Chain-breaking antioxidant activity of reduced forms ofmitochondria-targeted quinones, a novel type of geroprotectors
Abstract
Thechain-breaking antioxidant activities of reduced form of novel type ofgeroprotectors, mitochondria-targeted quinones (QH2) havequantitatively been measured for the first time. To this end, the chainperoxidation of methyl linoleate (ML) in Triton micelles was used as akinetic testing model. The studied QH2 were lipophilictriphenylphosphonium cations conjugated by an aliphatic linker to anantioxidant, i.e. a ubiquinol moiety (MitoQH2) or plastoquinolmoiety (SkQH2). The antioxidant activity was characterized bythe rate constant k1 for the reaction between QH2 andthe lipid peroxyl radical (LO2·) originatedfrom ML: QH2 + LO2· → HQ· + LOOH. Allthe tested QH2 displayed a pronounced antioxidant activity. Theoxidized forms of the same compounds did not inhibit ML peroxidation. Thevalue of k1 for SkQH2 far exceeded k1 forMitoQH2. For the biologically active geroprotectors SkQ1H2,the k1 value found to be as high as 2.2 × 105 M-1s-1,whereas for MitoQH2, it was 0.58 × 105 M-1s-1. The kineticbehavior of QH2 suggested that SkQ1H2 can rathereasily diffuse through lipid-water microheterogeneous systems.
Introduction
The oxidative stress caused by reactive
oxygen species (ROS) is assumed to significantly contribute to aging and
numerous age-related pathologies. Mitochondria are known as a place, where the most intensive ROS production
can occur. In the recent years, mitochondria-targeted antioxidants has been
developed [1-4]. Research was the series of papers published by our group in
1969-1970, where mitochondria-addressed penetrating synthetic cations were
described and the idea to use these cations as "electric locomotives" targeting non-charged compounds to mitochondria
was put forward [5,6]. In the late nineties, Murphy and coworkers initiated
the practical realization of this idea [1,7-9]. They synthesized and tested
several mitochondria-targeted antioxidants conjugated to the lipophilic
alkyltriphenylphosphonium cations. The ubiquinone moiety linked to
triphenylphosphonium cation by C10 aliphatic chain, MitoQ (Figure 1), seemed to be the most promising [1,4,9].
In 2005, an attempt was undertaken in our group to
replace the ubiquinone moiety in MitoQ by plasto-quinone. As a result, a series
of mitochondria-targeted antioxidants named SkQ has been synthesized [2,10].
There were two main reasons for this modification [1]. Plastoquinone playing in
chloroplasts the same role of an electron carrier as ubiquinone does in mitochondria
always operates under conditions of oxidative stress (elevated
oxygen concentration and an intensive ROS production) [2]. It was reported
[11-13] that the reactivity of the "tailless" plastoquinol analogs to the
peroxyl radicals was indeed higher than that of natural ubiquinols. The advantage
of mitochondria-targeted quinones of SkQ type over MitoQ was recently
demonstrated by using several biological models. In particular, it was found
that very low doses of SkQ1 (nmol/kg per day) prolong life of podospora,
ceriodaphnia, drosophila and mice. In mice, SkQ1 doubled median lifespan
arrested development of such traits of the senescence process as involution of
thymus and decline of other immunity mechanisms; osteoporosis; disappearance
of regular estrous cycles in females, cataract, retinopathies, balding,
catinies, hypothermia, chromosome aberrations, peroxidation of lipids and
proteins, etc. [10,14-20].
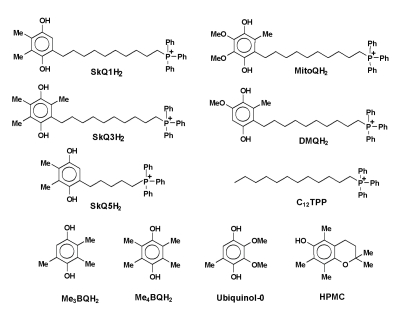
Figure 1.
The structure of the mitochondria-targeted
hydroquinones and other phenolics studied in this work.
Until
recently, the reactivity of the mitochondria-targeted antioxidants has, in
fact, not been quantitatively determined. This was done in the present paper.
The structure of the compounds studied is presented in Figure 1. The
chain-breaking antioxidant activity was characterized by the rate constant for
reaction of QH2 with the lipid peroxyl radical, LO2•, formed from ML or cardiolipin:
LO2• + QH2¾→ LOOH + QH• k1 [1] which
competes with the reaction of chain propagation of lipid peroxidation
LO2• + LH (+O2) ¾→ LOOH + LO2• k2[2].
Results
Figure 2 shows that SkQ1 is almost completely reduced to
SkQ1H2 by NaBH4. For SkQ1, the m/z value was found to be
537.08, which corresponds to the theoretically calculated one. As expected, the
m/z value for SkQ1H2 proved to be 539.1, i.e. m/z increased by two
units as compared with that for SkQ1. Similar results were also obtained for
the reduction of other mitochondria-targeted quinones.
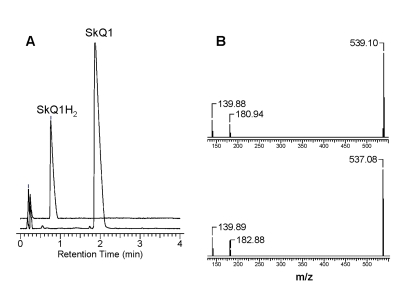
Figure 2. The reduction of
SkQ1 by NaBH4 as studied by UPLC-MS-MS analysis. (A) -
Reverse-phase HPLC chromatograms before and after the addition of NaBH4.
(B) - MS/MS spectra of SkQ1 before reduction (at the bottom) and after
reduction (at the top). Details of the protocol are given in the text.
The
non-inhibited oxidation of ML in Triton micelles is a chain process, which
rate, R0, was found to be proportional to [ML] and square root of
[AAPH] (not shown) as it was reported in our preceding papers [21,22]. Such
relationships are also inherent in the lipid peroxidation in other aqueous
microheterogeneous systems [23-25]. They correspond to the "classic" kinetic scheme with
bimolecular chain termination [26,27].
AAPH + LH + (O2) ¾→ LO2• + products RIN (0)
LO2• + LH + ¾→ LOOH + L• k2 [2] L• + O2¾→ LO2• k3 [3]
LO2• + LO2•¾→ products 2k4 [4] All the tested QH2
displayed a pronounced chain-breaking antioxidant activity as this is
exemplified by Figure 3 for SkQ1H2. When SkQ1H2
was added, the rate of oxidation, R, dramatically decreased. As SkQ1H2
was progressively consumed due to reaction [1], R increased with time and
eventually reaches the level of non-inhibited oxidation. As a result, the
pronounced induction period was observed (Figure 3A).
![The effect of 5 μM SkQ1H2 on the
kinetics of oxygen consumption caused by oxidation of 20 mM ML in micellar
solution of 50 mM Triton X-100 in 50mM phosphate buffer, pH 7.4, 37 °C. Oxidation was initiated by 3
mM AAPH. (A) [O2] trace; arrow shows addition of SkQ1H2.
(B) plot A in the axes of Eq. 7.](/article/100049/figure/F3/large)
Figure 3. The effect of 5 μM SkQ1H2 on the
kinetics of oxygen consumption caused by oxidation of 20 mM ML in micellar
solution of 50 mM Triton X-100 in 50mM phosphate buffer, pH 7.4, 37 °C. Oxidation was initiated by 3
mM AAPH. (A) [O2] trace; arrow shows addition of SkQ1H2.
(B) plot A in the axes of Eq. 7.
Quantitatively
similar [O2] traces were observed with all the other tested QH2
as well as with α-tocopherol and its synthetic analog
6-hydroxy-2,2,5,7,8-pentamethylchromane (HPMC). As for C12TPP, a
compound that has no hydroquinone moiety (Figure 1), it did not display any
inhibiting activity (not shown). Meanwhile, oxidized form of SkQ1 showed a weak
inhibition of ML oxidation, but only during a very short period of time (Figure 4).
Most likely, the inhibition is caused in this case by a minor contamination
of SkQ1H2 to SkQ1. A similar effect was also observed with other
mitochondria-targeted Q. This suggests that mitochondria-targeted quinones by
themselves do not act as a chain-breaking antioxidant.
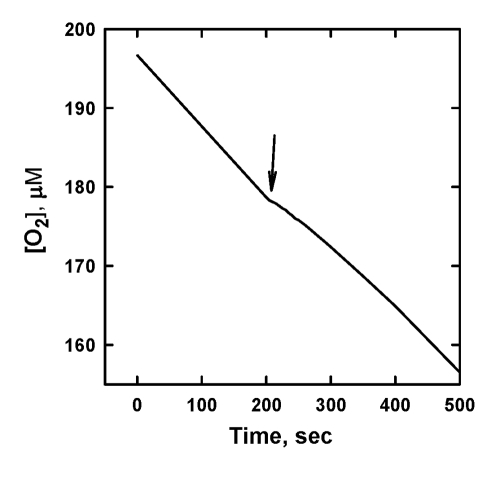
Figure 4. The effect of
addition of 10 μM SkQ1 on the
kinetics of oxygen consumption during the oxidation of 20 mM ML in 50 mM
micellar solution of 50 mM Triton X-100 in 50mM phosphate buffer, pH 7.40,
37 °C, initiated by
3 mM AAPH. Arrow shows the moment when SkQ1 was added.
The reduced forms of
mitochondria-targeted quinones studied in this work are p-hydroquinones. Acting
as chain-breaking antioxidants during the chain peroxidation of styrene
p-hydroquinones, "tailless" analogs of mitochondria-targeted antioxidants show
a very high inhibiting activity [11], sometimes comparable with that of α-tocopherol (k1 = 3.3 × 106M-1s-1 [26]).
For instance, k1 for Me3BQH2 was found to be
as much as 2.2 × 106M-1s-1 (Table 1). The
behavior of p-hydroquinones in such a system does not differ from that of
monophenolic antioxidants [26,27]. The situation dramatically changes when
going to the peroxidation
of ML in aqueous micelles [12,28]. The matter is that p-hydroxy-substituted
phenoxyl radicals QH• formed in reaction [1] having, as a rule, pK less
than 5 [29] undergo fast deprotonation at neutral pH: QH•¾→ Q•- + H+ [5] with the
formation of semiquinone anion, Q•-, which reacts
readily with molecular oxygen, forming O2•- [30,31]: Q•- + O2¾→ Q + O2•- [6] In turn, O2•- may react with oxidation substrate and QH2,
most likely in its protonated form, HO2•. Both reactions result in a decrease in the
inhibitory activity of QH2 [28]. SOD removes O2•-and thus arrests the mentioned undesirable reactions
with the participation of O2•- (HO2•). This was a reason why SOD was always added to our
system.
Table 1.
Kinetic parameters characterizing the antioxidant activity of the reduced
forms of mitochondria-targeted quinones and their analogs in micellar solution of 50 mM
Triton X-100, 50 mM phosphate buffer, pH 7.4, at 37 °C. Oxidation of ML or cardiolipin
was initiated by AAPH.
Notes: nd - not determined;
a structures of QH2 are given in Figure 1;
b figures in brackets are the number of independent experiments;
c ML is replaced by cardiolipin;
d determined during styrene oxidation in the bulk.
|
QH2a |
k1/k2b |
k1 × 105, M-1s-1 |
|
SkQ1H2 |
3670 ± 280 (7)
1980 ± 170 (3)c |
2.2 ± 0.2
nd
|
|
SkQ3H2 |
2720 ± 210 (4)
|
1.6 ± 0.1
|
|
SkQ5H2 |
2670 ± 180 (5)
|
1.6 ± 0.1
|
|
MitoQH2 |
970 ± 55 (6)
520 ± 37 (3)c |
0.58 ± 0.03
nd
|
|
DMQH2 |
1260 ± 85 (4)
|
0.76 ± 0.5
|
|
Me3BQH2 |
2170 ± 130 (4)
|
1.3 ± 0.1
23d |
|
Me4BQH2 |
5020 ± 380 (3)
|
3.0 ± 0.2
|
|
Ubiquinol-0
|
700 ± 45 (3)
|
0.42 ± 0.03
4.4d |
|
α-tocopherol
|
1170 ± 70 (4)
|
0.70 ± 0.04
|
|
HPMC
|
8680 ± 700 (4)
|
5.2 ± 0.4
|
The [O2] traces recorded during the
induction period of the inhibited oxidation of ML were used to determine k1.
On the base of a reductive kinetic scheme, which includes reactions (0), [1],
[2], and [4], the following equation can
be deduced [11,12] where
[LH] is the concentration of the oxidation substrate (in our case ML). Figure 3B depicts the original [O2] trace (Figure 3A) in the axes of Eq.
[7]. It is seen that the plot of F vs. time is a straight line as predicted by
Eq. [7]. The kinetic behavior of all the other QH2 studied proved to
be was similar. The value of k1/k2 can be calculated from
the slope of this straight line by using Eq. 7. It should be noted that this
way of calculation of k1/k2 does not require the
knowledge in RIN and the starting concentration of QH2.
The values of k1/k2 are listed in Table 1. The
absolute values of k1were
calculated from k1/k2 assuming k2 = 60 M-1s-1 [22].
The k1 values are also listed in Table 1.
With two QH2, SkQ1H2
and MitoQH2, similar experiments were conducted by using the same
testing system, but with substituting ML by cardiolipin, the most oxidizable
phospholipid component in mitochondria membranes [32,33]. As seen from Figure 5, both [O2]
traces during the induction period of the inhibited oxidation and the plots of F vs. time are very
similar to those for ML. The value of k1/k2 was
calculated from the slope of the plot B (Figure 5) by using Eq. [7] assuming
that each molecule of cardiolipin contains four fatty acid residue with 87 %
linoleate in the cardiolipin sample used in this work (see
http://www.avantilipids.com). These data are also presented in Table 1. Unfortunately,
the absolute values of k1 could not be calculated, as k2
for the oxidation of cardiolipin has never been reported.
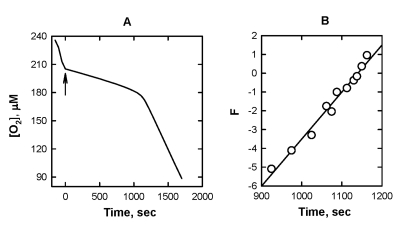
Figure 5. The effect of
addition of 10 μM SkQ1H2
on the kinetics of oxygen consumption during the oxidation of 2.6 mg mL-1 cardiolipin 50
mM micellar solution of 50 mM Triton X-100 in 50mM phosphate buffer, pH
7.40, 37 °C, initiated by
3 mM AAPH. Arrow shows the moment when SkQ1H2 was added.
Discussion
In
this paper, the reactivity of the reduced forms of the mitochondria-targeted
quinones as chain-breaking anti-oxidants has systematically been studied. As
may be seen from Table 1, the k1 value for SkQ1H2, SkQ3H2
and SkQ5H2 are significantly higher than that for MitoQH2.
This is in line with the data for simple "tailless" analogs of SkQ1H2
and MitoQH2, namely Me3BQH2, Me4BQH2
and Ubiquinol-0. The same
tendency was earlier observed when effects of "tailless" analogues on the chain
oxidation of styrene in bulk [11] and ML peroxidation in SDS micelles were
studied [12]. Possible reasons why
methyl-substituted p-hydroquinones are better antioxidants than
methoxy-substituted p-hydroquinones were described elsewhere [11,26]. In
brief, the effect under consideration is, the most probably, stereoelectronic
by its nature. The matter is that o-methoxy group forms H-bond with oxygen
belonging to the adjacent OH group. This causes the decrease in overlap between
p-type orbital of oxygen atom of OH-group and the aromatic π-electron cloud (the increase of the dihedral angle between the aromatic
ring and O - H bond). The latter results in strengthening O - H bond as
compared with that in o-methyl substituted QH2, where such an
intramolecular H-bond is absent.
Among
mitochondria-targeted QH2 studied in this work, SkQ1H2
showed the highest reactivity towards the lipid peroxyl radicals (Table 1).
This observation is in line with data obtained in our group by using several
biological models [2,10,14]. However, we recognize that the highest value of
k1 for SkQ1H2 is likely not the only reason for the
outstanding biological activity of SkQ1. It should be taken into account that k1
given in Table 1 are effective values and cannot be directly attributed to the
elementary reaction [1]. The genuine values of k1 can be determined
during the chain oxidation in non-polar media, for instance in styrene [11,34,35]. When going to the oxidation of fatty acid (ester) in bulk [12,36]and further to
the oxidation in aqueous micelles and liposomes [12,26,37], the experimentally
determined k1 values significantly decrease, nearly by one order of
magnitude (see data for ubiquinol-0, Table 1). A reason for such a reduction of
k1 was repeatedly discussed. The mentioned decrease in k1
is not specific of QH2. A similar effect has earlier been also
reported for the oxidation inhibited by monophenolics [25,26,37,38]. The
formation of H-bonds between the OH-group of phenolics and the carboxy-group of
ML has been suggested as the main reason for the k1 decrease when
going from the oxidation of non-polar hydrocarbon to that of fatty acid (ester)
[36]. Recently, hydrogen bonding between phenols and fatty acid esters was
directly observed by using the NMR technique [39]. Most likely, this is also
true for QH2 studied in this work. The further decrease in k1
when going from ML oxidation in bulk to that in aqueous micelles may be
explained by the additional formation of H-bonds between QH2 and
water molecules as this was earlier suggested for monophenolics [23,37,38].
A
general specific feature of reduced forms of the studied mitochondria-targeted
quinoles is that their reactivity is actually very close to that of their
"tailless" analogs (Table 1). This is in contrast to the couple "α-tocopherol having the long aliphatic chain its "tailless" analog HPMC.
The k1 value for α-tocopherol is nearly one order
of magnitude lower than that for HPMC (Table 1). This effect was reported to be
even more pronounced in the SDS micelles [23,37,38]. The essential feature of
our testing system and related microheterogeneous systems is that the
concentration of the antioxidants tested is much lower than that of the
oxidation substrate (in our case ML). While every micelle (microreactor)
contains several molecules of ML, only a few micelles contain an antioxidant.
Under these conditions, a fast LO2• reduction by an antioxidant is possible only if an
antioxidant is capable of fast transferring from one microreactor to another,
the characteristic time of this transfer being shorter than the time
of the occurrence of a single kinetic chain. The antioxidants with a rather
long aliphatic residue like α-tocopherol commonly do not meet
such a requirement [37]. The fact that the values of k1 for the
mitochondria-targeted quinols actually do not differ from that of their
"tailless" analogs (Table 1) means that all of them are capable of the fast
transfer from one microreactor to another. This is in line with a high reported
ability of SkQ and MitoQ to easily penetrate through biological membranes [14].
Materials and Methods
Methyl linoleate and Triton X-100 were
purchased from Sigma, heart bovine cardiolipin disodium salt was received from
Avanti PolarLipids. The water-soluble initiator 2,2'-azobis(2-amidinopropan)
dihydrochloride (AAPH) was obtained from Polysciences. NaH2PO4
and Na2HPO4 of the highest quality used to prepare buffer
solutions were purchased from Merck. The mitochondria-targeted
quinones, SkQ1, SkQ3, SkQ5, MitoQ, DMQ as
well as C12TPP (see Figure 1) were synthesized in the Mitoengineering Centre
of Moscow State University [2]. Trimethylhydroquinone (Me3BQH2)
was purchased from Aldrich; 2,3-dimethoxy-5-methyl-benzoqyuinone
(ubiquinone-0) was from Sigma; tetramethylbenzoquinone (Me4BQ) was
from EGA Chemie. All the other chemicals
were of highest available quality.
The reduced forms of the mitochondria-targeted quinones (QH2) were
produced by the reduction of corresponding quinones by NaBH4 in the
mixture of 50 mM NaH2PO4 (pH 5.0) with ethanol. This process
was under control of UPLC-MS-MS (see below). Reduced forms of ubiquinone-0 and tetramethylhydroquinone
(Me4BQH2) were produced by reduction of the quinones by
Zn powder [21]. The buffer solution (pH
7.40 ± 0.02) was prepared by mixing 50 mM solutions of NaH2PO4
and Na2HPO4. In turn, the solutions of the individual
sodium phosphates were prepared with doubly distilled water and were purged
from traces of transition metals by Chelex-100 resin (Bio-Rad).
HPLC-diode
array detection-electrospray ionization tandem mass spectrometry analysis
(UPLC-MS-MS) was performed using an ACQUITY system (Waters, Milford, MA, USA).
Chromatography was carried out using an ACQUITY BEH C18 column (2.1 x 50 mm,
1.7 μm) eluted with a gradient of 40-60% acetonitrile (4
min) and 20 mM acetic acid (pH 3.0) delivered at a flow rate of 0.5 mL per min.
UV-monitoring was performed at 280 mm. An injection volume of 11.2 μL (full loop) was used in all cases. A Quattro triple-quadrupole mass
spectrometer (Micromass-Waters) fitted with a Z-Spray ion interface was used
for analyses. Ionization was achieved using electrospray in a positive
ionization mode. The following conditions were found to be optimal for the
analysis of SkQ1: capillary voltage, 3.0 kV; source block temperature, 120°C; and
desolvatation gas (nitrogen) heated to 450°C and delivered at a flow rate of
800 L h-1; cone
voltage, 55 V; cone Gas Flow rate, 50 L h-1. MassLynx 4.0 software (Waters) was used for
processing.
The
standard testing system was composed of 50 mM buffer, pH 7.4, 50 mM Triton
X-100, 2-4 mM AAPH, 8-20 mM ML and 20 unit mL-1 SOD. In
some experiments, ML was replaced by cardiolipin. The kinetics of oxygen
consumption accompanied ML (cardiolipin) oxidation were studied with a
computerized 5300 Biological Oxygen Monitor (Yellow Springs Instruments Co.,
USA) with a Clark electrode as a sensor. The rate of oxidation was measured as
a slope of [O2] traces. Experiments were conducted at 37.0 ± 0.1 °C. ML was added to preliminarily thermostated micellar
solution of Triton X-100 and AAPH in buffer. Monitoring was started 3-5 min
after ML addition and the rate of non-inhibited oxidation (R0) was
measured. The tested compounds were then added to a reaction chamber under
steady monitoring as a stock solution by using a Hamilton micro-syringe. In
more detail, the protocol was described elsewhere [12,21,22].
Acknowledgments
Supported by Mitotechnology LLC, Russia Ministry of
Education and Science (grant "Leading Scientific Schools" N 5762.2008.4).
Conflicts of Interest
The authors in this manuscript have no conflict of interest to declare.
References
-
1.
Murphy
MP
and Smith
RAJ.
Targeting antioxidants to mitochondria by conjugation to lipophilic cations.
Annu Rev Pharmacol Toxicol.
2007;
47:
629
-656.
[PubMed]
.
-
2.
Skulachev
VP
A Biochemical Approach to the Problem of Aging: "Megaproject" on Membrane-Penetrating Ions. The First Results and Prospects.
Biochemistry (Moscow).
2007;
72:
1385
-1396.
[PubMed]
.
-
3.
Hoye
AT
, Davoren
JE
, Wipe
P
, Fink
MP
and Kagan
VE.
Targeting Mitochondria.
Acc Chem Res.
2008;
41:
87
-97.
[PubMed]
.
-
4.
Rocha
M
and Victor
VM.
Targeting antioxidants to mitochondria and cardiovascular diseases: The effects of mitoquinone.
Med Sci Monit.
2007;
13:
RA132
-145.
[PubMed]
.
-
5.
Liberman
EA
, Topaly
VP
, Tsofina
LM
, Jasaitis
AA
and Skulachev
VP.
Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria.
Nature.
1969;
222:
1076
-1078.
[PubMed]
.
-
6.
Severin
SE
, Skulachev
VP
and Yaguzhinsky
LS.
A possible role of carnitine in Transport of fatty acids through the mitochondrial membrane.
Biokhimiya.
1970;
35:
1250
-1257 (Russ).
[PubMed]
.
-
7.
Murphy
M P
Targeting bioactive compounds to mitochondria.
Trends Biotechnol.
1997;
15:
326
-330.
[PubMed]
.
-
8.
Murphy
MP
and Smith
RAJ.
Drug delivery to mitochondria: the key to mitochondrial medicine.
Adv Drug Deliv Rev.
2000;
41:
235
-250.
[PubMed]
.
-
9.
Smith
RAJ
, Kelso
GF
, James
AM
and Murphy
M P.
Targeting coenzyme Q derivatives to mitochondria.
Meth Enzymol.
2004;
382:
45
-67.
[PubMed]
.
-
10.
Skulachev VP.
Method
of acting upon organism by targeted delivery of biologically active substances
into mitochondria, pharmaceutical composition for carrying out said method, and
compound used for the purpose.
World patent.
.
-
11.
Loshadkin
D
, Roginsky
V
and Pliss
E.
Substituted p-hydroquinones as a chain-breaking antioxidant during the oxidation of styrene.
Int J Chem Kinetics.
2002;
34:
162
-171.
.
-
12.
Roginsky
V
, Barsukova
T
, Loshadkin
D
and Pliss
E.
Substituted para-hydroquinones as an inhibitor of lipid peroxidation.
Chem Phys Lipids.
2003;
125:
49
-58.
[PubMed]
.
-
13.
Kruk
J
, Jemiola-Rzeminska
M
and Strzalka
K.
Plastoquinol and α-tocopherol quinol are more active than ubiquinol and α-tocopherol in inhibition of lipid peroxidation.
Chem Phys Lipids.
1997;
87:
73
-80.
.
-
14.
Antonenko
YN
, Avetisyan
AV
, Bakeeva
LE
, Chernyak
BV
, Chertkov
VA
, Domnina
LV
, Ivanova
OY
, Izyumov
DS
, Khailova
LS
, Klishin
SS
, Korshunova
GA
and Lyamzaev
KG.
Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: synthesis and in vitro studies.
Biochemistry (Moscow).
2008;
73:
1273
-1287.
[PubMed]
.
-
15.
Bakeeva
LE
, Barskov
IV
, Egorov
MV
, Isaev
NK
, Kapelko
VI
, Kazachenko
AV
, Kirpatovsky
VI
, Kozlovsky
SV
, Lakomkin
VL
, Levina
SB
, Pisarenko
OI
and Plotnikov
EY.
Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 2. Treatment of some ROS- and age-related diseases (heart arrhythmia, heart infarctions, kidney ischemia, and stroke).
Biochemistry (Moscow).
2008;
73:
1288
-1299.
[PubMed]
.
-
16.
Agapova
LS
, Chernyak
BV
, Domnina
LV
, Dugina
VB
, Efimenko
AY
, Fetisova
EK
, Ivanova
OY
, Kalinina
NI
, Khromova
NV
, Kopnin
BP
, Kopnin
PB
and Korotetskaya
MV.
Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 3. Inhibitory effect of SkQ1 on tumor development from p53-deficient cells.
Biochemistry (Moscow).
2008;
73:
1300
-1316.
[PubMed]
.
-
17.
Neroev
VV
, Archipova
MM
, Bakeeva
LE
, Fursova
AZh
, Grigorian
EN
, Grishanova
AY
, Iomdina
EN
, Ivashchenko
ZhN
, Katargina
LA
, Khoroshilova-Maslova
IP
, Kilina
OV
and Kolosova
NG.
Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 4. Age-related eye disease. SkQ1 returns vision to blind animals.
Biochemistry (Moscow).
2008;
73:
1317
-1328.
[PubMed]
.
-
18.
Anisimov
VN
, Bakeeva
LE
, Egormin
PA
, Filenko
OF
, Isakova
EF
, Manskikh
VN
, Mikhelson
VM
, Panteleeva
AA
, Pasyukova
EG
, Pilipenko
DI
, Piskunova
TS
and Popovich
IG.
Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 5. SkQ1 prolongs lifespan and prevents development of traits of senescence.
Biochemistry (Moscow).
2008;
73:
1329
-1342.
[PubMed]
.
-
19.
Skulachev
VP
, Anisimov
VN
, Antonenko
YN
, Bakeeva
LE
, Chernyak
BV
, Erichev
VP
, Filenko
OF
, Kalinina
NI
, Kapelko
VI
, Kolosova
NG
, Kopnin
BP
and Korshunova
GA.
An attempt to prevent senescence: A mitochondrial approach.
Biochim Biophys Acta.
2009;
1797:
437
-461.
[PubMed]
.
-
20.
Plotnikov
EY
, Vasileva
AK
, Arkhangelskaya
AA
, Pevzner
IB
, Skulachev
VP
and Zorov
DB.
Interrelations of mitochondrial fragmentation and cell death under ischemia/reoxygenation and UV-irradiation: protective effects of SkQ1, lithium ions and insulin.
FEBS Lett.
2008;
582:
3117
-3124.
[PubMed]
.
-
21.
Roginsky
VA
and Barsukova
TK.
Kinetics of oxidation of hydroquinones by molecular oxygen. Effect of superoxide dismutase.
J Chem Soc Perkin 2 Trans.
2000;
N7:
1575
-1582.
.
-
22.
Roginsky
VA
Chain-breaking antioxidant activity of natural polyphenols as determined during the chain oxidation of methyl linoleate in Triton X-100 micelles.
Arch Biochem Biophys.
2003;
414:
261
-270.
[PubMed]
.
-
23.
Castle
L
and Perkins
MJ.
Inhibition kinetics of chain-breaking phenolic antioxidants in SDS micelles. Evidence that intermicellar diffusion rates may be rate-limiting for hydrophobic inhibitors such as alpha-tocopherol.
J Amer Chem Soc.
1986;
108:
6381
-6382.
.
-
24.
Barclay
LRC
, Locke
SJ
, MacNeil
JM
and VanKessel
J.
Quantitative studies of linoleate monomers sequestered in phosphatidylcholine bilayers. Absolute rate constant in bilayers.
Canad J Chem.
1985;
63:
2633
-2638.
.
-
25.
Barclay
LRC
, Locke
SJ
and MacNeil
JM.
Autoxidation in micelles. Synergism of vitamin C with lipid-soluble vitamin E and water-soluble Trolox.
Canad J Chem.
1985;
63:
366
-374.
.
-
26.
Barclay
LRC
and Vinqvist
MR.
Rappoport Z.
Phenols as antioxidants
The Chemistry of Phenols.
Willey
2003;
840
-907.
.
-
27.
Roginsky
VA
Phenolic Antioxidants: Efficiency and Reactivity.
1988;
Moscow
Nauka
Russian.
.
-
28.
Roginsky
VA
Superoxide dismutase enhances chain-breaking antioxidant capability of hydroquinones.
Free Radic Res.
2001;
35:
55
-62.
[PubMed]
.
-
29.
Landolt-Börnstein: Numerical Data and
Functional Relationships in Science and Technology - New Series. Group II, V. 13e.
1984;
Berlin, Heidelberg
Springer-Verlag
.
-
30.
Wardman
P
Bioreductive activation of quinones: Redox properties and thiol reactivity.
Free Radic Res Commun.
1990;
8:
219
-229.
[PubMed]
.
-
31.
O'Brien
PJ
Molecular mechanisms of quinone cytotoxicity.
Chem-Biol Interact.
1991;
80:
1
-41.
[PubMed]
.
-
32.
Lesnefsky
EJ
and Hoppel
CL.
Cardiolipin as an oxidative target in cardiac mitochondria in the aged rat.
Biochim Biophys Acta.
2008;
1777:
1020
-1027.
[PubMed]
.
-
33.
Ott
M
, Gogvadze
V
, Orrenius
S
and Zhivotovsky
B.
Mitochondria, oxidative stress and cell death.
Apoptosis.
2007;
12:
913
-922.
[PubMed]
.
-
34.
Litwinienko
G
and Ingold
KU.
Solvent effects on the rates and mechanisms of reaction of phenols with free radicals.
Acc Chem Res.
2007;
40:
222
-230.
[PubMed]
.
-
35.
Tikhonov
I
, Roginsky
V
and Pliss
E.
The chain-breaking antioxidant activity of phenolic compounds with different number of O-H groups as determined during the oxidation of styrene.
Int J Chem Kinet.
2008;
In press
.
-
36.
Roginsky
VA
Kinetics of oxidation of polyunsaturated fatty acid esters inhibited by substituted phenols.
Kinetics and Catalysis (Moscow).
1990;
31:
475
-481.
.
-
37.
Roginsky
VA
The inhibiting ability of lipid-soluble and water-soluble phenols at lipid peroxidation in micro-heterogeneous systems.
Biol Membr (Moscow).
1990;
4:
437
-451.
.
-
38.
Prayor
WA
, Strickland
T
and Church
DF.
Comparison of the efficiencies of several natural and synthetic antioxidants in aqueous sodium dodecyl sulfate micelle solutions.
J Amer Chem Soc.
1989;
110:
2224
-2229.
.
-
39.
Litwinienko
G
, Megiel
E
and Wojnicz
M.
Hydrogen bonding between phenols and fatty acid esters: 1H NMR study and ab initio calculations.
Org Lett.
2002;
4:
2425
-2428.
[PubMed]
.