Abstract
Oxygen
metabolism is thought to impact on aging through the formation of reactive
oxygen species (ROS) that are supposed to damage biological molecules. The
study of p66Shc, a crucial regulator of ROS level involved in
aging dysfunction, suggests that the incidence of degenerative disease and
longevity are determined by a specific signaling function of ROS other than
their unspecific damaging property.
What
we can learn from longevity mutants
The
reason why we age seems obvious: entropy increases. The reason why different
species are differently affected by passing of equal time should be apparent as
well: genetic and epigenetic variability. Organism modification with time has
been mainly explained by the production of free radicals as well as by
immunological theories of aging. However, what we still miss is a list of genes
responsible for aging; the study of these genes would tell us what aging is.
Senectus
ipsa morbus est (Old age is in itself
a disease), ancient romans said. However, the incidence of disease decreases in
the extreme elderly, when aging expression reaches its maximum, whereas
progeric syndromes associate to disease. Therefore, it is not clear whether
aging itself is a disease and how it would impact on life span in a protected
environment.
Our contribution to
this field arises from the study of p66Shc, the first protein identified whose deletion in mouse prolongs
life span and protects from a variety of aging-associated diseases without
showing apparent negative effects.
P66Shc is a redox signaller P66Shc
is a vertebrate protein. It is present in Xenophus, Botia Dario and mammals,
while it is absent in Saccaromyces, Drosophila or Caenorhabditis [1]. P66Shc
is one of three isoforms encoded by the ShcA locus [2].
The
other two isoforms, p46Shc and p52Shc, with molecular
weights of 46 and 52 KDa respectively, were first described as ‘adaptor'
proteins that specifically bind to phosphorylated tyrosines on the cytoplasmic
motif of growth factor receptors. Upon growth factor stimulation, p52Shc/p46Shc
proteins are rapidly and efficiently tyrosine-phosphorylated by all the
tyrosine kinase receptors tested in three major tyrosine residues, and recruit
the Grb2-Sos complex on the plasma membrane [3]. In turn
SOS, through its GEF activity, stimulates the conversion of the inactive Ras
GDP into an active Ras GTP that subsequently activates the mitogen-activated
protein kinase (MAPK) cascade. Recruitment of the Grb2/Sos complex by p52Shc/p46Shc
and membrane relocalization of Sos are events considered sufficient to induce
Ras activation [3]. The
hypothesis that Shc proteins are involved in the regulation of Ras is further
supported by the finding that over-expression of p52Shc/p46Shc
increases proliferative response and enhances MAP kinase and Fos activation
upon stimulation with EGF, GM-CSF and PDGF [2,4,5].
Notably, the shortest isoforms of Shc appeared early in evolution since their
orthologues have been found in flies and nematods [1].
At
molecular level, p66Shc, p52Shc and p46Shc
largely share the same amino acid sequence at the C-terminus including the Src
homologous type two domain (SH2), phosphotyrosine binding domain (PTB)
responsible for the binding to phosphorylated tyrosine, and a region highly
enriched in glycine and proline residues named collagen homologous (CH1) since
its homology with collagen protein [6]. The
peculiarity of p66Shc is an additional CH region (CH2) at its
N-terminus [2,4].
Despite
the high similarity p66Shc functionally differentiates from the
other ShcA isoforms. There is no indication that p66Shc activates
the Ras signaling pathway. Indeed, evidence for divergent regulation of p66Shc
versus p52Shc/p46Shc immediately emerged from studies
demonstrating that although p66Shc, like p52Shc /p46Shc,
is a target of receptor tyrosine kinases (EGFR, INSR, PDGFR) and binds the
Grb2/SOS complex [4,7,8], p66Shc
over-expression, unlike that of p52Shc/p46Shc, has a
negative effect on the Ras-MAPK-Fos pathway in response to EGF or cytokines in
lymphocytes [4,9]. In fact,
p66Shc has been shown to exert an inhibitory effect on the Erk
pathway, which is necessary for coordinated actin cytoskeleton polymerization [10], and normal
IGF-1 responsiveness of the MEK/ERK pathway in myoblasts [11]. How p66Shc
exerts this negative effect is not clear. It was proposed that it acts by
competing with p52Shc for Grb2 binding, sequestering the Grb2/Sos
complex and therefore terminating Ras signaling [11].
Finally, studies on p66Shc
knock down did not demonstrated any role for p66Shc in growth factor
response or Ras signaling whereas they revealed an unexpected function of p66Shc
in regulating intracellular redox balance and oxidative stress levels [12]. Indeed,
compared to WT, the amount of reactive oxygen species (ROS) was shown to be
decreased in p66Shc- depleted cultivated cells, as revealed by the
reduced oxidation of ROS sensitive probes as well as by the reduced
accumulation of endogenous markers of oxidative stress [9,13-17].
Likewise, p66Shc-/- mice show diminished levels of both systemic
(isoprostane) and intracellular (nytrotyrosines, 8-oxo-dG) oxidative stress [14,18,19].
Mechanisms
of p66 Shc - redox activity regulation
Basically,
intracellular ROS levels can be increased by three main mechanisms: reducing
ROS scavenging, increasing membrane oxidases activity, or by mitochondrial
respiratory chain leakage. P66Shc has been reported to act through
all of them. In fact, p66Shc silencing by RNAi or gene targeting
deletion was found to increase levels of superoxide dismutases and catalases in
a variety of cells. In particular, p66Shc appeared to decrease the
expression of ROS scavenging enzymes through the inhibition of FOXO transcription
factors [13] (Figure 1). In addition, p66Shc has been proposed to mask the growth factor
receptor bound protein Grb2 from Sos1, favoring the rac1-specific GEF activity
of Sos1, rac1 activation and triggering of NADPH membrane oxidase ROS
production [20] (Figure 1).
Finally,
a fraction of p66Shc has been observed within
the mitochondrial inter-membrane space (IMS) [16]. Notably, electrochemical
experiments demonstrated that the amino terminal portion of p66Shc
contains a redox active region able to mediate electron transfer from reduced
cytochrome c to molecular oxygen, thus producing hydrogen peroxide
(Figure 1).
As
reported, all proteins of the mitochondrial inter-membrane space are
synthesized in the cytosol and are then imported into the mitochondria [21]. Most of
them do not contain any cleavable sequences and are targeted to IMS by as yet
unidentified import signals.
The import of p66shc into mitochondrial IMS
is not still understood at a mechanistic level. However, a mechanism that
depends on p66Shc post-translational modifications, including serine
phosphorylation by stress kinases like Jnk-1 and Pkc-B and prolilisomerization
by Pin-1, has been described, which allows p66Shc increase within
the mitochondria during apoptosis [22]. A second
level of activation of p66Shc mitochondrial function is represented
by the effective amount of p66Shc within mitochondrial vesicles. In fact,
mitochondrial p66Shc has been observed to associate to a high
molecular weight complex of about 670 KDa and to the mitochondrial chaperon
mtHsp70 [23]. Notably,
treatment of cells with pro-apoptotic stimuli such as UVC or H2O2
induces the dissociation of this complex and the consequent release of
monomeric p66Shc,which is then free to react with
cytochrome c [23].
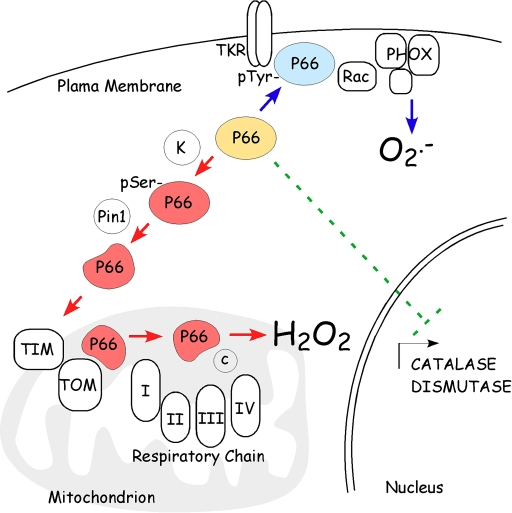
Figure 1. P66 Shc controls intracellular ROS metabolism at multiple sites. P66Shc
(in blue) stimulates ROS production by plasma membrane oxidases through the
association with membrane receptor and Rac activation of phagocitic
oxidases. Upon phosphorylation and consequent
Pin-1-mediated conformational changes, p66Shc (in red)
translocates, through the TIM/TOM mitochondrial import machinery, within
the mitochondrial inter-membrane space where it oxidizes reduced cytochrome
c and catalyzes the partial reduction of O2 to H2O2.
Then, p66Shc decreases the expression of ROS scavenging enzymes.
Interestingly,
p66Shc half-life increases upon apoptotic stimulation in a
p53-dependent way, thus linking the pro-apoptotic activity of p66Shc
to the p53 pathway [14].
Function
of p66 Shc - oxidative signal
Regardless of how p66Shc may
shift the intracellular redox balance towards oxidation, it appears that p66Shc
specifically evolved to increase intracellular ROS levels. In this view,
different functions have been assigned to p66Shc- produced ROS.
Initially it was reported that H2O2 produced by p66Shc
within the mitochondria induces the opening of the mitochondrial permeability
transition pore leading to swelling of the organelle [16]. The
consequent rupture of mitochondrial integrity then triggers the release of
various proapoptotic mitochondrial factors, including cytochrome c, into
the cytosol, where they activate the apoptotic
cascade leading to cell death [23]. Indeed,
p66Shc-/- cells have been demonstrated to be resistant to apoptosis
induced by a variety of different signals, including ultraviolet radiation,
staurosporine, growth factor deprivation, calcium ionophore, CD3-CD4
cross-linking and taxol [9,12,23].
Likewise, p66Shc-/- mice were found resistant to apoptosis induced
by paraquat, hypercholesterolemia, ischemia, angiotensin II, carbon
tetrachloride and ethanol [12,15,16,18].
Notably, p66Shc deletion in mice was shown to improve resistance to
hyperglycaemic damage in diabetic model of nephropathy and cardiovascular
diseases due the reduction of apoptosis and cell loss [24,25].
Recently,
another role for p66Shc - mediated ROS has been described in the
regulation of adipogenesis. In adipocytes, p66Shc was demonstrated
to be involved in insulin-induced gene expression regulation and triglyceride accumulation. In fat cells insulin
induces serine 36 specific phosphorylation of p66Shc thus
stimulating p66Shc ROS production, which, in turn, potentiates
insulin transduction signaling. Indeed, mutants unable to translocate to the
mitochondria and to produce H2O2 do not sustain insulin-dependent
signaling and triglyceride accumulation when reintroduced in p66Shc-/-
cells [17]. Interestingly, some phosphatases
inhibiting insulin signaling (e.g. PTEN) are inactivated by oxidation [26]. Thus, it appears that p66Shc-generated ROS play a crucial role in regulating insulin signaling and
fat development, likely through the modulation of these redox-sensitive
phosphatases. Indeed, p66Shc-/- mice are protected from diet-induced
obesity, suggesting that this molecular pathway regulates diet-associated fat
development [17]. But if p66Shc is
able to convert signals from the diet into variations of the intracellular redox
balance, affecting insulin sensitivity, critically, the process that
triggers adipogenesis following food intake should stem from the integration of
both intracellular (mitochondrial ROS production) and extracellular
(circulating insulin) signals [17] (Figure 2).
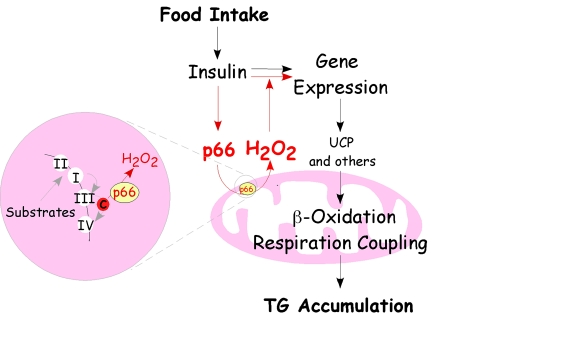
Figure 2. Regulatory circuit of p66 Shc-mediated fat development. The scheme
recapitulates the pathway of p66Shc that drives mitochondrial H2O2 and its relationship
with insulin receptor signaling leading to fat accumulation. Food intake determines
energetic substrate availability and insulin stimulates intracellular
transduction pathways that regulate gene transcription in order to favor
triglyceride accumulation. P66Shc-mediated ROS production is
directly boosted by insulin and in turn potentiates insulin receptor signaling,
suppresses the expression of uncoupling proteins and beta oxidation enzymes
leading to triglyceride accumulation.
Notably,
p66 Shc - produced H2O2 might control
intracellular signaling events also in tissues other than fat. In particular,
the response of myocytes and endothelial cells to glycaemia and ischemia, as
well as the renewal control of breast stem cells upon hypoxia, has been linked
to p66Shc- redox activity [24,27-29].
Therefore,
p66Shc behaves like an atypical signal transducer that tunes
membrane receptor signaling or intracellular glucose/oxygen sensing via the
regulation of intracellular re-dox balance.
P66Shc
impacts on overall energy metabolism and aging
Was
the p66Shc gene conserved during mammals development, in spite of
its deleterious effects on life-span and disease, because of p66Shc-
mediated ROS signaling function in fat tissues? P66Shc-/-
mice have reduced body weight, due to reduced fat mass of both white and brown
adipose tissues [17]. This
leanness is not explainable by changes in food intake, intestinal absorption of
nutrients or locomotor activity. Rather, it may reflect defective lipogenesis
in adipocytes, as suggested by the reduced lipid accumulation of p66Shc-/-
adipocytes transplanted into WT recipient mice [17]. However, this
interpretation of the mechanisms leading to decreased fat mass in p66Shc-/-
mice poses the question of how energy balance is maintained in the absence of
p66Shc, and why energy storage is reduced. As p66Shc-/-
mice showed increased basal body temperature and increased basal metabolic
rate, this suggests that increased uncoupled respiration in the fat
mitochondria of p66Shc-/- mice leads to increased energy
expenditure, which contributes to resistance to body weight gain [17].
Fat
has a crucial role in the thermoregulation of mammals. It protects from body
heat loss (thermoinsulation) and generates heat for the maintenance of body
temperature when animals are exposed to cold (thermo-genesis). Notably, p66Shc-/-
mice were found to be more sensitive to cold due to the reduced thermal
insulation effect of fat pads [17].
Therefore, adaptation to
cold as well as optimization of energy storage when food is available, both
altered in the lean p66Shc-/- mice, have been proposed as possible
evolutionary functions whose fitness pressure preserves the p66Shc
gene in mammals.
These findings of reduced adiposity in
p66Shc-/- mice might have important implications for the effect of
p66Shc on lifespan. Aging is associated with a pathological trait,
often associated with obesity (metabolic syndrome), which predisposes to
diabetes and cardiovascular diseases [30-34]. In
humans, these diseases strongly affect morbidity and mortality, especially
among the elderly [30,35].
Oxidative stress has been implicated in a number of chronic disease states
usually grouped under the umbrella of the metabolic syndrome [36-42], and it
is thought to contribute to the aging process [43]. It has
been hypothesized that the production of free radicals is dependent on
metabolic rate [44], and that
this may have an impact on the aging process. In p66Shc-/- mice,
like in caloric restriction and FIRKO mice, fat deposits are moderately decreased [17,45], suggesting that reduced
oxidative stress in p66Shc-/- mice might increase longevity through
the direct effect of reduced adiposity. Notably, p66Shc-/- mice are
more resistant to diabetes and have reduced risk of atherosclerosis and
cardiovascular damage upon HF-diet [18,25]. Therefore, the effect of p66Shc
on aging might be considered a sort of chronic decay like the metabolic
syndrome progression, although the contribution of the metabolic syndrome to
life span is still not clear (Figure 3).
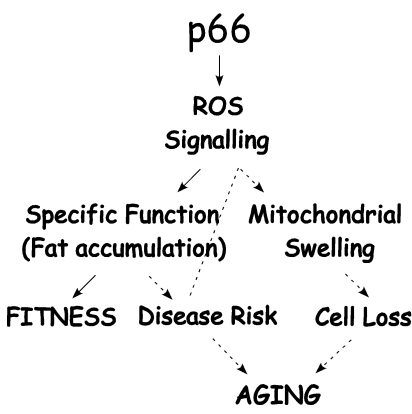
Figure 3. P66 Shc/ROS signaling determines fitness and aging associated dysfunctions. P66Shc/ROS
signals to specific functions that improve fitness whilst these same
functions may increase disease risk chronically (such as obesity related
disorders) and contribute to trigger p66Shc-mediated cell death.
Then, increased disease risk and cell loss rate contribute to aging dysfunctions.
The
life-prolonging action of caloric restriction (CR) offers an excellent chance
for investigating the connection between stress and aging. The anti-aging
action of CR can be viewed as "nutritional stress," because the
organism's reduced caloric intake seems to be a stimulatory metabolic response
for survival. Thus, as an omnipotent intervention, CR provides a unique
opportunity to probe the organism's ability to withstand age-related stress as
a survival strategy. Recent geriatric research has provided sufficient
experimental data supporting the anti-aging property of CR [46-48].
What
kills mammals is a "p66 Shc syndrome"
Finally,
the study of p66Shc confirms that very close links exist between
energetic metabolism, oxidative stress and aging. P66Shc represents
a clear example of an antagonistic pleiotropic function, which generates both
beneficial and detrimental phenomena in an organism.
Darwin
might say that aging expresses fitness (senectus robur est), at least as
much as one is able to face illness. However, it remains unclear whether aging
is also a disease or whether life span is regulated by energetic metabolism
disorders that could eventually result in lethal effects or sub-pathological
multiple dysfunctions.
In
a series of WT and p66Shc-/- very old moribund mice, significant
recurring cause of death were not identified. Indeed, it is known that in mice
as in humans even accurate autopsy might often remain "blank", in the
absence of masses, haemorrhages, abscesses or other evident septic conditions.
Mice presented only sporadic terminal emphysema (mainly in WT mice), occasional
lymphocytic pneumonia and very rare malignant tumors. On the other hand, it is
impossible to rule out other causes of death, such as cardiac fibrillation or
acute myocardial infarction, which score negative for morphological
investigation (unpublished data).
In
conclusion, whether aging determines life span through diseases or through the
acceleration of a fatal physiological decline remains puzzling. It is expected
that further, more intense investigations in the cause of death in mammals
might contribute to the solution.
P66Shc
story suggests that necessary regulators of oxygen and energetic metabolism may
be involved both in the onset of the acute phase of diseases and in the
induction of aging related detrimental changes that ultimately kill the
organism.
Acknowledgments
We thank Paola Dalton for the preparation of the manuscript. This work was supported by National Institute of
Health Grant 1P01AG025532-01A1.
References
-
1.
Luzi
L
, Confalonieri
S
, Di Fiore
PP
and Pelicci
PG.
Evolution of Shc functions from nematode to human.
Curr Opin Genet Dev.
2000;
10:
668
-674.
[PubMed]
.
-
2.
Pelicci
G
, Lanfrancone
L
, Grignani
F
, McGlade
J
, Cavallo
F
, Forni
G
, Nicoletti
I
, Grignani
F
, Pawson
T
and Pelicci
PG.
A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction.
Cell.
1992;
70:
93
-104.
[PubMed]
.
-
3.
Ravichandran
KS
Signaling via Shc family adapter proteins.
Oncogene.
2001;
20:
6322
-6330.
[PubMed]
.
-
4.
Migliaccio
E
, Mele
S
, Salcini
AE
, Pelicci
G
, Lai
KM
, Superti-Furga
G
, Pawson
T
, Di Fiore
PP
, Lanfrancone
L
and Pelicci
PG.
Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway.
Embo J.
1997;
16:
706
-716.
[PubMed]
.
-
5.
Lanfrancone
L
, Pelicci
G
, Brizzi
MF
, Aronica
MG
, Casciari
C
, Giuli
S
, Pegoraro
L
, Pawson
T
, Pelicci
PG
and Arouica
MG.
Over-expression of Shc proteins potentiates the proliferative response to the granulocyte-macrophage colony-stimulating factor and recruitment of Grb2/SoS and Grb2/p140 complexes to the beta receptor subunit.
Oncogene.
1995;
10:
907
-917.
[PubMed]
.
-
6.
Pelicci
G
, Dente
L
, De
Giuseppe A
, Verducci-Galletti
B
, Giuli
S
, Mele
S
, Vetriani
C
, Giorgio
M
, Pandolfi
PP
, Cesareni
G
and Pelicci
PG.
A family of Shc related proteins with conserved PTB, CH1 and SH2 regions.
Oncogene.
1996;
13:
633
-41.
[PubMed]
.
-
7.
Yokote
K
, Mori
S
, Hansen
K
, McGlade
J
, Pawson
T
, Heldin
CH
and Claesson-Welsh
L.
Direct interaction between Shc and the platelet-derived growth factor beta-receptor.
J Biol Chem.
1994;
269:
15337
-15343.
[PubMed]
.
-
8.
Skolnik
EY
, Lee
CH
, Batzer
A
, Vicentini
LM
, Zhou
M
, Daly
R
, Myers
MJ Jr
, Backer
JM
, Ullrich
A
and White
MF.
The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling.
Embo J.
1993;
12:
1929
-1936.
[PubMed]
.
-
9.
Pacini
S
, Pellegrini
M
, Migliaccio
E
, Patrussi
L
, Ulivieri
C
, Ventura
A
, Carraro
F
, Naldini
A
, Lanfrancone
L
, Pelicci
P
and Baldari
CT.
p66SHC promotes apoptosis and antagonizes mitogenic signaling in T cells.
Mol Cell Biol.
2004;
24:
1747
-1757.
[PubMed]
.
-
10.
Natalicchio
A
, Laviola
L
, De
Tullio C
, Renna
LA
, Montrone
C
, Perrini
S
, Valenti
G
, Procino
G
, Svelto
M
and Giorgino
F.
Role of the p66Shc isoform in insulin-like growth factor I receptor signaling through MEK/Erk and regulation of actin cytoskeleton in rat myoblasts.
J Biol Chem.
2004;
279:
43900
-43909.
[PubMed]
.
-
11.
Xi
G
, Shen
X
and Clemmons
DR.
p66shc negatively regulates insulin-like growth factor I signal transduction via inhibition of p52shc binding to Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 leading to impaired growth factor receptor-bound protein-2 membrane recruitment.
Mol Endocrinol.
2008;
22:
2162
-2175.
[PubMed]
.
-
12.
Migliaccio
E
, Giorgio
M
, Mele
S
, Pelicci
G
, Reboldi
P
, Pandolfi
PP
, Lanfrancone
L
and Pelicci
PG.
The p66shc adaptor protein controls oxidative stress response and life span in mammals.
Nature.
1999;
402:
309
-313.
[PubMed]
.
-
13.
Nemoto
S
and Finkel
T.
Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway.
Science.
2002;
295:
2450
-2452.
[PubMed]
.
-
14.
Trinei
M
, Giorgio
M
, Cicalese
A
, Barozzi
S
, Ventura
A
, Migliaccio
E
, Milia
E
, Padura
IM
, Raker
VA
, Maccarana
M
, Petronilli
V
, Minucci
S
and Bernardi
P.
A p53-p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis.
Oncogene.
2002;
21:
3872
-3878.
[PubMed]
.
-
15.
Zaccagnini
G
, Martelli
F
, Fasanaro
P
, Magenta
A
, Gaetano
C
, Di Carlo
A
, Biglioli
P
, Giorgio
M
, Martin-Padura
I
, Pelicci
PG
and Capogrossi
MC.
p66ShcA modulates tissue response to hindlimb ischemia.
Circulation.
2004;
109:
2917
-2923.
[PubMed]
.
-
16.
Giorgio
M
, Migliaccio
E
, Orsini
F
, Paolucci
D
, Moroni
M
, Contursi
C
, Pelliccia
G
, Luzi
L
, Minucci
S
, Marcaccio
M
, Pinton
P
, Rizzuto
R
and Bernardi
P.
Electron transfer between cytochrome c and p66Shc generates reactive oxygen species thattrigger mitochondrial apoptosis.
Cell.
2005;
122:
221
-233.
[PubMed]
.
-
17.
Berniakovich
I
, Trinei
M
, Stendardo
M
, Migliaccio
E
, Minucci
S
, Bernardi
P
, Pelicci
PG
and Giorgio
M.
p66Shc-generated oxidative signal promotes fat accumulation.
J Biol Chem.
2008;
283:
34283
-34293.
[PubMed]
.
-
18.
Napoli
C
, Martin-Padura
I
, de Nigris
F
, Giorgio
M
, Mansueto
G
, Somma
P
, Condorelli
M
, Sica
G
, De
Rosa G
and Pelicci
P.
Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet.
Proc Natl Acad Sci U S A.
2003;
100:
2112
-2116.
[PubMed]
.
-
19.
Francia
P
, delli
Gatti C
, Bachschmid
M
, Martin-Padura
I
, Savoia
C
, Migliaccio
E
, Pelicci
PG
, Schiavoni
M
, Luscher
TF
, Volpe
M
and Cosentino
F.
Deletion of p66shc gene protects against age-related endothelial dysfunction.
Circulation.
2004;
110:
2889
-2895.
[PubMed]
.
-
20.
Khanday
FA
, Yamamori
T
, Mattagajasingh
I
, Zhang
Z
, Bugayenko
A
, Naqvi
A
, Santhanam
L
, Nabi
N
, Kasuno
K
, Day
BW
and Irani
K.
Rac1 leads to phosphorylation-dependent increase in stability of the p66shc adaptor protein: role in Rac1-induced oxidative stress.
Mol Biol Cell.
2006;
17:
122
-129.
[PubMed]
.
-
21.
Herrmann
JM
and Neupert
W.
Protein transport into mitochondria.
Curr Opin Microbiol.
2000;
3:
210
-214.
[PubMed]
.
-
22.
Pinton
P
, Rimessi
A
, Marchi
S
, Orsini
F
, Migliaccio
E
, Giorgio
M
, Contursi
C
, Minucci
S
, Mantovani
F
, Wieckowski
MR
, Del Sal
G
, Pelicci
PG
and Rizzuto
R.
Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc.
Science.
2007;
315:
659
-663.
[PubMed]
.
-
23.
Orsini
F
, Migliaccio
E
, Moroni
M
, Contursi
C
, Raker
VA
, Piccini
D
, Martin-Padura
I
, Pelliccia
G
, Trinei
M
, Bono
M
, Puri
C
, Tacchetti
C
and Ferrini
M.
The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential.
J Biol Chem.
2004;
279:
25689
-25695.
[PubMed]
.
-
24.
Camici
GG
, Schiavoni
M
, Francia
P
, Bachschmid
M
, Martin-Padura
I
, Hersberger
M
, Tanner
FC
, Pelicci
P
, Volpe
M
, Anversa
P
, Luscher
TF
and Cosentino
F.
Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress.
Proc Natl Acad Sci U S A.
2007;
104:
5217
-5222.
[PubMed]
.
-
25.
Menini
S
, Amadio
L
, Oddi
G
, Ricci
C
, Pesce
C
, Pugliese
F
, Giorgio
M
, Migliaccio
E
, Pelicci
P
, Iacobini
C
and Pugliese
G.
Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress.
Diabetes.
2006;
55:
1642
-1650.
[PubMed]
.
-
26.
Lee
SR
, Yang
KS
, Kwon
J
, Lee
C
, Jeong
W
and Rhee
SG.
Reversible inactivation of the tumor suppressor PTEN by H2O2.
J Biol Chem.
2002;
277:
20336
-20342.
[PubMed]
.
-
27.
Sansone
P
, Storci
G
, Giovannini
C
, Pandolfi
S
, Pianetti
S
, Taffurelli
M
, Santini
D
, Ceccarelli
C
, Chieco
P
and Bonafe
M.
p66Shc/Notch-3 interplay controls self-renewal and hypoxia survival in human stem/progenitor cells of the mammary gland expanded in vitro as mammospheres.
Stem Cells.
2007;
25:
807
-815.
[PubMed]
.
-
28.
Rota
M
, LeCapitaine
N
, Hosoda
T
, Boni
A
, De
Angelis A
, Padin-Iruegas
ME
, Esposito
G
, Vitale
S
, Urbanek
K
, Casarsa
C
, Giorgio
M
, Luscher
TF
and Pelicci
PG.
Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene.
Circ Res.
2006;
99:
42
-52.
[PubMed]
.
-
29.
Bianchi
G
, Di Giulio
C
, Rapino
C
, Rapino
M
, Antonucci
A
and Cataldi
A.
p53 and p66 proteins compete for hypoxia-inducible factor 1 alpha stabilization in young and old rat hearts exposed to intermittent hypoxia.
Gerontology.
2006;
52:
17
-23.
[PubMed]
.
-
30.
Isomaa
B
, Almgren
P
, Tuomi
T
, Forsen
B
, Lahti
K
, Nissen
M
, Taskinen
MR
and Groop
L.
Cardiovascular morbidity and mortality associated with the metabolic syndrome.
Diabetes Care.
2001;
24:
683
-689.
[PubMed]
.
-
31.
Alexander
CM
, Landsman
PB
, Teutsch
SM
and Haffner
SM.
NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older.
Diabetes.
2003;
52:
1210
-1214.
[PubMed]
.
-
32.
Malik
S
, Wong
ND
, Franklin
SS
, Kamath
TV
, L'Italien
GJ
, Pio
JR
and Williams
GR.
Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults.
Circulation.
2004;
110:
1245
-1250.
[PubMed]
.
-
33.
Wilson
PW
, Meigs
JB
, Sullivan
L
, Fox
CS
, Nathan
DM
and D'Agostino
RB Sr.
Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study.
Arch Intern Med.
2007;
167:
1068
-1074.
[PubMed]
.
-
34.
Wilson
PW
, D'Agostino
RB
, Parise
H
, Sullivan
L
and Meigs
JB.
Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus.
Circulation.
2005;
112:
3066
-3072.
[PubMed]
.
-
35.
Lakka
HM
, Laaksonen
DE
, Lakka
TA
, Niskanen
LK
, Kumpusalo
E
, Tuomilehto
J
and Salonen
JT.
The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men.
Jama.
2002;
288:
2709
-2716.
[PubMed]
.
-
36.
Taniyama
Y
and Griendling
KK.
Reactive oxygen species in the vasculature: molecular and cellular mechanisms.
Hypertension.
2003;
42:
1075
-1081.
[PubMed]
.
-
37.
Diep
QN
, Amiri
F
, Touyz
RM
, Cohn
JS
, Endemann
D
, Neves
MF
and Schiffrin
EL.
PPARalpha activator effects on Ang II-induced vascular oxidative stress and inflammation.
Hypertension.
2002;
40:
866
-871.
[PubMed]
.
-
38.
Houstis
N
, Rosen
ED
and Lander
ES.
Reactive oxygen species have a causal role in multiple forms of insulin resistance.
Nature.
2006;
440:
944
-948.
[PubMed]
.
-
39.
Skalicky
J
, Muzakova
V
, Kandar
R
, Meloun
M
, Rousar
T
and Palicka
V.
Evaluation of oxidative stress and inflammation in obese adults with metabolic syndrome.
Clin Chem Lab Med.
2008;
46:
499
-505.
[PubMed]
.
-
40.
Landmesser
U
, Cai
H
, Dikalov
S
, McCann
L
, Hwang
J
, Jo
H
, Holland
SM
and Harrison
DG.
Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II.
Hypertension.
2002;
40:
511
-515.
[PubMed]
.
-
41.
Matsuzawa-Nagata
N
, Takamura
T
, Ando
H
, Nakamura
S
, Kurita
S
, Misu
H
, Ota
T
, Yokoyama
M
, Honda
M
, Miyamoto
K
and Kaneko
S.
Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity.
Metabolism.
2008;
57:
1071
-1077.
[PubMed]
.
-
42.
Kumashiro
N
, Tamura
Y
, Uchida
T
, Ogihara
T
, Fujitani
Y
, Hirose
T
, Mochizuki
H
, Kawamori
R
and Watada
H.
Impact of oxidative stress and peroxisome proliferator-activated receptor gamma coactivator-1alpha in hepatic insulin resistance.
Diabetes.
2008;
57:
2083
-2091.
[PubMed]
.
-
43.
Harman
D
The Free Radical Theory of Aging: Effect of Age on Serum Copper Levels.
J Gerontol.
1965;
20:
151
-3.
[PubMed]
.
-
44.
Sanz
A
, Pamplona
R
and Barja
G.
Is the mitochondrial free radical theory of aging intact.
Antioxid Redox Signal.
2006;
8:
582
-599.
[PubMed]
.
-
45.
Bluher
M
, Michael
MD
, Peroni
OD
, Ueki
K
, Carter
N
, Kahn
BB
and Kahn
CR.
Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance.
Dev Cell.
2002;
3:
25
-38.
[PubMed]
.
-
46.
Sohal RS,Weindruch R. Oxidative stress, caloric restriction, and aging.
Science.
1996;
273:
59
-63.
[PubMed]
.
-
47.
Masoro
EJ
Caloric restriction.
Aging.
1998;
10:
173
-174.
[PubMed]
.
-
48.
Bodkin
NL
, Ortmeyer
HK
and Hansen
BC.
Long-term dietary restriction in older-aged rhesus monkeys: effects on insulin resistance.
J Gerontol A Biol Sci Med Sci.
1995;
50:
B142
-147.
[PubMed]
.