Telomere length regulates ISG15 expression in human cells
Abstract
Endogenous genes regulated by telomere length have not previously been identified in human cells. Here we show that telomere length regulates the expression of interferon stimulated gene 15 (ISG15, 1p36.33). ISG15 expression (RNA and protein) increases in human cells with short telomeres, and decreases following the elongation of telomeres by human telomerase reverse transcriptase (hTERT). The short-telomere-dependent up-regulation of ISG15 is not mediated by replicative senescence/DNA damage signaling or type I interferons. In human skin specimens obtained from various aged individuals, ISG15 is up-regulated in a subset of cells in older individuals. Our results demonstrate that endogenous human genes can be regulated by the length of telomeres prior to the onset of DNA damage signals, and suggest the possibility that cell turnover/telomere shortening may provide a mechanism for adjusting cellular physiology. The upregulation of ISG15 with telomere shortening may contribute to chronic inflammatory states associated with human aging.
Introduction
The ends of human chromosomes (telomeres)
consist of many kilobases of the repeating DNA sequence TTAGGG, ending in ~18
to 600 nucleotides of single-stranded G-rich repeats [1-4]. This 3'
overhang is proposed to be inserted into the double-stranded DNA, forming a
local D-loop and an overall structure called a t-loop [5,6]. In
combination with the binding of telomeric proteins, this structure is thought
to hide the ends of the chromosomes from being recognized as double-strand
breaks needing repair by NHEJ, which would form dicentric chromosomes and cause
a mitotic catastrophe [7,8].
A combination of incomplete replication, processing
events and oxidative damage shortens telomeres with each round of cell
division. This shortening is prevented in the germline and certain stem cells [9] by the
presence of telomerase. Telomerase is a ribonucleo-protein. The RNA component
hTR (hTERC) [10] contains a
C-rich sequence that serves as a template for the addition of TTAGGG repeats
using the reverse transcriptase activity contained within the protein catalytic
subunit hTERT [11,12].
Telomerase activity is repressed in most human somatic tissues during
development [13], leading to
progressive telomere shortening with subsequent cell divisions. When telomeres
become short enough to produce inadequate telomeric protein binding or t-loop
packaging they generate a DNA damage signal, which causes the growth arrest
known as senescence or replicative aging [8]. Many other
stimuli can also induce an irreversible growth arrest which has historically
been called senescence even though the arrest is not telomere based [14].
In addition to protection of linear chromosome ends,
telomeres may also be involved in the regulation of gene expression. The
evidence comes from experiments in which a reporter gene inserted next to a
natural or an artificial telomere results in repression of expression of the
reporter gene, a phenomenon called telomere position effect (TPE). Telomere
position effect (TPE), first described in Drosophila, can result in the
silencing of genes positioned next to telomeres [15,16]. TPE
has been described in a variety of organisms, including
Saccharomyces cerevisiae [17],
Saccharomyces pombe [18],
Trypanosoma brucei [19,20],
Plasmodium falciparum [21],
mice [22] and humans [23,24].
In S. cerevisiae, there appears to be two different mechanisms of TPE [25].
"Classical" TPE is dependent on the SIR family of proteins,
and usually spreads in a continuous fashion for several kb into the
subtelomeric region. A second mechanism involving HAST domains (Hda1-affected
subtelomeric) influences the expression of genes ~10-25 kb from the telomeres.
There is evidence suggesting that both of these mechanisms may respond to
nutrient deprivation or stress, in which relief of TPE contributes to the
upregulation of a variety of subtelomeric genes (reviewed in [25]).
How telomere length might regulate gene
expression in mammals is completely unknown. The efficiency of TPE on model
reporters placed next to healed chromosomes in human cells varies with telomere
length [24]. In
contrast to yeast and parasites, where telomere length is thought to be
relatively constant in normal cells, telomere length decreases with age in
humans, raising the intriguing possibility that telomeric regulation of gene
expression might have a different function in mammals. Replicative senescence
has been shown to be associated with DNA damage signals from
"too-short" telomeres [26,27], so
there is no reason to suspect that TPE is involved in senescence. However,
there is currently no demonstrated mechanism by which cells monitor the length
of their telomeres prior to their becoming short enough to generate a DNA
damage signal. We have speculated that telomere length changes in TPE might be
a mechanism for using cell turnover to monitoring long periods of time (years
or decades) in order to coordinate life-history strategies in long-lived
organisms [28]. Similarly,
length-regulated TPE might be used to change gene expression in tissues
undergoing areas of chronically increased cell turnover due to inflammatory or
other processes, to adjust the physiological response over time. Either of
these hypotheses predicts that the number of genes regulated by telomere length
might be small, since it would not represent a general mechanism of gene
regulation used during development and normal physiology but only in special
circumstances.
In previous studies, reporter genes and artificially
truncated telomeres were used to demonstrate that telomere length could play a
role in the repression of reporter gene expression in mammals [22-24]. No
endogenous genes next to telomeres have yet been shown to be regulated by
telomere length in human cells. None of 34 telomere-proximal genes were found
to vary with telomere length when young and senescent human fibroblasts were
compared [29].
Telomere-proximal genes have been poorly represented in microarry chips because
the complicated repeat nature of the subtelomeric region delayed completion of
the human genome sequence to the very ends of the chromosomes until recently.
In order to perform a more comprehensive search for genes regulated by telomere
length, we constructed a microarray chip containing many newly identified
telomere-proximal genes. We examined gene expression patterns in a variety of
cell types in which we had manipulated telomerase in order to dissociate
telomere length changes from other confounding factors such as time in culture
and DNA damage signals from short telomeres. We here report the identification
of ISG15 (Interferon Stimulated Gene 15kda) as the first endogenous
human gene whose expression is regulated by telomere length. ISG15 is a
stress-response gene that may function as a tumor suppressor and contributor to
inflammatory responses [30]. This
raises many intriguing issues concerning the role of telomere length prior to
replicative arrest in the physiology of human aging.
Results
Identification of genes
up-regulated with telomere shortening
Table 1 lists a panel of human fibroblasts and mammary epithelial cells with
variations in telomere lengths used in the present studies. To examine the
correlation of gene expression and telomere shortening, we used a "Telo-Chip",
a customized microarray containing 1,323 potential subtelomeric genes (within
1,000 kilobase pairs from the telomeres) representing all 92 telomere ends. The
Telo-Chip also contained 92 random control genes, 12 housekeeping genes and 198
other genes (GEO Datasets, GSE6799). The initial screen was performed using
total RNA extracted from proliferating young cells with long telomeres (BJ-18,
HME31-26, and IMR90-26), cells proliferating with short telomeres (BJ-78,
HME31E6/7-69, IMR90-62, and IMR90E6/7-84), and cells proliferating with
experimentally elongated telomeres (BJ18hTERT-148, HME31hTERT-68, and
IMR90hTERT-134) (Table 1). Probes were hybridized to the Telo-Chips, and data
were analyzed with GeneSpring software. Approximately 24 genes that showed at
least a 1.5-fold increase in all the cells with short telomeres were further
examined using quantitative PCR (q-PCR).
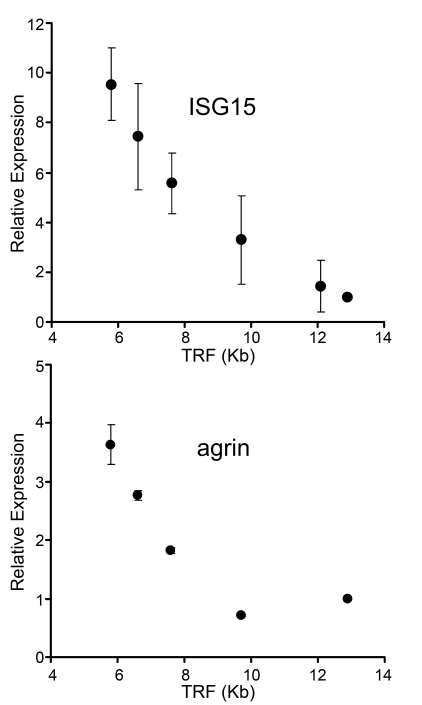
Figure 1. Up-regulation of gene expression with telomere shortening in human fibroblasts. mRNA levels of ISG15
and agrin were assayed by qantitative PCR
in human fibroblasts with different telomere lengths. Results from at least
three separate experiments are shown as means ± SEM. GAPDH was used as an
internal normalization control. All values were then normalized to the
level (=100%) of mRNA in young cells with long telomeres (PD18)
(see Table 1). Results show increase of ISG15 and agrin expression with telomere
shortening. Similar results for ISG15 were also obtained in IMR90 cells,
NHK and HME epithelial cells (Supplementary Figure 1).
A major problem with
previous efforts to identify TPE regulated genes is a failure to distinguish
telomere length effects from multiple confounding influences, such as the
length of time in culture, DNA damage signaling from short telomeres and clonal
succession [31] in
heterogeneous primary cultures. Only a single gene, interfereon stimulated gene 15
(ISG15), met our criteria for being regulated by telomere length when
examined using the series of BJ fibroblasts with different natural and
manipulated telomere lengths (see below). The up-regulation of ISG15 mRNA was
also observed in a human lung fibroblast cell line (IMR90) and in human breast
and kidney epithelial cell lines (HME31 and NHK) with short telomeres
(see Supplementary Figure 1). ISG15 is a ubiquitin-like molecule that can be conjugated to other
proteins (reviewed in [30,32]) to modify
their function [33]. Figure 1
compares the behavior of ISG15 with Agrin, one of many genes that
failed to meet our criteria. Agrin is a neuronal aggregating factor [34] and was
selected for illustrative purposes because it is located just
centromere-proximal to ISG15 on chromosome 1p36.33. The mRNA levels of
both ISG15 and agrin increase in human skin fibroblasts as their telomeres
shortened with population doublings between PD18 and PD83 in culture (Figure 1).
Up-regulation of agrin expression is associated with DNA damage signaling and/or replicative
senescence
As cells age, telomeres
shorten and the cells eventually enter replicative senescence due to
p53-mediated DNA damage signaling from the shortest telomeres [27]. This
raised the possibility that damage signaling contributed to the up-regulation
of gene expression we observed in cells with short telomeres. In order to
examine this question, we elongated the shortest telomeres by expressing hTERT
in human fibroblasts BJ13-141. BJ13 is a clone in which telomerase flanked by
loxP sites was expressed for seven doublings (beginning at population doubling
(PD) 85, approximately five doublings before the parental culture senesced).
After seven doubling hTERT was excised by cre-recombinase. The preferential
elongation of the shortest telomeres conferred an extra 50 doublings before
this clone senesced at PD145 with a telomere length of ~4 kb rather than the
usual ~6 kb length at senescence of normal BJ fibroblasts [35]. We
characterized the cells both shortly after re-introducing hTERT (early
expression, before significant telomere elongation had occurred) and again ~50
doublings later (long-term expression, after telomeres were significantly
elongated). The expression of the senescence marker SA-β galactosidase (Figure 2A) and γH2AX DNA damage foci (Figure 2B) increased as cells approached
senescence in both BJ-80 and BJ13-141 cells. Expressing hTERT rapidly
eliminated the SA-β-gal staining and reduced the γH2AX foci to the
levels of "young" cells (Figure 2A and B), even after only six
doublings (BJ13-141+6H) when the average telomere length was still close to
that of the cells at PD141 (Table 1), indicatingthe expression ofhTERT eliminated the DNA damage signaling
and replicative senescence induced by short telomeres. Similarly, the
expression of the p53 transcriptional target p21 was elevated in the two near
senescent cell types, and its expression also disappeared in cells with both
transient (BJ13-141+6H) and long-term (BJ13-141+53H) introduction of hTERT
(Figure 2C), further confirming the inhibition of DNA damage signaling from
short telomeres by the expression of hTERT.
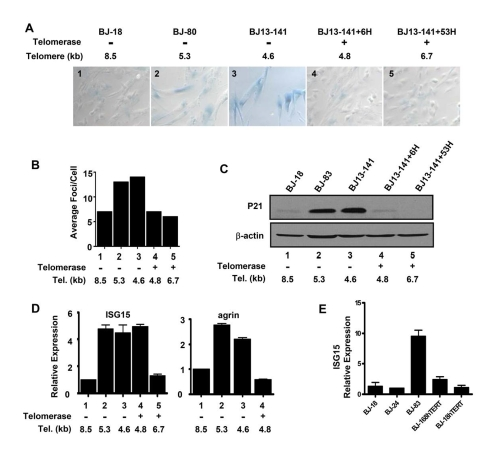
Figure 2. Replicative senescence and DNA damage signaling independent up-regulation of ISG15 expression in cells with short telomeres. (A)
Short and long-term expression of hTERT rescued cells from replicative
senescence. BJ cells with short telomeres (BJ-80 and BJ13-141) exhibited
significant increases in the number of SA-β-Gal positive cells;
whereas, the cells with long telomeres (BJ-18) did not show SA-β-Gal
staining. Exogenous telomerase rapidly eliminated senescent cells
(BJ13-141+6H, when only the shortest telomeres had been lengthened) as well
as after bulk telomere elongation had occurred (BJ13-141+53H). Rare fields
with an SA-β-Gal staining positive cell were selected for the last two
images to validate the staining procedure. The number in each image is a
key to the cell lines used in B-D. (B) γ-H2AX staining shows
that exogenous hTERT rapidly eliminates DNA damage signalling due to short
telomeres. Approximately 500 nuclei of each cell line were analyzed using
Metasystems software (Metasystems, Germany). (C) Western blot shows
that p21, a transcriptional target of DNA damage-induced p53 signaling,
rapidly disappeared following the introduction of telomerase to elongate
the shortest telomeres. (D) Q-PCR showing that ISG15
expression remained high in BJ cells rescued from replicative
senescence/DNA damage signaling after only a few doublings in the presence
of exogenous telomerase when telomeres were still short (ISG15, column 4),
while elongation of the telomeres after 53 doublings led to decreased
expression (ISG15, column 5). In contrast, elimi-nating replicative senescence/DNA damage following a short exposure
to telomerase caused a decrease in the expression of agrin (agrin, column
4). Agrin thus did not meet our criteria for telomere length regulation,
since its increase in old cells (agrin, columns 2&3) is secondary to
senescence and/or DNA damage (column 4). (E) BJ cells
overexpressing hTERT and having long telomeres express low levels of ISG15.
Table 1. Cell lines and strains with different telomere length and telomerase activity.
# Cell lines and strains used for Microarray analysis.
* TRAP = telomeric repeat amplification protocol. + telomerase positive and – telomerase negative.
† Telomere length was determined by Southern blot analysis (TRF). kb, kilobase, NA, not available.
§ PD = population doublings.
| Human
Cell Lines and Strains | Description | TRAP* | Telomere Length (kb)† |
| Skin
Fibroblasts | | | |
| | | |
|
BJ-18# |
PD
18§ |
-
|
12.9
|
|
BJ-24
|
PD
24
|
-
|
12.1
|
|
BJ-50
|
PD
50
|
-
|
9.7
|
|
BJ-72
|
PD 72
|
-
|
7.6
|
|
BJ-83# |
PD 83
|
-
|
5.8
|
|
BJE6/7-78
|
Expressing oncoproteins
E6/E7 to block p53/pRB signaling, PD 78
|
-
|
NA
|
| | | |
|
BJhTERT-168# |
Expressing hTERT, PD 168
|
+
|
13.4
|
|
BJ18hTERT-148
|
Expressing hTERT, PD 148
|
+
|
10.6
|
|
BJ13-141
|
Near senesence with very
short telomeres, PD 141
|
-
|
4.6
|
|
BJ13-141+6H
|
6 PD after introducing
hTERT into BJ13-141 cells to remove DNA damage signaling and replicative
senescence, still with short telomeres
|
+
|
4.8
|
|
BJ13-141+53H
|
53 PD after introducing
hTERT into BJ13-141 cells, with elongated telomeres, PD 194
|
+
|
6.7
|
|
BJB14-411
|
Minimal expression of
hTERT, short telomeres, PD 411
|
+
|
2.7
|
|
BJB14-411+5H
|
5 PD after introducing
hTERT into BJB14-411 cells to remove DNA damage signaling and replicative
senescence, still with short telomeres
|
+
|
2.7
|
|
BJB14-411+50H
|
50 PD after introducing hTERT into BJB14-411 cells, with
elongated telomeres
|
+
|
4.8
|
| Lung
Fibroblasts | | | |
|
IMR90-24.6# |
PD 24.6
|
-
|
9.9
|
|
IMR90-61.7# |
PD 61.7
|
-
|
6.8
|
|
IMR90E6/7-84.1# |
Expressing oncoproteins
E6/E7 to block p53/pRB
signaling, PD84.1
|
-
|
7.2
|
|
IMR90hTERT-124.6
|
Expressing hTERT, PD 124.6
|
+
|
13.3
|
| Mammary Epithelial
Cells | | | |
|
HME31-25.9# |
PD25.9
|
-
|
3.9
|
|
HME31E/6-69.3# |
Expressing oncoproteins
E6/E7 to block p53/pRB signaling, PD69.3
|
-
|
2.6
|
|
HME31hTERT-67.7# |
Expressing hTERT, PD67.7
|
+
|
3.7
|
We then examined the
expression of ISG15 and agrin in BJ13 cells early and late after expressing of
hTERT. The mRNA level of agrin was
up-regulated in cells with short telomeres (Figures 1B and 2D). However, the
expression of agrin decreased in BJ13-141+6H cells (Figure 2D), suggesting the
up-regulation of agrin in cells approaching senescence was due to DNA damage
signaling perhaps as part of replicative senescence. This pattern was typical
of most of the 22 other genes that did not fit our criteria for co-regulation
by telomere length. In contrast to agrin, in spite of the elimination of DNA
damage signaling and replicative senescence, ISG15 expression (mRNA in Figure
2, protein in Figure 3) remained at a high level in BJ13-141+6H cells,
indicating that the up-regulation of ISG15 expression in cells with short
telomeres was not due to DNA damage signaling and/or replicative senescence.
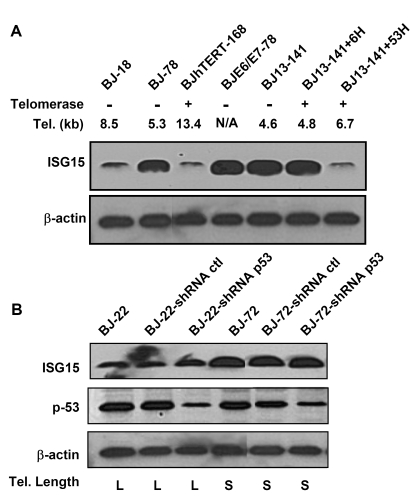
Figure 3. p53 is not involved in the up-regulation of ISG15 expression in cells with short telomeres. (A)
Western blot of ISG15 in human fibroblasts with different telomere lengths.
Both free and conjugated (data not shown) ISG15 increase with telomere
shortening (lanes 1 and 2) and in cells with short telomere (BJ13-141
before and after expressing telomerase for 6 doublings, lanes 5 and 6).
Expression of HPV16 E6, which degrades p53, had no effect on ISG15 protein
expression, while elongation of telomeres by the expression of telomerase
for 53 doublings returned ISG15 levels to baseline. β-Actin served as a loading
control. A typical result from three independent experiments is s shown. (B)
Western bolt analysis of ISG15 and total p53 protein in young and old human
fibroblasts with long and short telomeres, respectively. Stable expression
of shRNA led to significant (> 80%) reduction in the level of p53
protein compared to those in parental and mock infection cells in both
young and old cells. The reduction of p53 protein levels had no effect on
the expression of ISG15. β-actin served as
a loading control.
Telomere length is involved in the regulation of ISG15
expression
To examine the role of telomere length in the
regulation of ISG15 expression, we cultured the BJ13-hTERT cells for an
extended period to elongate the telomere length. After 53 population doublings
(BJ13-hTERT+53H), the average telomere length was elongated from 4.6kb (BJ13-141)
to 6.7kb (Table 1). With telomere elongation, the ISG15 expression decreased to
the level of young cells (Figure 2D, lane 5 and Figure 3A), sug-gesting that ISG15 expression is associated with
telomere length in human fibroblasts. This is further confirmed by the
observation that populations of BJ fibroblasts overexpressing hTERT that had
long telomeres (Table 1) expressed ISG15 at levels of young cells (Figure 2E
and 3A).
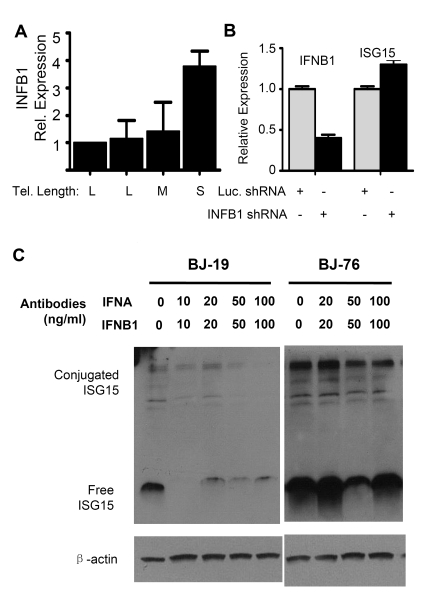
Figure 4. The up-regulation of ISG1 5 expression in cells with short telomeres does not depend on interferon beta1 (INFB1). (A) Q-PCR analysis
of mRNA levels of INFB1 in human fibroblasts with different telomere
length (BJ-18 to BJ-83). Results from at least three separate experiments
are shown as means ± SEM. GAPDH was used as an internal normalization
control. All values were then normalized to the level (=100%) of mRNA in
young cells (PD18) with long telomeres. Results show an increase of INFB1
expression with telomere shortening. (B) Stable knock down of INFB1
by shRNA in BJ cells with short telomeres did not reduce the expression of
ISG15. mRNA levels of ISG15 and INFB1 were quantified by q-PCR. (C)
Western blot showing that blocking antibodies to both IFN α and β
reduced the levels of both free and conjugated ISG15 in young BJ
fibroblasts, but failed to reduce expression in old cells with short
telomeres. Young (BJ-18) and old (BJ-76) cells were treated with neutralizing
antibodies against INFA and INFB1.
Up-regulation of ISG15
with telomere shortening is independent of the expression of p53 and type I
interferons
ISG15 expression can be regulated by
genotoxic stress [36-38]and type I interferons [32]. The
up-regulation of ISG15 in the BJ fibroblast series is not associated
with p53 expression and function as shown by knocking-down the expression of
p53 in both young and old BJ cells. Reducing p53 levels had no effect on ISG15
protein expression (Figure 3B). Similarly, blocking of p53 functions by
overexpression of E6 had no effect of ISG15 expression (Figure 3A, lane 4).
Q-PCR of interferon
α (INFA) showed no change in expression between young and old cells
(data not shown) while interferon β1 (INFB1) increased (Figure 4A).
Stably reducing INFB1 expression by ~60% in PD76 BJ cells using shRNA produced
no change in the expression of ISG15 (Figure 4B), indicating that the increase
in ISG15 expression in cells with short telomeres is not a result of increased
interferon expression. This is further confirmed by the fact that adding
blocking antibodies to both INFA and INFB1 to the medium failed to reduce ISG15
expression in old cells. However, the antibodies did reduce ISG15 expression in
young (PD19) BJ fibroblasts (Figure 4C), suggesting that much of the basal
level of ISG15 expression in young cells is secondary to the interferons they
secrete. However, the increase seen in cells with short telomeres is controlled
by an independent mechanism other than up-regulation of type I interferon
expression, since blocking antibodies failed to affect expression.
Up-regulation of ISG15
with aging in human skin biopsies
Significant up-regulation
in ISG15 protein expression with telomere shortening was also confirmed by
immunofluorescence staining (Figure 5A) in human skin fibroblasts. Intense
staining was observed in the cells with
short telomeres at PD78. Since telomeresshor- ten with in vivo aging
[39], we also
examined ISG15 expression in human skin tissues from donors of different ages (Figure 5B). The older adult group (ages 53-68) showed a significant increase in the
number of ISG15-positive cells in the dermis (Figure 5C), indicating that
up-regulation of ISG15 can occur in vivo.
Expression of ISG15 is not regulated by
"classical" TPE
ISG15 is
located on 1p36.33, about 1M base pairs from the telomere. In yeast, classical
TPE spreads in a continuous fashion from the telomere, so that all of the genes
between a TPE silenced gene and the telomere would also be silenced. We
examined eight other genes located between ISG15 and the telomere, and
none of them showed a correlation between gene expression and telomere length
in BJ cells (Supplementary Figure 2). Whether ISG15 is regulated by
telomeric looping or indirectly by other
telomere-length regulated genes remains to be determined.
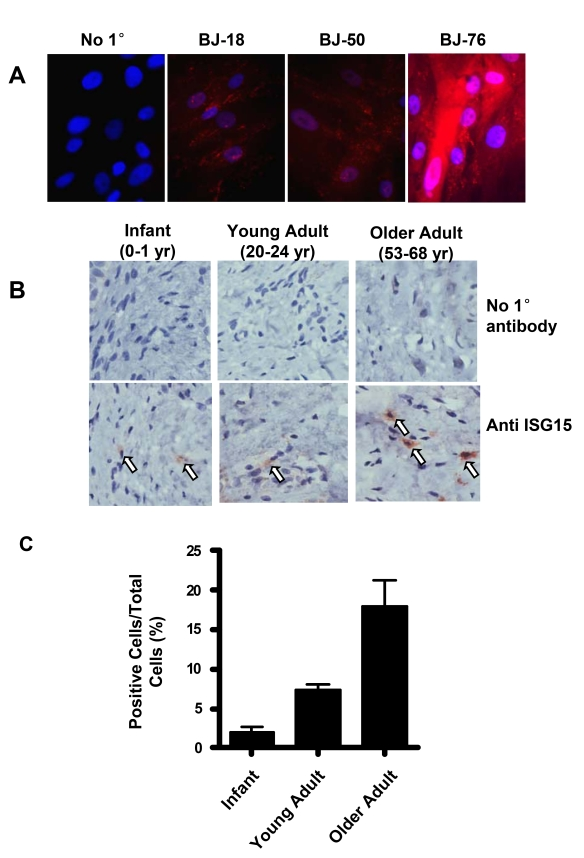
Figure 5. ISG15 is increased in human skin with aging. (A)
Immunofluorescence staining of ISG15 in BJ cells at different population
doublings. The negative sample was treated identically except no primary
antibody was added. Nuclei were stained with DAPI. Staining intensity
increases in cells with short telomeres. (B) Immunochemical staining
illustrating an age-dependent up-regulation of ISG15 expression in the
dermis of human skin tissues. 2-4 cases were examined in each group.
Infant, 0-1 year old; young adult, 20-24 year old; older adult, 53-68 year
old. No primary antibody was added to the negative control. (C)
Quantitation of the results of all the samples described above. 8-10 random
fields were counted for each sample.
Discussion
In this study, we analyzed more than 1,300
subtelomeric genes and open reading frames (ORFs) and another 300 genes of
interest using a customized microarray chip. Using young (long telomeres) and
old (short telomeres) human cells we found genes whose up-regulation is
associated with DNA damage and senescence when telomeres shorten, e.g. Agrin.
We also demonstrated that up-regulation of ISG15 with telomere shortening
was not due to genotoxic stress but correlated with telomere length, providing
evidence that in addition to protecting linear chromosome ends, telomeres are
also involved in the regulation of gene expression in human cells.
ISG15 [40] is the
founding member of a family of ubiquitin-like modifiers (Ubls) that include
SUMO, NEDD8, HUB1, APG12 and APG8 [41,42]. ISG15
has the structure of a di-ubiquitin molecule, and the E1 and E2 enzymes that
prepare ISG15 for conjugation [UBE1L [43] and
UBCH8/UBE2L6 [44]] are also
ubiquitin-conjugating enzymes. Although there appears to be common pathways
involving ubiquitin and ISG15, conjugation does not increase the degradation of
ISGylated proteins [45,46].
Although many targets of ISGylation have now been identified [45], the
consequences of modification by ISG15 are in most cases unknown. ISG15 is able
to inhibit viral release by blocking the neddylation of viral assembly proteins
[47]. In
addition, free ISG15 can be secreted [48] and there
is evidence that it has cytokine-like immuno-modulatory properties [49-51].
There are three properties of ISG15 that
make it particularly intriguing as a gene regulated by telomere length. Most of
the genes that are regulated in yeast by TPE can be characterized as being
related to stress/nutritional deprivation [25]. ISG15 is
expressed as part of the innate immunity/stress response pathway, and its regulation
by telomere length is consistent with a conservation of telomeric input into
the control of some aspects of the stress response. Secondly, secreted ISG15
may contribute to age-related inflammation. ISG15 can stimulate the production
of the proinflammatory interferon, IFNγ, in CD3+ cells [49,50].
Although IFNγ alone did not stimulate the proliferation and cytotoxicity
of NK cells in mixed cultures with CD3+ lymphocytes, their growth and
non-MHC-restricted killing activity was greatly increased following exposure to
free ISG15 [49].
Unconjugated ISG15 is also a chemotactic factor for neutrophils [51].
Collectively, these results suggest that secreted ISG15 could contribute to a
pro-inflammatory environment. It is well known that inflammation is associated
with a large number of age-related physiological conditions, such as sarcopenia
[52],
atherosclerosis and cardiovascular diseases [53,54]. In
addition, neurodegenerative diseases [55,56], renal
failure [57],
osteoporosis [58], and the
metabolic syndrome/diabetes [59] are
associated with inflammation, and it has been suggested that dysregulation of
inflammation is a fundamental aging mechanism [60-62].
The role of replicative aging in human aging has long
been debated, and the relatively low number of senescent cells in tissues from
elderly donors has been used as an argument that it is not relevant to
organismal aging. However, it is well established that telomere length declines
with age in a large number of different human tissues [39]. Since
ISG15 expression increases progressively with decreasing telomere length before
cells become senescent (Figure 1A), the effects of replicative aging/telomere
shortening on organ function could also be exhibited before cells became
senescent.
Finally, there is suggestive evidence that ISG15 can
function as a tumor suppressor [30].
Premalignant cells have to undergo many divisions before they become invasive,
and it is thought that the primary function of replicative aging is to limit
the number of available divisions as a brake against cancer formation [63].
Furthermore, hyperproliferation provides one of the conditions favoring the
development of malignancies. It is possible that the increased ISG15 expression
that accompanies proliferation induced telomere shortening functions to create
an internal or external environment that restricts tumor progression. Terminal
telomere shortening has been viewed as a "two-edged sword", since in
the absence of p53 very short telomeres can cause genomic instability and may
contribute to the formation of cancer. ISG15 regulation may be one mechanism by
which telomere shortening suppresses tumor formation prior to the telomeres
becoming sufficiently short to cause problems of genomic instability.
At least five genes between ISG15 and the
telomere were not regulated by telomere length (Supplementary Figure 2).
Discontinuous TPE can occur in yeast [64,65], and
DNA looping [66] might be
one mechanism that TPE could extend 1Mb from the telomere to the ISG15
gene without controlling the expression of the intervening genes.
Alternatively, telomere length could control ISG15 indirectly by
affecting a different telomeric gene that we have not yet identified.
Regardless of whether it is direct TPE or not, the consistent change in gene
expression that we observe when we manipulate telomeres establishes that ISG15
is regulated by telomere length.
The fact that only a single gene, ISG15, was
identified in this study is not unexpected. There are reasons to suspect
cell-type specific variation in the expression of telomere-length co-regulated
genes [67]. In the
absence of gene regulation by telomere length cells would lack the ability to
monitor telomere length prior to the point when they become so short that they
generate a DNA damage signal. The role of telomere shortening in monitoring
cell turnover or the passage of time may vary in different tissues. There may
be many other genes that are tissue-specific and only expressed in particular
cell types as a function of telomere length.
The finding that ISG15 expression correlates to the
telomere length in human cells suggests that telomeres are involved in the
regulation of gene expression and may be involved in broader physiological
functions beyond the protection of the linear ends of chromosomes. Shortening
of telomeres occurs with aging both in vivo and in cell culture. It will
be of great interest to explore the role of ISG15 in tumor suppression and age-associated
inflammatory condi-tions, and finally to identify additional genes and pathways
regulated by telomere length and how they impact human biology.
Methods
Microarray analysis.
Microarray
analysis was carried out at the UT Southwestern Medical Center at Dallas DNA
Microarray Core Facility (http://microarray.swmed.edu/).
Briefly, 60-70 nt oligo-nucleotides representing the selected genes were
synthesized (Operon Biotechnologies, Inc.) and printed on glass slides (Cat.
PXP-U 50B, Full Moon BioSystems, CA). Total RNAs isolated from human skin
fibroblast (BJ-19, BJ-78, BJ18hTERT-148 and BJhTERT-168), human lung
fibroblasts (IMR90-26.4, IMR90-61.7, IMR90E6/7-84.1 and IMR90hTERT-134.6) and
human mammary epithelial cells (HME31-25.9, HME31E6/7-69.3 and HME31hTERT-67.7)
were used to make fluorescence-labeled cRNAs for hybridization by following the
procedure provided by the Microarray Core Facility
(http://microarray.swmed.edu/protocols/General_spotted_array.htm). The slides were scanned
and the data were analyzed using GeneSpring software.
Quantativite PCR.
Quantatitive
PCR was carried out using the human Universal Probe Library (Cat. 04683633001)
and TaqMan Master (Cat. 04535286001) from Roche following the manufacture's
manual. The experiments were repeated 2-3 times, and the relative expression
level of each gene was normalized to the young cells with long telomeres
(BJ-18, IMR90-24.6, and HME31-25.9). The primers and probe for each gene were
selected by using ProbeFinder software provided by Roche
(https://www.roche-applied-science.com/sis/rtpcr/upl/adc.jsp). GAPDH was used as loading control.
Western blot analysis.
Western blot
analysis was carried out as
described [68]. Monoclonal
antibody against human ISG15 was generously provided by Dr. Ernest Borden (Cleveland Clinic Foundation, 1:1,000).
Antibody against p53 was purchased from Calbiochem (OP-43, 1:1,000), and
antibody against ß-actin was from Sigma (A1978, 1:20,000).
shRNAs.
The retroviral-vector based shRNA construct against
human p53 gene was provided by Dr. J.D. Minna (Hamon Center for Therapeutic
Oncology Research and Departments of Internal Medicine, Pharmacology,UT Southwestern
Medical Center at Dallas). The retroviral-vector based shRNA constructs against
human INFβ1 gene were purchased from OpenBiosystems (Cat.
RHS1764-97198161, RHS1764-97197488, and RHS1764-9206903).
Supplementary Materials
ISG15 expression in other cells lines. The expression of ISG15 in other cell lines with
long (young and hTERT expression) and short (old) telomeres.
PCR reagents was analyzed by q-PCR using probes from Roche Applied
Science. The relative levels are normalized to that in young
cells for each cell type. Telomere length was determined by
Southern blot analysis (TRF). (A) IMR 90 lung fibroblasts.
(B) HME31 mammary epithelial cells. (C) NHK human kidney epithelial cells.
Expression of other 1p subtelomeric genes in BJ cells with different telomere lengths.
q-PCR was used to examine eight genes between ISG15 and
the telomere. No signals were detected for genes XM_001127463,
XM_926974 and XR_015286, so only five genes are shown to compare
with ISG15. PCR reagents and probes were from Roche Applied
Science. GAPDH was used as an internal normalization control.
The relative levels are normalized to that in young BJ cells.
The numbers in parentheses indicate the distance of the gene
from the 1p telomere (A) ISG15, (B) NM_018948, (C) XR_015292,
(D) XR_017611, (E) NM_001005484, (F) XR_017612.
Acknowledgments
This work was supported by Public Health Service grant
AG07992 (W.E. Wright) from the National Institute on Aging, and grant HG 000567 from the National Institutes of Health and
The W.W. Smith Charitable Trust H0506 (H. Riethman), and grant NNJ05HD36G from
the National Aeronautics and Space Administration (J.W.Shay).
We thank Dr. Ernest Borden (Cleveland Clinic Foundation, Cleveland, OH) for providing the
antibody against human ISG 15.
Conflicts of Interest
The authors declare they have no financial conflicts
of interest.
References
-
1.
Moyzis
RK
, Buckingham
JM
, Cram
LS
, Dani
M
, Deaven
LL
, Jones
MD
, Meyne
J
, Ratliff
RL
and Wu
JR.
A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes.
Proc Natl Acad Sci U S A.
1988;
85:
6622
-6626.
[PubMed]
.
-
2.
Makarov
VL
, Hirose
Y
and Langmore
JP.
Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening.
Cell.
1997;
88:
657
-666.
[PubMed]
.
-
3.
Chai
W
, Shay
JW
and Wright
WE.
Human telomeres maintain their overhang length at senescence.
Mol Cell Biol.
2005;
25:
2158
-2168.
[PubMed]
.
-
4.
Zhao
Y
, Hoshiyama
H
, Shay
JW
and Wright
WE.
Quantitative telomeric overhang determination using a double-strand specific nuclease.
Nucleic Acids Res.
2008;
36:
e14
[PubMed]
.
-
5.
Griffith
JD
, Comeau
L
, Rosenfield
S
, Stansel
RM
, Bianchi
A
, Moss
H
and de Lange
T.
Mammalian telomeres end in a large duplex loop.
Cell.
1999;
97:
503
-514.
[PubMed]
.
-
6.
Murti
KG
and Prescott
DM.
Telomeres of polytene chromosomes in a ciliated protozoan terminate in duplex DNA loops.
Proc Natl Acad Sci U S A.
1999;
96:
14436
-14439.
[PubMed]
.
-
7.
Ferreira
MG
, Miller
KM
and Cooper
JP.
Indecent exposure: when telomeres become uncapped.
Mol Cell.
2004;
13:
7
-18.
[PubMed]
.
-
8.
Shay
JW
and Wright
WE.
Senescence and immortalization: role of telomeres and telomerase.
Carcinogenesis.
2005;
26:
867
-874.
[PubMed]
.
-
9.
Lee
HW
, Blasco
MA
, Gottlieb
GJ
, Horner
JW 2nd
, Greider
CW
and DePinho
RA.
Essential role of mouse telomerase in highly proliferative organs.
Nature.
1998;
392:
569
-574.
[PubMed]
.
-
10.
Feng
J
, Funk
WD
, Wang
SS
, Weinrich
SL
, Avilion
AA
, Chiu
CP
, Adams
RR
, Chang
E
, Allsopp
RC
and Yu
J.
The RNA component of human telomerase.
Science.
1995;
269:
1236
-1241.
[PubMed]
.
-
11.
Nakamura
TM
, Morin
GB
, Chapman
KB
, Weinrich
SL
, Andrews
WH
, Lingner
J
, Harley
CB
and Cech
TR.
Telomerase catalytic subunit homologs from fission yeast and humans.
Science.
1997;
277:
955
-959.
[PubMed]
.
-
12.
Meyerson
M
, Counter
CM
, Eaton
EN
, Ellisen
LW
, Steiner
P
, Caddle
SD
, Ziaugra
L
, Beijersbergen
RL
, Davidoff
MJ
, Liu
Q
, Bacchetti
S
, Haber
DA
and Weinberg
RA.
hEST2, the putative human telomerase catalytic subunit gene, is up- regulated in tumor cells and during immortalization.
Cell.
1997;
90:
785
-795.
[PubMed]
.
-
13.
Wright
WE
, Piatyszek
MA
, Rainey
WE
, Byrd
W
and Shay
JW.
Telomerase activity in human germline and embryonic tissues and cells.
Dev Genet.
1996;
18:
173
-179.
[PubMed]
.
-
14.
Itahana
K
, Campisi
J
and Dimri
GP.
Mechanisms of cellular senescence in human and mouse cells.
Biogerontology.
2004;
5:
1
-10.
[PubMed]
.
-
15.
Levis
R
, Hazelrigg
T
and Rubin
GM.
Effects of genomic position on the expression of transduced copies of the white gene of Drosophila.
Science.
1985;
229:
558
-561.
[PubMed]
.
-
16.
Hazelrigg
T
, Levis
R
and Rubin
GM.
Transformation of white locus DNA in drosophila: dosage compensation, zeste interaction, and position effects.
Cell.
1984;
36:
469
-481.
[PubMed]
.
-
17.
Gottschling
DE
, Aparicio
OM
, Billington
BL
and Zakian
VA.
Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription.
Cell.
1990;
63:
751
-762.
[PubMed]
.
-
18.
Nimmo
ER
, Cranston
G
and Allshire
RC.
Telomere-associated chromosome breakage in fission yeast results in variegated expression of adjacent genes.
Embo J.
1994;
13:
3801
-3811.
[PubMed]
.
-
19.
Horn
D
and Cross
GA.
A developmentally regulated position effect at a telomeric locus in Trypanosoma brucei.
Cell.
1995;
83:
555
-561.
[PubMed]
.
-
20.
Yang
X
, Figueiredo
LM
, Espinal
A
, Okubo
E
and Li
B.
RAP1 is essential for silencing telomeric variant surface glycoprotein genes in Trypanosoma brucei.
Cell.
2009;
137:
99
-109.
[PubMed]
.
-
21.
Scherf
A
, Hernandez-Rivas
R
, Buffet
P
, Bottius
E
, Benatar
C
, Pouvelle
B
, Gysin
J
and Lanzer
M.
Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum.
Embo J.
1998;
17:
5418
-5426.
[PubMed]
.
-
22.
Pedram
M
, Sprung
CN
, Gao
Q
, Lo
AW
, Reynolds
GE
and Murnane
JP.
Telomere position effect and silencing of transgenes near telomeres in the mouse.
Mol Cell Biol.
2006;
26:
1865
-1878.
[PubMed]
.
-
23.
Koering
CE
, Pollice
A
, Zibella
MP
, Bauwens
S
, Puisieux
A
, Brunori
M
, Brun
C
, Martins
L
, Sabatier
L
, Pulitzer
JF
and Gilson
E.
Human telomeric position effect is determined by chromosomal context and telomeric chromatin integrity.
EMBO Rep.
2002;
3:
1055
-1061.
[PubMed]
.
-
24.
Baur
JA
, Zou
Y
, Shay
JW
and Wright
WE.
Telomere position effect in human cells.
Science.
2001;
292:
2075
-2077.
[PubMed]
.
-
25.
Mondoux
M
and Zakian
VA.
Telomere Position Effect: silencing near the end. In: de Lange T, Lundblad V, Blackburn EH, editors. Telomeres. 2nd ed.
Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press;.
2006;
261
-316.
.
-
26.
d'Adda
di Fagagna F
, Reaper
PM
, Clay-Farrace
L
, Fiegler
H
, Carr
P
, Von
Zglinicki T
, Saretzki
G
, Carter
NP
and Jackson
SP.
A DNA damage checkpoint response in telomere-initiated senescence.
Nature.
2003;
426:
194
-198.
[PubMed]
.
-
27.
Zou
Y
, Sfeir
A
, Gryaznov
SM
, Shay
JW
and Wright
WE.
Does a sentinel or a subset of short telomeres determine replicative senescence.
Mol Biol Cell.
2004;
15:
3709
-3718.
[PubMed]
.
-
28.
Wright
WE
and Shay
JW.
Time, telomeres and tumors; is cellular senescence more than an anticancer mechanism.
Trends in Cell Biology.
1995;
5:
293
-296.
[PubMed]
.
-
29.
Ning
Y
, Xu
JF
, Li
Y
, Chavez
L
, Riethman
HC
, Lansdorp
PM
and Weng
NP.
Telomere length and the expression of natural telomeric genes in human fibroblasts.
Hum Mol Genet.
2003;
12:
1329
-1336.
[PubMed]
.
-
30.
Andersen
JB
and Hassel
BA.
The interferon regulated ubiquitin-like protein, ISG15, in tumorigenesis: friend or foe.
Cytokine Growth Factor Rev.
2006;
17:
411
-421.
[PubMed]
.
-
31.
Salk
D
, Au
K
, Hoehn
H
, Stenchever
MR
and Martin
GM.
Evidence of clonal attenuation, clonal succession, and clonal expansion in mass cultures of aging Werner's syndrome skin fibroblasts.
Cytogenet Cell Genet.
1981;
30:
108
-117.
[PubMed]
.
-
32.
Dao
CT
and Zhang
DE.
ISG15: a ubiquitin-like enigma.
Front Biosci.
2005;
10:
2701
-2722.
[PubMed]
.
-
33.
Okumura
F
, Zou
W
and Zhang
DE.
ISG15 modification of the eIF4E cognate 4EHP enhances cap structure-binding activity of 4EHP.
Genes Dev.
2007;
21:
255
-260.
[PubMed]
.
-
34.
Kummer
TT
, Misgeld
T
and Sanes
JR.
Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost.
Curr Opin Neurobiol.
2006;
16:
74
-82.
[PubMed]
.
-
35.
Steinert
S
, Shay
JW
and Wright
WE.
Transient expression of human telomerase extends the life span of normal human fibroblasts.
Biochem Biophys Res Commun.
2000;
273:
1095
-1098.
[PubMed]
.
-
36.
Polyak
K
, Xia
Y
, Zweier
JL
, Kinzler
KW
and Vogelstein
B.
A model for p53-induced apoptosis.
Nature.
1997;
389:
300
-305.
[PubMed]
.
-
37.
Hermeking
H
, Lengauer
C
, Polyak
K
, He
TC
, Zhang
L
, Thiagalingam
S
, Kinzler
KW
and Vogelstein
B.
14-3-3 sigma is a p53-regulated inhibitor of G2/M progression.
Mol Cell.
1997;
1:
3
-11.
[PubMed]
.
-
38.
Desai
SD
, Mao
Y
, Sun
M
, Li
TK
, Wu
J
and Liu
LF.
Ubiquitin, SUMO-1, and UCRP in camptothecin sensitivity and resistance.
Ann N Y Acad Sci.
2000;
922:
306
-308.
[PubMed]
.
-
39.
Blasco
MA
Telomeres and human disease: ageing, cancer and beyond.
Nat Rev Genet.
2005;
6:
611
-622.
[PubMed]
.
-
40.
Farrell
PJ
, Broeze
RJ
and Lengyel
P.
Accumulation of an mRNA and protein in interferon-treated Ehrlich ascites tumour cells.
Nature.
1979;
279:
523
-525.
[PubMed]
.
-
41.
Schwartz
DC
and Hochstrasser
M.
A superfamily of protein tags: ubiquitin, SUMO and related modifiers.
Trends Biochem Sci.
2003;
28:
321
-328.
[PubMed]
.
-
42.
Herrmann
J
, Lerman
LO
and Lerman
A.
Ubiquitin and ubiquitin-like proteins in protein regulation.
Circ Res.
2007;
100:
1276
-1291.
[PubMed]
.
-
43.
de Veer
MJ
, Holko
M
, Frevel
M
, Walker
E
, Der
S
, Paranjape
JM
, Silverman
RH
and Williams
BR.
Functional classification of interferon-stimulated genes identified using microarrays.
J Leukoc Biol.
2001;
69:
912
-920.
[PubMed]
.
-
44.
Moynihan
TP
, Ardley
HC
, Nuber
U
, Rose
SA
, Jones
PF
, Markham
AF
, Scheffner
M
and Robinson
PA.
The ubiquitin-conjugating enzymes UbcH7 and UbcH8 interact with RING finger/IBR motif-containing domains of HHARI and H7-AP1.
J Biol Chem.
1999;
274:
30963
-30968.
[PubMed]
.
-
45.
Malakhov
MP
, Kim
KI
, Malakhova
OA
, Jacobs
BS
, Borden
EC
and Zhang
DE.
High-throughput immunoblotting. Ubiquitiin-like protein ISG15 modifies key regulators of signal transduction.
J Biol Chem.
2003;
278:
16608
-16613.
[PubMed]
.
-
46.
Liu
M
, Li
XL
and Hassel
BA.
Proteasomes modulate conjugation to the ubiquitin-like protein, ISG15.
J Biol Chem.
2003;
278:
1594
-1602.
[PubMed]
.
-
47.
Sadler
AJ
and Williams
BR.
Interferon-inducible antiviral effectors.
Nat Rev Immunol.
2008;
8:
559
-568.
[PubMed]
.
-
48.
Knight
E Jr
and Cordova
B.
IFN-induced 15-kDa protein is released from human lymphocytes and monocytes.
J Immunol.
1991;
146:
2280
-2284.
[PubMed]
.
-
49.
D'Cunha
J
, Knight
E Jr
, Haas
AL
, Truitt
RL
and Borden
EC.
Immunoregulatory properties of ISG15, an interferon-induced cytokine.
Proc Natl Acad Sci U S A.
1996;
93:
211
-215.
[PubMed]
.
-
50.
Recht
M
, Borden
EC
and Knight
E Jr.
A human 15-kDa IFN-induced protein induces the secretion of IFN-gamma.
J Immunol.
1991;
147:
2617
-2623.
[PubMed]
.
-
51.
Owhashi
M
, Taoka
Y
, Ishii
K
, Nakazawa
S
, Uemura
H
and Kambara
H.
Identification of a ubiquitin family protein as a novel neutrophil chemotactic factor.
Biochem Biophys Res Commun.
2003;
309:
533
-539.
[PubMed]
.
-
52.
Roth
SM
, Metter
EJ
, Ling
S
and Ferrucci
L.
Inflammatory factors in age-related muscle wasting.
Curr Opin Rheumatol.
2006;
18:
625
-630.
[PubMed]
.
-
53.
Grimaldi
MP
, Vasto
S
, Balistreri
CR
, di
Carlo D
, Caruso
M
, Incalcaterra
E
, Lio
D
, Caruso
C
and Candore
G.
Genetics of inflammation in age-related atherosclerosis: its relevance to pharmacogenomics.
Ann N Y Acad Sci.
2007;
1100:
123
-131.
[PubMed]
.
-
54.
Libby
P
Inflammation and cardiovascular disease mechanisms.
Am J Clin Nutr.
2006;
83:
456S
-460S.
[PubMed]
.
-
55.
Griffin
WS
Inflammation and neurodegenerative diseases.
Am J Clin Nutr.
2006;
83:
470S
-474S.
[PubMed]
.
-
56.
Godbout
JP
and Johnson
RW.
Age and neuroinflammation: a lifetime of psychoneuroimmune consequences.
Neurol Clin.
2006;
24:
521
-538.
[PubMed]
.
-
57.
Csiszar
A
, Toth
J
, Peti-Peterdi
J
and Ungvari
Z.
The aging kidney: role of endothelial oxidative stress and inflammation.
Acta Physiol Hung.
2007;
94:
107
-115.
[PubMed]
.
-
58.
De
Martinis M
, Di Benedetto
MC
, Mengoli
LP
and Ginaldi
L.
Senile osteoporosis: is it an immune-mediated disease.
Inflamm Res.
2006;
55:
399
-404.
[PubMed]
.
-
59.
Esposito
K
, Giugliano
G
, Scuderi
N
and Giugliano
D.
Role of adipokines in the obesity-inflammation relationship: the effect of fat removal.
Plast Reconstr Surg.
2006;
118:
1048
-57; discussion 1058-1059.
[PubMed]
.
-
60.
Chung
HY
, Sung
B
, Jung
KJ
, Zou
Y
and Yu
BP.
The molecular inflammatory process in aging.
Antioxid Redox Signal.
2006;
8:
572
-581.
[PubMed]
.
-
61.
Franceschi
C
, Capri
M
, Monti
D
, Giunta
S
, Olivieri
F
, Sevini
F
, Panourgia
MP
, Invidia
L
, Celani
L
, Scurti
M
, Cevenini
E
, Castellani
GC
and Salvioli
S.
Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans.
Mech Ageing Dev.
2007;
128:
92
-105.
[PubMed]
.
-
62.
Morgan
TE
, Wong
AM
and Finch
CE.
Anti-inflammatory mechanisms of dietary restriction in slowing aging processes.
Interdiscip Top Gerontol.
2007;
35:
83
-97.
[PubMed]
.
-
63.
Shay
J
and Wright
W.
Hallmarks of telomeres in ageing research.
J Pathol.
2007;
211:
114
-123.
[PubMed]
.
-
64.
Fourel
G
, Revardel
E
, Koering
CE
and Gilson
E.
Cohabitation of insulators and silencing elements in yeast subtelomeric regions.
Embo J.
1999;
18:
2522
-2537.
[PubMed]
.
-
65.
Pryde
FE
and Louis
EJ.
Limitations of silencing at native yeast telomeres.
Embo J.
1999;
18:
2538
-2550.
[PubMed]
.
-
66.
Schedl
P
and Broach
JR.
Making good neighbors: the right fence for the right job.
Nat Struct Biol.
2003;
10:
241
-243.
[PubMed]
.
-
67.
Tham
WH
and Zakian
VA.
Transcriptional silencing at Saccharomyces telomeres: implications for other organisms.
Oncogene.
2002;
21:
512
-521.
[PubMed]
.
-
68.
Lou
Z
, O'Reilly
S
, Liang
H
, Maher
VM
, Sleight
SD
and McCormick
JJ.
Down-regulation of overexpressed sp1 protein in human fibrosarcoma cell lines inhibits tumor formation.
Cancer Res.
2005;
65:
1007
-1017.
[PubMed]
.