Identification of single nucleotide polymorphisms in the p21 (CDKN1A) gene and correlations with longevity in the Italian population
Abstract
Longevity in humans is determined by multiple environmental and genetic factors. We have investigated possible associations between longevity and Single Nucleotide Polymorphisms (SNPs) in the p21 (CDKN1A) gene, a stress-inducible senescence-associated cell cycle inhibitor, expression of which upregulates genes implicated in several age-related diseases. By sequencing the promoter and exons of p21 in genomic DNA of ten individuals over 90 years old, we have identified 30 SNPs, many of which had not been previously characterized. A cluster of minor alleles within the -4547/-3489 bp region did not alter the basal activity or p53 responsiveness of the p21 promoter. We then compared the frequency of 41 p21 SNPs between 184 centenarians and 184 younger subjects in the Italian population. Rare alleles of two exon-derived SNPs, rs1801270 and rs1059234, were significantly under-represented among the centenarians; no significant differences were found for 39 non-exonic SNPs. SNP rs1801270 causes Ser to Arg substitution at amino acid 31 and SNP rs1059234 leads to a nucleotide change in the 3'-untranslated region. Previous studies showed that the rare alleles of these two SNPs may play a role in cancer. These p21 alleles may be potentially detrimental to longevity and therefore are rare in centenarians.
Introduction
Longevity
in humans is believed to be a multifactorial condition to which both genetic
and environmental factors are
likely to contribute. Centenarians are often spared from
age-related diseases, such as cardiovascular disease, Alzheimer's disease,
diabetes mellitus, and cancer. The rate of aging and maximum lifespan varies
among species, and therefore it has been postulated to be at least in part
under genetic control [1-2]. Epidemiological data indicate the presence of a
strong familiar component of longevity that is largely determined by genetics.
Thus, progeroid syndromes of accelerated aging have known genetic causes [3-4].
A number of possible associations between longevity and allelic variants of
genes have been described. Estimates of the heritability of human lifespan vary
from 10-50% with the most common finding being that about a third of human
lifespan may be heritable. The rest is due to environmental exposure, accidents
and injuries, lifestyle and chance. Very long life, to beyond the age of 90
years, appears to have an even stronger genetic basis [5], which explains why
centenarians and near-centenarians tend to cluster in families.
Theories
on aging postulate that aging is a remodeling process, where the body of
survivors progressively adapts to internal and external damaging agents, to
which they are exposed over several decades. Thus,
stress response and adaptation mechanisms play a fundamental role in the aging
process and have an impact on individual lifespan. Centenarians'
capability to live such extraordinarily long lives is in large part due to
genetic variations that either affect the rate of aging or decrease the susceptibility
to age-associated diseases.
Some of the most promising candidate genes appear to be
those involved in stress response. An interesting
possible candidate is p21 (CDKN1A) which has been shown to be involved both in
stress response mechanisms and in the expression of genes implicated in
age-related diseases. p21 is best known as a stress-inducible
cyclin-dependent kinase inhibitor, which triggers cell growth arrest associated
with senescence and damage response. Some evidence suggests that the effects of
p21 inductionon gene expression in senescent cells may contribute
to the pathogenesisof cancer and age-relateddiseases.
In particular, p21 expression was found to upregulatemultiple genes
that have not only been associated with senescence but also implicatedin
age-related diseases, including Alzheimer'sdisease,
atherosclerosis, amyloidosis, arthritis and cancer, thus suggesting that
p21 induction by stress may play a causal role in these diseases [6]. The
role of p21 in cell senescence and its possible implication in the risk ofage-related diseases suggests that allelic variations in this gene may
have an impact on the lifespan. The goal of this study
was to identify p21 polymorphisms and to determine whether they may be
associated with longevity.
Results
Analysis strategy
To determine if any polymorphic variants of p21 that change either its amino acid sequence or
regulation of its transcription may be differentially represented in the long lived individuals (LLI),
we carried out this study in several steps. First, in the
pilot study, we sequenced the three exons of the p21 gene and a 5-kb stretch of
its promoter sequence in the DNA from ten LLI > 90 y.o. (Americans of
European descent) to identify SNPs in these regions. To determine if the
identified SNPs are specific for LLI, we then used the Sequenom SNP analysis
strategy to determine the frequencies of these SNPs in a population of 92
non-LLI individuals (Utah/CEPH population). Finally, in the ethnicity-matched
large scale analysis, we used the Sequenom strategy to determine the
frequencies of p21 SNPs in ethnically matched Italian
populations of 184 LLI and 184 non-LLI control subjects.
SNPs of the
p21 promoter identified by sequence analysis
As the first approach, we undertook the sequencing of the three p21
exons and a 5-kb sequence upstream of the p21 transcription start site in the
genomic DNA from ten LLI. Comparison with the human genome database sequence
revealed only one SNP within the three exons, an A->C transversion in codon 31 causing Arg -> Ser
substitution (rs1801270). This
SNP was previously known and the frequency of the minor allele among the LLI was 0.28 (5/18), which was similar to the minor allele frequency of 0.24 in the
general population (unstratified for ethnicity), reported at that time for this
SNP in the NCBI database. Promoter sequencing yielded a total
of 29 SNPs. Only six of the promoter SNPs had known frequencies
reported in the NCBI database, 17 others had been reported but not characterized,
and six other promoter SNPs had not been previously reported. To determine the
frequencies of the promoter SNPs in a non-LLI population, 25 of these SNPs were
assayed in 92 younger Utah/CEPH
individuals using Sequenom MassARRAY® system. The positions and allele frequencies for all the
SNPs identified in the promoter are presented in Table 1. Notably, we found ten SNPs that were strongly
associated with each other in the Utah/CEPH population (rs4711458, rs471459, rs4711461, rs4714002, rs471146, rs4714003,
rs56850951, rs10947623, rs12192827, rs12192877), in the region between -4547 bp and -3489 bp, where a
novel p53 binding site has been recently found
[7]. We have found that a cluster of minor alleles within the -4547/-3489 bp
region was more common in the ten LLI samples compared to the Utah/CEPH
population. The frequencies of the rare allele-carriers (almost all
heterozygotes) was 50% among the ten LLIs and
23% for the Utah/CEPH population. This
difference did not reach statistical significance (P< 0.158 t-test). No significant differences
between these two populations were found for the other SNPs in the promoter
region.
Table 1. Summary of statistics for SNPs identified in the pilot study.
| SNP | Location in the chromosome | Rare allele | Frequency of rare allele in LLI | Frequency of rare allele in controls | Common Allele |
| rs4711458 |
36749919
|
C
|
0.25
|
0.115
|
T
|
| rs4711459 |
36750002
|
C
|
0.25
|
0.115
|
T
|
| rs4711461 |
36750146
|
T
|
0.25
|
0.098
|
C
|
| rs4714002 |
36750164
|
T
|
0.25
|
0.885
|
G
|
| rs471146 |
36750168
|
A
|
0.25
|
0.904
|
G
|
| rs4714003 |
36750238
|
T
|
0.25
|
0.119
|
C
|
| rs56850951 |
36750380
|
T
|
0.25
|
NA
|
C
|
| rs10947623 |
36750814
|
A
|
0.22
|
0.118
|
G
|
| rs12192827 |
36750949
|
T
|
0.27
|
0.120
|
C
|
| rs12192877 |
36750977
|
A
|
0.25
|
0.080
|
C
|
|
CDKN1A11
|
36751056
|
G
|
0.10
|
0.070
|
A
|
|
CDKN1A12
|
36751203
|
G
|
0.25
|
NA
|
A
|
|
CDKN1A13
|
36751481
|
A
|
0.11
|
0.000
|
G
|
|
rs9394371
|
36751733
|
T
|
0.11
|
0.110
|
C
|
|
rs4135234
|
36752199
|
A
|
0.12
|
0.070
|
G
|
|
rs3829963
|
36752364
|
A
|
0.27
|
0.100
|
C
|
|
rs3829964
|
36752475
|
C
|
0.45
|
0.400
|
T
|
|
rs3829965
|
36752488
|
G
|
0.27
|
0.110
|
A
|
|
rs4135237
|
36752868
|
T
|
0.25
|
0.000
|
G
|
|
rs3829966
|
36752929
|
T
|
0.07
|
0.070
|
C
|
|
rs3829967
|
36752936
|
C
|
0.07
|
0.100
|
T
|
|
rs3829968
|
36752943
|
C
|
0.07
|
0.070
|
T
|
|
rs733590
|
36753181
|
C
|
0.06
|
0.000
|
T
|
|
rs762623
|
36753444
|
A
|
0.31
|
0.290
|
G
|
|
rs2395655
|
36753674
|
G
|
0.27
|
0.320
|
A
|
|
rs730506
|
36753946
|
C
|
0.11
|
0.190
|
G
|
|
rs4151702
|
36753966
|
C
|
0.11
|
0.200
|
G
|
|
rs4135239
|
36754331
|
C
|
0.13
|
NA
|
G
|
|
CDKN1A29
|
36754348
|
+C
|
1.00
|
NA
| |
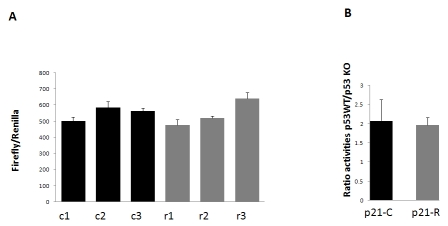
Figure 1. Activities of
the p21 promoter-luciferase constructs with the common (p21C-luc; black
bars) or rare (p21R-luc; grey bars) allele SNP cluster in the -4547/-3489
bp region. (A) Three independent plasmid preparations of each of p21C-luc or
p21R-Luc (R) were transfected into HCT116 wild type (WT) cells. Cells were
harvested 48 h after transfection, and firefly luciferase activity was
measured and normalized to Renilla luciferase expressed from a
co-transfected construct. The bars show mean and standard deviation for
triplicate transfections. (B) p21C-luc and p21R-luc plasmids were
transfected in parallel into HCT116 WT and p53-/- cell lines, in
triplicates as in A. The bars show mean and standard deviation of the ratio
of normalized luciferase activities achieved with the same plasmid in the
WT relative to p53-/- cells.
A
cluster of minor alleles in the p21 promoter does not alter its basal activity
and p53 responsiveness
Since ten rare SNPs within the region
between -4547 bp and -3489 bp,
comprising a p53 binding site, were found
at a higher frequency among the ten LLI relative to the Utah/CEPH population,
we were interested to determine if the presence of these minor alleles affects
the basal activity or p53 dependence of the p21 promoter. A 2.1-kb fragment containing a cluster of the minor
alleles in this region was amplified by PCR from the genomic DNA of one LLI and
cloned into the plasmid p21-PGL.4.10-luc-, which contains 5 kb of the p21
promoter driving the expression of the firefly luciferase reporter gene [7], replacing the common alleles in the corresponding promoter region. The resulting
plasmid was designated p21R-Luc. To
compare the basal activities of the rare-allele p21R-Luc and the common-allele p21-PGL.4.10-luc construct (designated
p21C-Luc in Figure 1), three independent preparations of each plasmid were
transfected into wild-type HCT116 colon carcinoma cells, together with a
Renilla luciferase expressing vector (normalization standard). Normalized
firefly luciferase expression from the two plasmids was indistinguishable,
indicating unaltered basal activity of the LLI-derived promoter (Figure 1A). To determine whether the two variants of the
p21 promoter could have a different response to p53, we transfected one
preparation of each plasmid into wild-type HCT116 cells and into the HCT116
derivative with the knockout of both p53 alleles [8]. The two plasmids showed
equal (two-fold) reduction in the promoter activity in p53-knockout cells
(Figure 1B), indicating that the LLI-derived promoter had essentially unaltered
response to p53.
Large
scale analysis of p21 SNPs correlations with longevity in the Italian population
A
comparison of allelic frequencies between the first two population samples that
we analyzed, ten LLI from Americans of European origin and younger individuals
of the Utah/CEPH population, is inevitably biased by the small sample size of
the LLI set and by historically limited variability in the founder pool of the
Utah population. Consequently the SNP frequencies could be affected not only by
longevity but could also have the founder pool as an uncontrolled confounder.
This is an issue typical in genetic association studies affected by the
phenomena of ‘stratification': the failure to adequately match the genetic
background of cases and controls. Therefore, to minimize this problem, in the
large scale analysis we only used DNA from centenarians (mean age 100.88±1.77 years) and younger (38.97±12.21 years) subject
populations of Italian origin (184 subjects each), selected for similar origins
in Central Italy and representing ethnically matched populations. We undertook this large case-control design
study to (i) verify frequencies of the SNPs identified in the pilot study, (ii)
create a haplotype map of 60,000 bp, and (iii) determine whether any specific SNPs and
haplotypes are associated with longevity. In addition to 17 of our SNPs
identified in the pilot study, 30 SNPs spanning the p21 gene were selected from
the SNP HapMap consortium database, for a total of 47 SNPs included in the
genotyping.
Table 2. Summary of statistics. Large scale analysis of Italian populations.
SNPs showing significant differences between the control and LLI populations are shown
in boldface; SNPs comprising the sixth haplotype block are italicized.
CHISQ=Chi Square, P=P value, OR=Odds
| SNP | Location in the chromosome | Rare allele | Frequency rare allele in LLI | Frequency rare allele in controls | Common Allele |
CHISQ
| P | OR |
|
rs6457931
|
36721790
|
G
|
0.450
|
0.447
|
T
|
0.006
|
0.936
|
1.014
|
|
rs1321312
|
36730852
|
G
|
0.155
|
0.198
|
C
|
1.790
|
0.181
|
0.746
|
|
rs4331968
|
36731221
|
T
|
0.299
|
0.282
|
A
|
0.200
|
0.655
|
1.086
|
|
rs9470367
|
36734910
|
C
|
0.323
|
0.321
|
G
|
0.004
|
0.949
|
1.012
|
|
rs6920453
|
36735461
|
T
|
0.228
|
0.207
|
C
|
0.382
|
0.537
|
1.134
|
|
rs9462209
|
36736020
|
G
|
0.466
|
0.411
|
T
|
1.452
|
0.228
|
1.254
|
|
rs4713999
|
36741047
|
A
|
0.413
|
0.459
|
G
|
1.098
|
0.295
|
0.829
|
|
rs1321309
|
36746614
|
T
|
0.440
|
0.400
|
C
|
0.965
|
0.326
|
1.177
|
|
rs4711459
|
36750002
|
C
|
0.153
|
0.139
|
T
|
0.200
|
0.655
|
1.115
|
|
rs4711461
|
36750146
|
T
|
0.163
|
0.136
|
C
|
0.826
|
0.364
|
1.238
|
|
rs4714003
|
36750238
|
T
|
0.086
|
0.092
|
C
|
0.072
|
0.789
|
0.921
|
|
CDKN1A
|
36750804
|
G
|
0.156
|
0.181
|
A
|
0.667
|
0.414
|
0.834
|
|
rs10947623
|
36750814
|
A
|
0.165
|
0.126
|
G
|
1.652
|
0.199
|
1.366
|
|
rs12192827
|
36750949
|
T
|
0.156
|
0.122
|
C
|
1.367
|
0.242
|
1.332
|
|
rs12192877
|
36750977
|
A
|
0.158
|
0.134
|
C
|
0.679
|
0.410
|
1.218
|
|
rs4135234
|
36752199
|
A
|
0.151
|
0.180
|
G
|
0.895
|
0.344
|
0.809
|
|
rs3829963
|
36752364
|
A
|
0.135
|
0.136
|
C
|
0.002
|
0.969
|
0.990
|
|
rs3829965
|
36752488
|
G
|
0.161
|
0.142
|
A
|
0.378
|
0.539
|
1.158
|
|
rs4135237
|
36752868
|
T
|
0.158
|
0.171
|
G
|
0.160
|
0.689
|
0.913
|
|
rs3829966
|
36752929
|
T
|
0.146
|
0.169
|
C
|
0.586
|
0.444
|
0.839
|
|
rs3829967
|
36752936
|
C
|
0.261
|
0.212
|
T
|
1.974
|
0.160
|
1.313
|
|
rs733590
|
36753181
|
C
|
0.452
|
0.458
|
T
|
0.025
|
0.875
|
0.973
|
|
rs762623
|
36753444
|
A
|
0.161
|
0.188
|
G
|
0.705
|
0.401
|
0.827
|
|
rs2395655
|
36753674
|
G
|
0.479
|
0.504
|
A
|
0.335
|
0.563
|
0.904
|
|
rs730506
|
36753946
|
C
|
0.245
|
0.252
|
G
|
0.035
|
0.852
|
0.964
|
|
rs4151702
|
36753966
|
C
|
0.257
|
0.254
|
G
|
0.008
|
0.930
|
1.017
|
| rs3176343 | 36758245 | A | 0.044 | 0.075 | G | 2.597 | 0.107 | 0.561 |
| rs3176344 | 36758525 | A | 0.018 | 0.043 | G | 3.069 | 0.080 | 0.396 |
| rs3176349 | 36759355 | T | 0.028 | 0.053 | G | 2.091 | 0.148 | 0.526 |
| rs1801270 | 36759949 | A | 0.043 | 0.093 | C | 5.412 | 0.020 | 0.437 |
| rs1059234 | 36761575 | T | 0.049 | 0.098 | C | 4.983 | 0.026 | 0.472 |
| rs876581 | 36763423 | A | 0.056 | 0.092 | G | 2.653 | 0.103 | 0.583 |
| rs6457938 | 36768431 | A | 0.309 | 0.290 | G | 0.255 | 0.614 | 1.096 |
| rs6457940 | 36771335 | A | 0.284 | 0.311 | C | 0.482 | 0.487 | 0.880 |
|
rs2145047
|
36771630
|
G
|
0.037
|
0.055
|
A
|
1.030
|
0.310
|
0.661
|
|
rs2894409
|
36774505
|
T
|
0.146
|
0.088
|
C
|
4.412
|
0.036
|
1.756
|
Table 3. Frequencies and P-values for the sixth p21 haplotype containing SNPs rs1801270 and rs1059234 in the centenarian and control Italian populations.
| Haplotype | Frequency LLI | Frequency
controls | P value | SNPs |
| Block
6 | | | | |
| FOUR SNP WINDOW | | | |
|
AGGA
|
0.03725
|
0.07798
| 0.03972 |
rs3176343|rs3176344|rs3176349|rs1801270
|
|
GGGA
|
0.01198
|
0.01181
|
0.98520
|
rs3176343|rs3176344|rs3176349|rs1801270
|
|
GGTC
|
0.02854
|
0.05105
|
0.17610
|
rs3176343|rs3176344|rs3176349|rs1801270
|
|
GAGC
|
0.01811
|
0.03977
|
0.12820
|
rs3176343|rs3176344|rs3176349|rs1801270
|
|
GGGC
|
0.90410
|
0.81940
| 0.00394 |
rs3176343|rs3176344|rs3176349|rs1801270
|
| SIX SNP WINDOW | | | |
|
AGGATA
|
0.03198
|
0.07607
| 0.02249 |
rs3176343|rs3176344|rs3176349|rs1801270|rs1059234|rs876581
|
|
GGGATA
|
0.01050
|
0.01284
|
0.80000
|
rs3176343|rs3176344|rs3176349|rs1801270|rs1059234|rs876581
|
|
GGTCCG
|
0.02906
|
0.05134
|
0.18560
|
rs3176343|rs3176344|rs3176349|rs1801270|rs1059234|rs876581
|
|
GAGCCG
|
0.01844
|
0.03970
|
0.13900
|
rs3176343|rs3176344|rs3176349|rs1801270|rs1059234|rs876581
|
|
GGGCCG
|
0.91000
|
0.82000
| 0.00211 |
rs3176343|rs3176344|rs3176349|rs1801270|rs1059234|rs876581
|
Of those markers, 45 had high
confidence calls on the platform and two markers (CDKN1A29 and rs4711458) were
excluded because of the low call-rate. Of the remaining SNPs, four (rs4711458, rs4714003, CDKN1A7, rs6920453) were eliminated because the genotype
frequencies were not consistent with
Hardy-Weinberg equilibrium in the control dataset or in the entire sample. The
association statistics for the remaining 41 SNPs are presented in Table 2.
Haplotype frequencies were estimated using a sliding window approach. Linkage
disequilibrium (LD) analysis of the p21 gene revealed the presence of seven
blocks of haplotype in the 60,000 bp region studied. Figure 2 shows a graphical
representation of the blocks identified. SNP
analysis revealed the presence of two minor alleles that were underrepresented
in the LLI compared to the non-LLI control populations, at SNPs rs1801270 and rs1059234.
Remarkably, these were the only two exonic SNPs of
all the SNPs analyzed. The above
mentioned rs1801270 consists of a base
change from AGC to AGA and amino acid changes from serine to arginine at codon
31 in exon 2. SNP rs1059234 (p21C70T) consists of a C to T change in the 3'
untranslated region of the p21 gene, 20 bp following the stop codon. Minor
allele frequencies of SNP rs1801270
were 0.093 and 0.043 in
controls and LLI respectively (P< 0.02 chi-test). The corresponding minor
allele frequencies for SNP rs1059234 were 0.098 and 0.049 (P< 0.026 chi-test). No
statistically significant frequency differences were observed between LLI and
controls for the other (non-exonic) SNPs analyzed.
SNPs
rs1801270 andrs1059234
are in LD and comprise the sixth LD block
in the gene (which includes SNPs rs3176343, rs3176344, rs3176349, rs1801270, rs1059234, rs876581,
rs6457938 and rs6457940). Table 3 shows analysis
of haplotype structure using tagSNP and estimated haplotype frequencies in this
sixth block. A 6-SNP haplotype comprising
rs1801270 andrs1059234 common
alleles (GGGCCG) is more prevalent in the LLI individuals compared to the
controls (91.0% vs 82.0%; p<0.002); the corresponding frequencies for the
4-SNP haplotype of common alleles are 90.4% vs. 81.9% (p<0.004). The
significance of these differences was confirmed by permutation analysis.
Discussion
In the present
study, we have investigated possible associations between longevity and SNPs in
the p21 (CDKN1A) gene, which plays a role in stress response and cell
senescence, and increased expression of which was shown to upregulate genes
implicated in several age-related diseases [6]. By sequencing the three exons
and 5 kb of the promoter region of p21, we have identified many previously unknown
or uncharacterized SNPs in the p21 promoter. We have tested the activity of the
promoter derived from an LLI and containing a cluster of minor alleles in the
region between -4547
bp
and -3489
bp,
where a novel p53 binding site has been recently identified [7] and found no
changes in the basal activity or p53 responsiveness of this promoter. It should
be noted, however, that there are many p53 independent physiological signals
that induce p21, where the response of the two alleles may potentially be different.
Interestingly the p21 promoter is induced by some signals involved in stress
response and inflammation (such as TGFβ,
INFγ, IL-6) that, as
discussed elsewhere, contribute to the pathogenesis
of many age-related diseases [9-11].
In a large
case-control design study, we have compared the frequency of 41 SNPs spanning
the p21 gene between large populations of LLI and younger Italian individuals.
Only two of 41 SNPs showed a statistically significant difference between the
two populations, and remarkably, these were the only two exon-derived SNPs. A
6-SNP haplotype comprising the common alleles of these two SNPs was strongly
overrepresented among the centenarians relative to the control population. One of these
exonic SNPs (rs1801270) changes the amino
acid sequence of p21 from Ser to Arg at codon 31, and the other (rs1059234) leads to a C->T
transition 20 nucleotides downstream of the stop codon in the 3' untranslated
region. Remarkably, several studies sug-gested that the rare alleles of these
two SNPs may play a role in different types of cancer [12-17]. In particular, Li et al. [12] have shown that, in non-Hispanic whites, the rare versions of the rs1801270 and
rs1059234 alleles are associated to an increased risk
susceptibility to squamous cell carcinoma, individually and in combination. In
addition, Mousses at al. [18] observed that the rare alleles of these two SNPs
were under-represented in breast cancer and sarcoma patients whose tumors
possessed somatic p53 mutations, as compared to tumors without p53 mutations,
suggesting that these alleles could influence p21 functions in a
p53-independent manner.
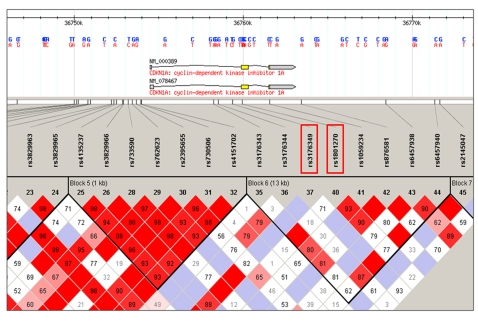
Figure 2. Haplotype blocks distrubution in the p21 gene generated by Haploview. The two SNPs
showing significant differences in frequency between the centenarians and
younger controls are bracketed. Every multimarker combination within this
block including the two SNPs is significant on the omnibus test for
frequency distribution among cases and controls. Table 3 gives the results
of the haplotype test.
According to the
data in the NCBI database (Entrez SNP), the allele frequency distribution of
the SNPs rs1801270
and rs1059234 is
highly variable and ethnic-specific.
The frequency of the rs1801270 minor allele varies from 0.021
(Europeans) to 0.47 (Asians) with African-Americans, Sub-Saharan Africans, and
Hispanics having intermediate values. Similar values are found for the rs1059234
rare allele, whose frequencies vary from 0.021 (Europeans) to 0.45 (Asians),
with African-Americans,
Sub-Saharan Africans, and Hispanics also having intermediate values. This high
ethnicity-related variability can greatly complicate the interpretation of
disease-associated studies, especially those conducted in multi-ethnic societies.
In our large-scale study, we have used a relatively homogenous Italian
population. Our results suggest that the presence of the rs1801270 and
rs1059234 rare alleles combined
may be detrimental to longevity and therefore negatively selected in Italian LLI.
Further large-scale studies
could be useful to compensate for genetic heterogeneity within the Italian
population and to clarify the potential role of these SNPs in limiting the
lifespan.
The
mechanisms underlying the
potential detrimental effect of the rare alleles of rs1801270 and rs1059234 are
presently unknown. The obvious hypotheses
are that the amino acid change at codon 31, which was proposed to abolish p21
phosphorylation at Ser 31 [19], could modulate its abilities to arrest the cell
cycle or to induce transcription of genes implicated in age-related diseases,
and that a nucleotide change in the 3' UTR could affect p21 mRNA stability or
translational efficiency. These possibilities remain to be tested in future
studies.
Longevity in humans can be defined as a
multifactorial condition to which both genetic and environmental factors are
likely to contribute. Twin studies have shown that genetic differences account
for about a quarter of the variance in adult human lifespan. Despite the challenges
of studying complex traits such as lifespan, studies have been reporting
alleles that were significantly associated with human longevity. One of the
best examples is APOE whose association has been reproduced consistently
[20-22]. The compression of morbidity hypothesis proposed by James Fries in
1980 [23] postulates that as the limit of human lifespan is approached, the
onset and duration of lethal impairment compresses toward the end of life. This
‘compression' is observed in the majority of centenarians who are often spared
from age-related diseases, specifically cardiovascular disease, Alzheimer
disease, diabetes mellitus, and cancer. To achieve their extreme age,
centenarians likely lack numerous gene variants that are associated with
age-related diseases and they may be more likely to carry protective variants
as well. Our finding that the frequency of specific minor alleles of p21 is
decreased among Italian centenarians lends additional support to this concept.
Materials and Methods
Subjects.
In
the pilot study we used ten DNA samples from LLI over 90 y.o. (white Americans
of European descent), including five females and five males. The DNA samples,
obtained from the NIA Aging Cell Repository DNA panel, were obtained from
CORIELL bank (Camden, NJ). The second group of samples comprised DNA
from 92 non-centenarian subjects, belonging to Utah/CEPH population, provided
by CORIELL bank. These samples were
initially collected from Utah residents with ancestry from northern and western
Europe. In the large case-control
study, 184 Italians with exceptional longevity (mean age, 100.88±1.77 years)
and a control group (38.97±12.21) were recruited by the Bologna group in
Central Italy, after checking for ethnicity and ancestor origins. The sex ratio
in the Italian samples was 7 female to 1 male in the centenarian group and 2:1
in the control group. A full socioeconomic, quality of life and health status
assessment was performed. Primary criteria for inclusion in the study were good
health (for centenarians, subjects categorized A or B according to Franceschi
et al. [24] were included), physical activity and absence of major diseases.
p21
genotyping.
We sequenced the three exons (68 bp, 450 bp,
1600 bp) and a 5 kb promoter region of the p21 (CDKN1A) gene in the DNA of ten
LLI. The exons and the promoter were amplified by PCR in overlapping fragments
of ~ 400 bp each. The PCR primer pairs are listed in Table 4, where p21pro.1 is
the furthest from the transcription start site. Primers were designed using the
primer3 software available at http://frodo.wi.mit.edu. The primers were
sized between 22-24 bases with a Tm of 69-71oC and a GC content of
40-60 %. The primers were checked for loops, hairpins and 3' complementarity.
The selected primers were synthesized by idtDNA (Coralville, IA). The
genomic DNA templates were added to a master mix containing 2 μl of each primer (10 μM), 5 μl Buffer
(New England Biolabs, Ipswich, MA), 2 μl of
Taq polymerase (Invitrogen), and water to the volume of 50 μl. The following PCR profile was used: preincubation
for 2 min at 96°C, 40 cycles of 30 sec at 95°C, 30 sec at the primer-specific
annealing temperature (Table 4) and 15 sec at 72°C, followed by final
incubation for 5 min at 72°C. Once
amplified, the fragments were purified with Millipore columns (Millipore
Billerica, MA) and then sequenced. Sequencing
was performed with ABI 3730 DNA analyzer, using a Big Dye protocol with Zymo
column-purified products. Complete sequences were aligned, assembled and
compared using the Clone Manager program. For verification, visual inspection
of sequence profiles for each candidate SNP was carried out. At least two
overlapping DNA templates amplified with different primers were used for
identification of each candidate SNP. In addition to the SNP candidate
approach, the latest data available on the HapMap were analyzed, in order to select
an appropriate number of tagSNPs to cover at least 90% of the genetic
information in the locus (r2=0.9 and MAF>0.05). The tagSNP and candidate SNP
sets were used for genotyping.
Table 4. Primer sequences and TM for amplifying the p21 gene.
| Name | Sequence | TM |
|
p21.exon1.R
|
AAGGCGAGCTCCCAGAAC
|
60°
|
|
p21seq.exon1.F
|
ACTGGGGGAGGAGGGAAGT
| |
|
p21seq.exon2.F
|
ACCAGCTGGAAGGAGTGAGA
|
60°
|
|
p21seq.exon2.R
|
GTCTTTGCTGCCTACTTGC
| |
|
p21seq.exon3.F1
|
TGCGGTGATGGATAAAATCA
|
58°
|
|
p21seq.exon3.R1
|
GAAAAGGAGAACACGGGATG
| |
|
p21seq.exon3.F2
|
TCCTAAGAGTGCTGGGCATT
|
60°
|
|
p21seq.exon3.R2
|
GCCCTTCTTCTTGTGTGTCC
| |
|
p21seq.exon3.F3
|
TCTTCTCCAGCTGGGCTCT
|
58°
|
|
p21seq.exon3.R3
|
CCCAAAAGCCCATTTATTTG
| |
|
p21pro1.r
|
GGGGCTGCCTATGTAGTGAA
|
58°+ dmso
|
|
p21pro1.F
|
GTGCCACAGTTCACAAGTGC
| |
|
p21pro2.f
|
TTTGCTTCTGGGCAGAACTT
|
58°
|
|
p21pro2.r
|
CAGAGCCAGGATGAATTGGT
| |
|
P21.pro.3.f
|
GATGTTGTTAGAGCCAGGAACAG
|
54°
|
|
P21.pro.3.r
|
ATCAAGGCATAAAAATTTCATTGTG
| |
|
P21pro4f
|
AAAAGGTTTTTGAATGAATGGATG
|
58.5°+dmso
|
|
P21pro4r.
|
AGAAGAGGCGGAACAAAGATAGAA
| |
|
P21pro5f.
|
CACGCCCGGCCAGTATATATT TTT
|
58 °+ dmso
|
|
P21pro5r.
|
GACAAAATAGCCACCAGCCTCTTCT
| |
|
p21pro6.f
|
CACCTTTCACCATTCCCCTA
|
58°
|
|
p21pro6.r
|
AGGGCTGGTTGTCAAATGTC
| |
|
p21pro7.f
|
TGCATGGTTGCAAACTTTTT
|
54°
|
|
p21pro7.r
|
TCACCTTTGCCTCCTTTCTG
| |
|
p21pro8.f
|
AGGTCAGCTGCGTTAGAGGA
|
58°
|
|
p21pro8.r
|
GGAAGGAGGGAATTGGAGAG
| |
|
p21pro9.f
|
GGAGGCAAAAGTCCTGTGTT
|
54°
|
|
p21pro9.r
|
ACATTTCCCCACGAAGTGAG
| |
|
p21pro10.f
|
TCTAGGTGCTCCAGGTGCTT
|
58° +dmso
|
|
p21pro10.r
|
CTGTGAACGCAGCACACAC
| |
|
p21pro11.f
|
CCGAAGTCAGTTCCTTGTGG
|
54°
|
|
p21pro11.r
|
GCTTCCTTGGGAACAAACTG
| |
The
SNP genotyping was performed using
Sequenom's chip-based matrix assisted laser desorption/ionization
time-of-flight MS (DNA MASSARRAY). This technology performs allele-specific
single-base primer extension reactions, which allow differentiation of
homozygous normal, heterozygous mutant and homozygous mutant samples (iPLEX
assay). The MassEXTEND primers anneal up to the polymorphic site and are
extended with one single base. The allele product masses depend on the SNP
allele base and thus they are easily distinguished with the mass spectrometer.
DNA of the Utah/CEPH population was analyzed as a service by Sequenom (San Diego, CA) on PCR-derived extension products from individual DNA
samples. Large-scale analysis of Italian samples was performed by the Bologna
group. Cases and controls were always
analyzed on the same chip to avoid potential artifacts caused by chip-specific
miscalls.
Plasmid constructs.
The
plasmid p21-PGL.4.10-luc containing the 5 kb p21 promoter comprising the common
alleles and driving firefly luciferase expression has been previously described
[7]. This plasmid was
used to replace a cluster of common SNP alleles in the promoter with the minor
alleles, contained within a fragment of ~2.1 kb, which was amplified by
double-round PCR from genomic DNA of a LLI. PCR was carried out using a
proofreading polymerase, Phusion™ Hot
Start High-Fidelity DNA Polymerase
(New England Biolabs, Ipswich, MA). The primed
template was pre-formed in the presence of 5X Phusion GC buffer (New England
Biolabs, Ipswich, MA)
and 200 μM of each dNTP, 0.5 μM primers, and 1
U of Phusion DNA polymerase. The template being GC-rich, 3% DMSO was added to
optimize the product yield. The samples were incubated as follows: preincubation for 30 sec at 98°C, 30 cycles of
10 sec at 98°C, 30 sec at 66°C and 3 min at 72°C and one final incubation for 5
min at 72°C. In the first round, the 5kb PCR product of the p21 promoter was
amplified using the following primers: p21-4997F TACAAACATTGGGTGGGGCGAGTC p21-R-44 CTCCGGCTCCACAAGGAACTGACTT In the second round, this PCR product was used as a template to generate
a PCR product of ~2.1 kb using the following primers: p21-4497F TACAAACATTGGGTGGGG CGAGTC p21-5R GACAAAATAGCCACCAGCCTCTTCT The latter PCR product was
digested with AatII and Sph restriction enzymes (New England
Biolabs), and cloned into p21-PGL.4.10-luc
plasmid digested with the same enzymes,
replacing the corresponding fragment containing the common alleles. The
resulting plasmid was sequence-verified and designated p21R-luc.
Promoter analysis by transient transfection.
HCT116
colon carcinoma cells, both wild type and p53-/- sublines [8] (a gift of Dr. B.
Vogelstein, Johns Hopkins University) were grown in DMEM with Earle's salts
supplemented with 10% FCS and 2 mM L-glutamine in a humidified 95% air 5% CO2
incubator. Cells were seeded in 12-well tissue culture plates for 24 h prior to
transfection. When 70% confluent, the cells were transfected with 1 μg of the indicated promoter-reporter plasmids,
together with pRL-TK Renilla luciferase expressing plasmid (Promega, Madison, WI) to normalize for transfection
efficiency, at a ratio of 10:1
test vector:standard vector.
Transfections were performed in triplicate, using FuGENE6 (Roche Molecular Biochemicals).
A precipitate was formed using 3 μl of FuGENE6/μg of transfected DNA and the transfection mixture was
diluted up to 1 ml with serum-free medium. After incubation at 22°C for 10 min,
the DNA/FuGENE6 mixture was added to cells. Cells were harvested 48 h after
transfection, and firefly and Renilla luciferase activities were measured.
Statistical analysis.
We tested
departurefrom Hardy-Weinberg equilibrium [25] in the controls by a 2 testusing P = 0.01 as threshold. This threshold was chosen basedon
anticonservativeness of this test as noted by Wigginton [25]. All SNPs (except
for rs4711458, rs4714003, CDKN1A7, rs6920453) were in Hardy-Weinbergequilibrium. Each
SNP was tested both with basic association testing based on comparing allele
frequencies between cases and controls (asymptotic and empirical p-value to
control for multiple testing), and with Conchran-Armitage trend test in a
dominant, recessive and general model.
Multi-locus
haplotype analysis was performed by using a sliding-window approach implemented
in PLINK [26] for multi-loci of 4 or 6 SNPs size. Multimarker haplo- types
have been estimated by using the E-M algorithm implemented in the software. An
LD map of the region has been produced by using the software Haploview [27] in
order to identify LD blocks within the typed markers. The haplotype blocks
identified have been tested in the same way, i.e. phased and used for frequency
estimation by E-M algorithm and chi-square testing of the frequencies.
Acknowledgments
We thank George Kampo for help with DNA sequencing,
Dr. Bert Vogelstein for HCT116 wild-type and p53-/- cell lines, and Dr. Eugenia
Broude for helpful discussions. This work was supported by NIH grants RO1
AG17921 and RO1 AG28687 (I.B.R.); the University of Bologna "Marco Polo"
fellowship (S.G.), "Ricerca Fondamentale Orientata 2007" (C.F. and S.S.),
Roberto and Cornelia Pallotti Legacy for Cancer Research (C.F. and S.S.),
"Progetti Strategici" 2006 (S.S.); Italian Ministry of University and Research
(MiUR) PRIN 2006 Project 2006061707 (C.F.) and 2006063387 (S.S.); and EU Grant
"PROTEOMAGE" FP6-518230 (C.F.).
Conflicts of Interest
I.B.R. is a founder of Senex Biotechnology, Inc., a
company that develops pharmaceuticals that prevent the induction of
disease-associated genes in senescent cells.
References
-
1.
Miller
RA
, Harper
JM
, Galecki
A
and Burke
DT.
Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice.
Aging Cell.
2002;
1:
22
-29.
[PubMed]
.
-
2.
Hekimi
S
and Guarente
L.
Genetics and the Specificity of the Aging Process.
Science.
2003;
299:
1351
-1354.
[PubMed]
.
-
3.
Martin
GM
Genetics and aging; the Werner syndrome as a segmental progeroid syndrome.
Adv Exp Med Biol.
1985;
190:
161
-170.
[PubMed]
.
-
4.
Fossel
M
Human aging and progeria.
J Pediatr Endocrinol Metab.
2000;
13:
1477
-81.
[PubMed]
.
-
5.
Perls
T
, Kunkel
LM
and Puca
AA.
The genetics of exceptional human longevity.
J Mol Neurosci.
2002;
19:
233
-238.
[PubMed]
.
-
6.
Chang
BD
, Watanabe
K
, Broude
EV
, Fang
J
, Poole
JC
, Kalinichenko
TV
and Roninson
IB.
Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases.
Proc Natl Acad Sci U S A.
2000;
97:
4291
-4296.
[PubMed]
.
-
7.
Saramäki
A
, Banwell
CM
, Campbell
MJ
and Carlberg
C.
Regulation of the human p21(waf1/cip1) gene promoter via multiple binding sites for p53 and the vitamin D3 receptor.
Nucleic Acids Res.
2006;
34:
543
-554.
[PubMed]
.
-
8.
Bunz
F
, Dutriaux
A
, Lengauer
C
, Waldman
T
, Zhou
S
, Brown
JP
, Sedivy
JM
, Kinzler
KW
and Vogelstein
B.
Requirement for p53 and p21 to Sustain G2 Arrest After DNA Damage.
Science.
1998;
282:
1497
-1501.
[PubMed]
.
-
9.
Carrieri
G
, Marzi
E
, Olivieri
F
, Marchegiani
F
, Cavallone
L
, Cardelli
M
, Giovagnetti
S
, Stecconi
R
, Molendini
C
, Trapassi
C
, De
Benedictis G
, Kletsas
D
and Franceschi
C.
The G/C915 polymorphism of transforming growth factor beta1 is associated with human longevity: a study in Italian centenarians.
Aging Cell.
2004;
3:
443
-448.
[PubMed]
.
-
10.
Olivieri
F
, Antonicelli
R
, Cardelli
M
, Marchegiani
F
, Cavallone
L
, Mocchegiani
E
and Franceschi
C.
Genetic polymorphisms of inflammatory cytokines and myocardial infarction in the elderly.
Mech Ageing Dev.
2006;
127:
552
-559.
[PubMed]
.
-
11.
Franceschi
C
, Capri
M
, Monti
D
, Giunta
S
, Olivieri
F
, Sevini
F
, Panourgia
MP
, Invidia
L
, Celani
L
, Scurti
M
, Cevenini
E
, Castellani
GC
and Salvioli
S.
Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans.
Mech Ageing Dev.
2007;
128:
92
-105.
[PubMed]
.
-
12.
Själander
A
, Birgander
R
, Rannug
A
, Alexandrie
AK
, Tornling
G
and Beckman
G.
Association between the p21 codon 31 A1 (arg) allele and lung cancer.
Hum Hered.
1996;
46:
221
-225.
[PubMed]
.
-
13.
Driver
KE
, Song
H
, Lesueur
F
, Ahmed
S
, Barbosa-Morais
NL
, Tyrer
JP
, Ponder
BA
, Easton
DF
, Pharoah
PD
, Dunning
AM; Studies in Epidemiology
and Risks
of Cancer Heredity (SEARCH) Team.
Association of single-nucleotide polymorphisms in the cell cycle genes with breast cancer in the British population.
Carcinogenesis.
2008;
29:
333
-341.
[PubMed]
.
-
14.
Li
G
, Liu
Z
, Sturgis
EM
, Shi
Q
, Chamberlain
RM
, Spitz
MR
and Wei
Q.
Genetic polymorphisms of p21 are associated with risk of squamous cell carcinoma of the head and neck.
Carcinogenesis.
2005;
26:
1596
-1602.
[PubMed]
.
-
15.
Chen
WC
, Wu
HC
, Hsu
CD
, Chen
HY
and Tsai
FJ.
p21 gene codon 31 polymorphism is associated with bladder cancer.
Urol Oncol.
2002;
7:
63
-66.
[PubMed]
.
-
16.
Facher
EA
, Becich
MJ
, Deka
A
and Law
JC.
Association between human cancer and two polymorphisms occurring together in the p21Waf1/Cip1 cyclin-dependent kinase inhibitor gene.
Cancer.
1997;
79:
2424
-2429.
[PubMed]
.
-
17.
Lukas
J
, Groshen
S
, Saffari
B
, Niu
N
, Reles
A
, Wen
WH
, Felix
J
, Jones
LA
, Hall
FL
and Press
MF.
WAF1/Cip1 gene polymorphism and expression in carcinomas of the breast, ovary, and endometrium.
Am J Pathol.
1997;
150:
167
-175.
[PubMed]
.
-
18.
Mousses
S
, Ozcelik
H
, Lee
PD
, Malkin
D
, Bull
SB
and Andrulis
IL.
Two variants of the CIP1/WAF1 gene occur together and are associated with human cancer.
Hum Mol Genet.
1995;
49:
1089
-1092.
[PubMed]
.
-
19.
Savas
S
, Ahmad
MF
, Shariff
M
, Kim
DY
and Ozcelik
H.
Candidate nsSNPs that can affect the functions and interactions of cell cycle proteins.
Proteins.
2005;
58:
697
-705.
[PubMed]
.
-
20.
Corder
EH
, Saunders
AM
, Strittmatter
WJ
, Schmechel
DE
, Gaskell
PC
, Small
GW
, Roses
AD
, Haines
JL
and Pericak-Vance
MA.
Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families.
Science.
1993;
261:
921
-923.
[PubMed]
.
-
21.
Schächter
F
, Faure-Delanef
L
, Guénot
F
, Rouger
H
, Froguel
P
, Lesueur-Ginot
L
and Cohen
D.
Genetic associations with human longevity at the APOE and ACE loci.
Nat Genet.
1994;
6:
29
-32.
[PubMed]
.
-
22.
Breitner
JC
and Miech
RA.
Sex and sociobiology: a role for APOE.
Neurobiol Aging.
1999;
20:
445
[PubMed]
.
-
23.
Fries
JF
Measuring and monitoring success in compressing morbidity.
Ann Intern Med.
2003;
139:
455
-459.
[PubMed]
.
-
24.
Franceschi
C
, Motta
L
, Valensin
S
, Rapisarda
R
, Franzone
A
, Berardelli
M
, Motta
M
, Monti
D
, Bonafé
M
, Ferrucci
L
, Deiana
L
and Pes
GM.
Do men and women follow different trajectories to reach extreme longevity? Italian Multicenter Study on Centenarians (IMUSCE).
Aging (Milano).
2000;
12:
77
-84.
[PubMed]
.
-
25.
Wigginton
JE
, Cutler
DJ
and Abecasis
GR.
A note on exact tests of Hardy-Weinberg equilibrium.
Am J Hum Genet.
2005;
76:
887
-893.
[PubMed]
.
-
26.
Purcell
S
, Neale
B
, Todd-Brown
K
, Thomas
L
, Ferreira
MA
, Bender
D
, Maller
J
, Sklar
P
, de Bakker
PI
, Daly
MJ
and Sham
PC.
PLINK: a toolset for whole-genome association and population-based linkage analyses.
Am J Hum Genet.
2007;
81:
559
-575.
[PubMed]
.
-
27.
Barrett
JC
, Fry
B
, Maller
J
and Daly
MJ.
Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005; 21: 263-265. linkage analysis.
American Journal of Human Genetics.
2007;
81:
559
-575.
[PubMed]
.