Introduction
Creatine kinase (CK) is an essential
enzyme found in tissues with periodic fluctuations in energetic requirements,
such as skeletal muscle, cardiac muscle and the brain [1]. CK catalyzes the
reversible transfer of the gamma phosphate from ATP to creatine forming
creatine phosphate (CrP) and ADP. The cycling of creatine and CrP play an
important homeostatic role as CK catalyzes the synthesis of ATP from CrP and
ADP when energy requirements are high, such as during exercise. During periods
of rest, creatine phosphate pools are replenished as CK catalyzes the reverse
reaction [1]. Within skeletal muscle cytosol, the majority of CK activity is
attributed to the homodimeric muscle
isozyme of CK (CKm); the brain isozyme, (CKb) is also found in muscle cytosol,
though at significantly lower concentrations [2].
Muscle
type CK has the unique property of binding with the M-line of sarcomere [3].
Its catalytic activity, which involves its function in muscle is elaborately
regulated. In its activated form acidification of the microenvironment
stimulates its binding with M-line proteins [4] where it supplies ATP coupled
with myofibrillar actin-activated Mg2+-ATPase [5,6]. In the
resting state it dissociates from the myofibril and catalyzes the formation of
phosphocreatine to reserve energy [4]. Recent studies have shown that a
negative regulation of CKm occurs through its oxidation O-CKm which is then
targeted for degradation via the ATP-ubiquitin-proteome system in muscle
cells. This oxidation occurs via the formation of an intrachain disulfide bond
between Cys74 and Cys146 [7]. Interestingly, circular
dichroism (CD) analysis, intrinsic fluorescence and ANS fluorescence have shown
that O-CK has decreased secondary structure, including increased hydrophobic
surface exposure. Functionally, the O-CKm showed a significant decrease in
enzyme activity and the loss of ability to interact with the M-line protein,
myomesin [7].
Reduction
of CKm activity may be a major contributor to the gradual loss of muscle
function associated with aging. Several lines of investigation have shown
age-related reductions in skeletal muscle oxidative capacity in rodents and
humans [8,9]. Additionally, recent
proteomic-liquid chromatography-tandem mass spectrometry (LC-MS/MS)
experiments have definitively shown that CKm is 3-nitrotyrosine (3-NT) modified
within aged skeletal muscle and a novel approach using the fluorescent probe 4,4-dianilino-1,1-binaphthyl-5,5-disulfonic acid (BisANS)
suggests that the three dimensional structure of CKm is altered during aging
[10-12]. Furthermore, in crude extracts prepared from human brains, reduced
activities for aged samples compared to young controls parallel the increases
of CKb carbonylation [13]. However, the consequences of oxidative modification
of CKm to its structure and function and its contribution to the age-related
decrease in skeletal muscle function is not understood.
Though a growing body of literature
suggests that CKm activity might be altered during aging, a detailed structure
and function analysis of oxidatively modifiedCKm
isolated from animals of different ages has not been performed. These
experiments are essential to demonstrate that the structure and function of
oxidatively modified CKm are alteredin aging
skeletal muscle. To address these issues we purified and characterized CKm from
the quadriceps of young, middle-aged and aged mice. Circular dichroism, limited
proteolysis, and enzyme kinetic analysis demonstrated reduced stability and
enzyme activity for CKm obtained from middle-aged and aged mice relative to
young mice. Interestingly, our fractionation of purified CKm revealed a
chromatographic shift of tyrosine nitrated CKm vs. unmodified as well as
carbonylated enzyme. Finally, as with the brain studies [13] the
age-associated reductions in function and stability correlated with levels of
protein nitration and carbonylation. In addition, the procedure of purification
of 3-NT modified CKm resulted in the identification of an apparent trimeric
form of CKm, suggesting that 3-NT modifycation may lead to the oligomerization
and aggregation of this enzyme.
Our
results indicate that there is an age-associated increase in nitrative modification
and carbonylation to CKm, that these modifications correlate with significant
decreases in activity and that these modifications may induce structural
changes that promote oligomerization and aggregation. Overall, these data
support a model of skeletal muscle aging where reduction of CKm activity may be
due to oxidative modifications that may contribute to diminished muscle
function.
Results
Purification
of CKm from young, middle-aged, and aged mouse quadriceps
To
directly examine age-related changes in protein structure and function, CKm was
purified from the quadriceps of young, middle-aged, and aged mice (Figure 1A).
An affinity Blue Sepharose chromatography procedure using a sequential
isocratic pH elution followed by a gradient pH elution, resulted in CKm that
was greater than 85% pure (Figure 1A, lanes 1-3). These samples, from all three
age groups, were used in the analysis of CKm enzyme activity and for
immunoblotting experiments that compared relative levels of 3-NT and carbonylation
modification. CKm protein that was greater than 95% pure (Figure 1A, lanes 4-6)
was obtained from all three age groups using an additional hydroxyapatite chromatography step (see Figure 5);
these samples were used in CD and limited proteolytic digestion studies.
Details of CKm purification are given in Methods.
Skeletal
muscle creatine kinase is 3-nitrotyrosine modified during aging
Western
blot analysis using a monoclonal anti-3-nitrotyrosine antibody was used to
compare levels of 3-NT modification within whole quadriceps extracts obtained
from six young (3-6 months), six middle-aged (12-14 months), and five aged
(20-24 months) mice (Figure 2A). A band with an apparent molecular weight of ~
45 kD exhibited a progressively increasing level ofnitration
from middle aged to aged samples compared to young samples. Densitometric
analysis of the 45 kDa bands shows significantly greater levels of 3-NT
immunoreactivitywithin the aged samples (Figure 2A; p<0.05). The anti-nitrotyrosine blot was re-probed with an anti-CKm
antibody (Figure 2B). CKm blots were superimposable with the 3-NT modified 45
kDa band, indicating that the modified
protein is CKm. The protein identity
was confirmed by 2-D gel electro-phoresis and mass spectrometry. Kanski et al.
[10,11] have shown that CKm is 3-NT modified within aged rat skeletal and
cardiac muscle. Our studies demonstrate higher levels of 3-NT modifications to
CKm in aged mouse muscle, relative to young and middle-aged samples (Figure 2C).
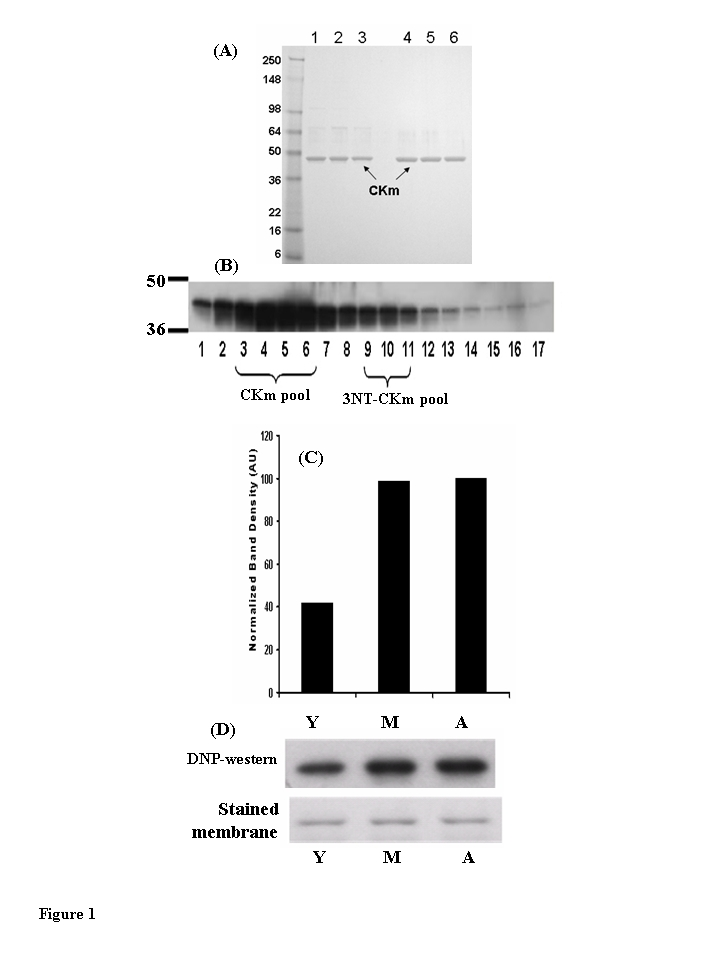
Figure 1. Muscleeeeee creatine kinase (CKm) purified from young (3-6 months), middle aged (12-14 months) and aged (20-24 months) mouse quadriceps. (A) Peak Blue Sepharose fractions of
purified CKm (1 μg) from young (lane 1), middle aged (lane 2) and aged (lane 3) mouse
muscle were resolved on a denaturing SDS gel and stained with Coomassie
Blue. These fractions are ~85% purified CKm and were used for enzyme
kinetic analyses. CKm within side fractions from the Blue Sepharose pH
gradient elution were pooled and purified to a single band using
hydroxyapatite chromatography. Lanes 4-6 represent samples from young,
middle-aged and aged mice, respectively. (B) Western blot analysis
of CKm levels in fractions eluted from a Blue Sepharose column, using an anti-creatine kinase type M antibody. (C) Densitometric
analysis demonstrates the increase in carbonylated CKm in quadriceps of
young, middle aged and aged mice. The peak level of carbonylation occurs
in muscle of middle aged mice. (D) immunoblot analysis of
carbonylated CKm in Blue Sepharose fractions [3-6], The carbonylated CKm
was identified by anti-DNP antibody.
Purified
CKm is carbonylated in an age-dependent manner
The
accumulation of oxidized proteins is a characteristic of the aged phenotype and
these age-related oxidative modifications have been shown to affect the
biological activity of the modified proteins [14-17]. Western blot analysis,
using 2,4-dinitrophenyl-hydrazine (DNP) to compare levels of carbonylation
within the Blue Sepharose purified CKm samples revealed that the carbonylated
protein is within protein fractions 3-6 (Figure 1B; 18, 19). Densitometric
analysis of the blot shows that purified CKm protein from middle-aged and aged
mice contains approxi-mately 2.5 times more carbonyl modifications relative to
CKm purified from young mice (Figure 1C,D). The observed higher levels of
carbonylation in the middle-aged and aged samples correlate with the observed
age-associated decreases in CKm activity and stability (see Figure 7).
Purified
CKm and glycogen phosphorylase are nitrated in an age-dependent manner
CKm
within solubilized muscle extract is 3-NT modified (Figure 2). Western blot analysis was used to
probe for 3-NT modification within the Blue Sepharose purified CKm samples
(Figure 3A). Analysis of fractions 3-6 (Figure 3B) revealed a single band
with an approximate molecular weight of 100 kDa that is 3-NT modified in an
age-dependent manner. The highest levels of modification of this protein
occurs in the aged muscle. In a
parallel experiment, the band identified as a 3-NT modified protein was excised
from a Coomassie Blue stained SDS-containing acrylamide gel. MALDI-TOF/TOF
mass spectrometry analysis identified the modified protein as glycogen
phosphorylase (Figure 3B; Table 1). The 3-NT modification of this protein
within aged rodent skeletal muscle has been previously observed [11]. The
anti-3-NT immunoblot of Fractions 3-6 was reprobed with an anti-CKm antibody
(Figure 3B, lower panel); these data show that the same Blue Sepharose purified
CKm samples (fractions 3-6) are not 3-NT modified. On the other hand,
immunoblot analysis revealed that fractions 9-12 contain 3-NT modified CKm
(Figure 3A). These data suggest that the nitrotyrosine modification may cause
a significant shift in the elution properties of 3-NT modified CKm. The data
also raise the question of whether the nitrated CKm is also carbonylated. To
address this we placed the pooled Blue Sepharose fractions 9-11 on a reverse
phase column to determine the levels of nitration vs. carbonylation. The
anti-nitrotyrosine and anti-DNP immunoblots in Figure 3C and 3D respectively
clearly show
a strong response to the anti-nitrotyrosine whereas the response to anti-DNP is
negligible. These results suggest that the 3-NT modified CKm may not be modified
by carbo-nylation and that the elution of the nitrated form is shifted away
from the elution of the carbonylated CKm.
Table 1. MALDI TOF/TOF Identification of 3-nitrotyrosine modified proteins.
|
Protein
| |
Mol. Mass
(theor./expt.)
|
Peptide
Count
|
Mascot Protein
Scorea
|
Expectation Valueb
|
|
Muscle glycogen phosphorylase [mus
musculus]
|
6755256
|
97.2/100
|
42
|
589
|
1.2 x 10-54 |
|
Creatine kinase, muscle [mus musculus]
|
6671762
|
43.0/130
|
16
|
597
|
1.9 x 10-55 |
The
level of alpha helical content is reduced in CKm from middle-aged and aged mice
The secondary structure content of CKm purified by Blue
Sepharose and hydroxyapatite fractionation from young, middle-aged, and aged
mice was compared using far-UV CD spectrometry (Figure 4A; Table 2). CKm CD
Spectra obtained for all three age groups show significant alpha helical
character, however less ellipticity was observed in the middle-aged and aged
samples relative to young CKm. The secondary structure composition of CKm
purified from the differently aged mice was estimated by interpreting CD spectra
using the SELCON3 program via the DICHROWEB server (www.cryst.bbk.ac.uk/cdweb/html/home.html) (Table 2); [20-22]. For example, sheet, turns and unordered protein
segments. FαR (young) shows that the regular α-helix content is 25%. Thus, in the aged sample there is
a decrease in α-helical structure to 0.193 (19.3%) as indicated by FαR (aged). Similarly the FβR and FβD which depict the percent regular
β-sheet indicates, as expected, an
age-associated increase in the distorted β-sheet. Furthermore, there is an increase in unordered
structure at the expense of the α-helix as indicated by the change in Fu from
0.276 (young) to 0.298) (aged). Relative to the young CKm sample, the
secondary structural composition of CKm from middle-aged and aged mice was
characterized by decreased alpha helical content with concomitant increases in
beta pleated sheet, turns and unordered protein segments.
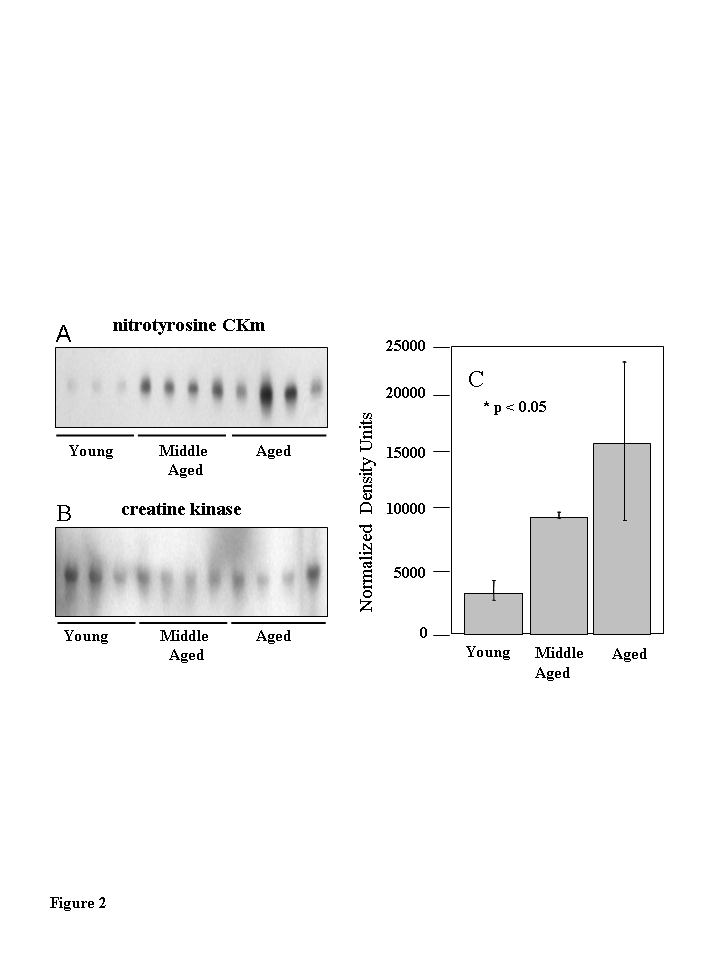
Figure 2. Muscle creatine kinase (CKm) is differentially 3-nitrotyrosine modified during aging.
Quadriceps muscle extracts (30 ?g) from six young
(3-6 months), six middle-aged (12-14 months) and five aged
(20-24 months) old mice were resolved by SDS PAGE and transferred
to a PVDF membrane. (A) Immunoblots probed with a
monoclonal anti-3-nitrotyrosine antibody reveal a nitrated 45 kDa
protein in middle aged and aged samples. (B) The immunoblots
in (A) were reprobed with an anti-creatine kinase type M
antibody which identifies the levels of CKm in the samples applied
to the gels in (A). (C) Densitometric analysis
demonstrates the progressive increase in nitration in the
aging muscle samples. The highest level of nitration is seen
in the aged muscle samples relative to the young samples.
Error bars depicted on the figure represent calculated standard
errors of mean. p < 0.05 in both cases.
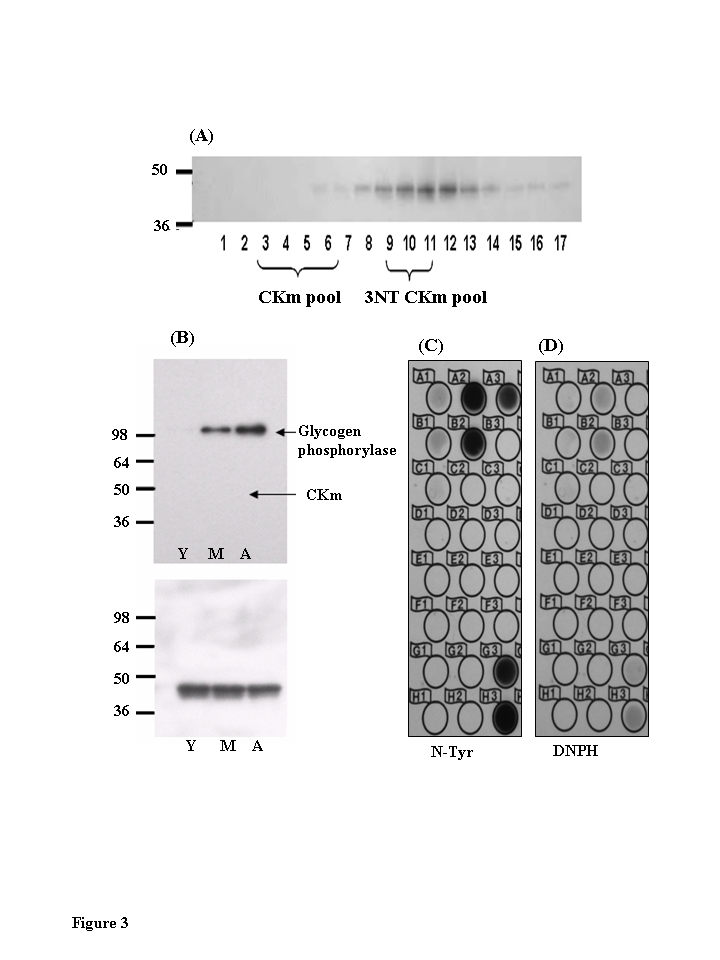
Figure 3. Chromatographic elution properties of 3-nitrotyrosine modified muscle creatine kinase are altered.
(A)
Immunoblots of Blue Sepharose fractions of CKm show that fractions 9-12 are
tyrosine nitrated. CKm (1 μg, > 85 % pure) was resolved
on a denaturing SDS polyacrylamide gel, transferred to a PVDF membrane, and
analyzed by immunoblot using anti-3NT-antibody. (B) Immunoblots of
Blue Sepharose fractions 3-7 shows that 3-NT CKm is not detected in these
fractions. Blots probed with an anti-3-nitrotyrosine antibody (top panel)
detected nitrated glycogen phosphorylase in middle aged and aged muscle.
However, the purified CKm in this fraction is not nitrated. Blots were
reprobed with an anti-CK antibody (3B, lower panel). (C) Fractions
9-11 were further fractionated by reverse phase chromatography. These
fractions were spotted on to PVDF membranes and analyzed for nitrated CKm
using anti-nitrotyrosine antibody, and carbonylated CKm using anti-DNPH
antibody (D).
Table 2. Predicted secondary structure content for CKm purified from differently agedmice.
|
FαR |
FαD |
FαTotal |
FβR |
FβD |
FβTotal |
FT |
FU |
Total
|
NRMSD
|
|
Young
|
0.250
|
0.159
|
0.409
|
0.090
|
0.068
|
0.158
|
0.162
|
0.274
|
1.002
|
0.274
|
|
Middle-Aged
|
0.236
|
0.158
|
0.394
|
0.096
|
0.071
|
0.167
|
0.172
|
0.274
|
1.011
|
0.308
|
|
Aged
|
0.193
|
0.144
|
0.337
|
0.095
|
0.073
|
0.168
|
0.187
|
0.298
|
0.990
|
0.285
|
CKm from
middle-aged and aged mice is charac-terized by increased susceptibility to
proteolysis
The stability of CKm purified from differently aged
mouse quadriceps was analyzed by limited chymotrypsin digestion and SDS PAGE
(Figure 4B). CKm purified from middle-aged and aged mice is digested
approximately 3.5 times faster than CKm purified from young mice. The rates of
proteolysis are given on Figure 4B. These results are consistent with several
studies that have documented the proteolytic resistance of CKm [23].
Furthermore, Zhao et al. [7] showed that structural alteration of oxidized CK
(O-CK) renders the enzyme more susceptible to proteolysis by both trypsin and
proteinase K. Regardless of age group, no proteolytic fragments were observed
during the limited chymotrypsin digestion whereas the amount of full length
protein decreases throughout the time course and the rates of proteolysis vary
among age groups. Taken with the results from our CD experiments (Figure 4A)
these data suggest that CKm purified from middle-aged and aged mice is less
resistant to proteolysis suggesting that it is structurally less stable than
CKm purified from young mice.
Two proteins with molecular weights of 130 kDa and 88
kDa are immunoreactive with an anti-CKm antibody
Fractions 3-6 of the Blue Sepharose
affinity column-linear pH gradient, shown by immunoblot to contain the unmodified CKm were fractionated by HA column chro- matography
(Figure 5A). These HA fractionations
from all three age groups were analyzed for 3-NT modified proteins by resolving
even numbered chromatography fractions on large format (26 wells) SDS polyacrylamide
gels, transferring the resolved proteins to PVDF membranes, and probing the
membranes with a monoclonal anti-3-nitrotyrosine antibody. Though 3-NT modified
proteins were detected, none of the bands had molecular weights consistent with
creatine kinase (data not shown). The anti-NT blots were stripped and
reprobed with an anti-CKm antibody. Short exposure of the blots allowed CKm to
be visualized within HA chromatography fractions 24-34 (Figure 5B, upper blot).
To allow detection of less abundant CKm species the blots were exposed for 5
minutes. At these longer exposure times two protein bands with apparent
molecular weights of 88 and 130 kDa were detected in HA fractions 26-32 (Figure 5B, lower blot). Throughout the rest of this manuscript, these proteins are
referred to as CKm 88 and CKm 130, respectively.
CKm
130 is 3-NT modified in an age-dependent manner
Anti-3NT
Western blots of Blue Sepharose chromatography fractions revealed several 3-NT
modified proteins with molecular weights ranging from 85-150 kDa (data not
shown). To examine the possibility that CKm 88 and CKm 130 kDa are present in
these high molecular weight 3-NT modified proteins, Blue Sepharose fractions
26-32 for each age group were pooled and further fractionated on a
mono-Q-Sepharose anion exchange column (Figure 6A). Entire chromatography fractions from all three age groups were scanned
for the 88 kDa and 130 kDa CKm proteins by resolving the even numbered
fractions on large format (26 wells) SDS polyacrylamide gels, transferring the
resolved proteins to PVDF membranes, and probing the membranes with an anti-CKm
antibody. CKm with a molecular weight of 45 kDa (the predicted molecular weight
of CKm) elutes from the column with a robust peak between 10 and 20 minutes
(Figure 6A). The 130 kDa CKm species was observed in fractions 26 and 28; the
88 kDa CKm species was not detected. Q-Sepharose fractions 26-28 were pooled
from all three groups, concentrated by centrifugal filtration, and analyzed for
3- NT modification by SDS PAGE/Western blotting (Figure 6B,
upper blot). Two proteins with apparent molecular weights of 130 and 100 KDa
are 3-NT modified in an age-dependent manner (Figure 6B). In a parallel experiment,
the bands that corresponded to the modified proteins were excised from a
Coomassie Blue stained SDS-PAGE acrylamide gel. MALDI-TOF/TOF mass spectrometry
analysis identified the modified proteins as CKm and glycogen phosphorylase
(Table 1). Densitometric analysis of 3-NT modified CKm 130 reveals a progressive
increase in 3-NT modification with age (Figure 6C).
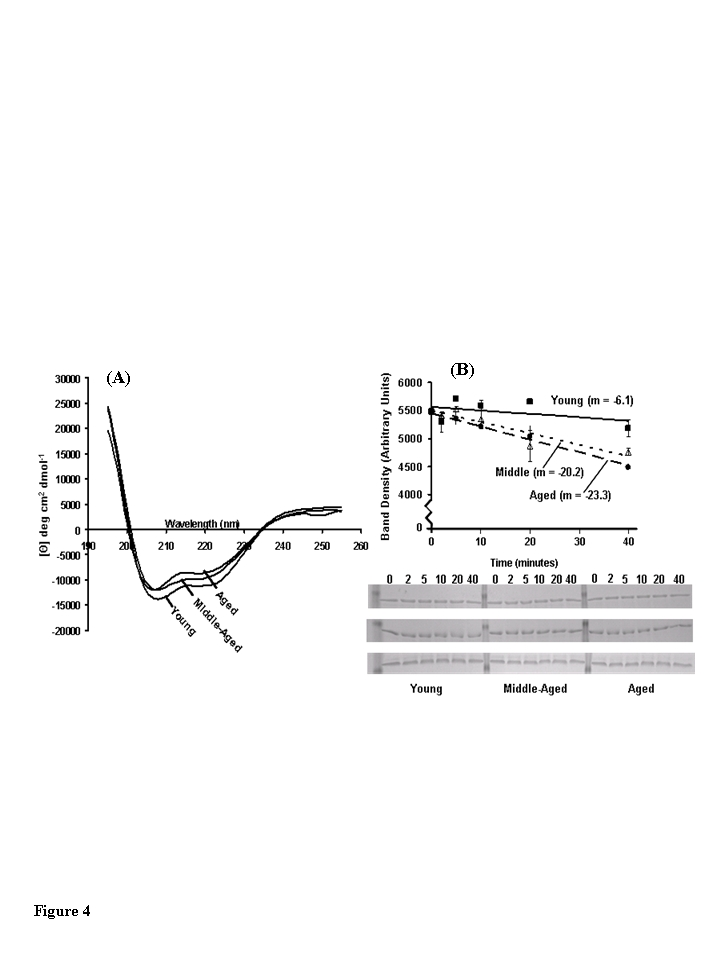
Figure 4. Structural analysis of muscle creatine kinase (CKm) purified from differently aged mouse quadriceps. (A) Far-UV CD spectra of CKm purified from
young, middle-aged and aged mice. All CD experiments were conducted at 25
˚C in 5 mM sodium phosphate buffer (pH 7.2) using hydroxyapatite
purified CKm (>95% pure) at a protein concentration equal to 10 μM. (B)
Limited chymotrypsin digestion of CKm purified from young, middle-aged and
aged mice. Chymotrypsin was added to each sample and the reaction was
quenched at 2, 5, 10, 20 and 40 minutes. Undigested CKm was used as the 0
minute time point. Time courses of proteolysis were constructed by
resolving time points by SDS PAGE and staining gels with Coomassie blue.
The abundance of undigested CKm was quantitated at each time point by
performing densitometry on the 45 kDa band that corresponded to undigested
CKm. Proteolysis experiments were repeated in triplicate and average
density values were plotted versus reaction time. Linear regression
analysis was used to plot best fit lines through the data and the slopes of
these curves are given on the figure. Error bars represented standard error
of mean calculated for each plotted value.
Steady state kinetic analysis of CKm shows
age-associated decreases in the kinetic parameter Vmax
The substrate dependence of CKm activity was
measured using a linked spectrophotometric assay system in the direction of
creatine and ATP production [24,25]. Plots of initial reaction velocities vs.
creatine phosphate and ADP concentrations exhibit hyperbolic Michaelis-Menton
kinetics for CKm purified from all three age groups (Figure 7). The parameters
KM and Vmaxwere extracted from Eadie-Hofstee plots (data not shown) of individual
kinetic experiments (Table 3) [26,27]. Michaelis-Menton constant (KM)
values measured for creatine phosphate and ADP agree well with previously
published values and do not vary between age groups [25]. However, the Vmaxdecreases with age. The Vmax of middle-aged CKm is
approximately 12.5% (13% decrease measured by creatine phosphate dependence and
12 % decrease measured by ADP dependence) less than the Vmax measured
for the young enzyme (creatine phosphate dependence, p<0.005; ADP dependence,
p<0.05). Interestingly, there were no statistically significant differences
between kinetic parameters measured for middle-aged and aged CKm.
Discussion
The decreased energy capacity of aging
skeletal muscle, coupled with recent proteomic experiments, implicates
diminished CKm function as a potential causative factor for age-related
sarcopenia [8-12]. Our structural studies using CD spectrometry and
limited chymotrypsin digestion are consistent with our hypothesis that the
structural alteration due to oxidative modification may be a factor that
affects CKm enzyme function. Although the CD spectra of young, middle-aged and
aged CKm show significant characteristics of alpha helical structure in all
three protein preparations [21,22,28], increases in beta-pleated sheet,
turns, and unordered segments that occur with age suggest structural changes
that are consistent with the observed decreases in enzyme activity.
Furthermore, unfolding transitions associated with increases in beta-sheet and
disordered segment content are associated with an increased tendency to
aggregate, suggesting that middle-aged and aged CKm may be more prone to
aggregation than young CKm [29,30]. Thus, we attribute the age-specific
aggregation of CKm to the unfolding indicative of the increased beta sheet
formation. Also, the limited chymotrypsin digestion of middle-aged and aged
CKm proceeded approximately 3.5 times faster than the digestion of the young.
This is consistent with the observation that structural alteration of O-CK
renders the enzyme more susceptible to proteolysis by both trypsin and
proteinase K [7]. In light of the CD data, and the fact that the rate of
limited chymotrypsin digestion increases 3.5-fold in modified CKm, we propose that
the different rates of proteolysis result from age-related decreases in native
state CKm stability.
The current study which directly examined the biochemical
properties of CKm from mouse quadriceps revealed statistically significant
decreases in Vmax, for middle-aged and aged CKm, relative to young
CKm and no change in the kinetic parameter KM. The biochemical
consequences of the age-dependent decreases in Vmax, reflect slower
rates of enzyme turnover in the middle-aged and aged muscle. Interestingly, no
statistically significant differences were observed between the middle-aged and
aged Vmax which suggests that even though the modifications continue
to increase with age, those modifications that affect enzyme function may have
occurred at middle age.
An underlying tenet of the Free Radical
Theory of Aging is that age-related increases in ROS production and the
concomitant increases in protein oxidation are gradual over a lifespan [31].
Moreover, proteins oxidized in vitro or in vivo often show decreased
activity and stability, though there is significant variability in the extent
of these changes [7,14-17]. Our data support the current interpretation of
the Free Radical Theory of Aging as increased levels of nitration and
carbonylation correlated with changes in function and structural features.
However, the middle-aged and aged CKm contained similar elevated levels of carbonyls, i.e.,
approximately 2.5 times more than young CKm, data which support a model of
muscle aging where the majority of this specific age-related modification un-expectedly
occurs by middle-age. Given the complexity of in vivo oxidative stress
and variability in the intrinsic ROS resistance of different proteins, it is
not surprising that some proteins may show differential levels of sensitivity
to oxidative modification at middle age.
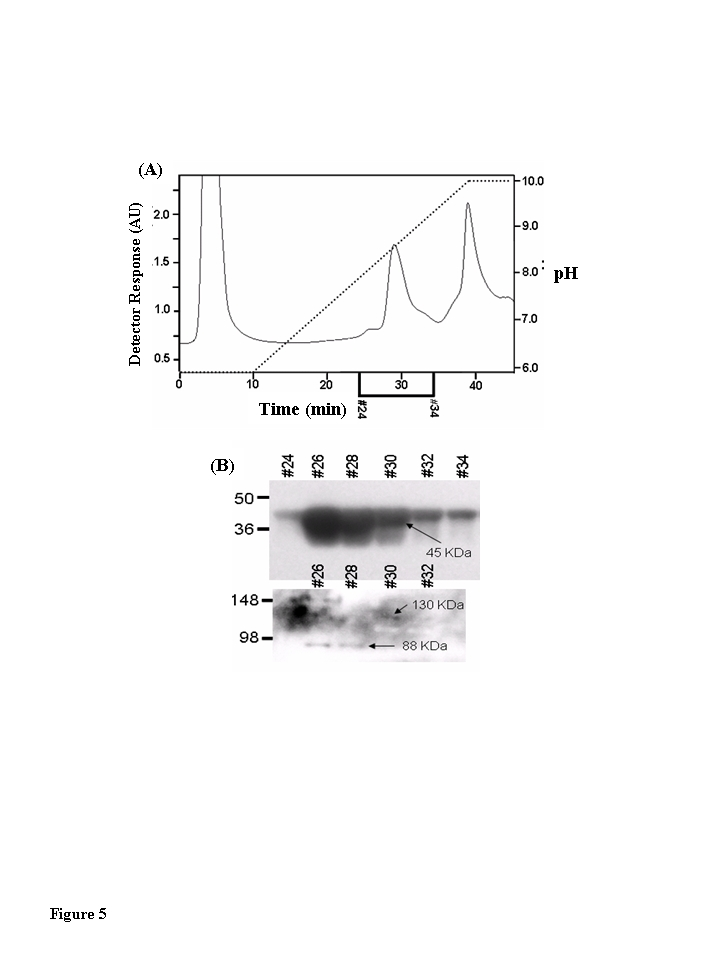
Figure 5. Muscle creatine kinase exists as 130 kDa and 88 kDa species in vivo. Blue Sepharose
affinity column fractions of cytosolic protein from quadriceps of young,
middle-aged, and aged mice that contained CKm were identified by Western
blotting. The samples were further fractionated by hydroxyapatite (HA)
chromatography. (A) HA fractionation of Blue Sepharose cytosolic
quadriceps fractions from Blue Sepharose fractionation. After application
of samples to the HA columns, the loaded columns were washed with 10 ml of
50 mM sodium phosphate pH 5.8 and developed with a 30 ml linear pH
gradient (pH 5.8 to pH 10.0). Flow rate equaled 1 ml/min throughout
purification and fractions were collected at a rate of one fraction per
minute. The chromatograph in this figure represents fractionation of the
middle-aged protein sample. Fractionation of the young and aged samples
yielded similar chromatographs. (B) Even numbered HA fractions
(26-34, 20 μl) were resolved
under denaturing conditions by SDS PAGE and transferred to a PVDF membrane.
Blots were probed with an antibody specific for CKm. A short exposure (15
second expo-sure, top blot) reveals that CKm (45 kDa) is abundant in
fractions 26-34. A longer exposure (5 minutes, bottom blot) reveals
additional CKm immunoreactive bands with higher molecular weights. A
species with an apparent molecular weight of 88 kDa is observed in
fractions 26 and 28, and a protein with an apparent molecular weight of 130
kDa is observed in fractions 30 and 32. The same high molecular weight CKm
proteins were also observed after Blue Sepharose fractionation of young and
aged mouse quadriceps samples.
Though the observed changes in structure and function
correlated with nitration and carbonyl levels, other covalent oxidative
modifications that result in altered structure and function support our
studies. Interestingly, the initial loss of GAPDH activity due to oxidative
nitrative stress has been shown to occur prior to the detection of its
nitration [32,33]. It has been proposed that this maybe due to oxidation of
cysteines of the GAPDH active site. It is possible therefore, that cysteine
oxidation may be a factor in the loss of CKm activity in middle aged muscle.
Oxidation of Cys74 and Cys146 which forms the intrachain
disulfide bond in oxidized CKm (O-CKm) causes dramatic structural changes that
affect the dimerization interphase and results in decreased catalytic activity,
structural instability, failure to interact with the M-line protein myomesin,
and ubiquitination [7]. The latter targets O-CKm for ATP-ubiquitin proteo-some
degradation and suggests that the generation of O-CKm is a negative regulatory
mechanism that may play a role in CKm turnover. Furthermore, Cys283 of
the active site is essential for catalysis and is a plausible site of oxidative
modification during aging [34]. In the O-CKm model, the orientation of Cys283
is altered which may be an additional cause for decreased catalytic activity.
These PTMs, strongly suggest that the structural alterations caused by
nitration and/or carbonylation that we have identified may be the cause for
loss of function in the aged muscle.
Table 3. Creatine kinase kinetic parameters.
| Creatine
Phosphate Dependence | ADP
Dependence |
|
KM
(mM)
|
Vmax
(sp. Activity)
|
KM
(mM)
|
Vmax
(sp. Activity)
|
|
CKyoung |
2.6 +/-
0.1
|
117 +/-
1.6**
|
66.7
+/- 11
|
98 +/-
2.2 *
|
|
CKmiddle |
2.3 +/-
0.2
|
102 +/-
2.9**
|
70.8
+/- 8.6
|
86 +/-
3.5 *
|
|
CKaged |
2.8 +/-
0.2
|
103 +/-
2.7
|
70.7
+/- 8.2
|
81 +/-
3.7
|
We and others have shown that CKm is 3-NT modified
within urea and detergent solubilized muscle extracts [10,11]. Our Blue Sepharose
fractionation confirmed the presence of 3-NT modified form of CKm under native
conditions, but interestingly it showed for the first time that this
modification altered the chromatographic properties of nitrated CKm as
indicated by the shift in its elution. While protein nitration is well
documented as a marker of oxidative stress it is also recognized that tyrosine
nitration affects both structure and function of the modified protein.
Nitrotyrosine shifts the pKa of the targeted region of the tyrosine ring
structure by approximately 3 pH units [35], and introduces steric and
electrostatic alterations in protein structure [36]. These altered
characteristics may explain the shift in elution of nitrated CKm in the Blue
Sepharose fractionation. Furthermore, our results also indicate that the
nitrated CKm fractions show very low levels of carbonylation suggesting that
the chromatographic shift may be due to structural changes caused by the
nitration. Formation of the age-specific, CKm immunoreactive 130 kDa protein
suggests that oxidative modification may cause structural changes that lead to
aggregation. The observed molecular weight of the protein and the fact that
mass spectrometry analysis did not produce significant search scores for other
proteins, suggest that this is an SDS-stable, trimeric form of CKm. We also
observed an 88 kDa protein by Western blot analysis, consistent with the
formation of an SDS-stable dimeric form. Perhaps the most likely structural
explanation for these species is a covalent cross-linking of two and three CKm
subunits, respectively, although there are reports of ROS-induced noncovalent
oligomers that are resistant to SDS denaturation [37]. To our knowledge this is
the first report of these age- CKm 88 and CKm 130 species.
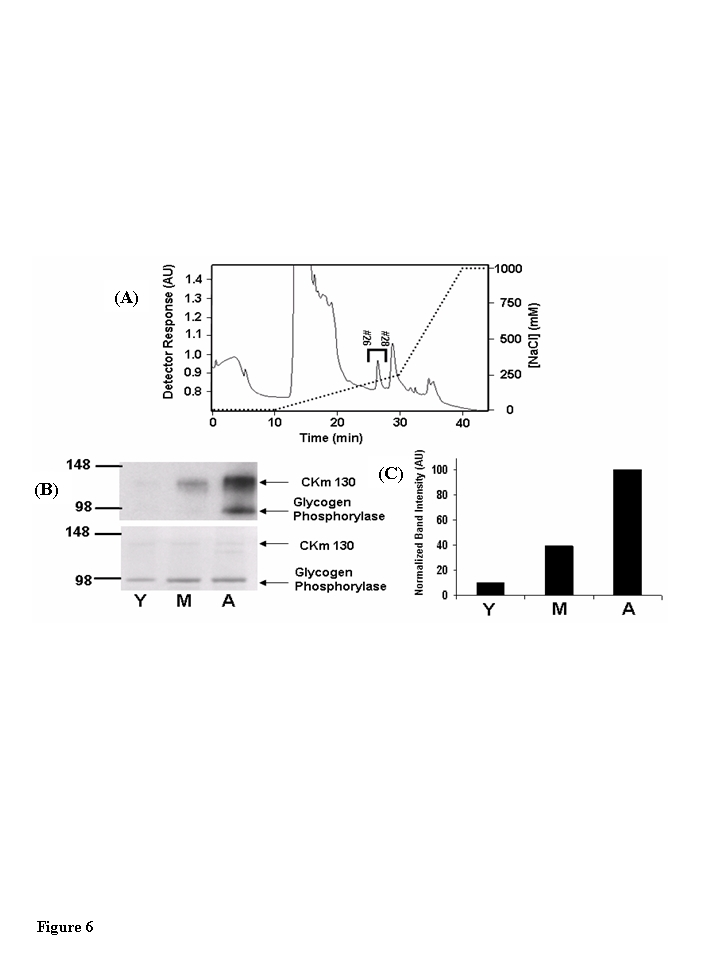
Figure 6. The 130 kDa CKm species is 3-nitrotyrosine modified in an age-dependent fashion. (A) Blue
Sepharose protein fractions #26-#32 (see Figure 5) that contained anti-CKm
immunoreactive proteins with apparent masses of 88 (CKm 88) and 130 (CKm
130) kDa were pooled from young, middle-aged and aged mouse samples and individually
loaded onto a 1 ml mono-Q-Sepharose column (Biorad Laboratories). After
application, samples were washed with 10 ml of 25 mM Tris pH 8.0, developed
with a shallow 20 ml linear NaCl gradient (0-250 mM NaCl in 25 mM Tris pH
8.0) followed by a steep 10 ml NaCl gradient (250 mM - 1M NaCl in 25 mM
Tris pH 8.0). Flow rate equaled 1 ml/min throughout purification and
fractions were collected at a rate of one fraction per minute. The above
chromatograph was obtained by fractionation of the middle-aged protein
sample, fractionation of the young and aged samples yielded similar
chromatographs. Fractions 26-28 (indicated on the figure) contained CKm
130. These fractions were pooled and analyzed for 3-nitrotyrosine
modification. (B) Pooled protein (0.5 μg) from young
(Y), middle-aged (M), and aged (A) Q-Sepharose fractionations were resolved
by SDS PAGE and transferred to a PVDF membrane. Blots probed with an
anti-3NT antibody [top blot, panel (B)] reveal that CK 130 is 3-NT modified
in an age dependent manner. The membrane was then stained with Coomassie
Blue [bottom blot, panel (B)] to normalize protein loading. (C)
Densitometry was used to compare the relative abundance of the 3-NT
modified form of CK 130 between age groups.
It is interesting that nitration of CKm
45 (monomer), CKm 88 (dimer) and CKm 130 (trimer) were observed to
significantly increase with age. Based on these observations, we hypothesize
that within the cell, the 3-NT modified CKm is affiliated with age-associated
protein aggregation. This contention is supported by a report which described
the use of a fluorescent probe (bis-ANS) to monitor protein conformation within
muscle extracts [12]. The low bis-ANS fluorescence quantum yields observed
within aged skeletal muscle samples are consistent with increased incidences of
CKm protein aggregation with age. It is likely that this is not a unique
observation for CKm but is a general consequence of age-related protein
oxidation and nitration [38-40]. Based on those observations we hypothesize
that (a) modified proteins may accumulate in aged tissue because of this
aggregation; (b) aggregation due to oxidative damage per
se is not catastrophic but may account for the decline in tissue function; (c)
these low levels of aggregated proteins may act as "seeds" and increase
aggregation in catastrophic misfolded protein syndromes; (d) these low levels
of aggregated proteins may elicit a misfolded protein stress response that
would account for the stabilization of the age-associated increase in
state-of-chronic stress [41,42].
While
our study has examined some of the structural and functional consequences of
oxidative modification of CK, there are other potential effects of age-related
oxidative modification. One area which remains to be examined is the
possibility that carbonylation and/or nitration may alter protein-protein
interactions of CK. It has been reported that a subpopulation consisting of
approximately 5-10% of the muscle isoform of CK associates with the M line area
of the sarcomere [43].
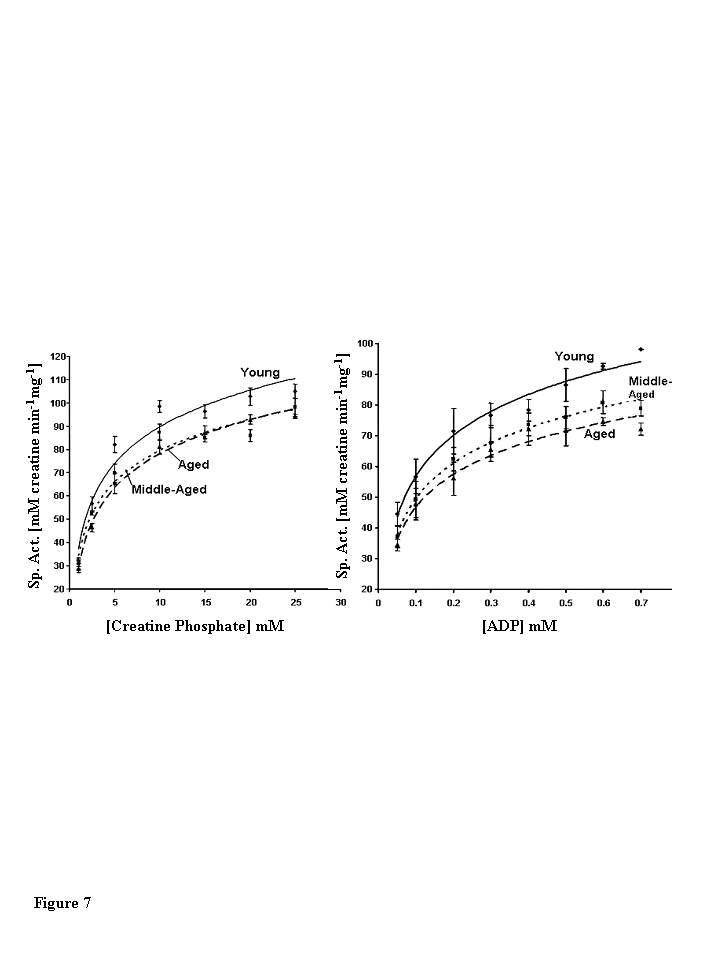
Figure 7. Steady state kinetic analysis of creatine phosphate. (A)
Creatine phosphate and (B) ADP dependence of muscle creatine kinase
purified from young (diamonds, solid line), middle-aged (squares, small
dashed lined), and aged (triangles, large dashed line) mice. The data
presented in the figure are the average of four independent experiments for
creatine phosphate and three independent experiments for ADP. Nonlinear
least squares regression analysis was used to plot best fit curves through
the data. Error bars represent the standard error of mean calculated for
each data point.
This
positions muscle CK in proximity to the myosin ATPase, potentially to provide a
ready pool of ATP to power muscle contraction [6]. Mutagenesis experi-ments
have shown that two pairs of lysine residues in the amino terminal of CK are
critical for the association of CK with the M-line area. Endogenous brain-type
CK expressed in skeletal muscle does not associate with the M line; however,
insertion of the lysine pairs from CKm into the amino terminal of brain-type CK
confers the ability to associate with the M line [3].
Lysine is a target of carbonylation. We
have shown an age-dependent increase in the degree of carbonylation of CKm. It
is possible that the amino terminal lysines which are critical for the
association of CKm with the M line may be targets of age-related carbonylation,
which in turn could affect the ability of CK to bind to the M line. This
potential loss of protein-protein interaction, in addition to the decreased
enzymatic activity which we have shown in this paper, could have the effect of
decreasing the local concentration of ATP available to the sarcomere in aged
skeletal muscle, leading to decreased muscle function. Similarly, since Tyr14
and Tyr20 are nitrated in aged rat skeletal muscle [11] this
modification of the CKm amino terminal domain also alters the ability to bind
to the M line thereby decreasing local concentration of ATP available to
the sarcomeres and decrease function of aged muscle [4,5,7]. Alternatively,
the recent observation that O-CKm causes a major change in orientation of Lys25
and Lys116 may also account for the loss the ability of O-CKm to
bind to the M-band [7].
Proteomics
experiments have identified a growing list of proteins that are differentially
modified by carbonylation or nitration during aging [10,11,43-46]. These
approaches provide valuable insight in that they identify specific proteins
whose functions may be altered. In this study we attempt to further understand
the biochemical consequences and biological significance of the age-related
oxidation and nitration of CKm. We observed higher levels of carbonylation
within middle-aged and aged CKm samples, relative to young CKm which is
affiliated with a significant reduction in function by middle-age. Unlike
carbonylation of the protein, 3-NT modification of the CKm appears to increase
throughout lifespan and we hypothesize that this modification is affiliated
with altered protein function. It is possible that the tyrosine nitration of
CKm only occurs on molecules that are already substantially oxidized. Kanski et
al [11] have mapped the sites of modification to Tyr14 and Tyr20
in rat skeletal muscle CKm. Inspection of the solvent accessibility of these
residues within the rabbit enzyme (pdb
identifier: 2CRK) using the GETAREA server
(www.scsb.utmb.edu/cgi-bin/get_a_form.tcl)
revealed that though Tyr14is on the outside of the protein, Tyr20 is partially buried
having a solvent accessible surface area of only 21.4 % [20,43].
Perhaps, the
age-related oxidation of CKm induces partial unfolding, where higher levels of
reactive nitrogen species within aged muscle leads to tyrosine nitration
which induces an unfolded conformation that is no longer soluble.
Our
studies suggest that the structural changes of the nitrated Ckm in aged
skeletal muscle may play an important role in its aggregation. However, since aggregated
proteins are normally cleared by autophagy, our studies also suggest that CKm
accumulation may be due to an attenuated autophagy in aged skeletal muscle.
Furthermore, since the accumulation of misfolded and aggregated proteins is
known to play a major role in neurodegeneraction [51], we propose that
aggregation and loss of function of CKm in aged skeletal muscle may play a role
in age-associated skeletal muscle frailty such as sarcopenia and
musculoskeletal disorders.
Overall,
the results from this study suggest that reduction in CKm activity could
contribute to the decreased oxidative capacity of aged skeletal muscle.
Additionally, another enzyme involved in energy availability within muscle,
glycogen phosphorylase, is 3-NT modified in an age-dependent manner. Sharov et
al. [48] have reported a decrease in glycogen phosphorylase isolated from aged
rat muscle that correlated with an age-dependent increase in 3-NT modification.
The study did not examine the properties of the enzyme from a middle-aged
sample but our results show significant 3-NT modification of glycogen
phosphorylase (GP) obtained from middle-aged mouse quadriceps. Based on these
data, we predict that GP activity would be significantly decreased by
middle-age as well, though the level of reduction in activity would likely be
less than the approximate 30% decrease reported for aged GP. Given the
importance of CKm and GP, it seems increasingly likely that oxidative and
nitrative modification leads to reductions in function of these two key
enzymes, decreasing the availability of essential energy metabolites which
directly contribute to the aged skeletal muscle phenotype.
Methods
Materials
. Tris-HCl,
ADP, alpha D-glucose, NADP, creatine phosphate, dithiothreitol, hexokinase, and
glucose-6-phosphate dehydrogenase were purchased from Sigma-Aldrich. Imperial
Coomassie Blue protein stain was obtained from Pierce. All other reagents were
from standard suppliers and were at least reagent grade. All solutions were
prepared in Milli-Q (Millipore) doubly deionized water.
Mice.
Young (3-6
months), middle-aged (12-14 months) and aged (20-24 months) male C57BL/6 mice from the National
Institute on Aging colonies (Bethesda, MD) were obtained through Charles River
Laboratories (Wilmington, MA). Mice were maintained with a 12 hour light/dark
cycle and fed ad libitum on a standard chow diet for at least one week
before use. Mice were sacrificed by cervical dislocation. All mice used in this
study were free of tumors or any other gross pathological conditions. The
quadriceps muscles were harvested and snap frozen in liquid nitrogen until
analysis.
Total
quadriceps tissue extracts.
Whole
protein extracts were prepared from the quadriceps muscles of six young (3-6
months), six middle-aged (12-14 months), and five aged (20-24 months) C57BL/6
male mice. Whole muscle extracts were prepared from individual samples by
grinding the quadriceps with an abrasive resin (PlusOne Sample Grinding Kit,
Amersham Biosciences) in urea/CHAPS buffer (8M urea, 4% CHAPS) following the
manufacturer's recommendations. After homogenization, insoluble material was
cleared with a 30 minute centrifugation (8,000 x g) and the supernatants were
retained for immunoblot analysis. Protein was quantified with the Bradford protein assay (Biorad), using BSA as a standard.
Preparation
of soluble protein extracts from quadriceps.
Quadriceps
muscles obtained from five young, five middle-aged and five aged C57BL/6 male
mice were pooled and homogenized with a whirling Polytron blender in a nondenaturing
buffer (50 mM sodium phosphate (pH 5.8), 1mM DTT, 0.4 mM EDTA and 1mM PMSF).
Insoluble material was cleared with a 30 minute centrifugation (8,000 x g) and
the supernatant was retained for protein purification.
Purification
of creatine kinase from young, middle-aged and aged mouse quadriceps.
All column
chromatography steps were performed on a dual pump HPLC system (ESA
Biosciences) equipped with a UV-Vis detector (UV-Vis Model 528, ESA
Biosciences) and a Gilson FC 204 fraction collector. The purification of CKm
was based on previously published methods with slight modifications [49]. Soluble
protein from young, middle-aged, and aged mice quadriceps were applied to a 5 ml Blue Sepharose affinity column
(Amersham Biosciences, HiTrap Blue HP). The resin was washed with 25 ml of
mobile phase (50 mM sodium phosphate, pH 5.8) and protein eluted with 50 mM
sodium phosphate (pH 8.5). Fractions containing CKm, as determined by Western
blot, were pooled, diluted 1 to 10 in 50 mM sodium phosphate (pH 5.8) and
reapplied to the Blue Sepharose column. The column was washed and CKm was
eluted with a 50 ml linear pH gradient (pH 5.8 to pH 10.0). Flow rate
throughout Blue Sepharose chromatography equaled 1 ml/min and fractions were
collected at a rate of one fraction every minute. Peak fractions, containing
CKm, were greater than 85% pure as determined by densitometry and were pooled
and saved for kinetic and protein immunoblot analysis that compared levels of
carbonylation and 3-NT modification. Side fractions, approximately 50% pure,
were pooled and applied to a 2 ml Bio-Scale ceramic hydroxyapatite (HAP) column
(Bio-Rad Laboratories). After the column was washed with 10 ml of low
phosphate buffer (5mM sodium phosphate, pH 7.4), protein was eluted with a linear
sodium phosphate (pH 7.4) gradient (5mM - 150 mM). Throughout HAP
chromatography, the flow rate equaled 0.5 ml/min and fractions were collected
at a rate of one fraction every minute. Peak fractions, used in circular
dichroism and limited proteolysis studies, were greater than 95% pure as
determined by densitometry following SDS PAGE. Concentrations of purified CKm
samples were determined by optical density measurements at 280 nm using an
extinction coefficient of 0.876 ml•mg-1•cm-1 [50].
Enrichment
of high molecular weight CKm protein species.
Soluble quadriceps protein from all three age groups
was fractionated on a 5 ml Blue Sepharose affinity column (Amersham
Biosciences, HiTrap Blue HP). After sample loading, the column was washed with
10 ml of 50 mM sodium phosphate pH 5.8 and developed with a 30 ml linear pH
gradient (pH 5.8 to pH 10.0). Flow rate equaled 1 ml/min throughout
purification and fractions were collected at a rate of one fraction per minute.
Fractions containing anti-CKm immuno-reactive proteins with apparent masses of
88 and 130 kDa were pooled from young, middle-aged and aged mouse samples and
individually loaded onto a 1 ml mono-Q-Sepharose column (Bio-Rad Laboratories).
After application, the column was washed with 10 ml of 25 mM Tris (pH 8.0),
developed with a shallow 20 ml linear NaCl gradient (0-250 mM NaCl in 25 mM
Tris pH 8.0), followed by a steep 10 ml NaCl gradient (250mM - 1M NaCl in 25 mM
Tris pH 8.0). The flow rate equaled 1 ml/min throughout purification and
fractions were collected at a rate of one fraction per minute.
Creatine kinase activity assay.
Creatine
kinase activity was assayed in the direction of creatine and ATP production
using a linked spectrophotometric assay [24,25]. Final concentrations for the
assay were: alpha D-glucose (15
mM), ADP (50-700 μM), MgCl2 (9.0 mM), NADP (1.3 mM), creatine
phosphate (1-25 mM), DTT (9.0 mM), Hexokinase (2.5 mU/ml), and
Glucose-6-phosphate dehydrogenase (2.5 mU/ml). All reagents were prepared in 50
mM Tris-HCl, pH 7.4. All CKm activity measurements were made at 25˚C on a
Beckman Coulter DU530 spectrophotometer.
Enzyme
kinetics.
CKm activity, as a
function of substrate concentrations, was measured by varying creatine
phosphate between 1 mM and 25 mM and ADP between 50 μM and 700 μM.
Initial reaction velocities were determined by measuring the initial change in
absorbance at 340 nm and converting the data to units of specific activity
(μmols creatine min-1mg-1) using an extinction
coefficient of 6220 M-1cm-1. Four and three independent
experiments were performed, for substrate and co-factor, respectively and the
kinetic parameters, KM and Vmax, were calculated from
Eadie-Hofstee plots obtained from individual kinetic experiments [26,31].
Standard errors of mean were also calculated for each parameter.
SDS
PAGE and western blot analysis.
Proteins were resolved on denaturing 4-20% gradient
gels (PAGE Gold precast gels, Cambrex Corporation) and transferred to Immobolin
PVDF membranes (Millipore) at 50 V for two hours. Membranes were blocked with
5% nonfat milk in TBS-T and probed with primary antibodies. A monoclonal anti-3-nitrotyrosine
(Upstate Biotechnology) antibody and a polyclonal anti-creatine kinase M (Santa
Cruz Biotechnology) antibody were used to detect 3-nitrotyrosine modified
proteins and the muscle isotype of creatine kinase, respectively. Blots were
visualized with appropriate horse radish peroxidase conjugated secondary
antibodies (Alpha Diagnostic) used in conjunction with a chemi-luminescent
substrate (Immobilon Western Blot reagent, Millipore). Kodak BioMax MR film was
used to visualize specific antibody binding. Exposed films of immunoblots were
digitized using a MultImage imaging system (Alpha Innotech Corporation) and
quantified by densitometry using AlphaEase software (Alpha Innotech
Corporation). Statistical analysis was performed by comparing normalized
immunoblot band densities using the 2-tailed t-test. P-values less than 0.05
were considered statistically significant.
Detection
of oxidized creatine kinase.
The relative abundance of oxidative modifications
(carbonyls) in young, middle-aged and aged CKm preparations were
determined using the Oxyblot kit (Intergen Company; 18, 19). Carbonyls within
protein samples were detected following the manufacturer's recommendations with
slight modification. Briefly, 1 μg of CKm purified from each age group
(Blue Sepharose pools, > 85% pure) was derivatized with
2,4-dinitrophenylhydrazine for exactly 10 minutes. After derivatization
reactions were quenched, samples resolved by SDS-PAGE, and transferred to a
PVDF membrane. Blots were developed using a primary antibody that is specific
for the 2,4-dinitrophenylhydrazone (DNP) moiety and blots visualized with an
appropriate HRP-conjugated secondary antibody. Blots were quantified by
densitometry and the Coomassie Blue stained membrane was used to normalize for
sample loading variation.
Circular
dichroism.
CKm samples (HAP purified, > 95 % pure)
from all three age groups were dialyzed versus 5 mM sodium phosphate buffer (pH
7.2) and each sample diluted to a concentration of 10 μM in preparation
for circular dichroism (CD) analysis. CD wavelength scans were made at 25˚
C in a 0.1 cm path length cuvette in the far-UV region (195 - 255 nm). CD
measurements were made on an Aviv (Aviv Instruments) Model 215 CD Spectrometer.
CD spectra were acquired by averaging three scans and subtracting buffer
absorbance. The secondary structure composition within each sample was
estimated using the program SELCON3 as accessed via the DICHROWEB server at
(www.cryst.bbk.ac.uk/cdweb/html/home.html)
[21,22,28].
Limited
proteolysis.
Limited proteolytic digestions of CKm were performed
on hydroxyapatite purified samples (> 95% pure) by adding chymotrypsin
(Worthington Chemicals) to a concentration of 1/20 (w/w) and allowing the
reaction to proceed at room temperature for 2, 5, 10, 20, and 40 minutes. At
the indicated time points, a 1 μg aliquot was removed and diluted into 5X SDS
sample loading buffer. Proteolysis was quenched by boiling the samples for 5
minutes. Undigested CKm was used as the "0 minute" time point for all three age
groups. Time courses were then resolved by SDS PAGE and proteins visualized by
Coomassie Blue staining. All proteolytic time courses were repeated three
times. Densitometric calculations were used to quantify the relative abundance
of undigested CKm throughout the time courses.
Protein identification.
Protein bands were excised from Coomassie Blue
stained gels and prepared for Matrix-Assisted Laser Desorption Ionization
Time-of-Flight mass spectrometry (MALDI-TOF) analyses. Gel pieces were
incubated with trypsin (20 μg/ml in 25 mM ammonium bicarbonate, pH 8.0; Promega
Corp.) at 37˚C for 6 hours. The digested sample (1 μL) was deposited onto
the MALDI plate and allowed to dry. Matrix (1 μL;
alpha-cyano-4-hydroxycinnamic acid; Aldrich Chemical Company) was then applied
on the sample spot and allowed to dry. MALDI-TOF/TOF MS was performed using an
Applied Biosystems model 4700 Proteomics Analyzer for peptide mass
fingerprinting and MS/MS analysis. Following MALDI MS analysis, MALDI MS/MS
was performed on several ions from each sample spot. Applied Biosystems GPS
software was used in conjunction with MASCOT to search the NCBI database for
protein identification. Protein match probabilities were deter-mined using
expectation values and MASCOT protein scores.
Calculation
of solvent accessible surface area of CKm residues.
The solvent
accessible surface area (SASA) of each amino acid of CKm was calculated by
submitting the atomic coordinates of the rabbit CKm crystal structure (pdb
identifier: 2CRK) to the GETAREA server
(www.scsb.utmb.edu/cgi-bin/get_a_form.tcl)
[20,43]. Default parameters were used for calculating the percent solvent
accessibility of each residue.
Acknowledgments
This
publication was supported by U.S.P.H.S. grant 1P01 AG021830 awarded by the
National Institute on Aging (JP). The University of Texas Medical Branch
Claude D. Pepper Older Americans Independence Center, P60AAG12583, The Sealy
Center on Aging, University of Texas Medical Branch, and a grant from the Clayton
for Research. J.E.N. would like to
thank the Kempner Foundation and the National Institutes of Environmental
Health Sciences Training Grant (T32-07254) for additional fellowship support.
We wish to acknowledge the University of Texas Medical Branch Proteomics core
facility for protein identification.
Conflicts of Interest
The
authors declare no conflict of interests.
References
-
1.
McLeish
MJ
and Kenyon
GL.
Relating structure to mechanism in creatine kinase.
Crit Rev Biochem Mol Biol.
2005;
40:
1
-20.
[PubMed]
.
-
2.
Eppenberger
HM
, Dawson
DM
and Kaplan
NO.
The comparative enzymology of creatine kinase.
J Biol Chem.
1967;
242:
204
-209.
[PubMed]
.
-
3.
Hornemann
T
, Stolz
M
and Wallimann
T.
Isoenzyme-specific interaction of muscle-type creatine kinase with the sarcomeric M-line is mediated by NH(2)-terminal lysine charge-clamps.
J Cell Biol.
2000;
149:
1225
-1234.
[PubMed]
.
-
4.
Hornemann
T
, Kempa
S
, Himmel
M
, Hayess
K
, Furst
DO
and Wallimann
T.
Muscle-type creatine kinase interacts with central domains of the M-band proteins myomein and M-protein.
J Mol Biol.
2003;
332:
877
-887.
[PubMed]
.
-
5.
Wallimann
T
, Schlosser
T
and Eppenberger
HM.
Function of M-line-bound creatine kinase as intramyofibrillar ATP regenenera-tor at the receiving end of the phosphorylcreatine shuttle in muscle.
J Biol Chem.
1984;
259:
5238
-5246.
[PubMed]
.
-
6.
Ventura-Clapier
R
, Veksler
V
and Hoerter
JA.
Myofibrillar creatine kinase and cardiac contraction.
Mol Cell Biochem.
1994;
133-134:
1225
-1244.
.
-
7.
Zhao
T-J
, Yan
Y-B
, Liu
Y
and Zhou
H-M.
The generation of oxidized form of creatine kinase is a negative regulation on muscle creatine kinase.
J Biol Chem.
2007;
282:
12022
-12029.
[PubMed]
.
-
8.
Taylor
DJ
, Kemp
GJ
, Thompson
CH
and Radda
GK.
Ageing: Effects on oxidative function of skeletal muscle in vivo.
Mol Cell Biochem.
1997;
174:
321
-324.
[PubMed]
.
-
9.
Pastoris
O
, Boschi
F
, Verri
M
, Baiardi
P
, Felzani
G
, Vecchiet
J
, Dossena
M
and Catapano
M.
The effects of aging on enzyme activities and metabolite concentrations in skeletal muscle from sedentary male and female subjects.
Exp Gerontol.
2000;
35:
95
-104.
[PubMed]
.
-
10.
Kanski
J
, Behring
A
, Pelling
J
and Schoneich
C.
Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: effects of biological aging.
Am J Physiol Heart Circ Physiol.
2005;
288:
H371
-H381.
[PubMed]
.
-
11.
Kanski
J
, Hong
SJ
and Schoneich
C.
Proteomic analysis of protein nitration in aging skeletal muscle and identification of nitrotyrosine-containing sequences in vivo by nanoelectrospray ionization tandem mass spectrometry.
J Biol Chem.
2005;
280:
24261
-26266.
[PubMed]
.
-
12.
Pierce
A
, deWaal
E
, VanRemmen
H
, Richardson
A
and Chaudhuri
A.
A novel approach for screening the proteome for changes in protein conformation.
Biochem.
2006;
45:
3077
-3085.
[PubMed]
.
-
13.
Smith
CD
, Carney
JM
, Starkreed
PE
, Oliver
CN
, Stadtman
ER
, Floyd
RA
and Markesbery
WR.
Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer-Disease.
Proc Natl Acad Sci.
1991;
88:
10540
-10543.
[PubMed]
.
-
14.
Rothstein
M
Roy AK and Chatterjee B.
Changes in enzymatic proteins during aging
Molecular Basis of Aging.
1984;
N.Y. Acad. Press
209
-232.
.
-
15.
Oliver
CN
, Ahn
BW
, Moerman
EJ
, Goldstein
S
and Stadtman
ER.
Age-Related-Changes in Oxidized Proteins.
J Biol Chem.
1987;
262:
5488
-5491.
[PubMed]
.
-
16.
Zhou
JQ
and Gafini
A.
Exposure of rat muscle phosphoglycerate kinase to a nonenzymatic Mfo system generates the old form of the enzyme.
J Gerontol.
1991;
46:
B217
-B221.
[PubMed]
.
-
17.
Szweda
LI
and Stadtman
ER.
Iron-Catalyzed Oxidative Modifica-tion of Glucose-6 Phosphate Dehydrogenase from Leuconostoc-Mesenteroides - Structual and functional Changes.
J Biol Chem.
1992;
267:
3096
-3100.
[PubMed]
.
-
18.
Levine
RL
, Wehr
N
, Williams
JA
and Stadtman
ER Shacter E.
Determination of carbonyl groups in oxidized proteins.
Methods Mol Biol.
2000;
99:
15
-24.
[PubMed]
.
-
19.
Levine
RL
and Stadtman
ER.
Oxidative modification of proteins during aging.
Exp Gerontol.
2001;
36:
1495
-1502.
[PubMed]
.
-
20.
Fraczkiewicz
R
and Braun
W.
Exact and efficient analytical calculation of the accessible surface areas and their gradients for macromolecules.
J Comput Chem.
1998;
19:
319
-333.
.
-
21.
Lobley
A
, Whitmore
L
and Wallace
BA.
DICHROWEB: an interactive website for the analysis of protein secondary structure from circular dichroism spectra.
Bioinformatics.
2002;
18:
211
-212.
[PubMed]
.
-
22.
Whitmore
L
and Wallace
BA.
DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data.
Nucleic Acids Res.
2004;
32:
W668
-W673.
[PubMed]
.
-
23.
Wyss
M
, James
P
, Schlegel
J
and Wallimann
T.
Limited proteolysis of creatine-kinase - implications for 3-dimensional structure and for conformational substrates.
Biochem.
1993;
32:
10727
-10735.
[PubMed]
.
-
24.
Rosalki
SB
An improved procedure for creatine phosphokinase determination.
J Lab Clin Med.
1967;
69:
696
-705.
[PubMed]
.
-
25.
Basson
CT
, Grace
AM
and Roberts
R.
Enzyme-Kinetics of a highly purified mitochondrial creatine-kinase in comparison with cytosolic forms.
Mol Cell Biochem.
1985;
67:
151
-159.
[PubMed]
.
-
26.
Eadie
GS
The inhibition of cholinesterase by physostigmine and prostigmine.
J Biol Chem.
1942;
146:
85
-93.
.
-
27.
Hofstee
BJH
Non-inverted versus inverted plots in enzyme kinetics.
Nature.
1959;
184:
1296
-1298.
[PubMed]
.
-
28.
Sreerama
N
, Venyaminov
SY
and Woody
RW.
Estimation of the number of alpha-helical and beta-strand segments in proteins using circular dichroism spectroscopy.
Protein Science.
1999;
8:
370
-380.
[PubMed]
.
-
29.
Dong
AC
, Prestrelski
SJ
, Allison
SD
and Carpenter
JF.
Infrared Spectroscopic Studies of Lyophilization-Induced and Temperature-Induced Protein Aggregation.
J Pharm Sci.
1995;
84:
415
-424.
[PubMed]
.
-
30.
Chi
EY
, Krishnan
S
, Randolph
TW
and Carpenter
JF.
Physical stability of proteins in aqueous solution: Mechanism and driving forces in nonnative protein aggregation.
Pharm Res.
2003;
20:
1325
-1336.
[PubMed]
.
-
31.
Stadtman
ER
and Levine
RL.
Protein oxidation.
Ann N Y Acad Sci.
2000;
899:
191
-208.
[PubMed]
.
-
32.
Buchczyk
DP
, Grune
T
, Sies
H
and Klotz
LO.
Modifications of glyceraldehyde-3-phosphate dehydrogenase induced by increasing concentrations of peroxynitrite: early recognition by 20S proteasome.
Biol Chem.
2003;
384:
237
-241.
[PubMed]
.
-
33.
Schroeder
P
, Klotz
LO
, Buchczyk
DP
, Sadik
CD
, Schewe
T
and Sies
H.
Epicatechin selectively prevents nitration but not oxidation reactions of peroxynitrite.
Biochem Biophys Res Comm.
2001;
285:
782
-787.
[PubMed]
.
-
34.
Buechter
DD
, Medzihradszky
KF
, Burlingame
AL
and Kenyon
GL.
The active-site of creatine kinase-affinity labeling of cysteine-282 with N-(2,3-epoxypropyl)-N-amidinoglycine.
J Biol Chem.
1992;
267:
2173
-2178.
[PubMed]
.
-
35.
Souza
JM
, Peluffo
G
and Radi
R.
Protein tyrosine nitration-functional alteration or just a biomarker.
Free Rad Biol Med.
2008;
45:
357
-366.
[PubMed]
.
-
36.
Savvides
SN
, Scheiwein
M
, Bohme
CC
, Arteel
GE
, Karplus
PA
, Becker
K
and Schirmer
RH.
Crystal structure of the antioxidant enzyme glutathione reductase inactivated by peroxynitrite.
J Biol Chem.
2002;
277:
2779
-2789.
[PubMed]
.
-
37.
Chapman
ALP
, Winterbourn
CC
, Brennan
SO
, Jordan
TW
and Kettle
AJ.
Characterization of non-covalent oligomers of proteins treated with hypochlorous acid.
Biochem J.
2003;
375:
33
-40.
[PubMed]
.
-
38.
Hyun
DH
, Gray
DA
, Halliwell
B
and Jenner
P.
Interference with ubiquitination causes oxidative damage and increased protein nitration: implications for neurodegenerative diseases.
J Neurochem.
2004;
90:
422
-430.
[PubMed]
.
-
39.
Agbas
A
, Zaidi
A
and Michaelis
EK.
Decreased activity and increased aggregation of brain calcineurin during aging.
Brain Research.
2005;
1059:
59
-71.
[PubMed]
.
-
40.
Hawkins
CL
and Davies
MJ.
The role of reactive N-bromo species and radical intermediates in hypobromous acid-induced protein oxidation.
Free Rad Biol Med.
2005;
39:
900
-912.
[PubMed]
.
-
41.
Hsieh
C-C
and Papaconstantinou
J.
The effect of aging on p38 signaling pathway activity in the mouse liver and in response to ROS generated by 3-nitropropionic acid.
Mech Aging Dev.
2002;
123:
1423
-1435.
[PubMed]
.
-
42.
Hsieh
C-C
and Papaconstantinou
J.
Thioredoxin-ASK1 complex levels link mitochondrial ROS activation for the p38 MAPK pathway in aged mouse livers.
FASEB J.
2006;
20:
259
-268.
[PubMed]
.
-
43.
Turner
DM
and Walker
JB.
Enhanced ability of skeletal muscle containing cyclocreatine phosphate to sustain ATP levels during ischemia following beta-adrenergic stimulation.
J Biol Chem.
1987;
262:
6605
-6609.
[PubMed]
.
-
44.
Rabek
JP
, Boylston
WH
and Papaconstantinou
J.
Carbonylation of ER chaperone proteins in aged mouse liver.
Biochem Biophys Res Comm.
2003;
305:
566
-572.
[PubMed]
.
-
45.
Reverter-Branchat
G
, Cabiscol
E
, Tamarit
J
and Ros
J.
Oxidative damage to specific proteins in replicative and chronological-aged Saccharomyces cervisiae - Common targets and prevention by calorie restriction.
J Biol Chem.
2004;
279:
31983
-31989.
[PubMed]
.
-
46.
Sharma
R
, Nakamura
A
, Takahashi
R
, Nakamoto
H
and Goto
S.
Carbonyl modification in rat liver histones: decrease with age and increase by dietary restriction.
Free Rad Biol Med.
2006;
40:
1179
-1184.
[PubMed]
.
-
47.
Rao
JK
, Bujacz
G
and Wlodawer
A.
Crystal structure of rabbit muscle creatine kinase.
Febs Lett.
1998;
439:
133
-137.
[PubMed]
.
-
48.
Sharov
VS
, Galeva
NA
, Kanski
J
, Williams
TD
and Schoneich
C.
Age-associate tyrosine nitration of rat skeletal muscle glycogen phosphorylase b: characterization by HPLC-nanoelectrospray-tandem mass spectrometry.
Exp Gerontol.
2006;
41:
407
-416.
[PubMed]
.
-
49.
Fisher
SE
and Whitt
GS.
Purification of the creatine-kinase isozymes of the green sunfish (Lepomis-Cyanellus) with Blue Sepharose C1-6B.
Anal Biochem.
1979;
94:
89
-95.
[PubMed]
.
-
50.
Kuby
S
, Noda
L
and Lardy
H.
Adenosinetriphosphate-creatine transphosphorylase. I. Isolation of the crystalline enzyme from rabbit muscle.
J Biol Chem.
1954;
209:
191
-201.
[PubMed]
.
-
51.
Komatsu
M
, Waguri
S
, Chiba
T
, Murata
S
, Iwata
JI
, Tanida
I
, Ueno
T
, Koike
M
, Uchiyama
Y
, Kominami
E
and Tanaka
K.
Loss of autophagy in the central nervous system cuases neuro-degeneration in mice.
Nature.
2006;
441:
880
-884.
[PubMed]
.