Abstract
Hematopoietic stem cells (HSC) give rise to an enormous number of blood cells throughout our life. In contrast their number of cell divisions preceding senescence is limited underin vitro culture conditions. Here we consider the question whether HSC can rejuvenate indefinitely or if the number of cell divisions is restricted. We have developed a multi-compartmental model for hematopoietic differentiation based on ordinary differential equations. The model is based on the hypothesis that in each step of maturation, the percentage of self-renewal versus differentiation is regulated by a single external feedback mechanism. We simulate the model under the assumption that hematopoietic differentiation precedes the six steps of maturation and the cells ultimately cease to proliferate after 50 divisions. Our results demonstrate that it is conceivable to maintain hematopoiesis over a life-time if HSC have a slow division rate and a high self-renewal rate. With age, the feedback signal increases and this enhances self-renewal, which results in the increase of the number of stem and progenitor cells. This study demonstrates that replicative senescence is compatible with life-long hematopoiesis and that model predictions are in line with experimental observations. Thus, HSC might not divide indefinitely with potentially important clinical implications.
Introduction
Human and animal tissues are continuously
renewed by somatic stem cells. A decline of stem cell function will inevitably
impair the regenerative potential and result in the aging process of the organism
[1].
Hematopoietic stem cells (HSC) give rise to all lineages of blood cells. At
least some of their progeny has to retain stem cell function in order to
maintain the stem cell pool. It is still controversial if this self-renewal
with regard to the differentiation potential also implies that HSC can
rejuvenate indefinitely or if they are destined to age as other somatic cells
do [2]. This might also have im-plications for HSC transplantation: A child that receives an allogeneic transplant from an elderly donor will eventually have a hematopoietic
system of age that exceeds the expected maximal age of humans. Under normal
conditions, there are no signs of anemia in elderly people although the
capacity for hematopoietic recovery under stress conditions appears to
gradually decline [3]. Aging
affects the immune system and the number of lymphocytes significantly decreases
[4].
Transplantation experiments indicated that this myeloid skewing of differentiation
potential with age is due to intrinsic changes in older HSC [5,6].
Hematopoiesis is a multi-step process, in
which a relatively small population of HSC gives rise to all types of blood
cells. The current understanding is based on a hierarchical tree in which each lineage of blood cells proceeds
through a chain of maturation stages, which are sequentially traversed. At least six different compartments
have been proposed although so far experimental data do not provide a precise
distinction between these stages (Figure 1A): 1) A small subset of HSC is
capable of long-term repopulation upon transplantation (LT-HSC). These cells
can be enriched by their immunophenotype as CD34+CD38-CD90+
and they are usually quiescent or very slow dividing [7-11]. 2) The next compartment comprises short-term repopulating stem
cells (ST-HSC) that sustain hematopoiesis only for a limited time of several
weeks or months after transplantation and these may correspond to a CD34+CD38-
phenotype [12]. 3) Multipotent progenitors
cells (MPC; such as common myeloid progenitor cells) are included in
the CD34+CD38+ cell fraction. In the later stages CD34
expression is absent and lineage specific markers are expressed. There is
evidence that maturation proceeds via 4) committed progenitor cells (CPC; such
as the granulocyte-macrophage colony-forming cells), 5) precursor cells with
single-lineage potential (such as the granulocyte-progenitors) and ultimately
6) mature cells with a limited lifetime (such as granulocytes). Upon every cell
division, some progeny cells have to maintain the stem cell pool
(self-renewal), whereas the others proceed to the next maturation compartment
(differentiation). There is evidence, that the relation between self-renewal
and differentiation is controlled by asymmetric cell divisions and this
correlates with asymmetric cell division kinetics of the progeny cells (Figure 1B) [13-15].
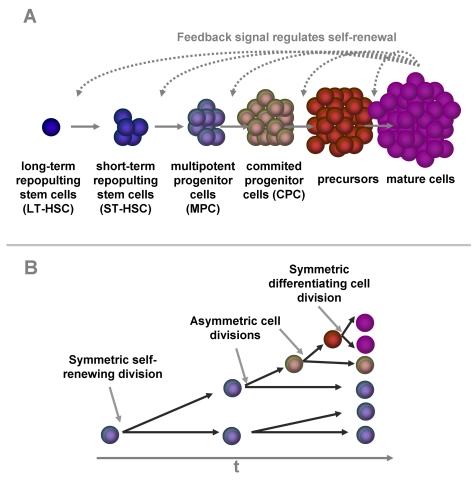
Figure 1. Self-renewal and differentiation in hematopoiesis.
Hematopoietic
differentiation is a multi-step process. A small group of long term
repopulating hematopoietic stem cells (LT-HSC) replicates very slowly. The
down-stream compartments are more and more committed to a specific linage
and replicate at faster rates. Some of the progeny have to self-renew to
keep the pool of hematopoietic stem and progenitor cells. Our model is
based on the hypothesis this percentage of self-renewal versus
differentiation is regulated by a feedback mechanism that is related to the
number of mature cells in the blood (A). There is evidence, that the
dual function of self-renewal and differentiation is regulated by
asymmetric cell divisions where one daughter cell retains the stem cell
function whereas the other differentiating cell becomes a faster
proliferating precursor cell. Alternatively, cells can undergo symmetric
cell divisions to produce either two identical, self-renewing cells or two
differentiated daughter cells (B).
Research on aging in adult stem cells is
limited by the methods available for their identification, purification and
culture expansion. However, all other cell
types, including hematopoietic cells, fibroblasts and mesenchymal stromal cells
enter a senescent state after a certain number of cell divisions. Within about
30 to 50 population doublings, the cells enlarge and become more granular with
an irregular cell shape [16,17].
Ultimately they irrevocably stop dividing although they
remain metabolically active and can be maintained in this state for years. It
has been demonstrated that similar cell enlargement can also be induced under
growth stimulation when the cell cycle is blocked: cells senesce if expression
of p21 is induced ectopically and this is accompanied by beta-galactosidase
staining, cellular hypertrophy, increased levels of cyclin D1 and active TOR
(target of rapamycin, also known as mTOR) [18]. Notably,
the loss of proliferative potential can be decelerated by rapamycine indicating
that senescence can be pharmacologically suppressed [19].
The phenomenon of replicative senescencewas first described in the 1960s by Leonard Hayflick [20]. Since
then, it is debated if reaching the so-called "Hayflick limit" might be related to the aging of the whole organism.
Human hematopoietic system accounts for an estimated
output of more than 1011 cells per day and approximately 4 x 1015
cells over a life time [21,22]. In theory this cell number could be reached from an individual
cell by 52 cell divisions but the question remains if replicative senescence is
really compatible with sustained hematopoiesis and how this affects the
relationship of stem cells with their more differentiated counterparts.
In our previous work, we have described
multi-compartment models to investigate possible mecha-nisms of regulation and
stabilization of blood cell production, following perturbations such as bone
marrow transplantation. We have demonstrated using mathematical modeling that feedback control of the frequency of self-renewal of
HSC is most essential for ematopoietic reconstitution following transplantation
[23]. This model
was now adapted to determine the effect of replicative senescence on
hematopoietic development. The aim of this study was to determine if a
limitation to 50 cell divisions is compatible with hematopoiesis over an entire
life-span and how this affects the ratio of stem cells number to that of their
differentiated progeny.
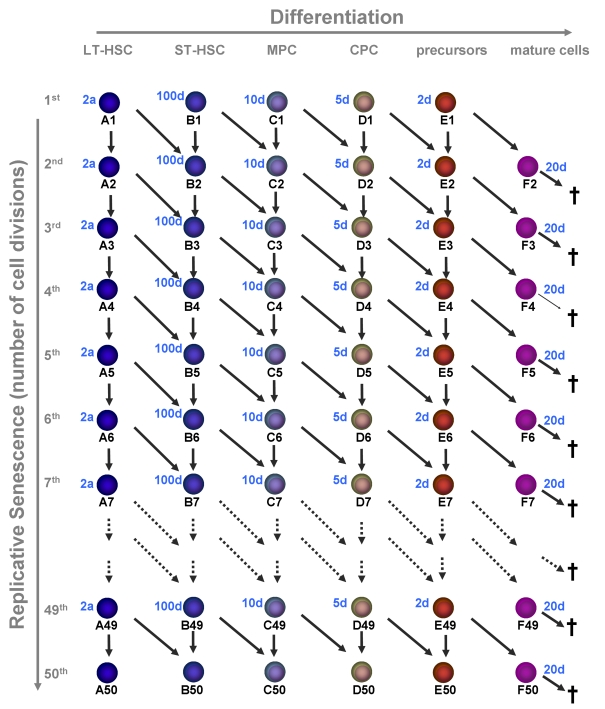
Figure 2. Replicative senescence in hematopoietic development.
In this model we
have addressed the question if hematopoiesis is compatible with a
restriction in cell divisions (e.g. 50 cell divisions). Upon each division
the daughter cells may either remain on the same maturation level or
proceed to the next step of differentiation. Proliferation rates increase
upon differentiation and the estimated times are indicated for each
maturation step. Mature cells are post-mitotic and die after 20 days.
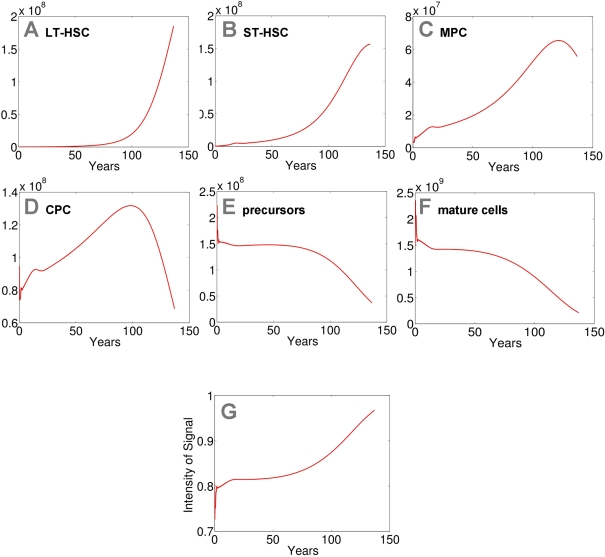
Figure 3. Modeling of replicative senescence in hematopoiesis.
Cell numbers
of the different compartments are plotted over a time course of 140 years
(A: LT-HSC; B: ST-HSC; C: MPC; D: CPC; E: precursors; F: mature cells). The
plots A-F
depict the dynamics of the cells. In addition the progression of the signal
is demonstrated (G).
Input cell numbers were chosen close to the local equilibrium. Our model
demonstrates that, under the assumptions on model parameters, hematopoiesis
can be maintained for more than 100 years with a restriction to 50 cell
divisions. However, the number of mature cells declines over time and the
feedback signal increases correspondingly. Therefore the percentage of
self-renewal increases resulting in a higher number of stem cells and
progenitor cells.
Results
We have developed
a mathematical model describing the dynamics of cells of n different differentiation stages under the
restriction that the number of possible cell divisions is limited by an
arbitrary number m (see Methods for the details of the model).
We have solved numerically the model with n=6 stages of maturation (long-term
repopulating stem cells (LT-HSC), short-term repopulating stem cells (ST-HSC),
multipotent progenitor cells (MPC), committed progenitor cells (CPC),
precursors, and mature cells) andm=50
possible cell divisions (Figure 2). We assume that, neither proliferation rates nor
self-renewal fractions, depend on the number
of cell generation but they only depend on the stage of differentiation. For
the initial cell numbers at birth (0 years) we have chosen cell numbers close
to the steady states value of the model without
replicative senescence (LT-HSC = 105; ST-HSC = 5x105;
MPC = 4 x 106; CPC = 8 x 107; precursors = 1.5 x 108;
mature cells = 1.5 x 109).
As anticipated a prerequisite for this
model is that LT-HSC are very slow dividing. Otherwise, the stem cell pool is
rapidly depleted. This might explain why ST-HSC cannot maintain hematopoiesis
for a long time. We have chosen a
proliferation rate of LT-HSC of once every two years with a maximum number of
50 cell divisions to adapt the results to a human life-time. Furthermore, the
rate of HSC self-renewal (a1) has to be larger than the
corresponding rates for the other compartments
(a1>ai for i=2,3,4,5). Otherwise, the
compartment with the highest self-renewal potential takes over the stem cell
function, whereas all up-stream compartments including the HSC compartment
eventually become extinct. This has also been described in our previous work.
For numerical simulations we have chosen the same maximal self-renewal rates as
in our previous work [23] (LT-HSC = 0.7;
ST-HSC = 0.65; MPC = 0.65; CPC = 0.65; precursors = 0.55; mature cells do not
divide). Under these
assumptions our model demonstrates that hematopoiesis can be sustained over a
life-time with only 50 cell divisions. However, hemato-poiesis does not reach a
steady state. The number of mature cells slowly declines after about 50 years
and after 140 years hematopoiesis ceases to take place.
Loss of mature cells is partly compensated by an
increased feedback signal that enhances the self-renewal rates. Hence, the
number of mature cells inversely correlates with the intensity of the signal
that increases over the years as some of the cells in the stem cell fraction reach the Hayflick limit. Therefore, other than
anticipated, the number of stem and progenitor cells increases with aging.
Hence, elderly people have a higher number of stem cells whereas their
remaining number of cell divisions is more restricted
(Figure 3).
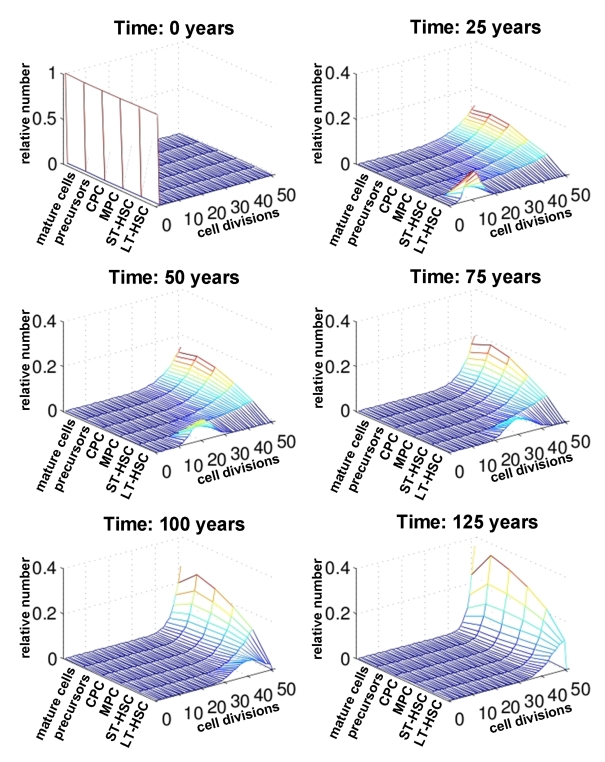
Figure 4. Number of cell divisions over time. For each compartment of differentiation the relative number of cells is plotted against the number of cell divisions (0 to 50). The distribution is compared at different time points (0, 25, 50, 75 and 100 years). This indicates that changes upon aging are more prominent in the stem cell compartment than in mature cells.
Subsequently, we
have analyzed the distribution of the number of cell divisions for each
maturation step at different ages (0 years, 25 years, 50 years, 75 years, 100
years and 125 years; Figure 4). Under the assumptions of our model all cells
can undergo 50 cell divisions counting from the time of birth of the
individual. As expected, the number of cell divisions in the LT-HSC compartment
decreases continuously with age. However, for the more differentiated
compartments the majority of cells already performed more than 45 cell
divisions after only a few years. Hence, the effects of replicative senescence
upon aging are most obvious in the stem cell compartment. Therefore,
age-associated changes should be observed in adult stem cells rather than their
differentiated progeny.
Discussion
This study demonstrates that a limitation of the
number of cell divisions is consistent with normal human hematopoiesis.
Furthermore, the system of cells undergoing replicative senescence does not
necessarily have a positive steady state. During aging the feedback signal
increases to sustain the number of mature cells. Hence, the quantity of
progenitor cells increases whereas their quality with regard to long-term
proliferation decreases. The model presented here does not distinguish between
different lineages of differen-tiated blood cells precursors, a simplification,
which assumes that all the lineages have the same proliferation and maturation
structure and dynamics. Also the numbers of compartments and the maximum number
of cell divisions have been arbitrarily chosen. It
might be speculated, that the proliferation rate decreases with the increase in
the number of cell divisions. For simplicity this has not been considered in
our model but it would result in a similar reduction of the regenerative
potential.
Other models have described aging of differentiated
epithelial tissue as the consequence of replicative senescence of progenitor
cells only [24,25].
Further-more, it has been suggested that hematopoiesis proceeds through a
significantly higher number of different compartments
and that about 31
mitotic events separate the HSC from the mature cells [26]. To our knowledge, the present study provides the first model
that treats replicative senescence and maturation of stem cells as two
independent processes. It is based on the
assumption that stem cells are quiescent or very slow dividing. Indeed, it is
commonly accepted, that LT-HSC are quiescent or very slow dividing and that
they can be enriched by slow division kinetics [8,10,11,14,15].
Recently, Wilson and co-workers have demonstrated
that there was a dormant-fraction of HSC that divided only five times during
the lifetime of mice and especially that these dormant HSC display repopulating
activity following a serial transplantation [9]. For the human system there are no clear biological data
available for the proliferation rate of LT-HSC or for the number of maximum
cell divisions. Clearly, slow divisions kinetics reduce the risk of mutagenesis
and defects during cell division [27]. These slow division kinetics are also a
prerequisite to maintain hemato-poiesis in our model. Otherwise the stem cell
pool might rapidly be depleted, even if a significantly higher number of
population doublings was assumed.
In the
murine transplantation model the potential for engraftment decreases after serial
transplantations. The reconstituting ability declines continuously within 4 to
5 transfers [28,29]. Various studies have indicated that the
functional ability of HSC in the repopulation model significantly declines with
an increasing donor age [30]. It has
been suggested that HSC from older mice have a significantly lower cycling
activity than those isolated from younger mice [31]. There
are several differences between different mouse strains and it needs to be
verified if observation from the murine system can be extrapolated to humans.
However, the data indicate that there are cell intrinsic changes in stem and
progenitor cells during aging. At least some of these changes might be
attributed to a lower number of remaining cell divisions.
Furthermore, competitive
repopulation assays in murine transplantation models demonstrated that bone
marrow of older mice had a higher number of ST-HSC and progenitor cells [32,33]. This is in line with similar in vitro experiments using
the cobblestone area forming cell (CAFC) assay as a surrogate assay for
primitive progenitor cells that demonstrated that their number increased about
four-fold with age [6,34,35]. Initially this was not expected, as it has been
anticipated that aging may be caused by the depletion of stem and progenitor
cells. Interestingly, this increase of stem and progenitor cells also manifests
in this study: during aging the number of mature cells declines slightly and
correspondingly the feedback signal for self-renewal increases. Therefore, the
number of stem and progenitor cells increases during aging.
The molecular mechanisms that trigger aging or
senescence of HSC are still unknown. Shortening of telomeres was proposed as a
biological clock that determines the number of cell replications. The idea of telomere erosion after about 50 cell divisions
might be easily introduced in into this model. In fact, there have been
reports that telomeres in HSC from bone marrow and peripheral blood are shorter than in those from peripheral blood [36]. There have also been reports that the length of telomeres
decreases as a function of age [22]. We have analyzed telomere length in human CD34+ HPC
and there was a tendency for shortening of telomeres with age although it was
not significant [37]. There is increasing evidence, that progressive shorten-ing of
the telomeres is not the only underlying mechanism and that it might represent
an effect rather than the cause of aging [38-40]. Other causal molecular events and stochastic mechanisms
also are compatible with this model. It has been suggested that senescence is
triggered e.g. by DNA damage, accumulation of the cyclin-dependent kinase
inhibitor p16INK4a or oxidative stress [1,41,42]. Alternatively, aging of HSC might be
influenced by the cellular microenvironment in the bone marrow - the so called
stem cell niche [2,43]. We have demonstrated that replicative senescence of
mesenchymal stromal cells (MSC) affects their hematopoieisis supportive
function [44]. By regulation of the proliferation rate
and maintenance of HSC in a quiescent state the stem cell niche would play a
central role in counteracting the replicative senes-cence.
Recently, we have described gene expression changes in
CD34+ hematopoietic progenitor cells (HPC) from healthy donors of
different age (0 years to 73 years). Various genes revealed significant gene
expression changes indicating that our stem and progenitor cells are not
protected from aging [37].
Interestingly, these changes are related to gene expression changes displayed
in long-term culture of MSC from human bone marrow [17]. The concordance of age-related changes in HPC and of
replicative senescence in MSC provides further evidence that our stem and
progenitor cells undergo a similar process also in vivo.
Our model demonstrates that replicative
senescence in the hematopoietic system is conceivable, and the results are
compatible with various observations such as i) slow proliferation rate of HSC,
ii) cell-intrinsic changes during aging, and iii) increasing number of stem and
progenitor cells. Therefore, the
possibility that the number of cell divisions for stem and progenitor cells is
restricted requires careful consideration. It might have implications for the
proliferative stress, such as after chemotherapy and for the long-term
performance after stem cell transplantation with transplants from elderly
donors.
Methods
Derivation of the
mathematical model.
The model presented in this paper is a modified version of
the discrete compartmental model with feedback (henceforth called the DCF
model) proposed in [23]. The novelty of the model presented here is the assumption
that replicative senescence distinguishes among subpopulations of different
generations (DCF model with senescence). The model is based on two major
assumptions: 1) in analogy to the DCF model, hematopoiesis is considered as a
process during which cells traverse a finite number of subsequent discrete
stages of differentiation and 2) it is assumed that each cell is able to
perform a fixed finite number of cell divisions before it loses the ability to
divide. In general we consider n differentiation stages
and mdivisions that a cell can undergo.
As in the DCF models behaviour
of each cell type is described by three parameters: 1) proliferation rate
describing how often, on the average, a cell divides per unit of time; 2) the
fractions of self-renewal and differentiation during cell division and
3) the death rate equal to the fractions of a specified cell subpopulation
which dies per unit of time.
Differentiation and
Senescence represent two indepen-dent dimensions.
The model describes the following
scenario: After division a cell gives rise to two progeny cells. Cell divisions
can be symmetric or asymmetric. Therefore, we assume that on the average the
fraction a of progeny cells remains at the
same stage of differentiation as the parent cell, while the 1 - a fraction of the progeny cells differentiate, i.e.
transfers to the higher differentiation stage. To account for the finite number
of cell divisions (equal to m) we divide each stage of differentiation into m
partitions. The partitions are numbered from 0 to m and the index of the
partition indicates how many divisions the cells performed since the starting
time t = 0. We refer to this number as a generation number. Thus, the
progeny cells always belong to the next generation compared tothe parent cell, independently from their
differentiation fate after division.
Treating the cell cycle as a well-mixed
tank, the scenario described can be modeled by a system of m + 1 times n ordinary differential equations (ODEs).
Denote by ci.j(t) (1 ≤ i≤ n, 0 ≤ j ≤
m) the size of the jth partition of the i stage of differentiation at time
t, i.e., the amount of cells which belong at time t to the ith stage of
differentiation and have performed j divisions since the starting time t=0. Denote the proliferation rate of the subpopulation ci,j
at time t by pi,j(t), the fraction
(probability) of self-renewal by ai,j(t) and the death rate
by d(t).
In the following the time evolution  of ci,j is
described.
of ci,j is
described.
Modeling of primitive stem cells.
Starting with c1,0 ; the flux to mitosis at
time t is given by p1,0(t)c1,0(t) and the flux to cell
death is given by d1,0(t)c1,0(t). Since c1,0
denotes the number of stem cells that have divided 0 times, there exists no influx to
this compartment. Therefore
 .
.
For c1,j, 0 < j < m; there exists
additionally the influx to
this compartment given by
2a1,j-1(t)p1,j-1(t)c1,j-1(t). Here p1,j-1(t)c1,j-1(t) describes the number of stem cells that have divided j-1
times and entered division at time t. After division a fraction of 2a1,j-1(t)p1,j-1(t)c1,j-1(t) belongs to the stem cells that have
undergone j divisions. The remaining 2(1-a1,j-1(t))p1,j-1(t)c1,j-1(t) progeny cells belong to c2,j, i.
e., to the class of cells of the second stage of differentiation that have
performed j divisions. Summarizing, we obtain the following equation,
 0 < j < m.
0 < j < m.
Modeling of mature cells.
Since cells
of the subpopulation c1,m do not divide (i.e., p1,m = 0),
there exists only the influx due to division of cells of the population c1,m-1
and the out flux due to death. The corresponding equation reads,

Modeling of all other maturation steps.
In a similar way, for
the cells of ith stage of differentiation
with 0 ,
0 < i < n.
,
0 < i < n.
Let 0 < j < n.
After division the number of  cells belong to the subpopulation
ci,j+1 (self-renewing cells at the i stage of differentiation)
and
cells belong to the subpopulation
ci,j+1 (self-renewing cells at the i stage of differentiation)
and  cells belong to the subpopulation
ci+1,j+1 (differentiating cells). Influx to ci,j from
ci,j-1 due to self-renewal is given by
cells belong to the subpopulation
ci+1,j+1 (differentiating cells). Influx to ci,j from
ci,j-1 due to self-renewal is given by
 and influx from ci-1,j-1 due to differentiation
is given by
and influx from ci-1,j-1 due to differentiation
is given by  . Therefore, we obtain for
0 < i < n, 0 < j < m,
. Therefore, we obtain for
0 < i < n, 0 < j < m,

Since cells of subpopulation ci,m do not divide, the corresponding equation reads
 0 < i < n.
0 < i < n.
Since mature cells are post-mitotic, there
exists no out flux due to division in the subpopulations cn,j,
 .
Therefore,
.
Therefore, 

 .
.
Modeling
of feedback regulation.
As in the DCF model, we assume that hematopoiesis is
regulated by extra-cellular signaling molecules, such as cytokines. The level
of the signal depends on the level of mature cells, and is modeled using the
equation

This dependence can be justified using a
quasi-steady state approximation of the plausible dynamics of the cytokine
molecules, [23].
The quasi-steady state approximation is based on the assumption that cytokine
metabolism takes place on a different (faster) time scale than cell cycle. The
above expression reflects the heuristic assumption that signal intensity
achieves its maximum under absence of mature cells and decreases asymptotically
to zero if the number of mature cells increases. The signal intensity satisfies
is  . We have previously demonstrated that the regulation
of hematopoiesis is much more efficient (and can be achieved in the clinically
relevant time scale) only if the feedback mechanism regulates the fraction of
self-renewal and differentiation [23].
Regulation of the proliferation rate is not sufficient for that purpose.
Therefore, in the reminder of this paper we assume that cell parameters depend
on s in the following manner:
. We have previously demonstrated that the regulation
of hematopoiesis is much more efficient (and can be achieved in the clinically
relevant time scale) only if the feedback mechanism regulates the fraction of
self-renewal and differentiation [23].
Regulation of the proliferation rate is not sufficient for that purpose.
Therefore, in the reminder of this paper we assume that cell parameters depend
on s in the following manner:  is constant in time, i.e.,
is constant in time, i.e.,  , and
, and  , where
, where  and
and  are non-negative constants. Death rates
are non-negative constants. Death rates  are assumed to be constant in time. In numerical simulations k was set as 1.6 x 10-10 in analogy to our previous
work [23].
are assumed to be constant in time. In numerical simulations k was set as 1.6 x 10-10 in analogy to our previous
work [23].
Model Equations.
The complete model is given by the following system of ODEs.

 for
for  ,
,

 for
for  ,
,
 for
for  and
and  ,
,
 for
for  ,
,

 for
for  ,
,
with  .
.
Acknowledgments
The
authors would like to thank Prof. Anthony Ho and Prof. Willi Jäger for
mentoring the WIN Kolleg Project. This work was
supported by the German Research Foundation DFG (HO 914/7-1), the German
Ministry of Education and Research (CB-HERMES), the Academy of Sciences
and Humanities, Heidelberg (WIN-Kolleg) and the Stem Cell Network North-Rhine
Westphalia. No competing financial interests
exist.
Conflicts of Interest
The authors in this manuscript have no conflict of interests to declare.
References
-
1.
Ho
AD
, Wagner
W
and Mahlknecht
U.
Stem cells and ageing. The potential of stem cells to overcome age-related deteriorations of the body in regenerative medicine.
EMBO Rep.
2005;
6 Spec No:
S35
-S38.
[PubMed]
.
-
2.
Wagner
W
, Horn
P
, Bork
S
and Ho
AD.
Aging of hematopoietic stem cells is regulated by the stem cell niche.
Experimental Gerontology.
2008;
43:
974
-980.
[PubMed]
.
-
3.
Globerson
A
Hematopoietic stem cells and aging.
Exp Gerontol.
1999;
34:
137
-146.
[PubMed]
.
-
4.
Linton
PJ
and Dorshkind
K.
Age-related changes in lymphocyte development and function.
Nat Immunol.
2004;
5:
133
-139.
[PubMed]
.
-
5.
Kim
M
, Moon
HB
and Spangrude
GJ.
Major age-related changes of mouse hematopoietic stem/progenitor cells.
Ann N Y Acad Sci.
2003;
996:
195
-208.
[PubMed]
.
-
6.
Dykstra
B
and de Haan
G.
Hematopoietic stem cell aging and self-renewal.
Cell Tissue Res.
2008;
33:
91
-101.
[PubMed]
.
-
7.
Hosen
N
, Park
CY
, Tatsumi
N
, Oji
Y
, Sugiyama
H
, Gramatzki
M
, Krensky
AM
and Weissman
IL.
CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia.
Proc Natl Acad Sci U S A.
2007;
104:
11008
-11013.
[PubMed]
.
-
8.
Morrison
SJ
and Weissman
IL.
The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype.
Immunity.
1994;
1:
661
-673.
[PubMed]
.
-
9.
Wilson
A
, Laurenti
E
, Oser
G
, van der Wath
RC
, Blanco-Bose
W
, Jaworski
M
, Offner
S
, Dunant
CF
, Eshkind
L
, Bockamp
E
, Lio
P
, Macdonald
HR
and Trumpp
A.
Hematopoietic Stem Cells Reversibly Switch from Dormancy to Self-Renewal during Homeostasis and Repair.
Cell.
2008;
135:
1118
-1129.
[PubMed]
.
-
10.
Brummendorf
TH
, Dragowska
W
, Zijlmans
JM
, Thornbury
G
and Lansdorp
PM.
Asymmetric cell divisions sustain long-term hematopoiesis from single-sorted human fetal liver cells.
J Exp Med.
1998;
188:
1117
-1124.
[PubMed]
.
-
11.
Wagner
W
, Ansorge
A
, Wirkner
U
, Eckstein
V
, Schwager
C
, Blake
J
, Miesala
K
, Selig
J
, Saffrich
R
, Ansorge
W
and Ho
AD.
Molecular evidence for stem cell function of the slow-dividing fraction among human hematopoietic progenitor cells by genome-wide analysis.
Blood.
2004;
104:
675
-686.
[PubMed]
.
-
12.
Zubair
AC
, Kao
G
, Daley
H
, Schott
D
, Freedman
A
and Ritz
J.
CD34(+) CD38(-) and CD34(+) HLA-DR(-) cells in BM stem cell grafts correlate with short-term engraftment but have no influence on long-term hematopoietic reconstitution after autologous transplantation.
Cytotherapy.
2006;
8:
399
-407.
[PubMed]
.
-
13.
Ho
AD
and Wagner
W.
The beaty of asymmetry - asymmetric divisions and self-renewal in the hematopoietic system.
Current Opinion in Hematology.
2007;
14:
330
-336.
[PubMed]
.
-
14.
Giebel
B
, Zhang
T
, Beckmann
J
, Spanholtz
J
, Wernet
P
, Ho
AD
and Punzel
M.
Primitive human hematopoietic cells give rise to differentially specified daughter cells upon their initial cell division.
Blood.
2006;
107:
2146
-2152.
[PubMed]
.
-
15.
Huang
S
, Law
P
, Francis
K
, Palsson
BO
and Ho
AD.
Symmetry of initial cell divisions among primitive hematopoietic progenitors is independent of ontogenic age and regulatory molecules.
Blood.
1999;
94:
2595
-2604.
[PubMed]
.
-
16.
Smith
JR
and Pereira-Smith
OM.
Replicative senescence: implications for in vivo aging and tumor suppression.
Science.
1996;
273:
63
-67.
[PubMed]
.
-
17.
Wagner
W
, Horn
P
, Castoldi
M
, Diehlmann
A
, Bork
S
, Saffrich
R
, Benes
V
, Blake
J
, Pfister
S
, Eckstein
V
and Ho
AD.
Replicative Senescence of Mesenchymal Stem Cells - a Continuous and Organized Process.
PLoS ONE.
2008;
5:
e2213
[PubMed]
.
-
18.
Demidenko
ZN
and Blagosklonny MV. Growth stimulation leads to cellular senescence when the cell cycle is blocked.
Cell Cycle.
2008;
7:
3355
-3361.
[PubMed]
.
-
19.
Demidenko
ZN
, Zubova
SG
, Bukreeva
EI
, Pospelov
VA
, Pospelova
TV
and Blagosklonny
MV.
Rapamycin decelerates cellular senescence.
Cell Cycle.
2009;
8:
1888
-1895.
[PubMed]
.
-
20.
Hayflick
L
The limited in vitro lifetime of human diploid cell strains.
Exp Cell Res.
1965;
37:
614
-636.
[PubMed]
.
-
21.
Lansdorp
PM
Stem cell biology for the transfusionist.
Vox Sang.
1998;
74 Suppl 2:
91
-94.
[PubMed]
.
-
22.
Vaziri
H
, Dragowska
W
, Allsopp
RC
, Thomas
TE
, Harley
CB
and Lansdorp
PM.
Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age.
Proc Natl Acad Sci U S A.
1994;
91:
9857
-9860.
[PubMed]
.
-
23.
Marciniak-Czochra
A
, Stiehl
T
, Ho
AD
, Jaeger
W
and Wagner
W.
Modeling of Asymmetric Cell Division in Hematopoietic Stem Cells - Regulation of Self-Renewal is Essential for Efficient Repopulation.
Stem Cells Dev.
2009;
18:
377
-385.
[PubMed]
.
-
24.
Op
den BJ
, Musters
M
, Verrips
T
, Post
JA
, Braam
B
and van
RN.
Mathematical modeling of vascular endothelial layer maintenance: the role of endothelial cell division, progenitor cell homing, and telomere shortening.
Am J Physiol Heart Circ Physiol.
2004;
287:
H2651
-H2658.
[PubMed]
.
-
25.
Wang
Y
, Aguda
BD
and Friedman
A.
A continuum mathematical model of endothelial layer maintenance and senescence.
Theor Biol Med Model.
2007;
4:
30
[PubMed]
.
-
26.
Dingli
D
, Traulsen
A
and Pacheco
JM.
Compartmental architecture and dynamics of hematopoiesis.
PLoS ONE.
2007;
2:
e345
[PubMed]
.
-
27.
Rossi
DJ
, Seita
J
, Czechowicz
A
, Bhattacharya
D
, Bryder
D
and Weissman
IL.
Hematopoietic stem cell quiescence attenuates DNA damage response and permits DNA damage accumulation during aging.
Cell Cycle.
2007;
6:
2371
-2376.
[PubMed]
.
-
28.
Ito
K
, Hirao
A
, Arai
F
, Takubo
K
, Matsuoka
S
, Miyamoto
K
, Ohmura
M
, Naka
K
, Hosokawa
K
, Ikeda
Y
and Suda
T.
Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells.
Nat Med.
2006;
12:
446
-451.
[PubMed]
.
-
29.
Ogden
DA
and Mickliem
H.
S. The fate of serially transplanted bone marrow cell populations from young and old donors.
Transplantation.
1976;
22:
287
-293.
[PubMed]
.
-
30.
Chen
J
Senescence and functional failure in hematopoietic stem cells.
Exp Hematol.
2004;
32:
1025
-1032.
[PubMed]
.
-
31.
de Haan
G
, Nijhof
W
and Van
Zant G.
Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity.
Blood.
1997;
89:
1543
-1550.
[PubMed]
.
-
32.
Morrison
SJ
, Wandycz
AM
, Akashi
K
, Globerson
A
and Weissman
IL.
The aging of hematopoietic stem cells.
Nat Med.
1996;
2:
1011
-1016.
[PubMed]
.
-
33.
Chambers
SM
, Shaw
CA
, Gatza
C
, Fisk
CJ
, Donehower
LA
and Goodell
MA.
Aging hematopoietic stem.
PLoS Biol.
2007;
5:
e201
[PubMed]
.
-
34.
de Haan
G
and Van
Zant G.
Dynamic changes in mouse hematopoietic stem cell numbers during aging.
Blood.
1999;
93:
3294
-3301.
[PubMed]
.
-
35.
Sudo
K
, Ema
H
, Morita
Y
and Nakauchi
H.
Age-associated characteristics of murine hematopoietic stem cells.
J Exp Med.
2000;
192:
1273
-1280.
[PubMed]
.
-
36.
Engelhardt
M
, Kumar
R
, Albanell
J
, Pettengell
R
, Han
W
and Moore
MA.
Telomerase regulation, cell cycle, and telomere stability in primitive hematopoietic cells.
Blood.
1997;
90:
182
-193.
[PubMed]
.
-
37.
Wagner
W
, Bork
S
, Horn
P
, Krunic
D
, Walenda
T
, Diehlmann
A
, Benes
V
, Blake
J
, Huber
FX
, Eckstein
V
, Boukamp
P
and Ho
AD.
Aging and Replicative Senescence Have Related Effects on Human Stem and Progenitor Cells.
PLoS ONE.
2009;
4:
e5846
[PubMed]
.
-
38.
O'Hare
MJ
, Bond
J
, Clarke
C
, Takeuchi
Y
, Atherton
AJ
, Berry
C
, Moody
J
, Silver
AR
, Davies
DC
, Alsop
AE
, Neville
AM
and Jat
PS.
Conditional immortalization of freshly isolated human mammary fibroblasts and endothelial cells.
Proc Natl Acad Sci U S A.
2001;
98:
646
-651.
[PubMed]
.
-
39.
Di Donna
S
, Mamchaoui
K
, Cooper
RN
, Seigneurin-Venin
S
, Tremblay
J
, Butler-Browne
GS
and Mouly
V.
Telomerase can extend the proliferative capacity of human myoblasts, but does not lead to their immortalization.
Mol Cancer Res.
2003;
1:
643
-653.
[PubMed]
.
-
40.
Lansdorp
PM
Telomeres, stem cells, and hematology.
Blood.
2008;
111:
1759
-1766.
[PubMed]
.
-
41.
Janzen
V
, Forkert
R
, Fleming
HE
, Saito
Y
, Waring
MT
, Dombkowski
DM
, Cheng
T
, DePinho
RA
, Sharpless
NE
and Scadden
DT.
Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a.
Nature.
2006;
443:
421
-426.
[PubMed]
.
-
42.
Kiyono
T
, Foster
SA
, Koop
JI
, McDougall
JK
, Galloway
DA
and Klingelhutz
AJ.
Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells.
Nature.
1998;
396:
84
-88.
[PubMed]
.
-
43.
Ju
Z
, Jiang
H
, Jaworski
M
, Rathinam
C
, Gompf
A
, Klein
C
, Trumpp
A
and Rudolph
KL.
Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment.
Nat Med.
2007;
13:
742
-747.
[PubMed]
.
-
44.
Walenda
T
, Bork
S
, Horn
P
, Wein
F
, Saffrich
R
, Diehlmann
A
, Eckstein
V
, Ho
AD
and Wagner
W.
Co-Culture with Mesenchymal Stromal Cells Increases Proliferation and Maintenance of Hematopoietic Progenitor Cells.
J Cell Mol Med.
2009;
Epub ahead of print
.