Introduction
Once a neuron is born, it loses its
capacity for cell division and differentiates, contributing uniquely to the
plasticity of the basic wiring pattern that defines a neuronal system. The
preservation of this pattern is necessary for the overall generation and
storage of memories, as well as the acquisition of other higher brain skills.
Differentiated neurons appear to be irreversibly post-mitotic, perhaps because
a hypothetical cell division would result in cytoskeletal and synaptic
disruption in order to prepare the cell for mitosis and cytokinesis, which
would in turn impair neuronal connectivity and function. Hence, it is
reasonable to hink that, once a neuron differentiates, it resides out of the reach
of cell division control. However, this notion was
first questioned when some researchers surprisingly observed that neuronal
programmed cell death was accompanied by the expression of cell cycle markers.
Specifically, cyclins and cyclin-dependent kinases (CDKs), key components of
the cell cycle machinery (see Figure 1) were found upregulated after exposure
to severe conditions, such as oxidative stress and trophic factors deprivation [1-10]. Based on
the premise that "neurons do not divide", the notion that has emerged from this
evidence is that activation of a neuronal cell cycle does exist but it is
abortive, the final result being the initiation of apoptosis. As we discuss
below, this aberrant phenotype has also been postulated as a mechanism of
neuronal loss in neurodegenerative diseases, particularly Alzheimer's disease
(AD).
Regulation
of the cell cycle
The
cell cycle of eukaryotic cells comprises four main successive phases: G1 phase
(first gap), S phase (DNA synthesis), G2 phase (second gap) and M phase
(mitosis) (Figure 1). Transition between the different phases and subsequent
progression through the mitotic cycle is driven by a group of protein kinases
whose activity is central to this process, the cyclin-dependent kinase (CDKs),
and requires the binding of their activating partners cyclins, whose levels of
expression varies throughout the cycle.
During G1 phase, mitogenic signals, such as extracellular
growth factors or intercellular contact, trigger the activation of D-type
cyclins that, jointly with CDK4 or CDK6, phosphorylate the retinoblastoma
protein (Rb), inhibiting its affinity to bind the transcriptor factor E2F- 1. E2F-1 is released and directs the transcription of
specific genes that code for proteins required in the next stages of the cell
cycle. In late G1, an increase in cyclin E-CDK2 activity ensures the G1/S
transition by completing Rb phosphorylation and irreversibly committing cells
to enter the division process. Throughout S phase, cyclin A-CDK2 phosphorylates
various substrates allowing DNA replication. After completion of S phase, DNA
replication ceases and cells enter the G2 phase of the cycle. CDK2 is then
replaced by CDK1 that associates with cyclin A and regulates the
phosphorylation of proteins specific to the G2 and M phases of the cell cycle
together with cyclin B-CDK1, that appears in late G2 and triggers the G2/M
transition. Cyclin A is degraded and the system is reset, re-establishing the
requirement for mitogenic cues to induce D-type cyclins for the next cycle. In
M phase, cells physically divide originating two separate daughter cells
(reviewed in [11]).
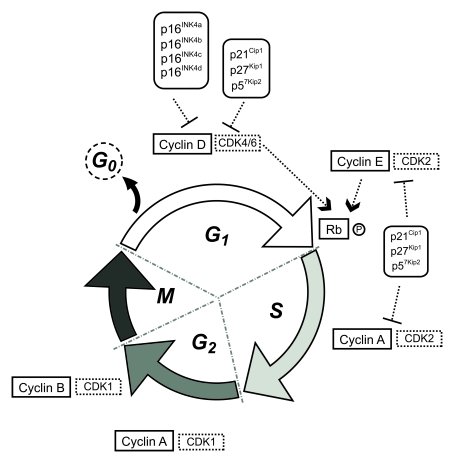
Figure 1. Schematic representation of the eukaryotic cell cycle.
CDK activity is regulated through posttranslational
modifications and subcellular translocations of specific CDK inhibitors
(CDKIs), which are organized in two families, INK4 and Cip/Kip. The INK4 family
(inhibitors of cyclin D-dependent kinases) consists of four members: p16INK4a,
p15INK4b, p18INK4c and p19INK4d, and the
Cip/Kip family (inhibitors of cyclin D-, cyclin E-, and cyclin A-dependent
kinases) comprises p21Cip1, p27Kip1 and p57Kip2.
Two important checkpoints (G1/S and G2/M) coordinate
CDKs activity and control the order and timing of cell-cycle transitions
ensuring that DNA replication and chromosome segregation are completed
correctly before allowing further progress through the cycle. The checkpoints
allow alternative decisions between progression, growth arrest or induction of
apoptosis. (See [12] for a
detailed review addressing the regulation of the cell cycle in proliferating
cells).
Differentiated
neurons express cell cycle proteins
Neurogenesis,
the birth of differentiated, functional neurons, takes place at two germinal
compartments that line the lateral ventricles - the ventricular zone (VZ) and
the subventricular zone (SVZ). Most neurons are originated prenatally through a
process of migration to shape a complex pattern of layers. The deep layers are
formed from earlier-born neurons originated in the VZ, while later-generated
neurons from the SVZ occupy higher layers [13]. The
journey is meant to cease proliferation and start neuronal differentiation.
However, although terminally differentiated neurons seem to irreversibly
withdraw from division, expression of cell cycle proteins is not completely
silenced. Thus, cytoplasmic cyclin D1 was detected in mature neurons associated
to the CDKIs p21Cip1 and p27Kip1, suggesting an
impairment of its nuclear transport and a possible role in cell cycle
withdrawal [14-16]. Indeed,
cyclin D1 is downregulated [17], but also
becomes predominantly cytoplasmic, in neuronal progenitor cells undergoing
terminal differentiation [18]. Similarly,
cyclin E expression was identified in the cytoplasm of postmitotic neurons [19,20]. More recently,
Thomas Arendt's lab reported that, within the
neocortex of the adult mouse, there is constitutive expression of cyclins D, E,
A and B; of CDKs 4, 2 and 1; and of their inhibitors p16INK4a, p15INK4b,
p18INK4c, p19INK4d, p21Cip1, p27Kip1
and p57Kip2 [21]. Furthermore,
CDKs were found to be properly complexed to cyclins and exhibit kinase
activity.
These findings have led to speculate
that, in the absence of detectable neuronal cell division, there may be
additional, cell cycle independent roles for cell cycle regulators in adult
neurons. Indeed, there is evidence to suggest that cyclins and CDKs may
participate in synaptic plasticity [22,23] and
neuronal differentiation [24,25].
Similarly, CDH1 and APC (anaphase-promoting complex), which are found
ubiquitously expressed in the nuclei of terminally differentiated neurons [26], and form a
complex involved in cellular division at the end of mitosis and G1 through
cyclin B degradation, also appear to play a role in regulating axonal growth
and patterning in the developing brain [27].
Furthermore, CDK5, a cyclin-dependent kinase whose exact role in the cell
cycle, if any, still remains elusive, is highly active in postmitotic neurons
and is involved in the coordination of complex neuronal properties including
synaptic plasticity, learning and memory (reviewed in [28]).
Thus,
the presence of cell division mediators in differentiated neurons where the
cell cycle is absent is well documented, and it does not appear to be the consequence
of abnormal regulatory events. Rather, it appears as if at least some cell
cycle proteins have adapted to life in a non-dividing neuron by learning and
taking up additional, cell cycle-independent roles that are presumably crucial
to neuronal function. The use of mouse conditional knockout models of these
proteins should help us to unveil both the identity and importance of these
putative functions.
Cell
cycle abnormalities in differentiated neurons
There
is also a substantial body of evidence pointing to a role for neuronal cell
cycle proteins in the modulation of stress-induced apoptosis through a
mechanism involving the initiation of a cell cycle. For example, rat cerebellar
granule neurons plated in culture medium without trophic factors, such as
brain-derived neurotrophic factor (BDNF), undergo apoptosis but also present
up-regulated expression of both mRNA and protein levels of cyclin D1.
Immunostaining confirmed cyclin D1 immunoreactivity prior to cell shrinkage and
nuclear condensation. Furthermore, blocking the cell cycle with the CDKs
inhibitors ciclopirox, mimosine and olomoucine was sufficient to suppress
immunoreactivity and, more importantly, cell death [6]. Herrup et
al. showed that two mouse neurological mutants, staggerer (sg/sg) and
lurcher (+/Lc), that model the absence of trophic support in the brain, present
significant numbers of cerebellar granule cells and inferior olive neurons
degenerating after elevation of Cyclin D and proliferating cell nuclear antigen
(PCNA) levels and bromodeoxyuridine (BrdU) incorporation [1]. RNA
alphavirus Sindbis-driven expression of p16INK4a, p21Cip1
and p27Kip1, and of dominant negative forms of CDK4 and CDK6,
protected rat primary neuronal cultures from apoptosis evoked by withdrawal of
nerve growth factor (NGF) [2] and neuronal
death as a result of DNA-damaging agents treatment, such as camptothecin, AraC
and UV radiation [3]. The CDK
inhibitors flavopiridol and olomoucine also protected the neurons from these
conditions, suggesting that these cell cycle elements might mediate death
signalling as a result of DNA-damaging environments [4]. Kruman et
al. hypothesized that cell cycle reentry is a critical component of the DNA
damage response in postmitotic neurons. Suppression of ataxia telangiectasia
mutated (ATM), a component of DNA damage-induced checkpoint, by caffeine and
wortmannin, attenuated both cell cycle reentry and apoptosis triggered by the
genotoxic compounds etoposide, methotrexate, and homocysteine [7].
Oxidative
stress-related cell death has also been associated with apparent cell cycle
induction in post-mitotic neurons. Induction of cyclin B prior to the
commitment of neurons to both dopamine- and peroxide-triggered apoptosis was
reported in primary cultures of post-mitotic sympathetic neurons. Both neuronal
death and rise in cyclin B were inhibited by antioxidant treatment [5].
In
summary, the evidence available to us suggests that exposure of post-mitotic
neurons to a wide range of stress stimuli triggers the expression of cell cycle
proteins as part of a well regulated programmed cell death response. The most
widely accepted scenario is that, in response to stress signals, neurons can be
driven into the cell cycle but their array of cell cycle proteins may not
suffice to allow for its completion, leading to a situation in which the cell
cannot reverse course or complete division, rendering it non-functional and
ready to trigger a programmed cell death response. In other words, neurons may
have learned to translate stress signals into an irreversibly damaging
incomplete cell cycle from which the cell has no choice but to trigger
apoptosis. It is also noteworthy in this context that, despite the
well-characterized presence of active apoptotic pathways in both in vitro and
animal models of AD, the presence of classic apoptotic pathways in the human AD
brain is not universally accepted [29]. Thus, it
remains formally possible that the cell cycle-linked cell death response in AD,
although well documented, may differ in nature from classic apoptosis pathways.
Additional support for this notion is
provided by the demonstration of a direct causality link between overexpression
of cell cycle mediators and neuronal death. Kranenburg et al showed that
artificial elevation of cyclin D1 was sufficient to induce apoptosis and could
be inhibited by the CDKI p16INK4 [30]. More
recently, McShea et al. used adenoviral-mediated expression of c-myc and
mutationally active ras oncogenes to force non-dividing cortical neurons into
the cell cycle leading to their death [31]. Transgenic
mouse models characterized by conditional expression of the simian virus 40 T
antigen oncogene in postmitotic neurons clearly presented a neurodegenerative
phenotype, consequence of forced cell cycle activation [32].
Nevertheless,
even if cell cycle activation is a sine qua non for apoptosis in
neurons, we still do not know whether the low constitutive levels of cell cycle
proteins in neurons may exist to facilitate a fast response to stress or their
presence simply reflects their role in unrelated functions.
Loss
of neuronal cell cycle control in AD
If
exposure to stress may trigger an abortive cell cycle in neurons, it is
reasonable to ask whether such mechanism may exist in the AD brain, which is
exposed to a wide range of stress stimuli. Substantial, although mostly
descriptive, evidence suggests that this is indeed the case. Cyclins, CDKs and
other cell cycle proteins are expressed in the AD brain [9,33-36]. In
addition, Ranganathan et al. reported high levels of hyperphosphorylated
Rb and observed altered subcellular distribution of E2F-1 to the cytoplasm [37] in brain
and spinal cord tissues from Alzheimer's disease (AD). In another study,
phosphorylated histone H3, a key component involved in chromosome compaction
during cell division, was found increased in the cytoplasm of hippocampal
neurons in AD, rather than within the nucleus as in actively dividing cells [38]. Cdk7, an
activator of major cyclin-CDK complexes, constantly expressed during the cell
cycle and indispensable for cell cycle progression, is also upregulated in
susceptible hippocampal neurons of AD patients [39].
Further
experiments from the Herrup's lab went further in their approach to the study
of the neuronal cell cycle and, using fluorescent in situ hybridization,
demonstrated that a significant fraction of the hippocampal pyramidal and basal
forebrain neurons in AD have fully or partially replicated four separate
genetic loci on three different chromosomes [40]. Mosch et
al.[41] also
quantified the DNA amount of identified cortical neurons in AD and reported a
population of cyclin B1-positive tetraploid neurons that had entirely passed
through a functional interphase with a complete DNA replication. These
experiments are particularly important because, unlike evidence showing the
presence of cell cycle markers in neurons, which could be dismissed as
epiphenomena of suspect physiological relevance, they demonstrate that the DNA
replication machinery is functional and capable of completing S phase in
post-mitotic neurons.
Interestingly, CDK inhibitors p16INK4a, p15INK4b,
p18INK4c and p19INK4d have also been found abnormally
expressed in the temporal cortex and in pyramidal neurons of the hippocampus of
AD patients [42-44]. An
increase in the cytoplasmic levels of p27Kip1 was also
identified in vulnerable neurons from individuals with histopathologically
confirmed AD [45]. The signifycance
of these findings is not immediately obvious. One could argue that expression
of these inhibitors occurs as a defence mechanism against the untimely
activation of cell cycle initiators. However, that would run counterintuitive
to the notion that initiation of an abortive cell cycle is an adaptive response
to stress. Clearly, much of the nature of cell cycle events in neurons, whether
in response to stress situations or in basal conditions, is far from being
understood.
Interestingly, although DNA replication
and entry into S phase can be demonstrated to occur in dying neurons,
progression through M phase has never been reported. Although the presence of
binucleated neurons has been recently reported [46], no
condensed chromosomes, formation of a mitotic spindle-like structure, or
cytokinesis have ever been described, consistent with the idea that susceptible
neurons may be arrested at the G2/M transition before they die. Therefore,
activation of CDK1 at G2 might be a rate-limiting step before neurons undergo
apoptosis. Indeed, activated CDK1 can phosphorylate and activate the pro-apoptotic
BAD protein [47], thus
providing a direct link between the cell cycle apparatus and the cell death
machinery in neurons. It is also reasonable to suggest, in our opinion, that
neuronal apoptosis at the G2 stage may simply be the result of permanent loss
of ability to undergo chromosome segregation and cytokinesis due to a highly
specialized cytoskeleton. In other words, cytoskeletal commitment to the
plasticity of neuronal shape may come at the expense of its inability to
dismantle dendrite and axonal structures to commit to mitotic spindle formation
and cytokinesis. Indeed, the microtubule associated protein tau, which is phosphorylated
during this phase of the cell cycle in a mitotic-competent cell, has also been
consistently reported to be abnormally phosphorylated in AD and colocalizes
with cell cycle regulators [32,33,45,48-50].
Moreover, tau can be phosphorylated by CDK1 [51] and
CDK1-like protein [52,53].
Therefore, abnormally increased levels of tau phosphorylation could be
explained in the context of an unsuccessful attempt to modulate G2 neuronal
architecture and prepare it for mitosis, leading to programmed cell death.
Mechanisms
of neuronal cell cycle reentry. Lessons from familial AD
Taken
together, the available evidence pointing to a role for an abortive cell cycle
in neurodegeneration in AD is reasonably strong. Nevertheless, the question
remains: what mechanisms do neurons use to enter the cell cycle in the first
place in response to a stress signal? If this is an adaptive response, there must
be a well-defined molecular pathway that triggers an entry into an apoptotic
cell cycle. Although nothing is known in this respect, some clues can be
obtained from studies of familial AD (FAD) cases that, perhaps not
surprisingly, also display cell cycle abnormalities [54-56].
Mutations
in the genes for amyloid precursor protein (APP) and presenilins (PS1, PS2)
associated to FAD lead in all cases to aberrant production of Aβ peptides [57], which in
turn exacerbate cell cycle-related neuronal death [58-61]. In addition,
increased Rb phosphorylation and E2F1 levels are measurable in areas
surrounding a subset of Aβ-containing plaques [62].
Interestingly, Copani et al. reported that, unexpectedly, the reparative DNA
polymerase β may act as a death signal when erratically expressed by
differentiated neurons exposed to Aβ [63]. In short,
exposure of post-mitotic neurons to the Aβ levels present in the AD brain
may trigger a signalling pathway leading to the initiation of an abortive
neuronal cell cycle.
Mutations
in Presenilin 1 (PS1) account for the majority of all FAD cases, and one of its
functions is precisely the APP γ-secretase-dependent cleavage responsible
for Aβ generation. However, PS1 is a multifunctional protein and
participates in many other signalling pathways, involving Notch, MEK/ERK,
PI3K/Akt, β-catenin and others (reviewed in [64]). Relevant
to the present discussion, PS1 is involved in β-catenin
proteolysis, coupling its stepwise phosphorylation by PKA and GSK3-β prior
to degradation [65-67]. Thus,
in the absence of PS1 or in the presence of PS1 FAD mutations, this function is
impaired and β-catenin is translocated to the nucleus, leading to
hyperproliferation in mitotically competent cells [66-68], and
tumorigenesis in peripheral tissue lacking PS1 [69]. Data from
our lab points to a β-catenin-dependent aberrant cell cycle reactivation in
cultured primary neurons from mice harbouring the knock-in PS1 mutation M146V
(PS1 KIM146V), as determined by increased BrdU incorporation. This
accelerated entry into the cell cycle appeared to be abortive, initiating an
apoptotic response. Furthermore, treatment with quercetin, a disruptor of the β-catenin/TCF transcription complex, reduced cyclin D1 levels and
reversed the cell cycle/cell death phenotype, consistent with a role for β-catenin
in this cell cycle-driven apoptosis [70]. Thus, it
is possible that the elevated levels of β-catenin that are
present in the PS1 FAD brain accelerate cell cycle entry simply by upregulating
cyclin D1 transcription. In further support of this notion, we found that
levels of cyclin D1 are elevated in the hippocampus of PS1 FAD patients when
compared to sporadic AD patients and non-demented controls (Currais, Hortobagyi
and Soriano, unpublished results).
Recently,
Repetto et al. demonstrated a critical role for PS1 in the trafficking and
turnover of the epidermal growth
factor receptor (EGFR), a key signaling receptor tyrosine kinase [71]. As with
β-catenin, mutations that enhance EGFR expression can serve as oncogenic
signals that promote hyperplasia and neoplastic transformation in human
tissues, including skin. EGFR is important for development of the nervous
system and maintenance of neural stem cells growth and differentiation.
However, excess of EGF induces neuronal death, and strong EGFR immunoreactivity
has been detected in neurites surrounding neuritic plaques in AD. Thus, the
authors hypothesize that activation of EGFR and β-catenin pathways by the
loss of PS1 can mutually reinforce each other and may contribute to neurodegeneration
and aberrant cell cycle re-entry by stabilizing both EGFR and β-catenin
while simultaneously driving Aβ42 deposition (discussed in [71]).
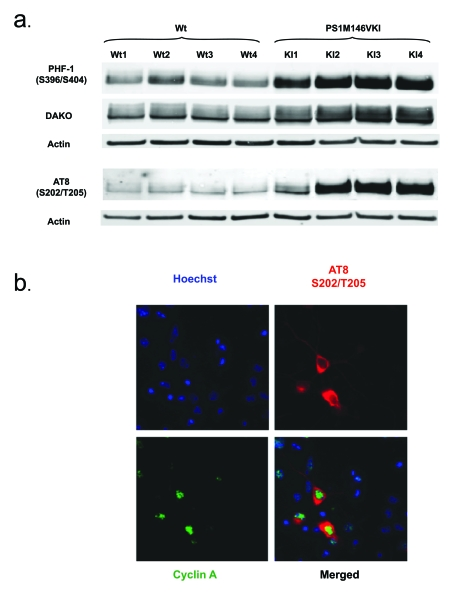
Figure 2. (a) Tau
accumulates and is hyperphosphorylated at S202/T205 and S396/S404 in
primary neurons from PS1 M146V mice compared to wild-type controls. Shown is a Western blot analysis of Triton
X-100 soluble lysates. Antibodies used were AT8 (phosphorylated S202/T205),
PHF-1 (phosphorylated S396/S404) and DAKO (total tau); (b) Tau
phosphorylation at S202/T205 is detectable exclusively in neurons
expressing cyclin A, highlighting the importance of tau phosphorylation
dynamics in the neuronal cell cycle.
Interestingly,
and consistent with the notion that a highly specialized cytoskeleton may be
the origin of cell cycle-driven apoptosis by simply preventing a cycling neuron
from undergoing chromosome segregation and cytokinesis, we have found profound
abnormalities in tau homeostasis in our PS1 FAD mouse model. Specifically, tau
is hyperphosphorylated in mitotic epitopes in these mice (Figure 2a) and,
perhaps more importantly, nuclear expression of cyclin A appears to correlate
with the tau phosphorylation at S202/T205 (Figure 2b).
In
summary, although the molecular events in a neuron converting a stress stimulus
into a signal to enter an abortive cell cycle remain unknown, results from
experiments using PS1 FAD models point to the accidental triggering of
oncogenic pathways (i.e. aberrant expression of cyclin D1 and EGFR). In that context,
tau hyperphosphorylation could be interpreted as a by-product of the attempt by
the affected neuron to achieve a mitosis-ready configuration. If this is
representative of what occurs in the more widespread non-familial AD cases, we
would favour the hypothesis that, rather than an abortive cell cycle being an
early event in a regulated cell death response to stress, upregulation of cell
cycle proteins in the AD brain may simply reflect the activation of oncogenic
pathways that cannot be translated into cell division because of impaired
cytoskeletal dynamics, rendering the cell dysfunctional and ready to be
eliminated by apoptosis. In further support of this notion, work from the Smith
lab has shown that forcing post-mitotic neurons to re-enter the cell cycle
through the expression of MYC results in tau changes similar to those seen in
AD neurons. More importantly, MYC expression in forebrain neurons of a
transgenic model results in cell death and cognitive deficits [31,72]
Concluding
remarks
After differentiation, neurons become
post-mitotic, acquiring a structural and functional plasticity at the apparent
expense of a permanent exit from the cell cycle. Therefore, the expression of
cell cycle markers in the adult brain has always been a subject of
controversial debate. Clearly, although neurons are terminally differentiated
cells, they do express a wide range of cell cycle proteins and are known to be
capable of replicating their DNA, although no cases of a neuronal cell division
have ever been reported. This, together with the finding that the expression of
cell cycle proteins is necessary to execute apoptosis in response to certain
stress signals, has led to the proposition that a neuronal cell cycle does exist
and is part of a well-regulated response to stress signals. Whether this
interpretation is correct will probably depend on the nature of the initial
signal triggering a neuron into the cell cycle in the first place. The fact
that cell cycle proteins in neurons are capable of performing non-cell cycle
functions and that, at least in PS1-associated FAD, oncogenic signals are
readily generated, argue, in our opinion, for a neuronal cell cycle being no
different from other oncogenic signals in proliferative cells. The reason for
the absence of neuronal division and, indeed, tumors of neuronal origin, would
simply reflect the impossibility of a fully mature neuronal cytoskeleton to
revert to a mitosis-ready configuration. Clearly, more research is needed before
we can begin to understand the physiological and pathogenic implications of a
neuronal cell cycle.
The authors of this manuscript have no conflict of
interests to declare.