mice deficient in Atg7 expression within pancreatic β cells demonstrate altered mitochondria. (A) Western blot analysis of purified
pancreatic islets obtained from Atg7F/+:Rip2-CRE or Atg7F/F:Rip2-CRE
mice demonstrating the relative expression of Atg7, p62 and actin (loading
control). (B) Intracellular insulin levels (mean +/- SEM) in
pancreatic tissue of 8-9 week old Atg7F/+:Rip2-Cre (n=4 mice) or
Atg7F/F:Rip2-Cre mice (n=5 mice). The slight reduction in
insulin levels in the Atg7F/F:Rip2-Cre mice was not significant
when compared to the control. (C) Pancreatic sections of control
Atg7F/+:Rip2-Cre or Atg7F/F:Rip2-Cre mice were
stained for non-β cell components within the islets with the
simultaneous use of anti-glucagon, anti-somatostatin, and anti-polypeptide
antibodies. (D) Serial sections were used to visualize β cells
with an anti-insulin antibody. Eight week old mice lacking autophagy in
β cells have qualitatively similar levels of α, δ, and polypeptide producing cells
within their islets, as well as similar levels of β cells when
compared to control mice. (E) Electron micrographs demonstrating the
accumulation of swollen, dysmorphic mitochondria within the Atg7-deficient
β cells. (F) Isolated islets from control and Atg7-/-
mice were assessed for fold +/- SEM changes in basal respiration (Atg7F/+:Rip2-Cre
isolated islets=1), and for oxygen consumption in the presence of
oligomycin (0.5 μM) or FCCP (0.5 μM). Results are normalized to islet
protein concentration and are from n=4 mice per genotype. (G)
Impaired glucose tolerance in Atg7F/F:Rip2-Cre mice. Blood
glucose measurements were made in 8-10 week-old control mice Atg7F/+:Rip2-Cre
(n=10 mice) or Atg7F/F:Rip2-Cre mice (n=8 mice) following the
IP injection of D-glucose (1 g/kg). Data represent the mean +/- SEM. *p≤0.05;
**p≤0.01.
We next asked what the role of continuous oxidative
stress was in this model of β cell dysfunction. We randomized knockout or
control mice beginning at age 4 weeks to treatment with or without NAC. As
expected, when compared to control animals, mice with conditional ablation of
Atg7 accumulated increased levels of p62 within their islets (Figure 6A).
Treatment with NAC did not noticeably affect this accumulation in conditionally
ablated animals (0/18 islets p62 positive in Atg7F/+:RIP2-Cre mice;
22/24 islets p62 positive in Atg7F/F:RIP2-Cre mice and 17/17 islets
p62 positive in Atg7F/F: RIP2-Cre mice treated with NAC; in random
slides obtained from n=3 mice per condition). In addition, as an in situ
marker of oxidative stress, we measured nitrotyrosine levels which are known to
increase and contribute to the diabetic phenotype [20]. As noted,
levels of nitrotyrosine were markedly elevated in Atg7 deficient islets and in
contrast to our observations with p62, treatment with NAC was very effective in
reducing the observed increase (Figure 6 B, C).
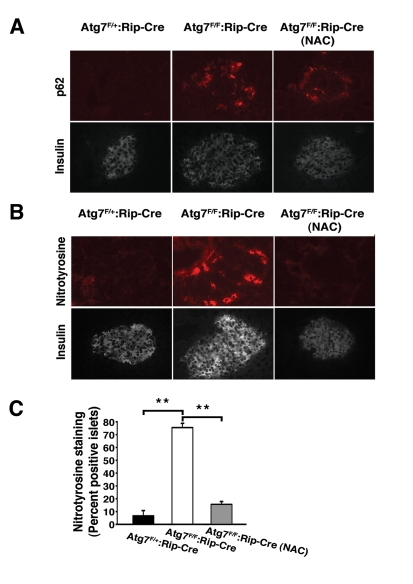
Figure 6. In vivo treatment with NAC reduces oxidative stress within pancreatic β cells.
(A) Atg7F/+:Rip2-Cre
or Atg7F/F:Rip2-Cre mice that were untreated or treated with the
antioxidant NAC for 12 weeks. At 16 weeks of age, mice were sacrificed and
serial sections of pancreatic tissue were analyzed for p62 and insulin or (B)
nitrotyrosine and insulin. (C) Quantification of nitrotyrosine
staining in islets of control mice, Atg7-deficient animals or
Atg7-deficient mice treated for 12 weeks with NAC (n=3 animals per group).
Graph represents the mean+/- SEM. **; p≤0.01.
We next asked whether reducing the levels
of oxidative stress by itself was sufficient to ameliorate the physiological
impairment observed with conditional deletion of Atg7. Littermates were
randomized after weaning to treatment with or without NAC and subsequently
assessed at age 16 weeks. Consistent with continuous oxidative stress playing a
causative role in the underlying physiology, and in contrast to untreated Atg7F/F:RIP2-Cre
mice, NAC treated Atg7F/F:RIP2-Cre
mice had a glucose tolerance response that was indistinguishable from
control mice (Figure 7A). This protection was also seen at later time points,
although the overall degree of rescue appeared to be reduced as the mice aged
(data not shown). The observed differences in glucose homeostasis were not a
result of reduced peripheral insulin sensitivity as insulin tolerance tests
were comparable for all four groups tested (data not shown). Furthermore, mice
lacking Atg7 within their β cells develop a defect in glucose-stimulated
insulin secretion, and this defect was not observed in conditionally ablated
mice treated with an antioxidant (Figure 7B).
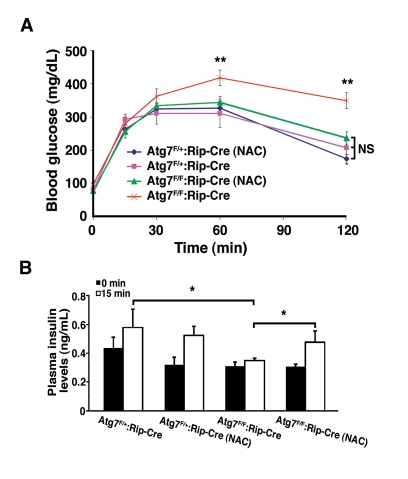
Figure 7. NAC treatment of Atg7 deficient mice prevents the development of a glucose intolerance phenotype. (A) Male Atg7F/+:Rip2-Cre (n=6
mice), Atg7F/+:Rip2-Cre (+NAC; n=6 mice), Atg7F/F:Rip2-Cre
(n=11 mice) or Atg7F/F:Rip2-Cre (+NAC; n=11 mice) were fasted
overnight and subsequently injected with 1 g/kg D-glucose. Serum glucose
levels were measured and the untreated β cell Atg7 deficient mice were
found to be statistically different at the indicated time points, while the
other three groups of mice were statistically indistinguishable over the 2
hr timecourse. (B) Insulin levels were determined by tail vein blood
sampling at time 0 and 15 min following glucose administration: Atg7F/+:Rip2-Cre
(n=6 mice), Atg7F/+:Rip2-Cre (+NAC; n=4 mice), Atg7F/F:Rip2-Cre
(n=8 mice) or Atg7F/F:Rip2-Cre (+NAC; n=5 mice). Data are
represented as the mean +/- SEM. * p≤0.05; ** p≤0.001.
Discussion
Using a variety of cellular and in vivo models,
we demonstrate that impairment of autophagy leads to the accumulation of
damaged and dysfunctional mitochondria and a corresponding increase in
intracellular ROS levels. In a model of autophagy deficiency occurring within
the pancreatic β cell, we further demonstrate that the overall physiological impairment in glucose tolerance and insulin secretion
can be significantly ameliorated by the simple addition of an antioxidant.
While assessment of autophagic flux by p62 expression suggests that NAC
treatment does not directly affect autophagy, the ultimate improvement of
glucose tolerance and glucose-stimulated insulin secretion suggests that at
least for this in vivo model, continuous oxidative stress plays an
important pathophysiological role.
A very recent report has suggested that oxidative
stress may also play a role in the alterations in innate immunity observed in
autophagy deficient cells [21]. In
particular, ROS appeared to mediate the increase in interferon secretion and
resistance to viral infection seen in Atg5-/- cells. The form of
autophagy studied in these experiments is quite specialized and involves the
delivery of viral nucleic acids to the endosome rather than the standard
situation where cargo is delivered to the lysosome. Similarly, it should be
noted that in this context, Atg5 deficient cells demonstrate increased
interferon protection and increased protection from viral infection rather than
the usual situation where autophagy disruption results in a loss of function
phenotype. Interestingly, in this recent study, Atg5-/- MEFs
exhibited an approximate 2-fold increase in the number of mitochondrial
genomes per cell, while for presently unclear reasons, we did not observe a
similar increase in mitochondrial number in our Atg7-/- MEFs (see Figure 2C).
Based on these recent in vitro
observations regarding Atg5 deletion in cells and our in vivo
observations regarding Atg7 deletion in the pancreas, it is tempting to
speculate that a rise in ROS levels may be a universal downstream mediator of the
positive or negative alterations seen in autophagy deficient tissues.
Nonetheless, other evidence suggests that such a broad conclusion is unlikely
to be always correct. For instance, while the accumulation of ubiquitin
positive protein aggregates in inclusion bodies are prominent features in Atg5
and Atg7 deficient neurons and cardiomyocytes, these changes are not observed
in T lymphocytes or dendritic cells that lack Atg5 expression [10, 11, 14, 22, 23].
This may reflect fundamental differences in the role of autophagy in rapidly
dividing versus postmitotic cells. Similarly, even within a single tissue or
organ, the effects of abrogating or inhibiting autophagy cannot always be
easily predicted. For instance, deletion of Atg5 in adult mice leads to the
spontaneous appearance of contractile dysfunction, while the same deletion
performed early in cardiogenesis does not result in any basal myocardial
phenotype [14]. Even less
straightforward are observations that while cardiac specific deletion of Atg5
results in an animal that is less able to withstand myocardial pressure
overload, heterozygous deletion of beclin, another essential autophagy gene,
results in mice with the seemingly opposite cardiac phenotype [14, 24]. Thus,
the downstream mediators and ultimate consequences of inhibiting autophagy may
vary widely depending on the strategy used to disrupt autophagic flux and in
what tissue or organ the disruption occurs.
Our data suggests that antioxidant treatment with NAC
is particularly beneficial in preventing the glucose intolerance phenotype
observed following deletion of Atg7 within β cells. Pancreatic secretion
of insulin is well known to be sensitive to changes in the cellular redox state
and overall mitochondrial function. Given our results on the metabolic profile
of Atg7-/- MEFs cultured in the presence of NAC, it is tempting to
think that in the setting of Atg7 deletion, the in vivo use of
antioxidants interrupts the ‘vicious cycle' of mitochondrial generated ROS
inducing further mitochondrial damage. These observations suggest that
antioxidant targeted therapy might be beneficial for at least a subset of the
growing number of conditions where deficiency or impairment of autophagy is
thought to contribute.
Methods
Mice and cells.
Atg7F/F mice have been previously described [9] and were
crossed with either MCK-Cre mice (Jackson Laboratory) or RIP2-Cre mice (Jackson
Laboratory) to generate mice with a conditional deletion of Atg7 within skeletal
muscle or pancreatic β cells. For genotype analysis, tissues
were digested overnightat 55 °C with Gitschier buffer (67 mM
Tris-HCL,pH 8.8, 16.6 mM ammonium sulfate, 6.5 mM MgCl2,
0.5% TritonX-100, 1% - mercaptoethanol, 100
μg/ml proteinase K). Samples were incubated at 95 °C for 5 min, shaken for 20
min with an Eppendorf thermomixer, and centrifuged at 16,100 x g for 2
min. Three primers 5'- TGGCTGCTACTTCTGCAA
TGA TGT-3', 5'- GCAAGCTCACTAGGC TG CAGAACC-3', and 5'-GGTCCA GAGTCCGGTCTC GG-3'
were used to detect the flox Atg7 allele in genomic DNA.
Cre-mediated
recombination was assessed by PCR analysis of genomic DNA using primers 5'- GGTCTGGCAG TAAAAACTATC-3' and
5'-GTGA AACAGCATTGCTGTCACTT-3',
and 5'-GGGTCC CA AAGGCCGCC-3' and
5'-GGATAGTTTTTACT GCCAGAC CGC-3' for Rip2-CRE and MCK-CRE, respectively [25, 26].
Similar to what has been recently described [12, 13], we observed no obvious
phenotypic differences between Atg7+/+, Atg7+/+: MCK-
Cre, Atg7+/+:Rip2-Cre, Atg7F/F, Atg7F/+:MCK-Cre
or Atg7F/+:RIP-Cre mice, and so we predominantly present the latter
two genotypes as representative controls in this study.
For chronic NAC treatment, randomized 4- week old mice
were treated with 1 g/L of NAC in the drinking water for the indicated duration
of the study. All animal experiments were conducted in accordance with the
guidelines of the Animal Care and Use Committee, National Heart Lung and Blood
Institute, NIH.
Mouse embryonic fibroblasts were prepared from E12-E14
day old embryos using standard methods. MEFs were prepared following a cross
between Atg7F/F mice with a transgenic mouse line carrying the Cre
recombinase under the control of the adenoviral promoter EIIa that is known to
be broadly expressed in the developing embryo [27]. PCR
analyses using primers, 5'- GCTG CTACTTC TGCAATGATGT-3' and 5'GCAAGCTCACTAGG CTGCAGAACC-3', were used to detect the wild-type Atg7
gene and primers, 5'- GCTGCTAC TTCTGCAAT GATGT-3' and 5'- ATG GTAC
ATGCTAAGCCTCTGGAC-3', were used to detect the deleted Atg7 gene. MEFs were
cultured in growth medium consisting of Dulbecco's Modified Eagle's medium
(DMEM; Invitrogen) supplementedwith 15% fetal bovine serum (FBS),
50 units/ml penicillin and 50 μg/ml streptomycin. For in vitro NAC
treatment, freshly thawed primary MEFs at passage 2 were placed in growth
medium supplemented with 500 μM NAC for 10 days with media being changed every
other day.
Glucose tolerance test, insulin measurement and islet
isolation.
Overnight fasted male mice
were given intraperitoneal injections of D-glucose (20% solution; 1 g/kg body
weight) and blood glucose was determined using a one-touch Ascensia Elite
glucometer (Fisher). Tail vein blood was collected at 0 and 15 min following
glucose injection and plasma insulin levels were measured with a rat insulin
radioimmunoassay kit according to the manufacturer's recommendations
(Millipore).
For pancreatic intracellular insulin measurement,
small sections of pancreas were digested with ethanol/acid buffer (25 ml
absolute ethanol, 8.3 ml ddH2O, and 0.5 ml concentrated HCl)
overnight at 4°C as previously described [30]. Insulin
was measured with a Rat/Mouse Insulin ELISA Kit Insulin (Crystal Chem Inc.).
Tissue weight was used to normalize the intracellular insulin levels.
Pancreatic islets were isolated using a
standard protocol [31]. Briefly,
the pancreas was perfused with a solution of 0.5 mg/ml Collagenase Type V
(Sigma) dissolved in Hanks Balanced Salt Solution (HBSS) containing calcium and
magnesium (Mediatech Inc). The digestion was performed at 37°C for 15-20 min after which the collagenase was neutralized with HBSS
supplemented with 1% FBS. The collagenase treated pancreas was then
sequentially filtered through 1.5 mm and 0.8 mm metal mesh filters. Islets
were subsequently enriched by centrifugation in Histopaque 1077 (Sigma) and
hand-picked under direct light microscopic visualization.
Isolated mitochondrial and intact metabolic studies
.
Purified
mitochondria were isolated from freshly harvested skeletal muscle using
standard methods [28]. Briefly, isolated
skeletal muscle were rapidly harvested, washed and minced in ice-cold Ionic
Medium (100 mM surcrose, 10 mM EDTA, 100 mM Tris-HCL, 46 mM KCl, pH 7.4). The
tissues were digested with 5% Proteinase Type XXIV (Sigma) in Ionic Medium for
5 min on ice and the protease was subsequently inactivated by the addition of
Ionic Medium supplemented with 0.5% BSA. Samples were then homogenizedwith
a glass-Teflon motorized homogenizer and the mitochondrial fraction isolated by
differential centrifugation. Mitochondria were subsequently washed twice and
then resuspended in Suspension Medium (230 mM mannitol, 70 mM sucrose, 0.02 mM
EDTA, 20 mM Tris-HCl, 5 mM K2HPO4, pH 7.4) prior to
functional assessment. Succinate (5 mM), rotenone (1 μM), Antimycin A (0.25
μM) and ADP (1 mM) were used to assay complex II dependent respiration.
Measurement of intact cellular respiration was
performed using the Seahorse XF24 analyzer (Seahorse Bioscience Inc.). Primary
MEFs were plated at a density of 40,000 cells/well on XF24 tissue culture
plate. Purified pancreatic islets were isolated and cultured overnight in DMEM
supplemented with 10% FBS, 50 units/ml penicillin and 50 μg/ml streptomycin.
Two-three hours prior to respiration measurements, 50-100 islets were
transferred to poly-L-lysine coated XF24 tissue culture plate. Prior to the
respiration assay, primary MEFs or islets were rinsed and cultured in DMEM
running medium (8.3g/L DMEM (Sigma), 200 mM GlutaMax-1 (Invitrogen), 100mM
sodium pyruvate (Sigma), 25 mM D-glucose (Sigma), 63.3 mM NaCl (Sigma), and
phenol red (Sigma), adjust pH to 7.4 with NaOH) according to manufacturer's
protocol. Oxygen consumption was measured under basal conditions, in the
presence of the mitochondrial inhibitors oligomycin (0.5 μM) or antimycin A
(0.25 μM), or in the presence of the mitochondrial uncoupler FCCP (0.5 μM or 1
μM)
to assess maximal oxidative capacity. Lactate measurements were made by
determining the change in extracellular pH over time, as previously described [29]. Oxygen
consumption and lactate measurements were normalized to cell number for primary
MEFs and protein concentration for pancreatic islets.
Histology and Immunohistochemistry.
Histological analysis was performed on 8-10 week old
and 16-24 week old mice. Skeletal muscle or pancreatic tissue was isolated from
Atg7+/+:MCK-Cre, Atg7F/+:MCK-Cre, and Atg7F/F:MCK-Cre,
or Atg7+/+:RIP2-Cre, Atg7F/+:RIP2-Cre, and Atg7F/F:RIP2-Cre
mice, respectively. Tissues were fixed in 10% formalin and paraffin embedded
tissue sections were used for subsequent hematoxylin and eosin (H&E)
staining. Immunohistochemistry analysis with anti-insulin (DAKO), anti-glucagon
(Sigma), anti-somatostatin (DAKO), and antipolypeptide (Millipore) antibodies
was performed using standard protocols. Cryostat sections were fixed with 4%
paraformaldehyde in PBS prior to immunohistochemical analysis with either a
nitrotyrosine antibody (Millipore) or p62 antibody (Progen Biotechnik). For
quantification purposes, an islet was considered to stain positive for p62 or
nitrotyrosine if greater than 5 cells within the islet exhibited positive
staining. Rhodamine or Cy2 conjugated secondary antibodies (Jackson
Immunological Research Laboratories) were used for visualization. Electron
micrographs were performed on ultrathin sections of tissues that were fixed in
2 or 2.5% glutaraldehye plus 1% paraformaldehyde, post-fixed with 1% OsO4,
stained en bloc with 1% uranyl acetate and embedded in Embed 812 (Electron
Microscopy Sciences) using standard methods. The sections were
stained with lead citrate and uranyl acetate before viewing.
Western Blot Analysis.
Primary MEFs or isolated pancreatic islets were lysed
with NonidetP-40 lysis buffer (1.0% Nonidet P-40, 50 mM Tris-HCl pH
7.4,150 mM NaCl, 5 mM EDTA) supplemented with protease inhibitor
tablet (Roche) and phosphatase inhibitors (1 mM Na3VO3, 1
mM β-glycerolphosphate, 10 mM NaF) for 15 min on ice prior to
clarificationby centrifugation at 16,100 x g for 15 min at 4
°C. Skeletal muscle was suspended in homogenizing buffer (25 mM Tris. HCl pH
7.4, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.1% SDS, 10% glycerol, 1 mM DTT,
0.5 % sodium deoxycholic acid) supplemented with protease and phosphatase
inhibitors and homogenized using a tissue lyser (Qiagen). Protein concentration
was determined with the Pierce BCA assay Kit (Thermo Fisher Scientific). Protein
lysates were resolved on precast Tris-Glycine SDS gels (Invitrogen) and
transferred onto nitrocellulose. Immunoblot analysis was performed with
antibodies directed against Atg7 (ProSci Inc and Sigma), actin (Sigma), the
Complex I component NDFS3 (NADH-ubiquinone oxidoreductase, MitoSciences), the
Complex II component FP (flavoprotein subunit of complex II, MitoSciences), and
the Complex III component Core1 (Ubiquinol-cytochrome-c reductase complex core
protein 1, MitoSciences).
For detection of mitochondria complexes on
native blue gels, purified mitochondria from skeletal muscle were resolved on Native
PAGE Novex Bis-Tris gels according to the manufacturer's instructions
(Invitrogen). Briefly, 1 mg of a purified mitochondria pellet was resuspended with
100 μl of 1x NativePAGE buffer and 12.5 μl of 10% maltoside (Sigma). The
samples were incubated on ice for 30 min with frequent vortexing before
pelleting by centrifugation at 16,100 x g for 10 min at 4°C. After centrifugation, 100 μl of supernatant was transferred to a
new tube containing 6.3 μl of Coomassie blue additive and samples (approximately
50 μg) were then resolved by Native PAGE Novex Bis-Tris gel electrophoresis.
ROS measurements.
Primary MEFs were cultured on Nunc
Lab-Tek two chamber glass slides. As an internal control, each dual
chamber slide contained one well of WT MEFs and one well of Atg7-/- MEFs,
or one well of Atg7-/- MEFs and one well of Atg7-/- MEFs
that have been treated with 500 μM NAC for 4 days. Cells were incubated with
HBSS containing 50 μM 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein
diacetate, acetyl ester (CM-H2DCFDA; Invitrogen) for 30 min at 37°C. Cells were then rinsed and mounted with mounting medium (Vector
Labs) and visualized with a Leica SP1 confocal microscope as previously
described [32]. Several
random fields were taken of each genotype and mean fluorescence intensity was
calculated in three or more separate experiments with data from over 250 cells
using at least two independently isolated WT and Atg7-/- MEF cell
isolates. Attached cells were measured rather than MEF cell suspensions since
we have previously observed dramatic alterations in ROS levels when attached
cells are trypsinized [33].
Mitochondrial number.
The ratio of mitochondrial DNA to nuclear DNA was
determined as previously described [34]. In brief,
quantitative PCR analysis using SYBR green (Applied Biosystems) was performed
with 25 μg of isolated DNA (Qiagen). Mitochondrial DNA was assessed using
primers, 5'-CTCTTAT CCACGC TTCCGTTACG-3' and 5'-GATGGTGGTACTCCCGCT GTA-3' for
the mitochondrial-encoded ND1 gene. Nuclear DNA level was determined by
amplifying the genomic H19 locus using primers 5'-GTACCCACCTGTCGTCC-3'and
5'-GTCCACGAGACCAATGA CTG-3'. The relative amount of mitochondrial to nuclear
DNA was determined by normalized ND1 to H19 levels.
We are grateful to Patricia S. Connelly and Amie L. Batson
for assistance with electron microscopy, to Oksana
Gavrilova for help with islet isolation, to Sushil Rane for advice on
pancreatic analysis, to Michael Sack for help with quantification of
mitochondrial number, and to Teng Lu for technical assistance. J.J.W. was
supported by a NIGMS Pharmacology Research Associate (PRAT) Program fellowship
and the work done in laboratory of T.F. is supported by The Ellison Medical
Foundation and the NIH Intramural Program.