Evidence for the progression through S-phase in the ectopic cell cycle re-entry of neurons in Alzheimer disease
Abstract
Aberrant neuronal re-entry into the cell cycle is emerging as a potential pathological mechanism in Alzheimer disease (AD). However, while cyclins, cyclin dependent kinases (CDKs), and other mitotic factors are ectopically expressed in neurons, many of these proteins are also involved in other pathological and physiological processes, generating continued debate on whether such markers are truly indicative of a bona fide cell cycle process. To address this issue, here we analyzed one of the minichromosome maintenance (Mcm) proteins that plays a role in DNA replication and becomes phosphorylated by the S-phase promoting CDKs and Cdc7 during DNA synthesis. We found phosphorylated Mcm2 (pMcm2) markedly associated with neurofibrillary tangles, neuropil threads, and dystrophic neurites in AD but not in aged-matched controls. These data not only provide further evidence for cell cycle aberrations in AD, but the cytoplasmic, rather than nuclear, localization of pMcm2 suggests an abnormal cellular distribution of this important replication factor in AD that may explain resultant cell cycle stasis and consequent neuronal degeneration.
Introduction
Alzheimer disease (AD) is a
progressive and fatal neurodegenerative disease that is clinically characterized
by dementia and neurobehavioral deterioration
[1-4].
While the hallmark features of amyloid
plaques, neurofibrillary tangles (NFTs), and neuronal loss are well
established, the cause(s) of the disease remain elusive. Nonetheless, one
mechanism that is gaining increased prominence is the ectopic re-entry of
neurons into the cell cycle [5], which
accumulate cyclins, CDKs, and other mitotic factors [6-22]. While
neuronal cell cycle re-entry mediates AD-type changes [23] and is
linked with cell death [24-27], a
number of unanswered questions remain [28]. For
example, it is still unclear whether the presence of various cell cycle markers
represent a bona fide cell cycle or are they, instead, consequential to
other pathological processes (e.g., apoptosis). Also, if representative of cell
cycle, it is unclear why neurons do not progress and enter cytokinesis. One
fitting hypothesis is that some cells undergo hypermitogenic cell cycle arrest,
as an alternative to apoptosis, which would result in cell senescence and
survival [29].
The minichromosome maintenance proteins
are a eukaryotic family of six distinct protein subtypes (Mcm2-7) that are
necessary for DNA replication initiation and progression in the cell cycle [30]. During the
G1-phase of the cell cycle, the hexameric Mcm2-7 complex assembles at origins
of replication on nuclear DNA [31]. Once in
S-phase, the complex is phosphorylated by the Cdc7/Dbf4 kinase and the B-type
CDKs, and acting as the DNA helicase initiates DNA replication at origins and
allows progression of the replication forks [32-37]. The
assembly of the Mcm complex is tightly regulated, can occur only in G1 when the
activity of CDKs and Cdc7 is low, and is actively prevented once cells enter
S-phase till exit of mitosis when the activity of these kinases is high [38], such that
replication only occurs once per cell cycle. Expression of Mcm proteins is
restricted to actively cycling cells and is a good proliferation marker [39]. While in
budding yeast Mcm2-7 proteins shuttle in and out of the nucleus, human Mcms are
generally detected in the nuclear compartment [40,41].
Phosphorylation can occur at multiple sites, however phosphorylation of Mcm2 in
two adjacent sites Ser40 and Ser41, carried out in succession by CDKs and Cdc7,
strictly correlates with cells undergoing or having terminated DNA synthesis [42]. As such,
antisera specific for pSer40/41 Mcm2 phosphorylation provides an excellent
marker for the detection of cells in a late stage of the cell cycle.
In this study, we compared Ser40/41 Mcm2
phosphorylation in AD and aged-matched control brain. In AD, phosphorylated
Mcm2 localized to the cytoplasm of neurons, and strikingly with the
characteristic NFT. These findings further support the notion that neurons in
AD re-enter the cell cycle, pass through S-phase by activating the only two
essential S-phase promoting kinases, and provide evidence for aberrant
localization of an essential DNA replication protein.
Results
Phosphorylated Mcm2 protein at a CDK- and Cdc7-
dependent site is localized to the cytoplasm of AD neurons and targets
neurofibrillary tangles and amyloid plaques
The presence of pSer40/41 Mcm2 (pMcm2) protein was
detected using the immunocytochemistry methods discussed in the corresponding
section. All of the AD cases examined demonstrated significant accumulation of
pMcm2 in NFTs, dystrophic neurites, and neuropil threads (Figure 1B). In most
cases, glial nuclei were often stained, and in a small number of cases, some
pyramidal cell nuclei within the CA3 region showed significant pMcm2 reactivity
(Figure 1D, arrows). In similar areas in most control cases, no staining was
seen (Figure 1A), in a small number of aged control cases, pyramidal neuron
nuclei showed high pMcm2 protein levels (Figure 1C). In some of the aged
controls, a small number of pathological structures (NFT, neuropil threads,etc)
were labeled with the pMcm2 antisera (data not shown).
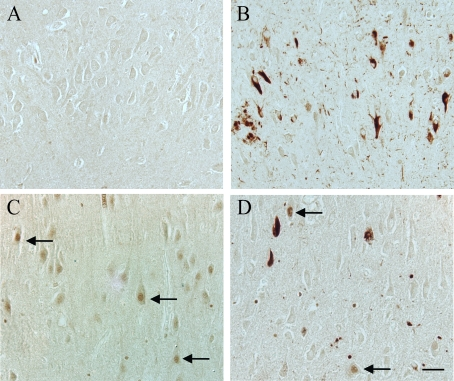
Figure 1. In an 87 year old AD case, hippocampal tissue sections demonstrate significant
localization of pMcm2 protein in NFT, dystrophic neurites, and neuropil
threads (B). In another AD case, in the CA3 region, in addition to
pathological structures, a few pyramidal neuron nuclei (arrows) have significant
pMcm2 accumulation (D). Most control cases, representative case age
61 years, demonstrate no neuronal staining for pMcm2 protein (A),
while a few older control cases demonstrate significant nuclear
immunolocalization in the pyramidal neurons (control case age 74 years, C)
Scale bar= 50 μm.
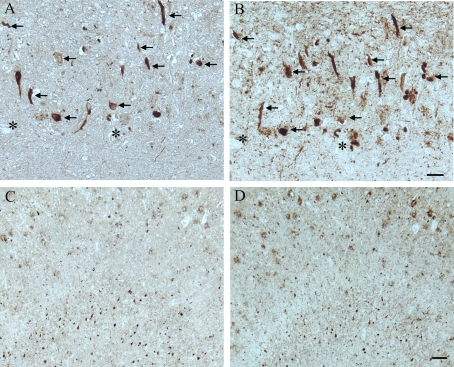
Figure 2. In another AD case, age 63, adjacent hippocampal
tissue sections demonstrate many of the AD-related pathological
structures (arrows) containing pMcm2 (A) are also positive
for hyper-phosphorylated tau (B) in the CA1 region.
Lower magnification of adjacent sections of the subiculum
shows the large number of NFT and plaques recognized by
pMcm2 (C) and AT8 (D). * denotes landmark vessel. Scale
bars= 50 μm (A,B), 100 μm (C,D).
All AD cases examined, both with formalin and
methacarn fixation, contained many immunoreactive NFT throughout the
hippocampus. Additionally, the binding of the anti-pMcm2 antibody to NFT within
AD brains was striking and showed some co-localization with phosphorylated tau
on adjacent sections of AD tissue In particular, many of the same NFT and
senile plaques demonstrated co-localization of tau with pMcm2 in all AD cases
(Figure 2). In Figure 3, the specificity of the antibody to pMcm2 protein was
confirmed by absorbing antibodies to pMcm2 with phosphorylated and non-phosphorylated
peptides. As expected, the phosphorylated peptide completely
absorbed the antibody producing no visible staining on the section (Figure 3C)
whereas the peptide lacking phosphorylation failed to absorb the antibody
(Figure 3B) and produced staining similar to that of the unabsorbed sample
(Figure 3A). Further confirmation of the specificity was obtained by treating
some sections with alkaline phosphatase to remove phosphate groups. Figure 4
shows that nearly all of the reactivity of the pMcm2 antisera is abolished
following dephosphorylation on adjacent sections with (Figure 4B) and without
(Figure 4A) alkaline phosphatase pretreatment.
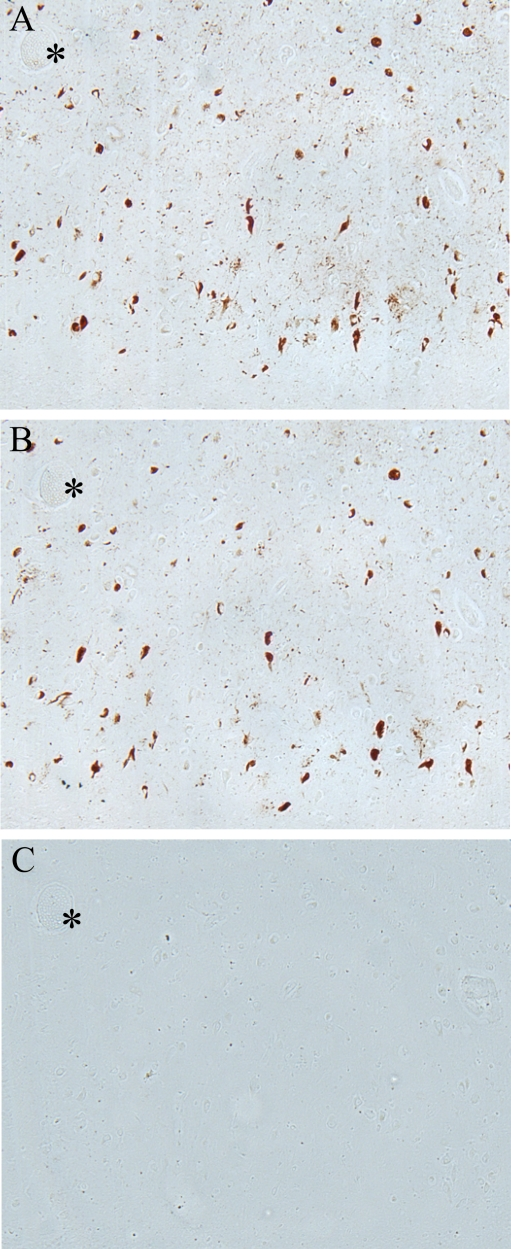
Figure 3. Adsorption
of pMcm2 antibody confirms specificity
to corresponding pMcm2 antigen. (A) AD hippocampal tissue stained
with pMcm2 antibody. (B) Adjacent section treated with pMcm2
antibody absorbed with non-phosphorylated Mcm peptide demonstrates similar
staining. (C) Adjacent section treated with pMcm2 antibody absorbed
with phosphorylated Mcm2 peptide demonstrates complete absorption. *
denotes landmark vessel.
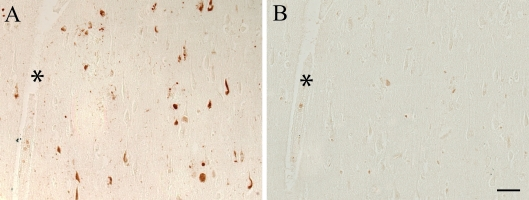
Figure 4. Pretreatment with alkaline phosphatase to remove phosphate
groups, results in elimination of pMcm2 reactivity (B) compared to an
untreated adjacent serial section of an AD case (A). * denotes
landmark vessel. Scale bar = 50 μm.
Discussion
In AD, multiple lines of evidence suggest that neurons
vulnerable to degeneration emerge from the post-mitotic, quiescent state and
are phenotypically suggestive of cells that are cycling, rather than being in
the normal, terminally differentiated, non-dividing state [43]. Such cell
cycle re-entry has not only been linked to cell death [44], but has
also been implicated in the hallmark pathologies of the disease, namely tau
phosphorylation and amyloid-β (Aβ) [23].
Nonetheless, despite the identification of a variety of cell cycle proteins in
AD, there remains controversy over whether these are truly indicative of a bona
fide reaction of the cell cycle or, instead, reflect the pleotrophic actions of
these protein markers [28]. Indeed,
proteins previously detected in AD such as Ki67, PCNA, cdc2, cdk4, BRCA1 and
pRb [9,44-49],
although noted regulators of the mitotic process, are also involved in neuronal
processes unrelated to the cell cycle such as DNA repair [50], apoptosis [51],
and oxidative stress [52].
Here, however, the detection of a key component of the DNA replication
machinery Mcm2, phosphorylated in the Cdk and Cdc7 dependent site Ser40/41 in
AD neuronal cytoplasm and NFT not only provides additional support for the cell
cycle hypothesis of AD [10], but
supports an authentic re-entrant phenotype associated with DNA replication [53]. Mcm2 is in
fact not expressed in non-proliferating tissues, as shown in neurons in
age-matched control brain, but it accumulates in G1 cells re-entering the cell
cycle. Dual phosphorylation of Mcm2 at serine 40 and serine 41, then requires the
activity of two kinases whose activity is upregulated in S-phase by the
periodic expression of regulatory subunits, Cyclin and Dbf4 [54].
Very intriguingly, pMcm2 in AD neurons, unlike in most
cancer cell lines [42], appears to
accumulate mostly in the cytoplasm suggesting further degree of deregulation of
the MCM complex in disease tissues that may explain the inability of neurons to
progress through cytokinesis.
The ectopic re-entry of neurons into the
cell cycle likely plays an important role mediating other aspects of AD
pathology. Specifically, the microtubule associated protein tau, in cases of
AD, exists in a highly phosphorylated form and composes the NFTs that burden
the diseased brain, and this increased phosphorylation of tau destabilizes
microtubular dynamics and results in neuronal dysfunction [55,56].
Interestingly, while cells are mitotically active, the cell cycle regulator
proteins CDKs initiate a similar phosphorylation of tau that precedes the
appearance of the NFTs [8] and suggests
a possible cause-effect relationship [23]. Similarlythe major protein component of senile plaques is a 4.2 kDa polypeptide
termed Aβ, which is derived from a larger precursor (APP) encoded on
chromosome 21. Attesting to the importance of this protein, mutations in the
APP gene are linked to the inevitable onset of familial AD [57]. Given the
probable role of mitotic re-entry in AD, it is notable that APP is upregulated
secondary to mitogenic stimulation [58] and that
APP metabolism is regulated by cell cycle-dependent changes [59].
Interestingly, Aβ itself is mitogenic in vitro [60,61] and
therefore may play a direct role in the induction and/or propagation of cell
cycle-mediated events in AD. Additionally, Aβ-mediated cell death, at
least in vitro, is dependent on the presence of various cell
cycle-related elements [62]. Most
importantly, the ectopic re-entry of neurons into the cell cycle was recently
shown to lead to cell death, gliosis, and cognitive deficits—all cardinal
features of AD [24].
In conclusion, our results provide further support for
the role of cell cycle re-entry in the initiation and progression and AD. As
such, cell cycle inhibitors present potential therapies for the disease [63].
Methods
Tissue.
Autopsy
tissue samples were obtained using a protocol approved by the Institutional
Review Board at University Hospitals of Cleveland. Hippocampal or cortical
tissue samples were obtained post mortem from patients (n = 10, ages 63-91
years, mean = 81.8 years) with clinically and histopathologically confirmed AD,
as well as from aged-matched controls (n = 8, ages 56-86 years, mean = 70.2
years) with similar post mortem intervals (AD: 2-31 h, mean = 14.5 h; controls:
5-27 h, mean = 15.6 h). All cases were categorized based on clinical and
pathological criteria established by CERAD and NIA consensus panel [64]. From the
clinical reports available to us, we found no obvious differences in agonal
status or other potential confounders between the groups. Tissue was fixed in
methacarn (methanol: chloroform: acetic acid; 6: 3: 1 v/v/v) at 4°C overnight
or in routine formalin. Following fixation, tissue was dehydrated through
ascending ethanol, embedded in paraffin, and 6-μm sections were cut.
Immunohistochemistry.
Tissue sections were deparaffinized in xylene,
hydrated through descending ethanol, and endogenous peroxidase activity was
quenched by 30 minute incubation in 3% hydrogen peroxide in methanol.
Non-specific binding sites were blocked with 30 minute incubation in 10% normal
goat serum. Sections of both AD and control were immunostained with rabbit
polyclonal antibody to Mcm2 phosphorylated at sites Ser40/41 (1:150) [42] or mouse
monoclonal antibody to tau (AT8 1:1000) recognizing phosphorylated tau
(Ser202/Thr205) (Pierce, Rockford, IL) to identify the location of neuronal
pathological structures. Absorption experiments were performed to verify the
binding of the Mcm2 Ser40/41 antibody to the appropriate phosphorylated
peptide. The primary antibody was incubated in 0.2mg/ml peptide containing 0 or
2 phosphates for 16 hours at 4°C prior to immunostaining. All sections were
immunostained using the peroxidase-antiperoxidase with 3-3'-diaminobenzidine as
co-substrate as previously described [65].
Acknowledgments
Work in the authors' laboratories is supported by the
National Institutes of Health (AG031364, AG030096, AG028679) and the
Alzheimer's Association.
Conflicts of Interest
Dr. Smith is, or has in the past been, a paid
consultant for, owns equity or stock options in and/or receives grant funding
from Canopus BioPharma, Medivation, Neurotez, Neuropharm, Panacea
Pharmaceuticals, and Voyager Pharmaceuticals. Dr. Perry is, or has in the past
been, a paid consultant for and/or owns equity or stock options in Takeda
Pharmaceuticals, Voyager Pharmaceuticals, Panacea Pharmaceuticals and Neurotez
Pharmaceuticals.
References
-
1.
Smith
MA
Alzheimer disease.
Int Rev Neurobiol.
1998;
42:
1
-54.
[PubMed]
.
-
2.
Folstein
MF
and Bylsma
FW.
Terry RD, Katzman R, Bick KL, Sisodia SS.
Noncognitive symptoms of Alzheimer disease
Alzheimer disease.
Philadelphia
Lippincott Williams & Wilkins
1999;
25
-37.
.
-
3.
Morris
JC
Terry RD, Katzman R, Bick KL, Sisodia SS.
Clinical presentation and course of Alzheimer disease
Alzheimer disease.
Philadelphia
Lippincott Williams & Wilkins
1999;
11
-24.
.
-
4.
West
MJ
, Kawas
CH
, Stewart
WF
, Rudow
GL
and Troncoso
JC.
Hippocampal neurons in pre-clinical Alzheimer's disease.
Neurobiol Aging.
2004;
25:
1205
-1212.
[PubMed]
.
-
5.
Zhu
X
, Lee
HG
, Perry
G
and Smith
MA.
Alzheimer disease, the two-hit hypothesis: an update.
Biochim Biophys Acta.
2007;
1772:
494
-502.
[PubMed]
.
-
6.
Zhu
X
, Raina
AK
and Smith
MA.
Cell cycle events in neurons. Proliferation or death.
Am J Pathol.
1999;
155:
327
-329.
[PubMed]
.
-
7.
Vincent
I
, Jicha
G
, Rosado
M
and Dickson
DW.
Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer's disease brain.
J Neurosci.
1997;
17:
3588
-3598.
[PubMed]
.
-
8.
Vincent
I
, Zheng
JH
, Dickson
DW
, Kress
Y
and Davies
P.
Mitotic phosphoepitopes precede paired helical filaments in Alzheimer's disease.
Neurobiol Aging.
1998;
19:
287
-296.
[PubMed]
.
-
9.
McShea
A
, Harris
PL
, Webster
KR
, Wahl
AF
and Smith
MA.
Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer's disease.
Am J Pathol.
1997;
150:
1933
-1939.
[PubMed]
.
-
10.
McShea
A
, Wahl
AF
and Smith
MA.
Re-entry into the cell cycle: a mechanism for neurodegeneration in Alzheimer disease.
Med Hypotheses.
1999;
52:
525
-527.
[PubMed]
.
-
11.
McShea
A
, Zelasko
DA
, Gerst
JL
and Smith
MA.
Signal transduction abnormalities in Alzheimer's disease: evidence of a pathogenic stimuli.
Brain Res.
1999;
815:
237
-242.
[PubMed]
.
-
12.
Vincent
I
, Rosado
M
and Davies
P.
Mitotic mechanisms in Alzheimer's disease.
J Cell Biol.
1996;
132:
413
-425.
[PubMed]
.
-
13.
Arendt
T
, Rodel
L
, Gartner
U
and Holzer
M.
Expression of the cyclin-dependent kinase inhibitor p16 in Alzheimer's disease.
Neuroreport.
1996;
7:
3047
-3049.
[PubMed]
.
-
14.
Smith
TW
and Lippa
CF.
Ki-67 immunoreactivity in Alzheimer's disease and other neurodegenerative disorders.
J Neuropathol Exp Neurol.
1995;
54:
297
-303.
[PubMed]
.
-
15.
Arendt
T
, Holzer
M
, Grossmann
A
, Zedlick
D
and Bruckner
MK.
Increased expression and subcellular translocation of the mitogen activated protein kinase kinase and mitogen-activated protein kinase in Alzheimer's disease.
Neuroscience.
1995;
68:
5
-18.
[PubMed]
.
-
16.
Nagy
Z
, Esiri
MM
, Cato
AM
and Smith
AD.
Cell cycle markers in the hippocampus in Alzheimer's disease.
Acta Neuropathol (Berl).
1997;
94:
6
-15.
[PubMed]
.
-
17.
Nagy
Z
, Esiri
MM
and Smith
AD.
Expression of cell division markers in the hippocampus in Alzheimer's disease and other neurodegenerative conditions.
Acta Neuropathol (Berl).
1997;
93:
294
-300.
[PubMed]
.
-
18.
Arendt
T
, Holzer
M
and Gartner
U.
Neuronal expression of cycline dependent kinase inhibitors of the INK4 family in Alzheimer's disease.
J Neural Transm.
1998;
105:
949
-960.
[PubMed]
.
-
19.
Zhu
X
, Raina
AK
, Boux
H
, Simmons
ZL
, Takeda
A
and Smith
MA.
Activation of oncogenic pathways in degenerating neurons in Alzheimer disease.
Int J Dev Neurosci.
2000;
18:
433
-437.
[PubMed]
.
-
20.
Zhu
X
, Rottkamp
CA
, Boux
H
, Takeda
A
, Perry
G
and Smith
MA.
Activation of p38 kinase links tau phosphorylation, oxidative stress, and cell cycle-related events in Alzheimer disease.
J Neuropathol Exp Neurol.
2000;
59:
880
-888.
[PubMed]
.
-
21.
Zhu
X
, Rottkamp
CA
, Raina
AK
, Brewer
GJ
, Ghanbari
HA
, Boux
H
and Smith
MA.
Neuronal CDK7 in hippocampus is related to aging and Alzheimer disease.
Neurobiol Aging.
2000;
21:
807
-813.
[PubMed]
.
-
22.
Zhu
X
, Raina
AK
, Perry
G
and Smith
MA.
Alzheimer's disease: the two-hit hypothesis.
Lancet Neurol.
2004;
3:
219
-226.
[PubMed]
.
-
23.
McShea
A
, Lee
HG
, Petersen
RB
, Casadesus
G
, Vincent
I
, Linford
NJ
, Funk
JO
, Shapiro
RA
and Smith
MA.
Neuronal cell cycle re-entry mediates Alzheimer disease-type changes.
Biochim Biophys Acta.
2007;
1772:
467
-472.
[PubMed]
.
-
24.
Lee
HG
, Casadesus
G
, Nunomura
A
, Zhu
X
, Castellani
RJ
, Richardson
SL
, Perry
G
, Felsher
DW
, Petersen
RB
and Smith
MA.
The neuronal expression of MYC causes a neurodegenerative phenotype in a novel transgenic mouse.
Am J Pathol.
2009;
174:
891
-897.
[PubMed]
.
-
25.
Hamdane
M
and Buee
L.
The complex p25/Cdk5 kinase in neurofibrillary degeneration and neuronal death: The missing link to cell cycle.
Biotechnol J.
2007;
2:
967
-977.
[PubMed]
.
-
26.
Wung
JK
, Perry
G
, Kowalski
A
, Harris
PL
, Bishop
GM
, Trivedi
MA
, Johnson
SC
, Smith
MA
, Denhardt
DT
and Atwood
CS.
Increased expression of the remodeling- and tumorigenic-associated factor osteopontin in pyramidal neurons of the Alzheimer's disease brain.
Curr Alzheimer Res.
2007;
4:
67
-72.
[PubMed]
.
-
27.
Neve
RL
and McPhie
DL.
Dysfunction of amyloid precursor protein signaling in neurons leads to DNA synthesis and apoptosis.
Biochim Biophys Acta.
2007;
1772:
430
-437.
[PubMed]
.
-
28.
Bowser
R
and Smith
MA.
Cell cycle proteins in Alzheimer's disease: plenty of wheels but no cycle.
J Alzheimers Dis.
2002;
4:
249
-254.
[PubMed]
.
-
29.
Blagosklonny
MV
Cell senescence and hypermitogenic arrest.
EMBO Rep.
2003;
4:
358
-362.
[PubMed]
.
-
30.
Bailis
JM
and Forsburg
SL.
MCM proteins: DNA damage, mutagenesis and repair.
Curr Opin Genet Dev.
2004;
14:
17
-21.
[PubMed]
.
-
31.
Bell
SP
and Dutta
A.
DNA replication in eukaryotic cells.
Annu Rev Biochem.
2002;
71:
333
-374.
[PubMed]
.
-
32.
Aparicio
T
, Ibarra
A
and Mendez
J.
Cdc45-MCM-GINS, a new power player for DNA replication.
Cell Div.
2006;
1:
18
[PubMed]
.
-
33.
Kudoh
A
, Daikoku
T
, Ishimi
Y
, Kawaguchi
Y
, Shirata
N
, Iwahori
S
, Isomura
H
and Tsurumi
T.
Phosphorylation of MCM4 at sites inactivating DNA helicase activity of the MCM4-MCM6-MCM7 complex during Epstein-Barr virus productive replication.
J Virol.
2006;
80:
10064
-10072.
[PubMed]
.
-
34.
You
Z
and Masai
H.
DNA binding and helicase actions of mouse MCM4/6/7 helicase.
Nucleic Acids Res.
2005;
33:
3033
-3047.
[PubMed]
.
-
35.
Masai
H
, You
Z
and Arai
K.
Control of DNA replication: regulation and activation of eukaryotic replicative helicase, MCM.
IUBMB Life.
2005;
57:
323
-335.
[PubMed]
.
-
36.
Lee
JK
and Hurwitz
J.
Processive DNA helicase activity of the minichromosome maintenance proteins 4, 6, and 7 complex requires forked DNA structures.
Proc Natl Acad Sci U S A.
2001;
98:
54
-59.
[PubMed]
.
-
37.
Kaplan
DL
, Davey
MJ
and O'Donnell
M.
Mcm4,6,7 uses a "pump in ring" mechanism to unwind DNA by steric exclusion and actively translocate along a duplex.
J Biol Chem.
2003;
278:
49171
-49182.
[PubMed]
.
-
38.
Blow
JJ
and Dutta
A.
Preventing re-replication of chromosomal DNA.
Nat Rev Mol Cell Biol.
2005;
6:
476
-486.
[PubMed]
.
-
39.
Gonzalez
MA
, Tachibana
KE
, Laskey
RA
and Coleman
N.
Control of DNA replication and its potential clinical exploitation.
Nat Rev Cancer.
2005;
5:
135
-141.
[PubMed]
.
-
40.
Krude
T
, Musahl
C
, Laskey
RA
and Knippers
R.
Human replication proteins hCdc21, hCdc46 and P1Mcm3 bind chromatin uniformly before S-phase and are displaced locally during DNA replication.
J Cell Sci.
1996;
109 ( Pt 2):
309
-318.
[PubMed]
.
-
41.
Rialland
M
, Sola
F
and Santocanale
C.
Essential role of human CDT1 in DNA replication and chromatin licensing.
J Cell Sci.
2002;
115:
1435
-1440.
[PubMed]
.
-
42.
Montagnoli
A
, Valsasina
B
, Brotherton
D
, Troiani
S
, Rainoldi
S
, Tenca
P
, Molinari
A
and Santocanale
C.
Identification of Mcm2 phosphorylation sites by S-phase-regulating kinases.
J Biol Chem.
2006;
281:
10281
-10290.
[PubMed]
.
-
43.
Obrenovich
ME
, Raina
AK
, Ogawa
O
, Atwood
CS
and Smith
MA.
Copani A and Nicoletti F.
Alzheimer disease - a new beginning, or a final exit?
The Cell Cycle and Neuronal Cell Death.
Georgestown, Texas
Landes Bioscience
2005;
79
-93.
.
-
44.
Smith
MZ
, Nagy
Z
and Esiri
MM.
Cell cycle-related protein expression in vascular dementia and Alzheimer's disease.
Neurosci Lett.
1999;
271:
45
-48.
[PubMed]
.
-
45.
Ueberham
U
, Hessel
A
and Arendt
T.
Cyclin C expression is involved in the pathogenesis of Alzheimer's disease.
Neurobiol Aging.
2003;
24:
427
-435.
[PubMed]
.
-
46.
Nagy
Z
Cell cycle regulatory failure in neurones: causes and consequences.
Neurobiol Aging.
2000;
21:
761
-769.
[PubMed]
.
-
47.
Ding
XL
, Husseman
J
, Tomashevski
A
, Nochlin
D
, Jin
LW
and Vincent
I.
The cell cycle Cdc25A tyrosine phosphatase is activated in degenerating postmitotic neurons in Alzheimer's disease.
Am J Pathol.
2000;
157:
1983
-1990.
[PubMed]
.
-
48.
Hoozemans
JJ
, Bruckner
MK
, Rozemuller
AJ
, Veerhuis
R
, Eikelenboom
P
and Arendt
T.
Cyclin D1 and cyclin E are co-localized with cyclo-oxygenase 2 (COX-2) in pyramidal neurons in Alzheimer disease temporal cortex.
J Neuropathol Exp Neurol.
2002;
61:
678
-688.
[PubMed]
.
-
49.
Yang
Y
, Mufson
EJ
and Herrup
K.
Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer's disease.
J Neurosci.
2003;
23:
2557
-2563.
[PubMed]
.
-
50.
Schwartz
EI
, Smilenov
LB
, Price
MA
, Osredkar
T
, Baker
RA
, Ghosh
S
, Shi
FD
, Vollmer
TL
, Lencinas
A
, Stearns
DM
, Gorospe
M
and Kruman
II.
Cell cycle activation in postmitotic neurons is essential for DNA repair.
Cell Cycle.
2007;
6:
318
-329.
[PubMed]
.
-
51.
Zhang
Y
, Qu
D
, Morris
EJ
, O'Hare
MJ
, Callaghan
SM
, Slack
RS
, Geller
HM
and Park
DS.
The Chk1/Cdc25A pathway as activators of the cell cycle in neuronal death induced by camptothecin.
J Neurosci.
2006;
26:
8819
-8828.
[PubMed]
.
-
52.
Clement
A
, Henrion-Caude
A
, Besnard
V
and Corroyer
S.
Role of cyclins in epithelial response to oxidants.
Am J Respir Crit Care Med.
2001;
164:
S81
-84.
[PubMed]
.
-
53.
Zhu
X
, Siedlak
SL
, Wang
Y
, Perry
G
, Castellani
RJ
, Cohen
ML
and Smith
MA.
Neuronal binucleation in Alzheimer disease hippocampus.
Neuropathol Appl Neurobiol.
2008;
34:
457
-465.
[PubMed]
.
-
54.
Jiang
W
, McDonald
D
, Hope
TJ
and Hunter
T.
Mammalian Cdc7-Dbf4 protein kinase complex is essential for initiation of DNA replication.
EMBO J.
1999;
18:
5703
-5713.
[PubMed]
.
-
55.
Lindwall
G
and Cole
RD.
Phosphorylation affects the ability of tau protein to promote microtubule assembly.
J Biol Chem.
1984;
259:
5301
-5305.
[PubMed]
.
-
56.
Alonso
AC
, Grundke-Iqbal
I
and Iqbal
K.
Alzheimer's disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules.
Nat Med.
1996;
2:
783
-787.
[PubMed]
.
-
57.
Hardy
J
Alzheimer's disease: the amyloid cascade hypothesis: an update and reappraisal.
J Alzheimers Dis.
2006;
9:
151
-153.
[PubMed]
.
-
58.
Ledoux
S
, Rebai
N
, Dagenais
A
, Shaw
IT
, Nalbantoglu
J
, Sekaly
RP
and Cashman
NR.
Amyloid precursor protein in peripheral mononuclear cells is up-regulated with cell activation.
J Immunol.
1993;
150:
5566
-5575.
[PubMed]
.
-
59.
Suzuki
T
, Oishi
M
, Marshak
DR
, Czernik
AJ
, Nairn
AC
and Greengard
P.
Cell cycle-dependent regulation of the phosphorylation and metabolism of the Alzheimer amyloid precursor protein.
EMBO J.
1994;
13:
1114
-1122.
[PubMed]
.
-
60.
McDonald
DR
, Bamberger
ME
, Combs
CK
and Landreth
GE.
beta-Amyloid fibrils activate parallel mitogen-activated protein kinase pathways in microglia and THP1 monocytes.
J Neurosci.
1998;
18:
4451
-4460.
[PubMed]
.
-
61.
Pyo
H
, Jou
I
, Jung
S
, Hong
S
and Joe
EH.
Mitogen-activated protein kinases activated by lipopolysaccharide and beta-amyloid in cultured rat microglia.
Neuroreport.
1998;
9:
871
-874.
[PubMed]
.
-
62.
Giovanni
A
, Wirtz-Brugger
F
, Keramaris
E
, Slack
R
and Park
DS.
Involvement of cell cycle elements, cyclin-dependent kinases, pRb, and E2F x DP, in B-amyloid-induced neuronal death.
J Biol Chem.
1999;
274:
19011
-19016.
[PubMed]
.
-
63.
Woods
J
, Snape
M
and Smith
MA.
The cell cycle hypothesis of Alzheimer's disease: Suggestions for drug development.
Biochim Biophys Acta.
2007;
1772:
503
-508.
[PubMed]
.
-
64.
Mirra
SS
, Heyman
A
, McKeel
D
, Sumi
SM
, Crain
BJ
, Brownlee
LM
, Vogel
FS
, Hughes
JP
, van
Belle G
and Berg
L.
The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease.
Neurology.
1991;
41:
479
-486.
[PubMed]
.
-
65.
Sternberger
LA
New York
Wiley
Immunocytochemistry.
1986;
.