MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8
Abstract
Senescence is a cellular program that irreversibly arrests the proliferation of damaged cells and induces the secretion of the inflammatory mediators IL- 6 and IL-8 which are part of a larger senescence associated secretory phenotype (SASP). We screened quiescent and senescent human fibroblasts for differentially expressed microRNAS (miRNAs) and found that miRNAs 146a and 146b (miR-146a/b) were significantly elevated during senescence. We suggest that delayed miR-146a/b induction might be a compensatory response to restrain inflammation. Indeed, ectopic expression of miR-146a/b in primary human fibroblasts suppressed IL-6 and IL-8 secretion and downregulated IRAK1, a crucial component of the IL-1 receptor signal transduction pathway. Cells undergoing senescence without induction of a robust SASP did not express miR-146a/b. Further, IL-1α neutralizing antibodies abolished both miR-146a/b expression and IL-6 secretion. Our findings expand the biological contexts in which miRNA-146a/b modulates inflammatory responses. They suggest that IL-1 receptor signaling initiates both miR-146a/b upregulation and cytokine secretion, and that miR-146a/b is expressed in response to rising inflammatory cytokine levels as part of a negative feedback loop that restrains excessive SASP activity.
Introduction
Cellular
senescence is a cell fate program
triggered by potentially oncogenic stimuli and stresses that prevent aged or abnormal cells from further
proliferation [1,2]. Several stimuli, including repeated proliferation,
growth stimulation coordinated with cell-cycle arrest, DNA damage and expression of activated oncogenes cause mammalian
cells to enter into the essentially irreversible growth senescent arrest and
acquire the morphological and behavioral features of senescent cells [3-5].
Senescent
cells have been shown to accumulate in a variety of aging tissues as well as
several premalignant and malignant lesions [1]. Because
cellular senescence eliminates the proliferative capacity of damaged cells it
is a potent tumor suppressing mechanism [1,6]. However
senescence also prevents the replacement of cells lost owing to age, injury or
apoptosis. Thus, the senescence response is likely a tradeoff between tumor
suppression and tissue regeneration. Senescence may therefore be considered an
example of evolutionary antagonistic pleiotropy, whereby a trait that confers a
selective advantage early in life (tumor suppression) may be retained even
though it also has deleterious effects later in life [7].
Senescent human cells exhibit numerous changes in gene
expression, many of which relate to the growth arrest [8]. Senescent
cells also develop a senescence-associated secretory phenotype (SASP) [9]. The SASP
is characterized by the secretion of a wide range of growth factors, cytokines,
extracellular matrix proteins and degradative enzymes, most of which can alter
the local tissue microenvironment [9-13]. The SASP is controlled in a modular fashion: for example,
the DNA damage response kinase ATM is required for the upregulation of some,
but not all, SASP factors [14]. Of particular interest
SASP is characterized by high level secretion of the cytokines, IL-6 and IL-8, which are key mediators of
inflammation. Inflammation is important for development of cancer as well as
many other age-related diseases [15].
Furthermore, IL-6 and IL-8 were recently shown to reinforce the senescent growth
arrest [15-17]. Thus,
understanding the mechanisms that regulate IL-6 and IL-8 in association with
senescence is important for understanding
biological processes as diverse as tumor suppression and the development of
age-related diseases, including cancer.
Recent studies have identified microRNAs (miRNAs) as
important regulators of diverse biological processes. miRNAs are ~22
nucleotide non-coding regulatory RNAs that are evolutionary conserved from
nematodes to humans [18,19].
Primary miRNAs are initially transcribed by RNA polymerase II as larger
precursors, which are then cleaved by a nuclear complex containing the
ribonuclease Drosha and DCGR8. The cleaved product is a hairpin RNA ~65
nucleotides in length known as the pre-miRNA [20]. The
pre-miRNA is further processed to the mature miRNA by the cytosolic enzyme
Dicer. The mature miRNA is then incorporated into the RNA-induced silencing
complex (RISC). The miRNA-RISC complex binds to target messenger RNAs (mRNAs),
often in the 3' untranslated regions, and either promotes mRNA degradation or
translational repression [21-23]. Each
miRNA has the potential to regulate the expression of multiple mRNA targets.
miRNAs regulate a broad range of phenotypes including embryonic
development, cell proliferation, differentiation and apoptosis [24-27]. miRNAs also control various activities of the immune
system [28-30]. Recent studies show
that miRNAs are important etiological or facilitating factors in the pathogenesis of several diseases, including
cancer, diabetes, rheumatoid arthritis, and Alzheimer's disease [31-35].
miRNAs have also been implicated in the control of
aging and cellular senescence. Mutation of miR-lin-4 in C.elegans dramatically
shortens life span [36].
Additionally members of the miR-34 family
of miRNAs were recently shown to suppress cell proliferation and be direct
targets of the p53 tumor suppressor protein which
is required for the senescence growth arrest [31,37].
Indeed, overexpression of miR-34a in
normal human IMR90 fibroblast caused a senescence growth arrest [37]. Similarly
the MDM2 inhibitor Nutlin3A induced miR-34 and senescence in human fibroblasts
via activation of p53 [38]. In mouse
embryonic fibroblasts (MEFs), miR-20a induced senescence, in this case by
upregulating the p16INK4A tumor suppressor protein [39]. Finally
ablation of Dicer in MEF's induced senescence by upregulating p53, indicating
that miRNAs play both positive and negative roles in regulating the senescence
arrest [40]. In contrast to a rising understanding of how miRNAs
modulate the senescence growth arrest, virtually nothing is known about whether
or how miRNAs regulate any component of the SASP.
Here we report, that the levels of two related miRNAs,
miR-146a and 146b (miR-146a/b), increase in senescent human fibroblasts in an
interleukin IL1α dependent manner, but only when high levels of IL-6 and
IL-8 secretion accompany senescence. In
the context of the SASP, we propose that increased expression of miR-146a/b
serves to restrain excessive secretion of the inflammatory cytokines IL-6 and IL-8,
thereby limiting senescence-associated inflammation.
Results
Induction of miR-146a and miR-146b by senescent human
HCA2 fibroblasts
In
screening arrays of known miRNAS for those that are differentially expressed by
quiescent versus senescent cells (C. Patil, manuscript in preparation), we
found that miR-146a and miR-146b were
expressed at significantly higher levels by senescent HCA2 cells, which are
normal human fibroblasts from neonatal foreskin. This was true whether
senescence was induced by a DNA damaging agent (bleomycin, which causes DNA
double strand breaks) (DS) or replicative exhaustion (RS). We validated the
array results for miR-146a expression by northern analysis. miR-146a was
readily detectable in senescent cells, but was undetectable in proliferating
(P) or quiescent (Q) cells (Figure 1A). In these and subsequent samples,
senescence was confirmed by the low percentage of proliferating
(BrdU-incorporating) cells and high percentage of cells that stained positive
for the senescence associated
β-galactosidase (SA-β-gal) (Figure 1; Experimental Procedures).
Because quiescent cells, which are not senescent but rather temporarily growth
arrested, expressed low to undetectable levels of miR-146a, as did
proliferating presenescent cells, we conclude that robust expression of
miR-146a is specifically associated with senescence and not simply with growth
arrest. In the experiments that follow, we used proliferating cells as a
negative control for miR-146a/b expression.
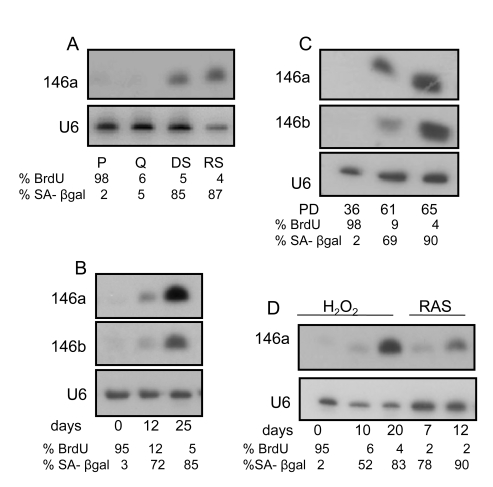
Figure 1. miR146a/b expression increases in senescent HCA2 fibroblasts. (A)
Northern blot analysis of total RNA prepared from proliferating (P),
quiescent (Q), damage (bleomycin)-induced senescent (DS) and replicatively
senescent (RS) HCA2 cells. We analyzed 10 μg of RNA from P, Q and DS
cells, but 5 μg of RNA from RS cells. After separation and transfer to
membranes, the blots were probed for miR-146a. Equal RNA loading was
confirmed by probing for the small RNA species U6. Values for the
percentage of cells incorporating bromodeoxyuridine (% BrdU) or expressing
the sensecence-associated beta-galactosidase (% SA-β-gal) are indicated
below each lane. (B) Northern blot analysis of RNA from DS cells.
Cells were harvested for RNA at the days indicated after cells were induced
to senesce by bleomycin. The blot was initially probed for miR-146a, then
stripped and reprobed for miR-146b. The proliferation levels (% BrdU) and
% cells that express the SA-β-gal are indicated. (C) Northern
blot analysis of replicatively senescencing cells. Cells were harvested at
the PD (population doubling level) indicated below the figure. The
proliferation levels (% BrdU) and % cells that express the SA-β-gal
are indicated. (D) Northern blot analysis of cells treated with H2O2
(0.1 mM for 2 h) or infected with the lentivirus expressing oncogenic RASV12. Cells were harvested for RNA at
the indicated days after treatment. The proliferation levels (% BrdU) and % cells that express the
SA-β-gal are indicated.
We
investigated the kinetics of induction of miR-146a/b expression following
induction of senescence by different stimuli. Following a senescence-inducing
dose of the DNA damaging agent bleomycin, miR-146a/b was first detected
approximately 12 days later (Figure 1B). By comparison, the SASP is first
evident 3-4 days after a senescence-inducing dose of DNA damage, and completely
established within 5-7 days after senescence induction [9,14] Thus,
the levels of miRNA-146a/b remained undetectable or very low during the
interval in which the SASP developed in response to DNA damage (not shown), and
were detected by northern analysis only several days later. By 25 days after
senescence was induced by DNA damage, the levels of miRNA-146a/b were maximal (Figure 1B). At this time point, the few cells that were able to repair the damage and
resume growth comprised only a small fraction of the population and thus the
population remained largely senescent with ~5% BrdU incorporation and 85% SA-β-gal
activity. We obtained similar results when we analyzed replicatively senescent
cells (Figure 1C). At PD 65, when the cells were nearly completely senescent
(4% BrdU incorporation, 90% positive for SA-β-gal), miR-146a/b expression
was higher than at PD 61, when the cells were less completely senescent ( 5%
BrdU incorporation, 69% SA-β-gal positive cells). Further, miR-146a/b
followed a similar expression pattern when we induced senescence by oxidative
stress (hydrogen peroxide treatment) (Figure 1D, left lanes) or the oncogene
RASV12 (to cause oncogene-induced senescence) (Figure 1D, right lanes). Across
all conditions tested, miRNA-146a/b expression remained low during the early
period of senescence, when other phenotypes (growth arrest, SA-β-gal expression,
and the SASP) were well underway, but rose to higher levels during a later
period after these senescence-associated phenotypes had been fully
established.
miR-146a/b
suppresses IRAK-1 expression and reduces IL-6 and IL-8 secretion in HCA2 fibroblasts
To
determine the role of miR-146a/b, in the phenotypes of human fibroblast, we
stably infected proliferating cells with either a control lentivirus or
lentiviruses expressing miR-146a or miR-146b (Figure 2A). The miR-146a/b
overexpressing fibroblasts displayed no obvious morphological alterations and
maintained a proliferation rate comparable to that of control cells (data not
shown). Thus, miR-146a/b did not induce a quiescence or senescence growth
arrest.
As
discussed above, senescent cells robustly secrete the inflammatory cytokines
IL-6 and IL-8. Recent reports identified miR-146a/b as negative regulators of
inflammatory cytokine expression during immune reactions and cancer cell
invasiveness [9,41,42]. We
therefore asked, whether miR-146a/b modulated the secretion of inflammatory
cytokines by senescent cells. First, we
determined the effect of miR-146a/b over-expression on IRAK1 and TRAF-6. These
proteins are established miR-146a/b targets and key downstream components of
the IL-1 and Toll-like receptor signaling cascades, which ultimately regulate
the expression of inflammatory cytokines such as IL-6 and IL-8 [41,42]. HCA2
fibroblasts that overexpressed miR-146a/b had markedly reduced levels of IRAK1
(Figure 2B). However, the levels of TRAF6 remained unaltered in these cells (Figure 2B). Thus, at least in HCA2 cells, miR-146a/b targeted IRAK1, but not TRAF-6.
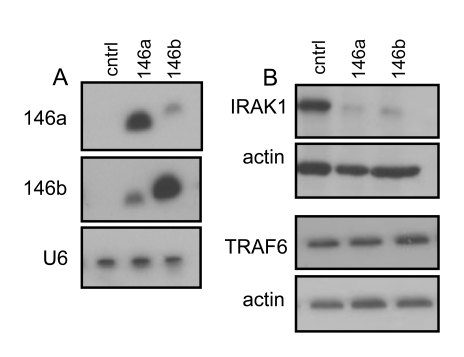
Figure 2. IRAK1 but not TRAF6 levels are reduced in HCA2 cells overexpressing miR146a and miR 146b. (A)
Northern blot analysis of total RNA prepared from control (insertless
virus-infected) HCA2 cells (cntrl), cells infected with a
miR-146a-expressing virus (146a) and cells infected with a
miR-146b-expressing virus (146b). 8 μg of total RNA was loaded in each lane. (B) Western blot analysis of
total protein lysates prepared from proliferating cells (cntrl, PD32), or
cells overexpressing miR-146a or
miR-146b, and analyzed for IRAK1 (top panel) and TRAF6 (bottom panel).
Actin protein levels served as a loading control.
Because overexpression of miR-146a/b in human MDA-MB-231 breast cancer cells, reduced the
levels of secreted IL-6 and IL-8 [41] and
since IRAK1 is a key mediator of the expression of IL-6 and IL-8, we compared
the basal levels of secreted IL6 and IL8 in control and miR-146a/b
overexpressing HCA2 cells. We collected conditioned medium (CM) from these
cells over a 24 h period and assayed the CM for IL-6 and IL-8 by western
analysis (Figure 3A, proliferating) and ELISA (Figure 3B, proliferating). We
observed a marked reduction in basal secretion of IL-6 and IL-8 in the
miR-146a/b overexpressing cells relative to control cells. We normalized these
measurements, against cell number and, where applicable, against the level of
secreted IGFBP3 (Figure 3A), a protein that is not influenced by miR-146a/b
expression [41].
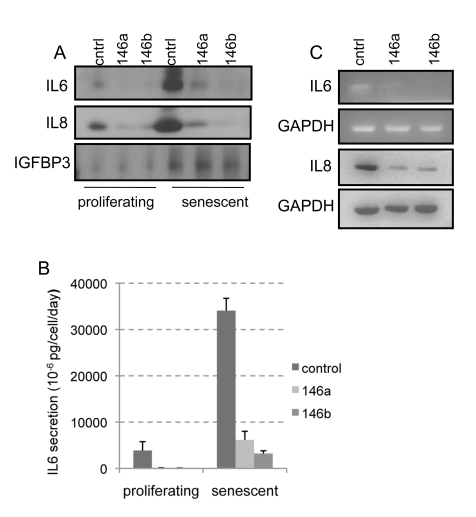
Figure 3. Overexpression of miR-146a/b suppresses basal and senescence-associated secretion of IL-6 and IL-8 in HCA2 cells. (A)
Western blot analyses of TCA-precipitated
proteins prepared from CM collected over 24 h from cells infected with the
lentivirus backbone (cntrl) or lentiviruses expressing miR-146a (146a) or
miR-146b (146b). The blot was analyzed for IL-6, IL-8 and IGFBP3. Equal
loading was based on cell number prior to collection of CM and IGFBP3
levels. Proliferating indicates cells described in Figure 2A. The same
cells were treated with bleomycin and CM was harvested 11 days later
(senescent). (B) IL-6 in CM from the cell populations described in
Fig 3A was measured by ELISA. The data are reported as 10-6 pg
per cell per day. (C) RT-PCR analysis of transcript levels of IL-6
and IL-8 in miR-146a/b-overexpressing cells. RNA collected from
proliferating cells was used as the control (cntrl).
To
determine whether miR-146a/b influenced the increased IL-6 and IL-8 secretion
that accompanies the senescence, we induced the miR146a/b overexpressing HCA2
fibroblasts to senesce by DNA-damage (bleomycin). Western and ELISA analyses
of CM collected 11 days after DNA damage showed that IL-6 and IL-8 secretion by
senescent miR-146a/b-overexpressing cells, was strikingly reduced compared to
control senescent cells (Figure 3A-B right panel). RT-PCR analysis (Figure 3C), showed that miR-146a/b reduced the levels of IL-6 and IL-8 transcripts,
indicating that miR-146a/b exerts these effects by decreasing transcription or
promoting mRNA degradation. Together, these observations establish that
miR146a/b expression is sufficient to negatively regulate IL-6 and IL-8
secretion in both pre-senescent and senescent human fibroblasts.
miR-146a/b
expression increases only in senescent human fibroblasts that have robust IL-6
secretion
To
determine whether miR-146a/b negatively regulates IL-6 and IL-8 secretion in
other human fibroblast strains, we examined BJ and IMR90 primary human
fibroblasts. BJ, like HCA2, fibroblasts are derived from neonatal foreskin,
whereas IMR90 fibroblasts are derived from fetal lung. Moreover, upon
senescence, BJ and HCA2 cells express a robust SASP, whereas IMR90 cells
express a less robust SASP, and, in particular, secrete less IL-6 and IL-8 [9]. We
confirmed that senescent IMR90 cells secreted about 8-fold lower levels of IL-6
than senescent BJ cells when CM were analyzed 11 days after induction of
senescence by DNA damage (bleomycin) (Figure 4A). Notably, miR-146a levels
were also substantially lower in senescent IMR90 compared to senescent BJ
cells; in fact, mi-146a was essentially undetectable by northern analyses in
senescent IMR90 cells but readily detectable in BJ cells (Figure 4B). Additionally,
replicatively senescent IMR90 cells also expressed undetectable levels of
miR-146a, assayed by northern blotting, whereas miR-146a expression was easily
detectable in near replicatively senescent BJ cells (Figure 4C). Likewise,
senescent cells of the strain WI-38, also derived from fetal lung and
exhibiting the low SASP characteristics of IMR90 [9], did not
express detectable levels of miR-146a/b (data not shown).
Because of the correlation between the
magnitude of the inflammatory cytokine component of the SASP and miR-146a/b
expression, we asked whether IMR90 might express higher levels of miR-146a
under conditions that induced higher inflammatory cytokine secretion. We
previously showed that RAS oncogene-induced senescence results in a more robust
SASP than damage-induced senescence [9]. We therefore
measured the levels of IL-6 secretion (Figure 4D) and miR-146a/b expression (Figure 4E) in IMR90 cells induced to senesce by oncogenic RAS. Oncogenic RAS
significantly increased both IL-6 and miR-146a in IMR90 cells. There was a
close parallel between miR-146a/b expression and robustness of inflammatory
cytokine secretion.
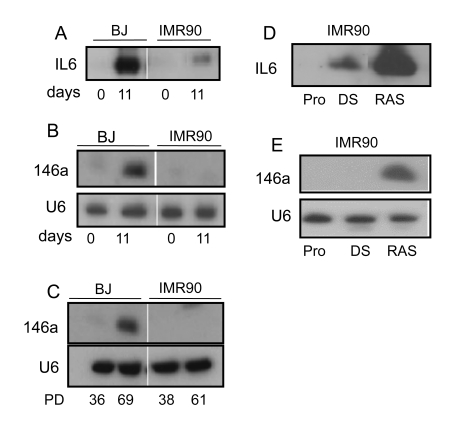
Figure 4. miR-146a/b increase in senescent fibroblasts that secrete high levels of inflammatory cytokines. (A) & (B) Proliferating BJ (PD
36) and IMR90 (PD 38) cells were treated with bleomycin to induce
senescence. CM and RNA were harvested 11 d later. (A) Western
analysis for secreted IL-6. (B) Northern analysis for miR-146a. (C)
Northern analysis for miR-146a levels in replicatively senescent BJ and
IMR90 cells. The PD levels at which cells were harvested for analysis is
given below each lane. BJ cells reach complete senescence after
approximately 70 PDs, whereas IMR90 cells are nearly completely senescent
by PD61. (D)
& (E) Proliferating IMR90 cells (PD40) were either untreated
(Pro), treated with bleomycin (DS) or infected with the lentivirus
expressing oncogenic RAS (RAS). CM and RNA were collected 11 days after
treatment or infection. (D) Western analysis for IL-6 in CM. (E)
Northern analysis for miR-146a and U6 (control) levels.
IL1-α
upregulates miR146a/b in senescent human fibroblasts
To
determine the mechanism that connects miR-146a/b expression and IL-6 and IL-8
secretion in human fibroblasts, we explored the role of IL-1 signaling which is a master regulator of inflammatory cytokine
secretion. Because IRAK1 is both a
primary target of miR-146a/b and an essential downstream component of the IL-1
receptor signaling system, we tested the IL-1 receptor ligands, IL-1α and
IL-1β, as strong candidates for ability to upregulate both miR-146a/b
expression and IL-6 and IL-8 secretion. IL-1β is a SASP component [9] and
senescent cells contain high levels of membrane-bound IL-1α have also been
observed in senescent cells (A. Orjalo, manuscript in preparation). We added
neutralizing antibodies against IL-1α or IL-1β to the culture medium
of HCA2 cells one day after treatment with the DNA damaging agent bleomycin,
and collected RNA samples 10 days later. Northern
analysis showed that neutralizing antibodies against IL- 1α not IL-1β
suppressed the senescence-associated upregulation of miR-146a (Figure 5A). In parallel, we assessed IL-6 secretion levels in CM
obtained in the presence of neutralizing antibodies. Again, neutralizing antibodies
against IL-1α, but not IL-1β, suppressed the senescence-associated
secretion of IL-6 (Figure 5B). Similar suppression was seen in the levels of
IL-8 (data not shown).
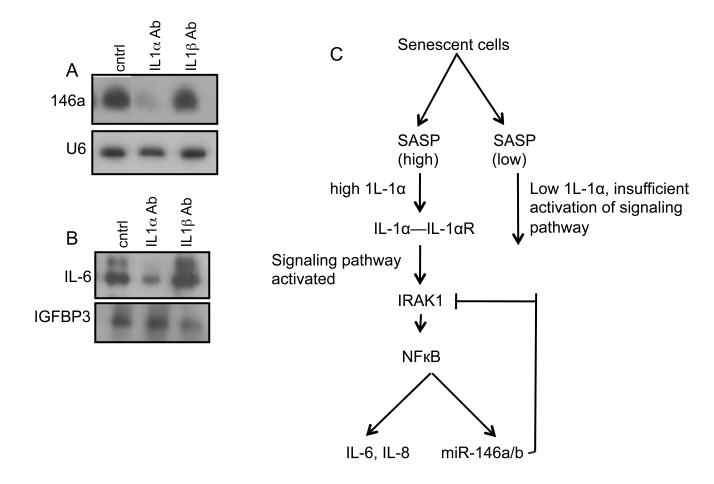
Figure 5. IL-1α upregulates miR-146a in senescent cells. (A)
Northern analysis for miR-146a levels in damage-induced senescent HCA2
cells treated with neutralizing antibodies against IL1-α and
IL1-β. HCA2 cells (PD 35) were used and induced to senesce by
treatment with bleomycin. Cells were harvested for RNA 11 days later.
Details of the procedure are described in ‘Experimental Procedures. (B)
Western analysis for IL-6 in damage-induced senescent HCA2 cells treated
with neutralizing antibodies to IL-1α and IL-1β. CM were
harvested 11 days after bleomycin treatment. (C) Model for the role
of miR-146a/b in senescent cells: In response to a high SASP (right
branch), IL-1α interacts with the IL-1α receptor (IL-1αR)
and the signaling pathway that involves IRAK1 is fully activated. This
activation leads to the well-documented activation of the transcription
factor NFкB and production of IL-6, IL-8 and also miRNA-146a/b.
miRNA-146a/b is a component of a negative feedback loop and acts to
downregulate the levels of IRAK1, hence restraining the levels of IL-6 and
IL8. However, in response to a low SASP (left branch), the signaling
pathway is not sufficiently activated. Thus there is a low level of IL-6
and IL-8 secretion and miRNA-146a/b is not upregulated.
These
findings suggest the following model (Figure 5C): When the SASP inflammatory
secretion levels are low (right branch), IL-1α levels and IL-1R signaling
are low. However, when inflammatory cytokine secretion is high, (left branch),
high IL-1R signaling activates the downstream kinase IRAK1, ultimately
resulting in activation of NFкB. This transcription factor stimulates
expression of the SASP cytokines, IL-6 and IL-8, as well as miR-146a/b. By
targeting the IRAK1 mRNA, miR-146a/b creates a negative feedback loop that
restrains IRAK1 signaling and limits senescence-associated cytokine production.
Discussion
The
role of miRNAs in promoting diverse cellular programs such as stem cell
maintenance, differentiation and apoptosis underscores their emerging
importance as regulators of myriad biological processes [24-27]. Here,
we demonstrate a role for miRNA146a/b in the
cell non-autonomous effects of cellular
senescence, a phenomenon linked to both cancer and aging.
Following
multiple forms of senescence-inducing treatments, miR146 levels increase
sharply in senescent HCA2 cells rising from nearly undetectable baseline
levels in proliferating and quiescent cells. miR-146a/b expression is
associated with a wide range of normal and pathological biology. These miRNAs
have been implicated in the biology of monocytic immune cells responding to
lipopolysaccharide stimulation, human lung alveolar epithelial cell line
responding to IL-1β induction, brain tissue from patients with Alzheimer's
disease and synovial tissue from patients with rheumatoid arthritis [34,35,43,44].
In the majority of these situations, miR-146a/b acts as a negative regulator of
inflammatory pathways. In the immune system, miR-146a/b upregulation provides
negative feedback on the innate immune system by targeting IRAK1 and TRAF6 [44]. These
proteins are key components of the IL-1 and Toll-like receptor signaling
system, of which NFкB activation is a primary downstream effector. In
agreement with earlier observations on immune cells, our previous work
demonstrated that miR-146a/b negatively regulates NFkB activity and the inflammatory
pathway, in breast cancer cells [41,42]. In
the context of senescence, the results presented here expand and corroborate
the role of miR-146a/b as negative regulators of inflammation and, in
particular, underscore its influence as a negative regulator of excessive IL-6
and IL-8 secretion, a hallmark of senescence.
HCA2 human fibroblasts overexpressing
miR-146a/b exhibited markedly decreased levels of IRAK1 as well as IL-6 and
IL-8, under both proliferating and senescent conditions. IL-6 and IL-8
expression was suppressed at the mRNA level, most likely a consequence of
diminished NFкB activation following miR-146a/b-mediated reduction in
IRAK1 protein levels [41]. In the
context of senescence, this regulation was a consequence of IL-1R signaling
specifically activated by IL-1α but not IL-1β. This specificity,
however, might be cell type-specific. In
studies of lung alveolar epithelial cells by Perry et al, stimulation
with IL-1β caused a rapid increase in miR-146a levels but this did not
impact the protein levels of either IRAK1 or TRAF6 [43]. However,
inhibitors of miR-146a did enhance the levels of the pro-inflammatory cytokines
IL8 and RANTES [43]. These studies suggest that miRNA-146a can function as
a negative regulator of inflammation at the level of translation suppression,
independent of the IL-1 signaling pathway involving IRAK1. In contrast,
miR-146a/b downregulated IRAK1 protein levels in human fibroblasts, and thereby
suppressed expression of IL-6 and IL-8, in agreement with a model whereby
miR-146a/b responds to robust activation of the IL-1α signaling pathway
and negatively regulates the IL-6 and IL-8 expression driven by that pathway (Figure 5C) [44].
We
found that regulation of miR-146a/b and the IL-6 and IL-8 inflammatory
components of the SASP requires signaling through the IL-1R pathway. Neutralizing antibodies against IL-1α sharply
decreased both IL-6 secretion and miR-146a production. In contrast,
neutralizing antibodies against IL-1β did not suppress inflammatory
cytokine secretion, suggesting that IL-1β secretion levels at senescence
are not sufficient to produce a significant level of IL-1R signaling. Taken together,
these results suggest the model shown in Figure 5C. In senescent cells, activation
of the IL-1 receptor signaling pathway is dependent upon sufficiently high
IL-1α levels. In HCA2 cells, a wide range of conditions that induce
senescence promote IL-1 receptor activation, whereas in IMR90 cells only
oncogene-induced senescence results in sufficiently intense SASP to promote
IL-6 and miR-146 expression; senescent fibroblasts that exhibit lower levels of
SASP secretion do not induce miR-146 (Figure 4).
Thus, miR-146a/b in senescent fibroblasts that express
a robust SASP negatively regulates IRAK1 protein levels, thereby dampening the
IL-1 receptor signaling pathway and hence the expression and secretion of
inflammatory molecules such as IL-6 and IL-8. This negative feedback loop
would serve to limit the deleterious effects of the SASP on surrounding
tissues. This safeguard may be particularly important when the local
concentration of SASP factors is high, for example when senescent cells
accumulate in premalignant nevi [45] or are
induced after exposure to chemotherapeutic agents [9].
Materials and Methods
Cells.
Early passage HCA2 human foreskin fibroblasts were
obtained from J Smith (University of Texas, San Antonio). Early passage BJ human
foreskin fibroblasts and IMR90 fetal lung fibroblast were obtained from ATCC.
Cells were cultured at 37° C in a 10% CO2 incubator in DMEM with 10%
fetal bovine serum. 293FT packaging cells (Invitrogen) were used to generate
lentivirus. We defined presenescent (proliferative) cells, as having undergone
fewer than 35 population doublings and having a 24 h BrdU labeling index of
~95%. Subconfluent cells (1500-4000/cm2) were made quiescent by
washing with serum-free medium and incubating in 0.2% serum for 4 d.
Proliferating cells were made replicatively senescent by serial passage in 10%
serum. For damage induced senescence, cells were plated at a density of
40000/cm2. Two days later the cells were treated with bleomycin 40
μg/ml for 2 h. On the 11th day or indicated day following treatment,
cells were stained for the senescence-associated β-gal (SA-β-gal)
marker [46] and DNA
synthesis was measured over a 24 h interval using a BrdU labeling kit ( Roche
Diagnostics). Cultures that had > 80% SA-β-gal positive cells and
≤ 4% BrdU positive cells were considered senescent.
Lentiviral constructs, viruses and infections.
The
lentiviral miR-146a/b expression vectors were constructed as described [41]. The
expression of miR-146a/b was under control of the cytomegalovirus promoter.
The lentivirus encoding oncogenic RAS V12 has been described [14]. 24 h
following lentiviral infection cells were placed under puromycin (1 μg/ml)
selection for 4 d.
Antibodies.
IRAK1 ( SC 5288 ), TRAF6 (SC 8409) and IGFBP3 (SC 9028) antibodies were
obtained from Santacruz Biotechnology, USA. IL-6 (AF-206-NA), IL-8 (MAB208),
IL-1α (MAB200) and IL-1β (MAB601) was obtained from R&D systems.
Northern blots.
Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA) and analyzed by
northern blotting. Briefly, RNA was separated on 15% TBE- urea polyacrylamide
gels (Invitrogen, Carlsbad, CA) and transferred onto Hybond Plus membranes (Amersham,
Piscataway, NJ) as previously described [47]. The blots
were probed with an antisense miR-146a DNA oligonucleotide, striped and
reprobed with an antisense miR-146b DNA oligonucleotide. Oligonucleotides were32P end-labeled. RNA loading was confirmed by probing for the small
RNA species, U6. Unless mentioned otherwise 10 μg of RNA was loaded in each
lane.
For Northern analysis of RNA harvested
from cells treated with
neutralizing antibodies, IL-1α
(0.6 μg/ml) and IL-1β (0.8 μg/ml) antibodies were added to the medium
one day after cells were induced to senescence by bleomycin. Fresh medium was
added 6 d later and the medium was replenished with another aliquot of the
neutralizing antibody at the same concentration. RNA was harvested 11 d after
bleomycin treatment.
RT PCR.
Total RNA was used for RT-PCR analysis of IL-6 and
IL-8 transcripts. Reverse transcription was done using Super Script II (Invitrogen,
Carlsbad,USA). Following reverse transcription the products were was amplified
for 30 cycles using appropriate primers.
Western Blots.
Total protein extracts were used for western analysis of IRAK1, TRAF6 and actin
(loading control). CM was prepared by washing cells 3 times in PBS and
incubating the cells in 0.2% serum in DMEM medium. CM was harvested 24 h
latter and protein precipitated with 15% TCA overnight. Loading was based on
equal cell number and where appropriate IGFBP3 was used as a loading control.
For treatment with neutralizing antibodies, IL-1α (0.6 μg/ml) and
IL-1β (0.8 μg/ml) was added 24 h prior to collection of CM.
ELISA.
CM was prepared as described above except cells were
incubated for 24 h in serum free DMEM. ELISA was performed using kits and
procedures from R&D (IL-6- #D06050).
Acknowledgments
We
thank Dr. Pierre Desprez for valuable comments and critical reading of the
manuscript. This work was supported by grants from the National Institutes of
Health (R37-AG09909, U54-CA0126540, U54-ES016655, P30-AG025708).
Conflicts of Interest
There is no conflict of interest for any of the authors.
References
-
1.
Campisi
J
Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors.
Cell.
2005;
120:
513
-522.
[PubMed]
.
-
2.
Campisi
J
and d'Adda
di Fagagna F.
Cellular senescence: when bad things happen to good cells.
Nat Rev Mol Cell Biol.
2007;
8:
729
-740.
[PubMed]
.
-
3.
Lundberg
AS
, Hahn
WC
, Gupta
P
and Weinberg
RA.
Genes involved in senescence and immortalization.
Curr Opin Cell Biol.
2000;
12:
705
-709.
[PubMed]
.
-
4.
Serrano
M
and Blasco
MA.
Putting the stress on senescence.
Curr Opin Cell Biol.
2001;
13:
748
-753.
[PubMed]
.
-
5.
Demidenko
ZN
and Blagosklonny
MV.
Growth stimulation leads to cellular senescence when the cell cycle is blocked.
Cell Cycle.
2008;
7:
3355
-3361.
[PubMed]
.
-
6.
Shay
JW
and Roninson
IB.
Hallmarks of senescence in carcinogenesis and cancer therapy.
Oncogene.
2004;
23:
2919
-33.
[PubMed]
.
-
7.
Kirkwood
TB
and Austad
SN.
Why do we age.
Nature.
2000;
408:
233
-238.
[PubMed]
.
-
8.
Fridman
AL
and Tainsky
MA.
Critical pathways in cellular sensecence and immortalization revealed by gene expression profiling.
Oncogene.
2008;
27:
5975
-5987.
[PubMed]
.
-
9.
Coppe
JP
, Patil
CK
, Rodier
F
, Sun
Y
, Munoz
DP
, Goldstein
J
, Nelson
PS
, Desprez
PY
and Campisi
J.
Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor.
PLoS Biol.
2008;
6:
2853
-2868.
[PubMed]
.
-
10.
Krtolica
A
, Parrinello
S
, Lockett
S
, Desprez
PY
and Campisi
J.
Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging.
Proc Natl Acad Sci U S A.
2001;
98:
12072
-12077.
[PubMed]
.
-
11.
Bavik
C
, Coleman
I
, Dean
JP
, Knudsen
B
, Plymate
S
and Nelson
PS.
The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms.
Cancer Res.
2006;
66:
794
-802.
[PubMed]
.
-
12.
Parrinello
S
, Coppe
JP
, Krtolica
A
and Campisi
J.
Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation.
J Cell Sci.
2005;
118:
485
-496.
[PubMed]
.
-
13.
Liu
D
and Hornsby
PJ.
Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion.
Cancer Res.
2007;
67:
3117
-31126.
[PubMed]
.
-
14.
Rodier
F
, Coppe
J-P
, Patil
CK
, Hoeijmakers
WAM
, Munoz
DP
, Raza
SR
, Freund
A
, Campeau
E
, Davalos
AR
and Campisi
J.
Persistent DNA damage signaling triggers senescence-associated inflammatory cytokine secretion.
Nature Cell Biology.
2009;
In press
.
-
15.
Coussens
LM
and Werb
Z.
Inflammation and cancer.
Nature.
2002;
420:
860
-867.
[PubMed]
.
-
16.
Acosta
JC
, O'Loghlen
A
, Banito
A
, Guijarro
MV
, Augert
A
, Raguz
S
, Fumagalli
M
, Da
Costa M
, Brown
C
, Popov
N
, Takatsu
Y
, Melamed
J
, d'Adda
di Fagagna F
, Bernard
D
, Hernando
E
and Gil
J.
Chemokine signaling via the CXCR2 receptor reinforces senescence.
Cell.
2008;
133:
1006
-1018.
[PubMed]
.
-
17.
Kuilman
T
, Michaloglou
C
, Vredeveld
LC
, Douma
S
, van
Doorn R
, Desmet
CJ
, Aarden
LA
, Mooi
WJ
and Peeper
DS.
Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network.
Cell.
2008;
133:
1019
-31.
[PubMed]
.
-
18.
Ambros
V
The functions of animal microRNAs.
Nature.
2004;
431:
350
-355.
[PubMed]
.
-
19.
He
L
and Hannon
GJ.
MicroRNAs: small RNAs with a big role in gene regulation.
Nat Rev Genet.
2004;
5:
522
-531.
[PubMed]
.
-
20.
Han
J
, Lee
Y
, Yeom
KH
, Nam
JW
, Heo
I
, Rhee
JK
, Sohn
SY
, Cho
Y
, Zhang
BT
and Kim
VN.
Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex.
Cell.
2006;
125:
887
-901.
[PubMed]
.
-
21.
Jing
Q
, Huang
S
, Guth
S
, Zarubin
T
, Motoyama
A
, Chen
J
, Di Padova
F
, Lin
SC
, Gram
H
and Han
J.
Involvement of microRNA in AU-rich element-mediated mRNA instability.
Cell.
2005;
120:
623
-634.
[PubMed]
.
-
22.
Valencia-Sanchez
MA
, Liu
J
, Hannon
GJ
and Parker
R.
Control of translation and mRNA degradation by miRNAs and siRNAs.
Genes Dev.
2006;
20:
515
-524.
[PubMed]
.
-
23.
Bhattacharyya
SN
, Habermacher
R
, Martine
U
, Closs
EI
and Filipowicz
W.
Relief of microRNA-mediated translational repression in human cells subjected to stress.
Cell.
2006;
125:
1111
-1124.
[PubMed]
.
-
24.
Ambros
V
MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing.
Cell.
2003;
113:
673
-6.
[PubMed]
.
-
25.
Brennecke
J
, Hipfner
DR
, Stark
A
, Russell
RB
and Cohen
SM.
bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila.
Cell.
2003;
113:
25
-36.
[PubMed]
.
-
26.
Chang
S
, Johnston
RJ Jr
, Frokjaer-Jensen
C
, Lockery
S
and Hobert
O.
MicroRNAs act sequentially and asymmetrically to control chemosensory laterality in the nematode.
Nature.
2004;
430:
785
-789.
[PubMed]
.
-
27.
Plasterk
RH
Micro RNAs in animal development.
Cell.
2006;
124:
877
-881.
[PubMed]
.
-
28.
Baltimore
D
, Boldin
MP
, O'Connell
RM
, Rao
DS
and Taganov
KD.
MicroRNAs: new regulators of immune cell development and function.
Nat Immunol.
2008;
9:
839
-845.
[PubMed]
.
-
29.
Schickel
R
, Boyerinas
B
, Park
SM
and Peter
ME.
MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death.
Oncogene.
2008;
27:
5959
-74.
[PubMed]
.
-
30.
Dahlberg
JE
and Lund
E.
Micromanagement during the innate immune response.
Sci STKE.
2007;
2007:
pe25
[PubMed]
.
-
31.
He
X
, He
L
and Hannon
GJ.
The guardian's little helper: microRNAs in the p53 tumor suppressor network.
Cancer Res.
2007;
67:
11099
-11101.
[PubMed]
.
-
32.
Poy
MN
, Eliasson
L
, Krutzfeldt
J
, Kuwajima
S
, Ma
X
, Macdonald
PE
, Pfeffer
S
, Tuschl
T
, Rajewsky
N
, Rorsman
P
and Stoffel
M.
A pancreatic islet-specific microRNA regulates insulin secretion.
Nature.
2004;
432:
226
-230.
[PubMed]
.
-
33.
Volinia
S
, Calin
GA
, Liu
CG
, Ambs
S
, Cimmino
A
, Petrocca
F
, Visone
R
, Iorio
M
, Roldo
C
, Ferracin
M
, Prueitt
RL
, Yanaihara
N
, Lanza
G
, Scarpa
A
, Vecchione
A
, Negrini
M
, Harris
CC
and Croce
CM.
A microRNA expression signature of human solid tumors defines cancer gene targets.
Proc Natl Acad Sci U S A.
2006;
103:
2257
-2261.
[PubMed]
.
-
34.
Lukiw
WJ
, Zhao
Y
and Cui
JG.
An NF-{kappa}B-sensitive Micro RNA-146a-mediated Inflammatory Circuit in Alzheimer Disease and in Stressed Human Brain Cells.
J Biol Chem.
2008;
283:
31315
-31322.
[PubMed]
.
-
35.
Nakasa
T
, Miyaki
S
, Okubo
A
, Hashimoto
M
, Nishida
K
, Ochi
M
and Asahara
H.
Expression of microRNA-146 in rheumatoid arthritis synovial tissue.
Arthritis Rheum.
2008;
58:
1284
-1292.
[PubMed]
.
-
36.
Boehm
M
and Slack
F.
A developmental timing microRNA and its target regulate life span in C. elegans.
Science.
2005;
310:
1954
-1957.
[PubMed]
.
-
37.
He
L
, He
X
, Lim
LP
, de Stanchina
E
, Xuan
Z
, Liang
Y
, Xue
W
, Zender
L
, Magnus
J
, Ridzon
D
, Jackson
AL
, Linsley
PS
, Chen
C
, Lowe
SW
, Cleary
MA
and Hannon
GJ.
A microRNA component of the p53 tumour suppressor network.
Nature.
2007;
447:
1130
-1134.
[PubMed]
.
-
38.
Kumamoto
K
, Spillare
EA
, Fujita
K
, Horikawa
I
, Yamashita
T
, Appella
E
, Nagashima
M
, Takenoshita
S
, Yokota
J
and Harris
CC.
Nutlin-3a activates p53 to both down-regulate inhibitor of growth 2 and up-regulate mir-34a, mir-34b, and mir-34c expression, and induce senescence.
Cancer Res.
2008;
68:
3193
-3203.
[PubMed]
.
-
39.
Poliseno
L
, Pitto
L
, Simili
M
, Mariani
L
, Riccardi
L
, Ciucci
A
, Rizzo
M
, Evangelista
M
, Mercatanti
A
, Pandolfi
PP
and Rainaldi
G.
The proto-oncogene LRF is under post-transcriptional control of MiR-20a: implications for senescence.
PLoS ONE.
2008;
3:
e2542
[PubMed]
.
-
40.
Mudhasani
R
, Zhu
Z
, Hutvagner
G
, Eischen
CM
, Lyle
S
, Hall
LL
, Lawrence
JB
, Imbalzano
AN
and Jones
SN.
Loss of miRNA biogenesis induces p19Arf-p53 signaling and senescence in primary cells.
J Cell Biol.
2008;
181:
1055
-1063.
[PubMed]
.
-
41.
Bhaumik
D
, Scott
GK
, Schokrpur
S
, Patil
CK
, Campisi
J
and Benz
CC.
Expression of microRNA-146 suppresses NF-kappaB activity with reduction of metastatic potential in breast cancer cells.
Oncogene.
2008;
27:
5643
-5647.
.
-
42.
Taganov
KD
, Boldin
MP
, Chang
KJ
and Baltimore
D.
NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses.
Proc Natl Acad Sci U S A.
2006;
103:
12481
-12486.
[PubMed]
.
-
43.
Perry
MM
, Moschos
SA
, Williams
AE
, Shepherd
NJ
, Larner-Svensson
HM
and Lindsay
MA.
Rapid changes in microRNA-146a expression negatively regulate the IL-1beta-induced inflammatory response in human lung alveolar epithelial cells.
J Immunol.
2008;
180:
5689
-5698.
[PubMed]
.
-
44.
Taganov
KD
, Boldin
MP
and Baltimore
D.
MicroRNAs and immunity: tiny players in a big field.
Immunity.
2007;
26:
133
-137.
[PubMed]
.
-
45.
Michaloglou
C
, Vredeveld
LC
, Soengas
MS
, Denoyelle
C
, Kuilman
T
, van der Horst
CM
, Majoor
DM
, Shay
JW
, Mooi
WJ
and Peeper
DS.
BRAFE600-associated senescence-like cell cycle arrest of human naevi.
Nature.
2005;
436:
720
-724.
[PubMed]
.
-
46.
Dimri
GP
, Lee
X
, Basile
G
, Acosta
M
, Scott
G
, Roskelley
C
, Medrano
EE
, Linskens
M
, Rubelj
I
and Pereira-Smith
O.
A biomarker that identifies senescent human cells in culture and in aging skin in vivo.
Proc Natl Acad Sci U S A.
1995;
92:
9363
-9367.
[PubMed]
.
-
47.
Scott
GK
, Goga
A
, Bhaumik
D
, Berger
CE
, Sullivan
CS
and Benz
CC.
Coordinate suppression of ERBB2 and ERBB3 by enforced expression of micro-RNA miR-125a or miR-125b.
J Biol Chem.
2007;
282:
1479
-1486.
[PubMed]
.