Review
The biochemistry and genetics of p53 function
p53 is a sequence-specific DNA-binding
protein and stress-activated transcription factor that controls the expression
of hundreds of genes implicated in a variety of physiological responses to
genome instability, virus infection and interferon production, DNA damage, metabolic
stresses such as hypoxia, and cytokine signaling. The vast numbers of gene products
mediating the p53 signal coordinately
promote many repair processes, some
of which include elimination of damaged proteins, DNA repair, ATP generation via oxidative
phosphorylation, organellar functions that maintain autophagy signaling and mitochondrial
function, the cell division cycle, and programmed cell death. The implications
of this stress-induced transcription re-programming by p53 is that cell and tissue
integrity can be maintained, thereby contributing to organism health and
viability.
Inactivating missense mutations in p53 are
very common in a wide range of human cancers, indicating a critical role for
p53 as a cancer suppressor in very distinct tissue microenvironments [1]. These
missense mutations reside predominantly in the core DNA-binding domain or
tetramerisation domain (Figure 1), and result in a p53 protein with an altered
conformation and attenuated sequence-specific DNA-binding function [2]. These
mutations thus suppress p53 transcription, reduce the cellular repair capacity,
and stimulate tumourigenesis. As p53 is a conformationally flexible and thermodynamically
unstable protein, biophysical studies have suggested there might be promise in
drug developments aimed at stabilizing the mutant p53 conformation into a wild-type
state, and re-engaging the p53 transcription program [3].
Transgenic technologies in mice have
supported biochemical and clinical data showing a critical role for the
DNA-binding function of p53 in cancer suppression. Animals null for p53 strikingly
develop cancer at an advanced rate [4]. By
contrast, deletion of many of the p53-inducible genes do not give the same
tumour incidence or tumour spectrum as p53-null animals [5], further
highlighting the role of p53 itself as a central hub in the integration of
tissue repair triggers. There is one intriguing exception: animals double null
for ataxia telangiectasia mutated (ATM) and the p53-inducible
gene p21 have a similar tumour spectrum and death incidence to the p53-null
animals [6]. This
suggests that ATM and p21 form a positive genetic circuit in the p53-dependent
cancer suppression mechanism.
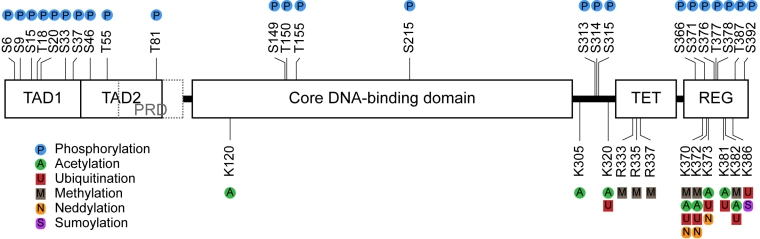
Figure 1. Sites of post-translational modifications on p53. Schematic
representation of the 393 amino acid domain structure of human p53 showing
the sites of post-translational modification including phosphorylation,
acetylation, ubiquitination, methylation, neddylation, and sumoylation.
Abbreviations: N-terminal transactivation domain (TAD); proline-rich domain
(PRD); tetramerisation domain (TET); C-terminal regulatory domain (REG);
arginine (R); lysine (K); serine (S); threonine (T).
In addition to a role for p53 in
cancer control, transgenic studies have also indicated that p53 can play a role
in aging-related processes that are triggered by telomere erosion or oxidative
damage to proteins, lipids or DNA, which in turn affect phenotypes including
neuromuscular coordination and longevity. The first such transgenic p53 animal
exhibited a genetic alteration that resulted in the constitutive production of
a C-terminal fragment of p53 that escaped degradation from its key negative
regulator, the E3 ubiquitin ligase MDM2. These animals exhibited aging
phenotypes including reduced longevity, osteoporosis, generalized organ
atrophy and a diminished stress tolerance [7]. A second transgenic study showing that enhanced
p53 function promotes aging utilized another truncated form of p53 with
mutations in the MDM2-binding domain [8]. An additional transgenic model displaying a
pro-aging phenotype had a BRCA1 mutation that constitutively activates p53 via
the enhanced endogenous DNA damage signals [9]. There is also some biochemical and clinical data
suggesting that p53 activation might play a role in human diseases of aging.
Recent reports have shown that p53 activation can trigger the pathways that
promote tau protein aggregation, which in turn is thought to reflect specific
stages in Alzheimer's disease [10]. Further, the activation of p53
by β-amyloid peptides might prove in vivo to either suppress the
accumulation of abnormal neurons by apoptotic pathways, or induce cell loss
resulting in attenuated brain functions associated with aging [11].
These studies summarized above are consistent with the
concept that elevated p53 activation might promote aging, which in turn seems
to fit well with the models that the evolution of p53 might have come about as
a trade-off between pathways that regulate longevity and maintain tissue
integrity. Too much p53 might promote more efficient cancer suppression at the
cost of elevated aging; whilst increasing longevity through reduced p53
function might result in elevated cancer development. However, other genetic
studies that alter p53 levels have not supported these interpretations.
Transgenic mice with either elevated p53 gene dosage [12] or
hypomorphic MDM2 function [13] have no
effect on the aging phenotype, although the animals have reduced cancer incidence,
which would be expected if p53 function was in fact elevated.
These two distinct outcomes have
been interpreted to indicate that when the p53 gene is under its normal
physiological control, aging programs are not necessarily engaged [14]. On the other hand, artificial
activation of p53 results in the abnormal production of a pro-aging phenotype,
suggesting that p53 promotes aging only under abnormal or pathological
circumstances [14]. This discrepancy has been
resolved in part by the most recent study in which animals with enhanced p19ARF and p53 were generated
that are under normal physiological control. These doubly transgenic mice
displayed en-hanced resistance to cancer and reduced aging characteristics,
including increased longevity [15], thus identifying a previously
unknown anti-aging signaling trigger in vertebrates. Two key p53-inducible gene
products that could play a role in this p53 anti-aging program are the
antioxidants Sestrins1 and 2 whose induction by the ARF-activation signal
presumably attenuates the accumulation of reactive oxygen species and
associated damaged cellular constituents that would normally promote aging [16]. Thus, an understanding of the
physiological factors that regulate the specific activity of p53 should shed
further light on the role of p53 in aging.
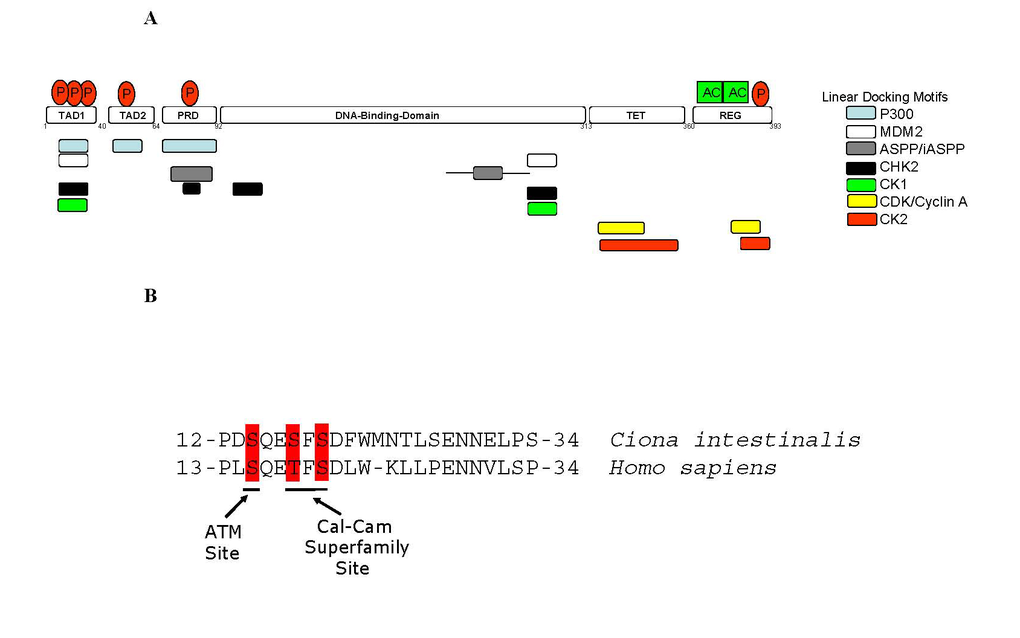
Figure 2. Linear Peptide Docking Sites in p53.
(A) Linear peptide docking
sites for enzymes that regulate p53 function. The N-terminus
is composed of three transactivation motifs,TAD1, TAD2, and Proline-repeat
domain (PRD). A key regulatory domain in the C-terminus (REG) contains the
acetylation motifs and phosphorylation site and flanks the Tetramerization
domain (TET). The overlapping, but distinct, linear polypeptide docking
motifs for p53 regulators include the acetyltransferase p300, the E3
ubiquitin ligase MDM2, iASPP, and the protein kinases including CDK, CK2,
CK1, and CHK2 are highlighted. (B) Conservation of key
phospho-acceptor sites between urochordate and human. The panel
highlights the conservation of amino acids and phospho-acceptor sites in
the BOX-I transactivation domain of p53 (TAD1 in Figure 2A) between human and urochordate
(Ciona intestinalis).
The ATM phospho-acceptor site at Ser15 and the Calcium Calmodulin kinase/CK1
phospho-acceptors sites at Thr18 and Ser20 are highlighted as indicated.
The biochemistry and genetics of p53 regulation
p53 protein function is regulated post-translationally
by coordinated interaction with signaling proteins including protein kinases,
acetyltransferases, methyl-transferses, and ubiquitin-like modifying enzymes
(Figure 1). The majority of the sites of covalent modification occur at
intrinsically unstructured linear peptide docking motifs that flank the
DNA-binding domain of p53 which play a role in anchoring or in allosterically activating
the enzymes that mediate covalent modification of p53 (Figure 2A). Such unstructured
linear domains are proving to be important in signaling control [17-19]. In
undamaged cells, p53 protein has a relatively short half-life and is degraded
by a ubiquitin-proteasome dependent pathway through the action of E3 ubiquitin
ligases including MDM2, PirH2, COP-1, and CHIP [20]. Following
stress, p53 is phosphorylated at multiple residues, thereby modifying its
biochemical functions required for increased activity as a transcription
factor. The biochemical functions include sequence-specific DNA binding and
protein-protein interactions. Acetylation of p53 is DNA-dependent, and this
modification facilitates chromatin remodeling and activation of p53 target gene
expression [21,22]. Of the
dozens of phospho-acceptor sites reported on p53 only three (Ser15, Thr18,
Ser20) are highly conserved between humans and urochordates (Figure 2B), the
latter being where a bona-fide p53-MDM2 axis has appeared in evolution. Especially
striking is the conservation of primary amino acid homology in the p53
transactivation domain between the invertebrate sea squirt and humans,
indicating that as yet undefined evolutionary selection pressures have
maintained this amino acid sequence at least since this urochordate lineage.
The only other highly conserved phosphorylation site in p53 is within the
C-terminus of p53 and is conserved only amongst vertebrates; the CK2 site at
Ser392. As such, we have focused our research on studying two of these highly
conserved phosphorylation sites in p53: the Ser20 site and the Ser392 site, as
they form a paradigm to facilitate our understanding of how phosphorylation
controls p53 function as a transcription factor. The many other sites of
covalent modification on p53 (Figure 1) also likely play important roles in p53
function or regulation, but there are relatively smaller amounts of genetic and
biochemical data describing the effects of these modifications on p53 function.
The Ser392 phospho-acceptor site is
located in the C-terminal regulatory domain (REG) in a flexible and
unstructured motif (Figures 1 and 2) whose phosphorylation by casein kinase 2
(CK2) stimulates the sequence-specific DNA-binding function of p53 [23]. This activation
of p53 presumably occurs by changes in the conformation of the DNA binding domain
that increases p53 thermostability as defined with biophysical studies using a phospho-mimetic
S392D mutant p53 protein [24]. This
Ser392 site is flanked by a sumoylation site [25] and a
cyclin A-docking site [26].
Phosphorylation at p53 Ser392 also increases after either UV or ionizing
radiation in cell lines, in mice spleenocytes in vivo, and in human skin
basal cell populations [27-29]. These
data are consistent with an activating rather than inhibitory role for phosphorylation
of this site on p53 function. Critically, substitution mutation of the murine
equivalent of Ser392 to Ala392 results in enhanced UV-induced skin cancer and
elevated carcinogen-induced bladder cancer in transgenic mice [30,31]. These
data identify a p53-activating kinase pathway whose attenuation could modify
aging-related diseases in squamous tissue like skin and bladder. Whether
phosphorylation of p53 at the Ser392 site plays a tumour suppressing role in other
cancer types remains to be determined.
The second highly conserved phospho-acceptor site,
Ser20, is located in the N-terminal transactivation domain (TAD) in an
unstructured linear motif (Figures 1 and 2) whose phosphorylation stabilizes
the binding of the transcriptional co-activator p300 by creating a
phospho-SDLxxLL docking motif [21,22,32].
The docking of p300 to this motif is required to promote DNA-dependent
acetylation of p53 at promoters, and hence transcriptional activation of p53
target genes. Mutation of Ser20 to Asp20, thereby mimicking constitutive
phosphorylation of p53 Ser20, results in a p53 with enhanced transcription function
in cell lines [33,34].
Further, as Ser20 site phosphorylation is elevated after DNA damage [35,36], these
data suggest that phosphorylation at p53 Ser20 forms a stimulatory rather than
an inhibitory signal for p53 activity. Transgenic mice with a phospho-acceptor
site mutation at the Ser20 equivalent in murine p53 have been shown to develop
spontaneous B-cell lymphoma [37], providing
evidence of the first spontaneous cancer-prone phenotype for a p53 covalent
regulatory site. Further, as B-cells from these transgenic mice exhibit
attenuated ionizing radiation-induced apoptosis in vitro [37], these data
highlight a central role for Ser20 site phosphorylation in p53-dependent
apoptotic activation in this cell type.
Together, these biochemical and genetic studies show
that phosphorylation can activate p53 function, although these studies do not
necessarily explain what selection pressures have maintained the integrity of
the Ser20 and Ser392 phospho-acceptor sites
during evolution in the urochordate-chordate lineage. Nevertheless, the
apparent cell- and damage-type specificity observed in post-translational
modification signaling pathways highlights the need to develop tissue-specific
experimental cancer models that reflect the physiological switches that can
activate p53, including changes in cytokoines like transforming growth factor
β (TGF-β) or interferons, metabolic stresses like hypoxia, glucose
starvation or acidification, external stresses including carcinogen damage to DNA,
and internal signals such as oncogene activation.
The enzymatic pathways that regulate p53
phosphorylation at Ser20
Although one of the key paradigms in the p53 field is
that p53 integrates diverse microenvironmental stresses into an outcome (Figure 3), the molecular mechanisms whereby these stresses activate p53 are only
beginning to be defined. DNA damage activation has been the most widely studied
signal input into p53. The checkpoint kinases 1 and/or 2 (CHK1/2) have been implicated as the ionizing radiation-induced p53
Ser20 site kinase(s) [38]. These
enzymes have evolved an allosteric docking site in the DNA-binding domain of
p53 (Figure 2A) that induces phosphorylation of p53 at Ser20 [39,40], and a
second interaction site for CHK2 was identified in the proline-rich domain
(PRD) of p53 [41]. Studies in
transgenic mice have shown that CHK2 is required to mediate the p53-dependent
response to ionizing radiation [42]. Although
these data indicate CHK2 is the most likely Ser20 site kinase for p53, genetic
screens have not supported this conclusion. The use of siRNA to CHK1 or CHK2
does not abrogate the damage-induced stabilization of p53 [43], and the
knockout of CHK2 in cancer cell lines does not compromise Ser20 site
phosphorylation [44]. Thus, the
ionizing radiation-induced kinase that targets the Ser20 site of p53 is still
undefined. In this study, we set out to identify the p53 Ser20 kinase(s)
induced by three very different stresses that are known to activate p53:
ionizing radiation, viral infection, and metabolic stress to determine whether
the p53 "integration" of distinct stress signals to this phospho-acceptor site
goes through the same or distinct kinase pathways.
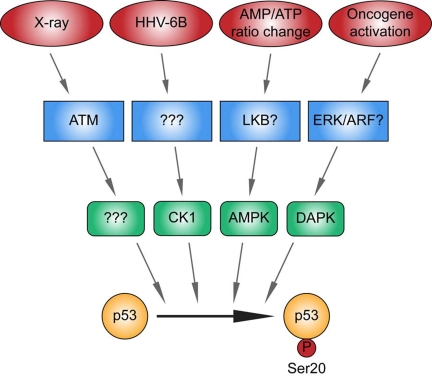
Figure 3. Different kinase signaling pathways link distinct stress signals to catalyze p53 phosphorylation at Ser20 in the TAD1 transactivation domain.
p53 is activated by distinct stresses, some of which include as indicated,
ionising radiation, viral infection, metabolic stress induced by an altered
AMP/ATP ratio, and oncogene activation. The X-ray-induced Ser20 site kinase
is ATM-dependent, but its identity is unknown (highlighted by "?"). CK1 is
the DNA virus HHV-6B-induced p53 Ser20 kinase, but the upstream sensor is
currently undefined (highlighted by "?"). The Ser20 site kinase induced by
an elevated AMP/ATP ratio is AMPK, and LKB is the likely upstream sensor.
DAPK-1 is the p53 Ser20 kinase induced by inappropriate oncogene
activation, and ERK or ARF are the likely upstream sensors. These data
support the formation of a model suggesting that the phosphorylation of p53
at Ser20 is triggered by distinct stress-responsive signaling cascades.
Future analysis will be required to determine the identity of all the
enzymes that mediate stress-induced phosphorylation at this site and
"integrate" the p53 response and developing disease models that deregulate
these signaling cascades.
Results
In attempts to define whether the activation of p53
Ser20 site kinase(s) induced by different stresses is triggered by the same or
different signaling pathways, we treated cells with specific kinase inhibitors
in combination with distinct stresses known to activate p53. We performed all
experiments using one cell culture model, namely the MOLT-3 cell line, which is
a human acute lymphoblastic leukaemia T-cell line. The MOLT-3 cell line was
first validated using ionizing radiation and kinase inhibitors specific for
CHK2, CHK1 and ATM. As a control consistent with siRNA screens for CHK2 [43], the
X-ray-induced Ser20 site phosphorylation of p53 was not attenuated by the CHK2
inhibitor (Figure 4A and B; lanes 6, 8, 10, 12 vs 5, 7, 9, 11). Further, the
CHK1 inhibitor SB218078 was equally unable to prevent Ser20 site
phosphorylation induced by X-rays (Figure 4C and D; lanes 6, 8, 10, 12 vs 5, 7,
9, 11). In fact, X-ray induced phosphorylation at Ser20 was elevated (Figure 4
C and D; lanes 6, 8, 10, 12 vs 4), and basal levels of p53 were stabilized by
the CHK1 inhibitor in the absence X-ray treatment (Figure 4C and D; lanes 5, 7, 9, 11 vs 3). However, this stabilized form of p53 in undamaged
cells was not phosphorylated at Ser20 (Figure 4C and D; lanes 5, 7, 9, 11).
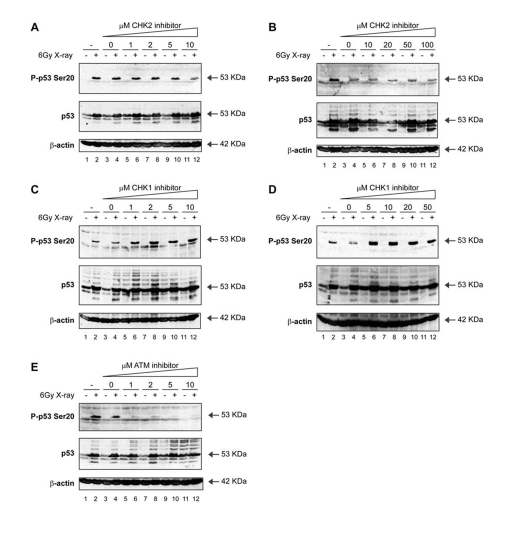
Figure 4. Activation of p53 by ionising radiation: effects of ATM-CHK pathway inhibitors on p53 phosphorylation. (A, B)
A CHK2 inhibitor does not attenuate Ser20 site phosphorylation of
p53 nor p53 induction mediated by treatment with
X-rays. MOLT-3 cells were treated with (even-numbered lanes)
or without (odd-numbered lanes) 6Gy X-ray and cultured for 4
hours after an initial 44-hour pre-treatment with: increasing
concentrations [1-10μM (A) or 10-100μM (B)] of the CHK2 inhibitor
(lanes 5-12), a DMSO solvent control (lanes 3-4), or a culture
medium control (lanes 1-2). Cell lysates were examined by Western
blotting with antibodies against the indicated proteins.
(C, D) A CHK1 inhibitor does not attenuate Ser20 site phosphorylation
of p53 nor p53 induction mediated by treatment with X-rays.
MOLT-3 cells were treated with (even-numbered lanes) or without
(odd-numbered lanes) 6Gy X-ray and cultured for 4 hours after
an initial 44-hour pre-treatment with: increasing concentrations
[1-10μM (C) or 5-50μM (D)] of the CHK1 inhibitor SB218078
(lanes 5-12), a DMSO solvent control (lanes 3-4), or a culture
medium control (lanes 1-2). Cell lysates were examined by Western
blotting with antibodies against the indicated proteins.
(E) An ATM inhibitor attenuates Ser20 site phosphorylation of p53,
but not p53 induction, mediated by treatment with X-rays. MOLT-3
cells were treated with (even-numbered lanes) or without
(odd-numbered lanes) 6Gy X-ray and cultured for 4 hours after
an initial 44-hour pre-treatment with: increasing concentrations
(1-10μM) of the ATM inhibitor KU-55933 (lanes 5-12), a DMSO
solvent control (lanes 3-4), or a culture medium control
(lanes 1-2). Cell lysates were examined by Western blotting
with antibodies against the indicated proteins.
These data are consistent with the recent study
showing that CHK1 loss can activate p53 [45] and that
CHK2 loss does not prevent Ser20 site phosphorylation [43].
Nevertheless, the treatment of cells with the specific ATM inhibitor KU-55933
resulted in a dose-dependent attenuation of X-ray-induced Ser20 site
phosphorylation (Figure 4E; lanes 6, 8, 10, 12 vs 4). These data indicate that
the X-ray-induced phosphorylation of p53 at Ser20 is ATM-dependent (Figure 3),
but since ATM consensus sites require an SQ core motif, it is not possible for
ATM to be the direct Ser20 site kinase.
Because the X-ray induced Ser20 kinase
was still undefined, we examined whether other kinase signaling pathways,
including casein kinase 1 (CK1), were involved. CK1 was identified as the human
herpesvirus 6B (HHV-6B)-induced protein kinase that targets the Ser20 site on
p53 [46]. Other DNA
and RNA viruses are also able to activate p53 function, consistent with the
intrinsic interferon-α/β responsiveness of the p53 pathway [47]. Whether
these other viruses also induce p53 phosphorylation at Ser20 is not fully
defined. As reported previously [46], the
treatment of HHV-6B infected cells with the specific CK1 inhibitor D4476
resulted in a dose-dependent attenuation of Ser20 site phosphorylation (Figure 5A; lanes 4, 6, 8, 10, 12, 14 vs 2). However, the CK1 inhibitor had no effect
on the X-ray-induced p53 Ser20 phosphorylation (Figure 5B; lanes 6, 8, 10, 12
vs 5, 7, 9, 11). Together, these data indicate that Ser20 site phosphorylation
is ATM-dependent after ionizing irradiation, but CK1-dependent after virus
infection (Figure 3).
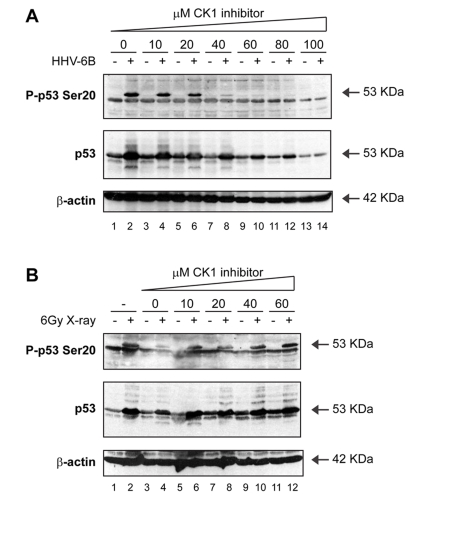
Figure 5. Activation of p53 by viral infection: effects of a CK1 inhibitor on p53 phosphorylation. (A) A CK1 inhibitor attenuates
Ser20 site phosphorylation of p53 and p53 induction mediated by HHV-6B
infection. MOLT-3 cells were infected with (even-numbered lanes) or without
(odd-numbered lanes) HHV-6B for 48 hours in the presence of increasing
concentrations (10-100μM) of the CK1 inhibitor D4476 (lanes 3-14) or a
DMSO solvent control (lanes 1-2). Cell lysates were examined by Western
blotting with antibodies against the indicated proteins. (B) A CK1 inhibitor does not attenuate Ser20 site
phosphorylation of p53 nor p53 induction mediated by treatment with X-rays.
MOLT-3 cells were treated with (even-numbered lanes) or without
(odd-numbered lanes) 6Gy X-ray and cultured for 4 hours after an
initial 44-hour pre-treatment with: increasing concentrations
(10-60μM) of the CK1 inhibitor D4476 (lanes 5-12), a DMSO solvent
control (lanes 3-4), or a culture medium control (lanes 1-2). Cell lysates
were examined by Western blotting with antibodies against the indicated
proteins.
We subsequently screened cells for other signals,
including hypoxia, glucose starvation, anoxia and perturbation of the AMP/ATP
ratio, which could trigger p53 phosphorylation at Ser20. Of these signals, the
most pronounced effect on Ser20 site phosphorylation was observed with the
compound Acadesine (AICAR; Figure 6A; lane 2 vs 1), which is known to activate
AMP-activated protein kinase (AMPK) by virtue of elevating the intracellular
AMP levels. We had previously identified AMPK in a candidate kinase screen as
an enzyme within the Calcium-Calmodulin kinase superfamily capable of targeting
p53 at Ser20 in vitro [40]. The
AICAR-mediated induction of Ser20 site phosphorylation was attenuated in a
dose-dependent manner by the treatment of cells with the AMPK inhibitor
Compound C (Figure 6A; lanes 6, 8, 10, 12 vs 2). On the other hand, the AMPK
inhibitor was unable to prevent Ser20 site phosphorylation induced by X-rays (Figure 6B; lanes 6, 8, 10, 12 vs 5, 7, 9,
11), indicating that AMPK is not the Ser20 site enzyme induced by X-rays.
Further, neither the CK1 inhibitor (Figure 6C), nor the ATM inhibitor (Figure 6D) abrogated the AICAR-induced p53 Ser20 phosphorylation (Figure 6C and D; lanes 6, 8, 10, 12 vs 5, 7, 9,
11). These data therefore confirm that
p53 Ser20 phosphorylation is ATM-dependent after X-rays, CK1-dependent after
virus infection, and AMPK-dependent after perturbation of AMP/ATP ratios
(Figure 3).
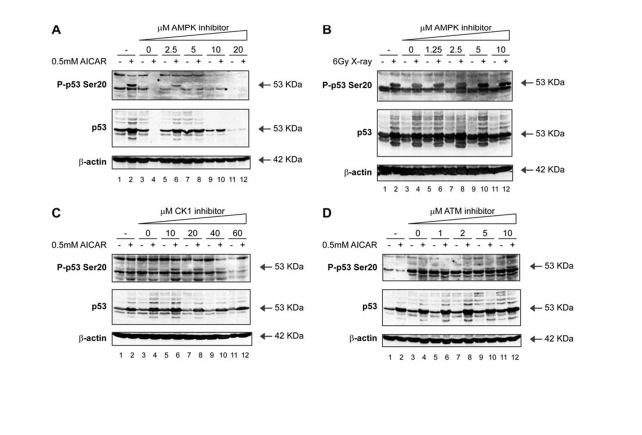
Figure 6. Activation of p53 by metabolic stress; effects of an AMPK inhibitor on p53 phosphorylation. (A) An AMPK inhibitor attenuates Ser20
site phosphorylation of p53 and p53 induction mediated by treatment with
AICAR. MOLT-3 cells were treated with (even-numbered lanes) or without
(odd-numbered lanes) 0.5mM AICAR for 24 hours after an initial 24-hour
pre-treatment with: increasing concentrations (2.5-20μM) of the AMPK
inhibitor Compound C (lanes 5-12), a DMSO solvent control (lanes 3-4), or a
culture medium control (lanes 1-2). Cell lysates were examined by Western
blotting with antibodies against the indicated proteins. (B) An AMPK
inhibitor does not attenuate Ser20 site phosphorylation of p53 nor p53
induction mediated by treatment with X-rays. MOLT-3 cells were treated with
(even-numbered lanes) or without (odd-numbered lanes) 6Gy X-ray and
cultured for 4 hours after an initial 44-hour pre-treatment with:
increasing concentrations (1.25-10μM) of the AMPK inhibitor Compound C
(lanes 5-12), a DMSO solvent control (lanes 3-4), or a culture medium
control (lanes 1-2). Cell lysates were examined by Western blotting with
antibodies against the indicated proteins. (C) A CK1
inhibitor does not attenuate Ser20 site phosphorylation of p53 nor p53
induction mediated by treatment with AICAR. MOLT-3 cells were treated with
(even-numbered lanes) or without (odd-numbered lanes) 0.5mM AICAR for 24
hours after an initial 24-hour pre-treatment with: increasing
concentrations (10-60μM) of the CK1 inhibitor D4476 (lanes 5-12), a
DMSO solvent control (lanes 3-4), or a culture medium control (lanes 1-2).
Cell lysates were examined by Western blotting with antibodies against the
indicated proteins. (D) An ATM inhibitor does not attenuate
Ser20 site phosphorylation of p53 nor p53 induction mediated by treatment
with AICAR. MOLT-3 cells were treated with (even-numbered lanes) or without
(odd-numbered lanes) 0.5mM AICAR for 24 hours after an initial 24-hour pre-treatment
with: increasing concentrations (1-10μM) of the ATM inhibitor KU-55933
(lanes 5-12), a DMSO solvent control (lanes 3-4), or a culture medium
control (lanes 1-2). Cell lysates were examined by Western blotting with
antibodies against the indicated proteins.
Together, these
data form a paradigm demonstrating that (i) distinct stresses, including
ionising radiation, virus infection and metabolic stress in the form of altered
AMP/ATP ratios, can induce p53 phosphorylation at Ser20; a site that can
stabilize p300 binding [21,22,32] and whose
mutation promotes the development of spontaneous B-cell lymphoma in transgenic
mice [37]; and (ii) the
induction of this phosphorylation depends upon distinct signals and kinase
pathways, namely ATM, CK1 and AMPK (Figure 3).
Model
Phosphorylation in the control of p53 function
A fundamental paradigm in p53 function is
that p53 "integrates" diverse stress signals towards a biological outcome. The
integration mechanism is undefined but presumably involves both inhibition of
p53's degradation pathway and activation of its transcription function. p53 is
controlled by a variety of post-translational mechanisms (Figure 1). Of the
many types of activating covalent modifications observed on p53,
phosphorylation has been the most well-studied both biochemically and
genetically. In this report, we have initiated a chemical biology screen to
determine the mechanisms underlying the integration of stress signals to p53
activation. The fundamental question that we set out to answer is whether one
common kinase pathway is able to target the Ser20 site within the
transactivation domain of p53 in response to various stresses, or whether
distinct kinases induced by different stresses are required to drive the same
mechanism. We have focused on the Ser20 site since it is the most highly
conserved phospho-acceptor site between urochordates and humans (Figure 2B)
with well-documented genetic and biochemical effects. Phosphorylation at Ser20
has the most striking effect on stabilizing the p300:p53 transcription complex
through interactions with multiple LxxLL peptide binding domains on p300 [21,22]. Ser20
phospho-peptides or phospho-mimetic peptides can inhibit DNA-dependent
acetylation of p53, showing an important role for this modification in driving
p53 acetylation [32]. Mutation
of the Ser20 site equivalent in mice to Ala20 gives rise to a spontaneous
tumour phenotype in transgenic animals [37], which
might, in part, explain its importance, as inferred from its high conservation
throughout evolution. In this study, we show that phosphorylation at the Ser20
site of p53 increases in response to distinct stresses, including ionizing
radiation, virus infection or metabolic stress, and we investigate the kinase
signaling pathways involved in this phosphorylation using small molecule kinase
inhibitors.
The ATM signal and aging
The phosphorylation of p53 at Ser20 after X-rays was
not attributed to CHK1 or CHK2 despite original data supporting this model [38]. In
addition, neither CK1 nor AMPK were the enzymes responsible for this
modification. However, an ATM-dependent pathway does drive X-ray induced Ser20
site phosphorylation (Figure 3), highlighting an important clue to the
identification of the X-ray-activated Ser20 site kinase. Transgenic mice with
phospho-acceptor site mutations at the murine equivalent of the Ser15 ATM target
site have been shown to exhibit an accelerated aging-associated phenotype,
along with an enhanced spontaneous development of late-onset lymphomas [48]. This
indicates that the Ser15 phospho-acceptor site is important for the tumour
suppression and anti-aging activity of p53, and implies that the kinases that
mediate the phosphorylation of this site, such as ATM, contribute to both the
tumour suppression and anti-aging activities of p53 [48]. In a
separate study, the p53 response to several forms of stress was found to
decline in various tissues of aging mice [49]. In
addition, the expression and activity of the kinase ATM was shown to be
decreased in older mice, again highlighting the importance of this kinase for p53 function [49]. This report also suggests that
decreased p53 function could, at least in part, explain the higher tumour
incidence in older individuals. Finally, ATM is
thought to be involved in telomere maintenance, and ATM-deficient cells undergo
telomere shortening and premature senescence [50].
The CK1 signal and aging
We had originally initiated biochemical
approaches to define the Ser20 kinase induced by DNA virus infection and
demonstrated that this phosphorylation is mediated by CK1 (Figure 3) [46]. Although
CK1 has not generated much interest in recent years due to the fact that it is
not regulated by reversible phosphorylation as are many classic
stress-activated enzymes, a recent study has shown that CK1 is the major enzyme
that mediates TGF-β-dependent activation of p53, however, the site of
phosphorylation is at Ser6/9 in the transactivation domain [51]. As CK1 is
presumably regulated by interacting proteins, it is therefore of interest to
understand how stresses as distinct as virus infection or TGF-β can
organize the CK1 interactome to target two different sites on p53. CK1 has also
been implicated in an aging-associated disease, namely Alzheimer's disease.
Indeed, the expression of CK1 has been shown to be up-regulated in the brain of
Alzheimer patients [52,53], and
CK1 has been implicated in the phosphorylation of the proteins tau and
β-secretase that have been linked to Alzheimer's disease [54,55]. More
recently, CK1 has been shown to be involved in the formation of the neurotoxic
peptide amyloid-β from amyloid precursor protein [56]. Given the
role of the ARF-p53 pathway in aging (reviewed in [14]) and the
likelihood that cytokines like TGF-β or interferons will play tissue-specific
roles in p53 modification, further examination of the role of CK1 in p53 aging
models would be intriguing.
The AMPK signal and aging
One of the key changes that occur intracellularly
after stress is ATP depletion and co-incident elevation in the ratio of
AMP/ATP. The enzyme AMPK senses this change and activates a signaling cascade
to reprogram the cellular response to stress. It is interesting that AMPK is
the enzyme that appears to target the Ser20 site of p53 after artificially-induced
changes in the AMP/ATP ratio using AICAR (Figure 3). In addition, this
metabolic stress-induced Ser20 site phosphorylation is CK1- and
ATM-independent, but is likely to be LKB-dependent [57]. AMPK
modulates several aging-associated processes, such as mitochondrial biogenesis,
obesity and decreased fatty acid oxidation, as well as insulin resistance
(reviewed in [58]). In
addition, AMPK activity has been shown to be decreased in aging rodent models [59,60]. AMPK
dysfunction could therefore be a key factor involved in the aging-associated
deficiencies in mitochondrial activity and metabolic regulation [58].
Do kinases modify the ARF-p53 anti-aging signal?
Other cellular stresses, including
aberrant oncogene activation and subsequent induction of ARF [61,62] or
extracellular signal-regulated kinases (ERKs) [63] and
death-associated protein kinase 1 (DAPK-1; Figure 3) [64-66] have not
been evaluated as of yet due to the lack of a common cell model that has an
active ARF pathway. However, given the role of ARF-p53 axis in regulating
longevity (reviewed in [14]), this
signal will be important to evaluate. In fact, recent studies have shown that
oncogene-induced senescence does not change p53 levels but increases its
specific activity [67], a phenomenon
that can be accomplished by p53 phosphorylation at specific regulatory sites.
Together, these data provide a paradigm
explaining how distinct stresses can activate p53 (summarized in Figure 3). In
a biochemical approach to identify candidate kinases, we had previously
identified many members of the calcium-calmodulin kinase superfamily, including
CHK1/2, DAPK-1 and AMPK as p53 Ser20 site kinases [40]. The identification
of CK1 as a major Ser20 site kinase was the first member outwith this superfamily
that could target this site on p53 [46]. However,
all these enzymes have a common biochemical requirement for a high affinity
docking site in the core DNA-binding domain of p53 to catalyse Ser20 site
phosphorylation in the transactivation domain [40,46]. Thus,
cells have evolved the ability to co-opt protein kinases that respond to
distinct signals to dock to the same site in the p53 DNA-binding domain and induce
Ser20 site phosphorylation. The fact that many of these enzymes including ATM, CK1
and AMPK can also modify pathways in cells linked to aging phenotypes
highlights a future direction for investigation aimed at understanding how
these kinase signaling pathways integrate into the ARF-p53 anti-aging pathway.
Methods
Chemicals, reagents and antibodies
. All
reagents were purchased from Sigma-Aldrich (Gillingham, UK), unless otherwise stated.
The AMPK inhibitor Compound C (or Dorsomorphin), the CHK1 inhibitor SB218078,
the CHK2 inhibitor, and the CK1 inhibitor D4476 were purchased from Merck
Chemicals (Nottingham, UK). The ATM inhibitor KU-55933 was a gift from KuDOS
Pharmaceuticals (Cambridge, UK). The DO-1 antibody to p53 was kindly provided
by B. Vojtesek (Masaryk Memorial Cancer Institute, Brno, Czech Republic). P-p53
Ser20 antibody to p53 phosphorylated at Ser20 was obtained from Santa Cruz Biotechnology (supplied by Insight Biotechnology,
Wembley, UK). Rabbit anti-mouse or swine anti-rabbit secondary antibodies were
obtained from Dako (Ely, UK).
Cell culture and treatments.
The
human acute lymphoblastic leukaemia T-cell line, MOLT-3, was cultured in IMDM (Invitrogen,
Paisley, UK) supplemented with 10% (v/v) foetal bovine serum (FBS; Autogen Bioclear,
Calne, UK). MOLT-3 cells were infected with HHV-6B strain PL-1 as previously
described [68].
Mock-infected and HHV-6B-infected MOLT-3 cells were treated with kinase
inhibitors (or DMSO solvent controls) concomitantly with infection, for 48
hours. Alternatively, MOLT-3 cells were pre-treated with kinase inhibitors (or DMSO
solvent controls) for 44 hours before exposure (or sham exposure) to 6Gy X-ray using
a cabinet X-ray machine (Faxitron X-Ray, Illinois, USA), and further culture
for 4 hours. Finally, MOLT-3 cells were pre-treated with kinase inhibitors (or
DMSO solvent controls) for 24 hours before treatment with 0.5mM Acadesine
(AICAR), or a DMSO solvent control, and further culture for 24 hours.
Cell lysis and Western blotting.
Cells
were harvested and lysed in urea lysis buffer [7M urea, 20mM HEPES (pH 7.6), 25mM
NaCl, 0.05% (v/v) Triton X-100, 0.1M dithiothreitol, 5mM NaF, 2mM Na3VO4, 2.5mM Na4P2O7, and 1 x
Complete Mini Protease Inhibitor Cocktail (Roche Diagnostics, Burgess Hill,
UK)] by incubation on ice for 30 minutes, followed by centrifugation at 13000g
for 10 minutes at 4°C. Protein lysates (40μg) were resolved by SDS-polyacrylamide
gel electrophoresis (PAGE) through 10% (w/v) tris-glycine gels and transferred
onto nitrocellulose membranes (Hybond ECL, GE Healthcare, Chalfont St Giles,
UK). Membranes were probed with primary antibodies, followed by secondary antibodies
conjugated to horse radish peroxidase (HRP). Bound antibody was detected by enhanced
chemiluminescence (ECL).
This work was funded by a Cancer Research UK Programme
Grant (Novel signaling pathways that control the tumour suppressor p53;
C483/A6354).
The authors in this manuscript have no conflict of
interest to declare.