Introduction
It
is generally accepted that the primary cause of functional impairment in muscle
is a cumulative failure to repair damage related to an overall decrease in
anabolic processes. Despite numerous theories and intensive research, the
principal molecular mechanisms underlying the process of muscle wasting are still unknown.
Current data point out that
muscle wasting is a multifactorial process and believed to be the result of both intrinsic factors, involving changes
in molecular and cellular levels, and
extrinsic ones, such as nutrition and exercise [1]. Among intrinsic factors,
the proteolytic systems have been postulated to be responsible for the protein breakdown. Calpain-, ubiquitin- and
caspase- mediated
protein degradation are the principal proteolytic pathways activated in several
pathologies, leading to myofiber degeneration, and impaired muscle
regeneration.
Calpains are
calcium-activated cysteine proteases that participate in various intracellular
signal transduction pathways mediated by Ca2+ [2], causing
disruption of the contractile tissue, mitochondrial swelling, sarcoplasmic
reticulum vacuolization, and sarcomeric alterations.
The
ubiquitin-proteasome pathway plays a key role in the turnover of muscle protein
and the pathway involves an enzymatic cascade starting with the ubiquitination
of muscle protein to be degraded by the 26S proteasome in a process that
unfolds the protein, releases ubiquitin, and
degrades the protein to small peptides and amino acids [3].
Caspases are a
family of cysteine proteases, representing central components of the apoptotic
machinery in several tissues [4].
Additionally, many other factors, including stress
oxidative damage and alteration in satellite cells activity may all contribute
to muscle wasting [5,6].
In designing therapies that can counteract muscle wasting it is
important to choose molecules able to maintain muscle mass, suppress muscle
loss and stimulate muscle regeneration. In this context, one of the potential
candidates is the insulin-like growth factor-1 (IGF-1), involved in several
anabolic process in skeletal muscle [7].
The molecular complexities of IGF-1 transcription
An impressive
body of knowledge has been accumulated since the IGF-1 locus was first
described, but surprisingly the potential diversity of roles played by
different IGF-1 isoforms has only recently been appreciated. As its name
implies, IGF-1 is similar to insulin in structure, with it shares a 50% amino
acid identity. However, unlike the insulin gene, the single-copy IGF-1 gene
locus encodes multiple proteins with variable amino- and carboxy-terminal amino
acid sequences (Figure 1). The amino acid sequence of the mature peptide differs
from that of insulin by retention of the C peptide, by a short extension of the
A chain to include a novel domain D, and by the presence of variable C-terminal
E peptides.
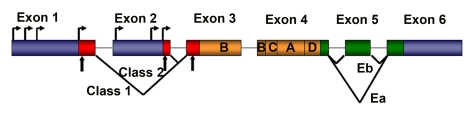
Figure 1. Schematic representation of rodent IGF-1 gene. The
rodent IGF-1 gene contains six exons (colored boxes), separated by five
introns (black lines). Both exons 1 and 2 contain multiple transcription
start sites (horizontal arrows). Translation initiation codons (AUG) are
located at exons 1, 2 and 3 (vertical arrows). Exons 1, 2 and 3 code for
the signal peptide of precursor IGF-1 (red boxes). Exons 5 and 6 each
encode distinct portions of the E-peptides (green boxes).
Although the IGF-1 gene is highly conserved in numerous
species, its relatively large size (>70 kb), and its complex transcriptional
and splicing pattern, have complicated its analysis.
The rodent IGF-1 gene contains six exons, separated by
five introns (Figure 1) [8]. Exons 1 and 2 encode distinct 5′UTRs, as well as
different parts of the signal peptide, and are therefore termed leader exons.
Exon 3 encodes 27 amino acids that are part of the signal peptide and common to
all isoforms, as well as part of the mature IGF-1 peptide.
Exon 4 encodes the rest of the mature
peptide and 16 amino acids of the amino-terminal region of the E-peptide, which
is also common to all IGF-1 mRNAs. Exons 5 and 6 encode two distinct
carboxy-terminal E-peptides and the 3′UTR.
Although IGF-1 transcripts are
not exclusively tissue-restricted, those that initiate at Exon 2 predominate in
the liver, are highly growth hormone responsive and as such are major endocrine
effectors of GH [9]. By contrast, transcripts initiating at Exon 1 are widely
expressed in all tissues, and are less effected by circulating growth hormone
levels, presumably performing autocrine or paracrine functions. The alternate
splicing at the 5' ends of these two IGF-1 transcripts generates different
signal peptides, which purportedly affects the precise N-terminal pro-peptide
cleavage site [9]. The function of the proteins encoded by these different
transcripts is widely debated but a cohesive picture has yet to emerge [10].
Elucidation of isoform function is also complicated by
alternate splicing at the 3' end of IGF-1 transcripts. This produces
variability in the length and amino acid sequence of the E peptide, and in the
length and base sequence of the 3'UTR. To date, two different splice patterns
have been documented in rodents (Figure 1). Each generates E peptides with a
common N-terminal 16 aa sequence, and alternate C-terminal sequences [8,11].
If Exon 4 splices to Exon 6 (the predominant pattern), the length of the 3'UTR
is highly variable, but in all cases the Ea peptide is generated with 19
additional amino acids. If Exon 4 splices to Exon 5 and 6, a variant known as
Eb is encoded, which is frameshifted relative to Exon 6 and therefore a
different 25 aa sequence is added to the common 16 aa encoded by Exon 4.
Although E peptide choice appears to be independent of
promoter use, Eb-containing transcripts are more abundant in liver, whereas
Ea-containing transcripts are widespread in extra-hepatic tissues. In addition,
the analysis of the amino acid structure of both E-peptides has revealed the
presence of two N-linked glycosylation sites only in the Ea peptide, but not in
the Eb peptide, suggesting that this post-translational modification is
involved in a biological action of the IGF-1 isoform [11].
The IGF-1Eb isoform is also up-regulated in muscles
subjected to stretch and has been named mechano growth factor (MGF) [12]. The
determination of E peptide function and fate awaits the availability of
epitope-specific antibodies, since it is unclear when or whether E peptides are
cleaved from the mature IGF-1 protein. Notably, E peptide splicing patterns are
different in the human gene [8], an anomaly that will need to be considered in
the future when translating the results of animal research into clinical
applications.
The importance of IGF-1 isoforms
Analyses
of transgenic mice expressing different IGF-1 isoforms have provided insight
into the role of IGF-1 signaling in the physiology of striated muscle [7]. The
fact that IGF-1 can act either as a circulating hormone or as a local growth
factor has confounded previous analyses of animal models in which transgenic
IGF-1 synthesized in extra-hepatic tissues was released into the circulation.
Thus, over-expression of one IGF-1 isoform in the heart prevented activation of
cell death in the viable myocardium after infarction, limiting ventricular
dilation, myocardial loading, cardiac hypertrophy, and diabetic cardiomyopathy,
supporting the notion that constitutive over-expression of IGF-1 in cardiomyocytes protects them from apoptosis and hypertrophy in the normal and pathological heart
[13,14]. However, in another study, over-expression of a different
IGF-1-transgene in the heart induced physiological cardiac hypertrophy that
progressed to maladaptive hypertrophy [15]. The discrepancies in these phenotypes
underscore the normal physiological difference between IGF-1 isoform function.
In addition, substantial evidence supports the involvement of IGF-1 in
mitogenesis and neoplastic transformation [16], suggesting that this signaling
pathway plays an important role in the process of tumor promotion. The
neoplastic potential of at least certain IGF-1 isoforms is an obvious concern
to be taken into account when designing IGF-therapeutic strategies for human
pathologies, where the specific role of each IGF-1 isoform must be viewed in
the appropriate tissue context.
Thus, restricting the action of
supplemental IGF-1 to the tissue of origin by use of a local IGF-1 isoform will
allow the assessment of its autocrine/paracrine role in skeletal muscle
throughout the life-span of the animal, exclusive of possible endocrine effects
on other tissues.
The effects of local isoform of
IGF-1 on muscle homeostasis
mIGF-1 and muscle aging
The prolongation of skeletal muscle strength in aging
and neuromuscular disease has been the objective of numerous studies employing
a variety of approaches.
IGF-1, involved in muscle growth and hypertrophy,
decline during postnatal life, raising the prospect that this decline
contributes to the progress of muscle atrophy in senescence, and limits the
ability of skeletal muscle tissue to effect repair or to regenerate.
To test this possibility we generated a transgenic mouse
in which the local isoform of IGF-1 (mIGF-1) is driven by MLC promoter
(MLC/mIGF-1) [17]. The MLC regulatory elements included in this construct
activate linked gene expression as early as E9.5 days in embryonic mouse
development, and expression continues to be high in the fastest Type IIb
fibers. Transgenic animals exhibits marked skeletal muscle hypertrophy with no
undesirable side effects such as tumor formation.
The increased muscle mass in
mIGF-1 transgenic mice was associated with augmented force generation compared
to age-matched wild type littermates [17]. Examination of two year-old animals
revealed that whereas wild type mice underwent characteristic muscle atrophy,
expression of the mIGF-1 transgene was protective against normal loss of muscle
mass during senescence [17]. Over-expression of the mIGF-1 transgene also
preserved the regenerative capacity of senescent muscle tissues stimulating
both the activity of satellite cells and the recruitment of circulating stem
cells [17,18] (Figure 2). We demonstrated that upon muscle injury, stem cells
expressing c-Kit, Sca-1, and CD45 antigens increased locally and the percentage
of the recruited cells were conspicuously enhanced by mIGF-1 expression [18].
More recently, we demonstrated that local expression of mIGF-1 accelerates the
regenerative process of injured skeletal muscle, negatively modulating the
inflammatory response [19]. These data indicate that mIGF-1 promote a
qualitative environment, guaranteeing a more efficient muscle regeneration
process. Thus mIGF-1 can overcome the normal inability of skeletal muscle to
sustain regeneration and repair and as such represents a potentially effective
gene therapeutic strategy to combat muscle wasting. This hypothesis was
supported by the demonstration that the action of mIGF-1 is not
dependent on life-long expression. Introduction of mIGF-1 somatically using an
Adeno-Associated-Viral (AAV) vector was sufficient to rejuvenate the leg
muscles of 27 month old mice, which exhibited the same mechanical force as legs
of younger mice, and did not develop the pathological characteristics of
senescent muscle [20].
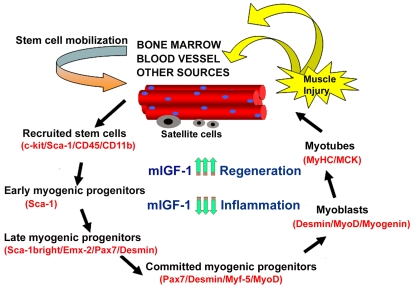
Figure 2. Model of stem cell-mediated muscle regeneration. (modified from ref. 18).
Muscle injury involves the activation of satellite cells and the
recruitment of circulating stem cells, which when penetrating the muscle
compartment receive myogenic signals and may contribute to muscle
regeneration and repair. This process is enhanced by mIGF-1 expression. By
modulating the inflammatory response and reducing fibrosis, supplemental
mIGF-1 creates a qualitatively different environment for sustaining more
efficient muscle regeneration and repair.
The importance of appropriate
IGF-1 isoform selection is further underscored by preliminary analysis of mouse
lines generated with a second IGF-1 transgene (cIGF-1), which differs from the
mIGF-1 only in a variant C-terminal peptide. These animals did not display
pronounced muscle hypertrophy but had increased levels of circulating IGF-1,
mild cardiac hypertrophy, an increased incidence of late onset neoplasia
(unpublished observation). Thus, the choice of isoform is critical to the
design of gene therapeutic strategies employing IGF-1.
mIGF-1 and muscular dystrophy
Muscular dystrophies are degenerative disorders
characterized by progressive weakness in specific muscle groups, persistent protein
degradation and alteration in the regenerative capacity of muscle satellite
cells [21]. Mutations in genes encoding proteins of the dystrophin-glycoprotein
complex (DGC) lead to alteration in muscle structure and cause muscular
dystrophy [21,22]. Without dystrophin, the DGC is unstable leading to an
increase in muscle damage. Different studies support the notion that loss of
the link between extracellular matrix and cytoskeleton represents the critical
parameter for the maintenance of the structural integrity of skeletal muscle [23].
A further complication that exacerbates muscular
dystrophy is the persistence of inflammation. In normal skeletal muscle, damage is followed by an inflammatory
response [24] involving multiple cell types that subsides after several days.
This transient inflammatory response is a normal homeostatic reaction to
myonecrosis and is necessary for efficient repair. However a persistent inflammatory response is observed in
dystrophic muscle, leading to an altered extracellular environment [25],
including an increased presence of inflammatory cells (e.g., macrophages) and
elevated levels of various inflammatory cytokines (e.g., TNF-alpha, TGF-beta).
Because it is clear that mIGF-1 can prevent aging-
related loss of muscle function, stimulates muscle regeneration and modulates
the inflammatory response in damaged muscle, it is possible that mIGF-1 can
prevent or diminish muscle loss associated with diseases.
To prove this hypothesis, we introduced mIGF-1 into the
mdx dystrophic animals (mdx/mIGF-1) [26]. By analyzing muscle morphology and
function in transgenic mdx/mIGF-1 mice we observed significant improvement in
muscle mass and strength, a decrease in myonecrosis, and a reduction in
fibrosis in aged diaphragms [26]. In particular, even though IGF-1 has been
shown to stimulate fibroblasts, there was a net decrease in fibrosis in
diaphragms of the mdx/mIGF-1 mice [26]. It may be that the efficient and rapid
repair of the mdx/mIGF-1 muscles prevents the establishment of an environment
into which the fibroblasts migrate. This is of particular relevance to the
human dystrophic condition where virtually all skeletal muscles succumb to
fibrosis.
Finally, signaling
pathways associated with muscle regeneration and protection against apoptosis
were significantly elevated [26]. These results suggest that a combination of
promoting muscle regenerative capacity and preventing muscle necrosis could be
an effective treatment for the secondary symptoms caused by the primary loss of
dystrophin.
In addition, another study demonstrated that
coinjection of the rAAV-microdystrophin and rAAV-mIGF-1 vectors resulted in
increased muscle mass and strength, reduced myofiber degeneration, and
increased protection against contraction-induced injury [27]. These results
suggest that a dual-gene combinatorial strategy could enhance the efficacy of
gene therapy of DMD and underscored the importance of rAAV vectors due to their
relative lack of immunologic and toxic side effect combined with their
potential for body-wide systemic gene delivery to muscle [27].
mIGF-1 and amyotrophic lateral sclerosis (ALS)
ALS is a progressive, lethal
neuromuscular disease associated with the degeneration of motor neurons,
leading to muscle atrophy and paralysis [28]. Although a significant proportion
of familial ALS results from a toxic gain-of-function associated with dominant
SOD1 mutations, the etiology of the disease and its specific cellular origins
have remained difficult to define.
Notably, restriction of SOD1
mutant expression selectively to post-natal motor neurons failed to produce
detectable sign of pathology or motor-neuron disease [29], suggesting that
other cell types may be involved in ALS-associated neurodegeneration. Indeed,
analysis of chimeras generated between wild type and SOD1 mutant mouse
embryonic cells revealed that wild type non neuronal cells in adult chimeric
animals extended the survival of SOD1 mutant motor neurons, suggesting that the
neurodegenerative action of mutant SOD1 may operate through a dominant
paracrine activity emanating from non neuronal cells [30].
Skeletal muscle is an untested
component in the motor neurodegenerative effects of SOD1 mutations. More
recently, we addressed this critical aspect of the pathogenesis of ALS,
demonstrating that skeletal muscle is a direct target of SOD1G93A-mediated
toxicity [31],
refocusing therapeutic strategies to attenuate motor neuronal degradation
towards skeletal muscle.
Adult muscle fibers are a source
of signals that influence neuron survival, axonal growth and maintenance of synaptic
connections. Among them IGF-1 has also been implicated in anabolism of nerve
tissue, promoting neuronal survival [7].
Recently, the potential
beneficial effect of human recombinant IGF-1 on ALS patients has been tested,
however the results were doubtful [32]. In particular, the subcutaneously
injection of IGF-1 did not show beneficial effects in ALS patients [32]. The
critical problem could be the failure to deliver the neurothophin effectively
to the target cells and tissue. Moreover, the IGF-1 system, as discussed above,
is complex, since multiple transcripts of the IGF-1 gene encode different
isoforms, which induce different cellular responses. This hypothesis was
supported by the evidences that either AAV-mIGF-1 mediated muscle delivery [33]
or localized
expression of co-inherited MLC/mIGF-1 transgene exclusively in the muscles of
SOD1G93A mouse [34,35] counteracts the symptoms of ALS and reduces
components of catabolism, activating satellite cell and markers of regeneration
[33-35]. The protective effects of muscle-restricted mIGF-1 against the
dominant action of mutant SOD1G93A stabilized also neuromuscular junctions and led to a
reduction in astrocytosis/inflammation in the spinal cord, enhancing motor
neuronal survival.
Work in the authors' laboratories has been supported by
Seventh Framework Programme-Myoage, Telethon, MDA, AIRC, AFM, MIUR and ASI.
The authors in this manuscript have no conflict of
interests to declare.