CXCL5 drives obesity to diabetes, and further
Abstract
We have recently shown that the CXCL5 chemokine is secreted by adipose tissue in the obese state. We demonstrated that adipose tissue-derived CXCL5 mediates insulin resistance in muscle. We speculate in this paper that CXCL5 could also mediate other obesity, and diabetes-derived pathologies, such as cardiovascular disease, retinopathy, or inflammatory bowel disease. In this scenario CXCL5 targeted therapy would prevent not only the development of type II diabetes in obese subjects, but also several other obesity-related co morbidities. Finally we propose to analyze the CXCL5 gene to find particular polymorphisms that could predict the development of type II diabetes in obese subjects.
The major environmental risk factors for
type II diabetes are obesity and a sedentary lifestyle [1], and the dramatic
increase in the rates of type II diabetes in recent years has been attributed,
primarily, to the striking rise in obesity worldwide [2]. Adipose tissue is
absolutely required for glucose homeostasis. Indeed, subjects with lipoatrophy
and transgenic animals that are engineered to lack adipose tissue are extremely
insulin resistant [3]. This seems therefore to indicate that storage of energy
in adipocytes favors insulin sensitivity. Adipose tissue dysfunction, which is
associated with obesity is the key factor of obesity-related insulin resistance
and type II diabetes. Since adipose tissue only contributes minimally to
glucose disposal, signaling pathways might exist from adipose tissue to muscle
and other insulin sensitive tissues. Both proteins and lipids have been
proposed as non-mutually exclusive signaling molecules, which can affect the
muscle. A first group of important mediators consists of fatty acids. Since the
original observation by Randle, it has been established that increased fatty
acid concentrations in the muscle decrease glucose metabolism (reviewed in [4]). A second class of mediators that
affect insulin sensitivity in both muscle and liver and which are derived from
adipose tissue are adipokines. Adipokines are factors secreted by the different
cell compartments of white adipose tissue (WAT), such as adipocytes or
macrophages, and were initially characterized as regulators of metabolic
processes, such as regulation of food intake, energy homeostasis, adipocyte
differentiation, or insulin sensitivity. Subsequently, it was found that
adipokines could modulate inflammatory processes. These adipokines include
WAT-specific factors, such as leptin, adiponectin, and well-known cytokines
secreted by several cell types, such as TNF-alpha, IL-6, IL-8, IL-1, or
monocyte chemoattractant protein-1 [5]. In our recent publication we identify
the CXCL5 chemokine as one of these signaling molecules secreted in adipose
tissue that have major implications in insulin sensitivity in muscle cells [6].
CXCL5
or epithelial neutrophil activating peptide (ENA-78) is a cytokine belonging to
the family of chemokines that is mainly implicated in the chemotaxis of
inflammatory cells through the generation of local concentration gradients [7,8].
It has been shown to be a recruiter of neutrophils and involved in their
activation. This C-X-C chemokine has been implicated in pulmonary disease, lung
cancer, arthritis, and other pathological states [7,9,10]. In our paper we
show that CXCL5 is a new chemokine secreted by adipose tissue resident
macrophages and that circulating CXCL5 is highly increased during obesity in
both mice and humans. CXCL5 is able to inhibit insulin action in muscle by activating the Jak/STAT/SOC signaling
pathway showing that CXCL5 can induce insulin resistance. Higher CXCL5 level is associated with insulin-resistant patients compared to
non-insulin-resistant obese patients. Moreover, CXCL5 is directly regulated by
TNFα in both adipose tissue and macrophages by NFκB activation,
suggesting that CXCL5 mediates the effects of TNFα in insulin resistance.
Most importantly, inhibition of signaling from CXCR2, which is the CXCL5
receptor, by injection of neutralizing anti-CXCL5 antibody or selective
antagonist to CXCR2 in insulin-resistant-obese mice improves both insulin
sensitivity and glucose clearance. In summary our data show that CXCL5 promotes
insulin resistance [6].
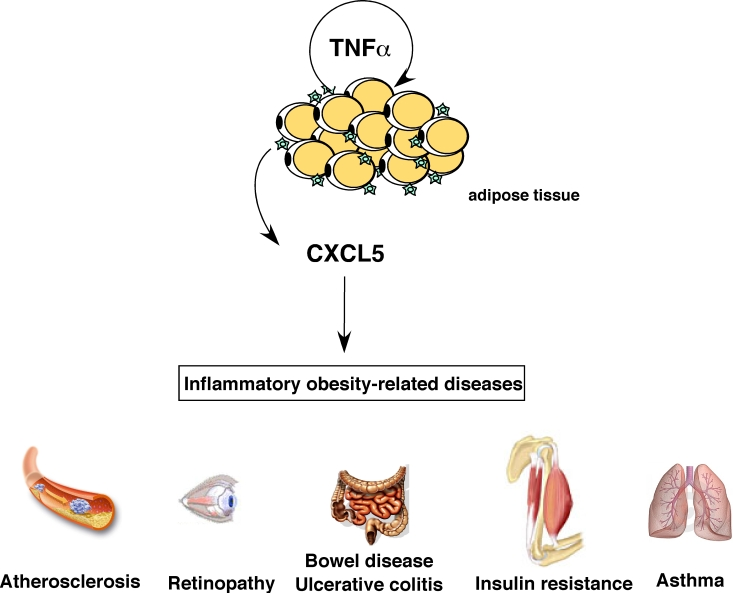
Figure 1. Role of CXCL5 in inflammatory obesity-related pathologies. CXCL5 is
produced in response to TNFα
by adipose tissue-resident macrophages and can trigger several
obesity-associated complications like asthma, atherosclerosis, bowel
disease, colitis, diabetes and retinopathy.
Implication of CXCL5 in other pathological conditions associated to
obesity-induced diabetes
In addition to insulin resistance, obese diabetic
patients are at high risk to develop associated pathologies, including, but not
limited to atherosclerosis, retinopathies, or other inflammatory diseases. This
is represented in figure 1. Interestingly, a major common feature of these
pathologies is inflammation. Since CXCL5 is an inflammatory factor, and since
its levels are increased in obese patients, we could speculate that CXCL5 is at
the origin of obesity- associated co-morbidities. Furthermore, the CXCL5
receptor CXCR2 is expressed in cells other than muscle cells, such as
endothelial, pulmonary, or intestinal epithelial cells. In this context, it is
interesting the recently suggested correlation between obesity and asthma [11].
Strikingly, exacerbation of asthma has been also correlated with increased
expression of both CXCL5 and its receptor CXCR2 [12].
Atherosclerosis is
another obesity-related risk factor in which CXCR2 could play an important
role. This receptor is found in macrophage-rich intimae in human
atherosclerotic lesions, and it has been shown to have a major impact on
macrophage accumulation in advanced lesions [13]. CXCR2 ligands, such as GRO-α participate in this macrophage accumulation and lesion progression,
although they might not have a causative role [14], but rather contribute to
disease progression. CXCL5 could also participate in this process.
Secondary to obesity-induced diabetes is the
development of retinopathy. Development of diabetic retinopathy is a
multifactorial process, and affects as much as 30% of type II diabetic
patients. Much of the damage of retinopathy results from leakage of retinal
blood vessels and inadequate retinal perfusion. [15] Sustained hyperglycemia in
diabetes affects various vasoactive factors, such as vascular endothelial
growth factor [16]. These factors, which are all interrelated, contribute to
development of structural and functional changes in diabetic retinopathy, such
as breakdown of the blood-retina barrier. Participation of CXCL5 in the
development of retinopathy was suggested by the increased levels of this
chemokine found in retinopathy diabetic patients [17].
Finally, but not limited to, CXCL5 could
be also involved in the development of obesity-related inflammatory bowel
disease. Although obesity has not been directly linked to the pathophysiology
of inflammatory bowel disease (IBD), increased macrophage numbers as well as
enhanced production of proinflammatory adipokines in obese patients may create
a favorable environment for disease progression in intestinal inflammation and
IBD [18]. Increased basal cytokine levels associated with obesity, both due to
increased adipocytes numbers and size may predispose to more severe outcomes in
IBD patients. Recent observations indicating that fat tissue is also associated
with immune responses also suggest a link between obesity and gut inflammation
[19]. The proinflammatory effects of CXCL5 are widely accepted. Furthermore,
it was shown that CXCR2 plays a crucial pathophysiological role in experimental
ulcerative colitis in mice [20]. In humans, a marked increase in ENA-78 has
been reported in ulcerative colitis patients [21], and has been shown to be localized
to colonic epithelial cells in IBD tissues [21,22]. Taken together, these
observations suggest that the increased CXCL5 circulating levels observed
during obesity could contribute to the development or progression of IBD.
Studies aiming to elucidate the role of WAT-secreted
CXCL5 in all these obesity-related pathologies are likely to be forthcoming in
the near future. Inhibiting CXCL5 secretion or function in obese individuals
not only ameliorate their insulin sensitivity, but could also decrease the risk
of developing other major obesity-related pathologies.
CXCL5 gene polymorphisms
It is now accepted that type II diabetes is, in part,
inherited. Family studies have revealed that first degree relatives of
individuals with type II diabetes are about 3 times more likely to develop the
disease than individuals without a positive family history of the disease
[23]. It has also been shown that concordance rates for monozygotic twins,
which have ranged from 60-90%, are significantly higher than those for
dizygotic twins. It is therefore clear that type II diabetes has a strong
genetic component. Candidate genes identified sofar include the nuclear
receptor PPARγ, the sulfonylurea receptor ABCC8, the potassium
channel Kir6.2, or the intracellular calcium-dependent cystein protease calpain
10. Taking into account the relative importance of CXCL5 in the development of
insulin resistance we can hypothesize that this chemokine could also be a type
II diabetes susceptibility gene. Indeed several polymorphisms in the CXCL5 gene
have been described. Interestingly, a -156G to C polymorphism in the promoter
of the gene has been associated to increased expression and plasma
concentration of CXCL5. It cannot be excluded that this or other activating
polymorphisms are overrepresented in type II diabetes and obese subjects.
Anti CXCL5-CXCR2 based therapies
Despite the list of new and classical agents designed
for the treatment of type II diabetes, such as
thiazolidinediones, biguanides, meglitinides, or sulphonylureas is increasingly
long, a major challenge remains because even using the more aggressive therapy,
glycemic control in type II diabetic patients may still deteriorate. Our study
may provide a new therapeutic target. We show that inhibition of the
CXCL5-CXCR2 axis, both by CXCR2 antagonists or CXCL5 blocking antibodies
decreases glycemia in mice models of diabetes. Long term treatments are
currently being evaluated in our laboratory. The most interesting feature of
this newly identified target is that is directed not only for insulin
resistance treatment, but could also target diabetes-associated co-morbidities.
It is interesting to notice, at some extent, similarities between other
insulin-sensitizing drugs, such as metformin and anti-CXCL5 therapy. Similar to
metformin, CXCL5 antagonism restores insulin sensitivity, has
anti-atherosclerosis effects, and could be even beneficial as anti-cancer
agent. From this perspective, anti-CXCL5 therapy could be also considered as
anti-aging therapy (reviewed in [24]). Safety studies of the tested molecules,
as well as discovery of new CXCR2 antagonists are guaranteed.
Acknowledgments
LF lab is supported by grants from Agenge Nationale de
la Recherche (ANR), Institut National du Cancer (INCA), and Fondation pour la
Recherche Médicale (FRM).
Conflicts of Interest
The authors of this manuscript have no conflict of
interests to declare.
References
-
1.
van
Dam RM
The epidemiology of lifestyle and risk for type 2 diabetes.
Eur J Epidemiol.
2003;
18(12):
1115
-1125.
[PubMed]
.
-
2.
Zimmet
P
, Alberti
KG
and Shaw
J.
Global and societal implications of the diabetes epidemic.
Nature.
2001;
414(6865):
782
-787.
[PubMed]
.
-
3.
Moller
DE
and Flier
JS.
Insulin resistance-mechanisms, syndromes, and implications.
N Engl J Med.
1991;
325:
938
-948.
[PubMed]
.
-
4.
Martin
G
, Schoonjans
K
, Staels
B
and Auwerx
J.
PPARg activators improve glucose homeostasis by stimulating fatty acid uptake in the adipocytes.
Atherosclerosis.
1998;
137:
75
-80.
.
-
5.
Qatanani
M
and Lazar
MA.
Mechanisms of obesity-associated insulin resistance: many choices on the menu.
Genes Dev.
2007;
21(12):
1443
-1455.
[PubMed]
.
-
6.
Chavey
C
, Lazennec
G
and Lagarrigue
S.
CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance.
Cell Metab.
2009;
9(4):
339
-349.
[PubMed]
.
-
7.
Walz
A
, Burgener
R
, Car
B
, Baggiolini
M
, Kunkel
SL
and Strieter
RM.
Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin 8.
J Exp Med.
1991;
174(6):
1355
-1362.
[PubMed]
.
-
8.
Walz
A
, Schmutz
P
, Mueller
C
and Schnyder-Candrian
S.
Regulation and function of the CXC chemokine ENA-78 in monocytes and its role in disease.
J Leukoc Biol.
1997;
62(5):
604
-611.
[PubMed]
.
-
9.
Wislez
M
, Philippe
C
and Antoine
M.
Upregulation of bronchioloalveolar carcinoma-derived C-X-C chemokines by tumor infiltrating inflammatory cells.
Inflamm Res.
2004;
53(1):
4
-12.
[PubMed]
.
-
10.
Walz
A
, Strieter
RM
and Schnyder
S.
Neutrophil-activating peptide ENA-78.
Adv Exp Med Biol.
1993;
351:
129
-137.
[PubMed]
.
-
11.
Strine
TW
, Balluz
LS
and Ford
ES.
The associations between smoking, physical inactivity, obesity, and asthma severity in the general US population.
J Asthma.
2007;
44(8):
651
-658.
[PubMed]
.
-
12.
Qiu
Y
, Zhu
J
, Bandi
V
, Guntupalli
KK
and Jeffery
PK.
Bronchial mucosal inflammation and upregulation of CXC chemo-attractants and receptors in severe exacerbations of asthma.
Thorax.
2007;
62(6):
475
-482.
[PubMed]
.
-
13.
Boisvert
WA
, Santiago
R
, Curtiss
LK
and Terkeltaub
RA.
A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice.
J Clin Invest.
1998;
101(2):
353
-363.
[PubMed]
.
-
14.
Boisvert
WA
, Rose
DM
and Johnson
KA.
Up-regulated expression of the CXCR2 ligand KC/GRO-alpha in atherosclerotic lesions plays a central role in macrophage accumulation and lesion progression.
Am J Pathol.
2006;
168(4):
1385
-1395.
[PubMed]
.
-
15.
Joussen
AM
, Poulaki
V
and Le
ML.
A central role for inflammation in the pathogenesis of diabetic retinopathy.
Faseb J.
2004;
18(12):
1450
-1452.
[PubMed]
.
-
16.
Adamis
AP
, Miller
JW
and Bernal
MT.
Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy.
Am J Ophthalmol.
1994;
118(4):
445
-450.
[PubMed]
.
-
17.
Meleth
AD
, Agron
E
and Chan
CC.
Serum inflammatory markers in diabetic retinopathy.
Invest Ophthalmol Vis Sci.
2005;
46(11):
4295
-4301.
[PubMed]
.
-
18.
Karagiannides
I
and Pothoulakis
C.
Obesity, innate immunity and gut inflammation.
Curr Opin Gastroenterol.
2007;
23(6):
661
-666.
[PubMed]
.
-
19.
Tilg
H
and Moschen
AR.
Adipocytokines: mediators linking adipose tissue, inflammation and immunity.
Nat Rev Immunol.
2006;
6(10):
772
-783.
[PubMed]
.
-
20.
Buanne
P
, Di Carlo
E
and Caputi
L.
Crucial pathophysiological role of CXCR2 in experimental ulcerative colitis in mice.
J Leukoc Biol.
2007;
82(5):
1239
-1246.
[PubMed]
.
-
21.
Keates
S
, Keates
AC
, Mizoguchi
E
, Bhan
A
and Kelly
CP.
Enterocytes are the primary source of the chemokine ENA-78 in normal colon and ulcerative colitis.
Am J Physiol.
1997;
273(1 Pt 1):
G75
-82.
[PubMed]
.
-
22.
Yang
SK
, Eckmann
L
, Panja
A
and Kagnoff
MF.
Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells.
Gastroenterology.
1997;
113(4):
1214
-1223.
[PubMed]
.
-
23.
Gloyn
AL
The search for type 2 diabetes genes.
Ageing Res Rev.
2003;
2(2):
111
-127.
[PubMed]
.
-
24.
Blagosklonny
MV
Validation of anti-aging drugs by treating age-related diseases.
Aging.
2009;
1:
281
-288.
.