Caveolin-1, cellular senescence and pulmonary emphysema
Abstract
Caveolae are vesicular invaginations of the plasma membrane. Caveolin-1 is the structural protein component of caveolae. Caveolin-1 participates in signal transduction processes by acting as a scaffolding protein that concentrates, organizes and functional regulates signaling molecules within caveolar membranes. Cigarette smoke, a source of oxidants, is an environmental hazard that causes pulmonary emphysema. Recently, we reported that the development of cigarette smoking-induced pulmonary emphysema was inhibited in caveolin-1 null mice, which do not express caveolin-1. We demonstrated that lack of caveolin-1 expression in lung fibroblasts dramatically inhibited premature senescence induced by oxidants contained in cigarette smoke. Mechanistically, we uncovered that premature senescence of lung fibroblasts induced by oxidative stress occurred through activation of an ataxia telangiectasia-mutated (ATM)/p53-depedent pathway following sequestration of the catalytic subunit of protein phosphatase 2A (PP2A-C), an inhibitor of ATM, by caveolin-1 into caveolar membranes. We propose caveolin-1 as a key player of a novel signaling pathway that links cigarette smoke to premature senescence of lung fibroblasts and development of pulmonary emphysema.
Caveolae and caveolin-1
Caveolae, 50-100 nm flask-shaped
invaginations of the plasma membrane, are found in many cell types, including
fibroblasts [1]. Caveolae
represent a subgroup of lipid rafts, which are microdomains of the plasma
membrane enriched in cholesterol, sphingolipids and glycosyl
phosphatidylinositol anchored proteins [2]. The
presence of the structural protein caveolin-1 drives the formation of the plasma
membrane invaginations and makes caveolae unique among lipid rafts. Caveolae
have been implicated in numerous cellular functions, including signal
transduction, cellular metabolism, vesicle trafficking, cholesterol
homeostasis, endothelial transcytosis, and tumor suppression [2-4].
Caveolin-1 acts as a scaffolding protein to compartmentalize and functionally
regulate signaling molecules within caveolar membranes [2].
Caveolin-1 regulates stress-induced premature
senescence (SIPS)
Several theories
have been proposed in the past to explain why and how living organisms can not
escape aging. The "free radical theory" of aging was proposed by Denham Harman
in the fifties and is based on the concept that normal aging occurs as the
result of tissue damages inflicted by reactive oxygen species (ROS) [5]. In support of
this theory, increased oxidative damage of DNA, proteins, and lipids have been
reported in aged animals [6]. Thus,
endogenous and exogenous stimuli may significantly increase oxidant levels within
the cell and induce a series of cellular damages.
Most cells cannot divide indefinitely due to a process
termed cellular senescence [7-13]. Growth
arrest is associated with well-defined biochemical alterations. These include
cell cycle arrest, increased p53 activity, increased p21Waf1/Cip1
and p16 protein expression, and hypo-phosphorylation of pRb [7-11].
Interestingly, subcytotoxic oxidative stress has been shown to accelerate the
induction of cellular senescence in a number of cell types in culture,
including fibroblasts [14-16]. Thus,
investigating the signaling machinery that regulates the ability of free
radicals to induce premature senescence in cell culture models will contribute
to a better understanding of the more complicated aging process.
Our group has demonstrated a key role of caveolin-1 in
the induction of cellular senescence. We showed that over-expression of
caveolin-1 in mouse embryonic fibroblasts was sufficient to induce premature
senescence, as demonstrated by cell cycle arrest in the G0/G1
phase of the cell cycle, a reduced proliferative lifespan, up-regulation of p21Waf1/Cip1,
development of senescence-like cell morphology and senescence-associated
increase in β-galactosidase activity [1,17]. We also
showed that caveolin-1 plays a direct role in oxidative stress-induced
premature senescence, as demonstrated by inhibition of SIPS in mouse embryonic
fibroblasts derived from caveolin-1 null mice, which do not express caveolin-1,
and NIH 3T3 cells harboring antisense caveolin-1 [1,18].
Since over-expression of caveolin-1 was sufficient to
induce premature senescence and caveolin-1 expression was required for SIPS, we
also asked whether free radicals had an effect on endogenous caveolin-1
expression. We found that sub-cytotoxic oxidative stress up-regulated
caveolin-1 protein expression through activation of the caveolin-1 gene
promoter in a p38 mitogen-activated protein kinase/Sp1-dependent manner [19].
What is the molecular mechanism underlying
caveolin-1-mediated SIPS? We found that caveolin-1 is a novel binding protein
for Mdm2, a negative regulator of p53. We showed that after oxidative stress
caveolin-1 sequestered Mdm2 away from p53, leading to stabilization of p53 and
up-regulation of p21Waf1/Cip1. Consistent with these data,
expression of a peptide corresponding to the Mdm2 binding domain of caveolin-1
was sufficient to up-regulate p53 and p21Waf1/Cip1 protein
expression and induce premature senescence. Thus, we propose caveolin-1 as a
signaling molecule whose ability to activate the p53 pathway is critical for
stress-induced premature senescence.
Our
results have been supported by studies showing that senescent human diploid
fibroblasts express higher levels of caveolin-1, as compared to younger human
diploid fibroblasts [20]. Up-regulation
of caveolin-1 was associated to a significant inhibition of EGF-stimulated
ERK-1/2 phosphorylation [20]. Caveolin-1 has
also been shown to play an important role in senescence-associated
morphological changes by regulating focal adhesion kinase activity and actin
stress fiber formation in senescent cells [21]. In addition,
it has been shown that replicative senescent cells re-enter the cell cycle upon
EGF stimulation after down-regulation of caveolin-1 [22]. Together,
these data indicate that caveolin-1 plays a key role in the signal transduction
events leading to cellular senescence.
Role of caveolin-1 in cigarette smoking-induced
pulmonary emphysema
Pulmonary emphysema is an age-related disease of the
lungs. It occurs after a prolonged period of cigarette smoking. Pulmonary emphysema is characterized by alveolar
destruction, airspace enlargement and reduction of alveolar capillary exchange
area. Because cigarette smoke is enriched
in potent oxidants, oxidative stress is believed to play a key role in the
pathogenesis of emphysema [23,24]. The classical concept of the pathogenesis of emphysema
was based on lung inflammation caused by cigarette smoke and environmental
pollutants, leading to a protease/antiprotease imbalance [25]. However,
cigarette smoke has been shown to promote premature senescence of lung
fibroblasts in culture [26]. In
addition, a reduced proliferation rate [27,28], lower
number of population doubling in culture [27], and
increased senescence-associated β-galactosidase
activity [29] were
observed in lung fibroblasts from patients with emphysema. Since fibroblasts play a structural role that is
necessary for proper lung integrity, the
presence of senescent fibroblasts may
affect tissue microbalance and structural maintenance of the lungs. In
addition, senescent cells can secrete matrix metalloproteases [30] and inflam-matory
cytokines [31,32] that
could enhance the protease/antiprotease
imbalance and fuel the abnormal inflammatory response in the lungs,
respectively. Thus, accumulation of senescent fibroblasts may contribute to the
development of pulmonary emphysema.
However, the molecular mechanisms linking cigarette smoke to premature
senescence of lung cells and emphysema remain to be fully identified.
We have recently shown that cigarette smoke extracts
induced premature senescence of lung fibroblasts in a caveolin-1-dependent
manner [33]. More
specifically, the number of senescent cells was dramatically reduced and the
up-regulation of p53 and p21Waf1/Cip1 was significantly inhibited in lung fibroblasts derived
from caveolin-1 null mice, which do not
express caveolin-1, after treatment with cigarette smoke extracts. Co-treatment with antioxidants prevented the ability
of cigarette smoke extracts to induce premature senescence of lung fibroblasts,
suggesting that oxidants contained in cigarette smoke extracts were responsible
for the observed senescent phenotype. We also identified a mechanism through
which oxidative stress induces premature senescence of lung fibroblasts. Free
radicals have been shown to activate the ATM protein kinase [34], a key activator of p53. We
found that sequestration of PP2A-C, an ATM inhibitor, into caveolar membranes
was required for the activation of ATM and up-regulation of p53 in wild type
fibroblasts upon oxidative stress [33]. Cigarette smoke extracts failed
to activate ATM and up-regulate p53 in caveolin-1 null lung fibroblasts [33].
Does a lack of caveolin-1 prevent cigarette
smoke-induced activation of p53 and premature senescence in vivo? When
caveolin-1 null mice were exposed to cigarette smoking for either 6 weeks or 6
months, premature senescence of lung fibroblasts and activation of the p53 pathway were significantly prevented, as
compared to wild type mice [33]. Because
exposure to cigarette smoking for 6 months has been shown to induce pulmonary
emphysema in mice, we examined the lung phenotype of caveolin-1 null mice
exposed to cigarette smoking for 6 months. We found that, in contrast to wild
type mice, the development of pulmonary emphysema was significantly inhibited
in caveolin-1 null mice [33]. Senescent
fibroblasts were observed in the lungs of wild type mice after only 6 weeks of
exposure to cigarette smoking while pulmonary emphysema was morphologically
detectable after 6 months of exposure. Considering that a lack of caveolin-1
prevented both premature senescence of lung fibroblasts and development of
pulmonary emphysema, we propose a model in which oxidants contained in
cigarette smoke induce premature senescence of lung fibroblasts in a
caveolin-1/ATM/p53-dependent manner and that senescent lung fibroblasts
contribute to the pathogenesis of pulmonary emphysema.
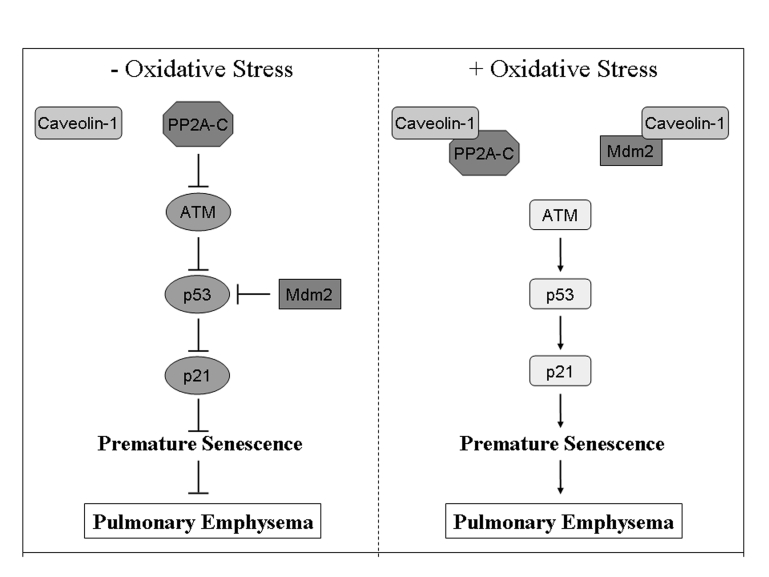
Figure 1. Schematic diagram summarizing the caveolin-1-dependent activation of the p53/p21 Waf1/Cip1/senescence pathway after oxidative stress.
In resting cells, PP2A-C-dependent inhibition of ATM prevents the
activation of p53. In addition, p53 is directly inhibited by binding to
Mdm2. Oxidative stress promotes the sequestration of PP2A-C and Mdm2 by
caveolin-1 leading to activation of p53 and its downstream target p21Waf1/Cip1,
and induction of premature senescence. Activation of the p53/p21Waf1/Cip1/senescence
pathway after oxidative stress is inhibited in cells lacking caveolin-1
expression. We suggest that activation of this pathway in lung fibroblasts
by oxidants contained in cigarette smoke contributes to the development of
pulmonary emphysema. Adapted from [33].
Activation of
ATM following the caveolin-1-mediated sequestration of PP2A-C may not be the
only mechanism employed by cigarette smoke to activate p53. As mentioned
earlier, we have shown that caveolin-1 activated the p53 pathway after
oxidative stress (hydrogen peroxide was used a source of free radicals in these
experiments) through an Mdm2-dependent pathway [18]. Although we
have not proved it directly, we speculate that oxidants contained in cigarette
smoke may activate p53 through both caveolin-1/ATM- and caveolin-1/Mdm2-dependent
mechanisms.
Lungs from
caveolin-1 null mice have marked hypercellularity resulting in thickening of
the alveolar wall and constriction of alveolar spaces [35]. This
hypercellularity can be correlated with the excessive proliferation of MEFs
derived from caveolin-1 null mice that is observed in cell culture models and
is consistent with data proposing caveolin-1 as a tumor suppressor [36]. The
hyperproliferation of lung cells observed in caveolin-1 null mice may
counterbalance alveolar destruction and airspace enlargement induced by
cigarette smoking, contributing to explain the lower number of senescent cells
and the milder emphysematous phenotype that we have observed in these mice.
Conclusive
remarks
Based
on our findings, we propose caveolin-1 as a novel upstream positive regulator
of p53 in the signaling pathway that leads to premature senescence of lung
fibroblasts upon oxidant stimulation and, eventually, to pulmonary emphysema.
We currently do not know whether cigarette smoke induces premature senescence
of other lung cell types, such as alveolar epithelial cells, which mediate
oxygen absorption. Since caveolin-1 is also endogenously expressed in these
cells, it is possible that caveolin-1 may also mediate oxidative stress-induced
premature senescence of lung epithelial cells in vivo and that senescent
alveolar epithelial cells may contribute to the development of pulmonary
emphysema. Thus, one may envision a therapeutic intervention aimed at lowering
caveolin-1 expression in lung cells for the treatment and/or prevention of the
tissue damages that are caused by cigarette smoking. However, given the role
that caveolin-1 plays as a tumor suppressor in certain forms of cancer, such as
breast cancer, and that most types of cancer are of epithelial origin, we can
not rule out the possibility that the indiscriminate down-regulation of
caveolin-1 expression in lung cells may limit emphysema but promote the
development of lung cancer. Therefore, a targeted down-regulation of caveolin-1
expression in lung fibroblasts may be a strategic approach to limit emphysema
without promoting tumor development.
Acknowledgments
This work was supported by grants from the National
Institute on Aging (R01-AG022548 and R01-AG030636) (to F.G.).
Conflicts of Interest
The authors of this manuscript have no conflict of
interest to declare.
References
-
1.
Volonte
D
, Zhang
K
, Lisanti
MP
and Galbiati
F.
Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts.
Mol Biol Cell.
2002;
13:
2502
-2517.
[PubMed]
.
-
2.
Razani
B
, Schlegel
A
and Lisanti
MP.
Caveolin proteins in signaling, oncogenic transformation and muscular dystrophy.
J Cell Sci.
2000;
113:
2103
-2109.
[PubMed]
.
-
3.
Williams
TM
and Lisanti
MP.
The Caveolin genes: from cell biology to medicine.
Ann Med.
2004;
36:
584
-595.
[PubMed]
.
-
4.
Hagiwara
Y
, Nishina
Y
, Yorifuji
H
and Kikuchi
T.
Immunolocaliza-tion of caveolin-1 and caveolin-3 in monkey skeletal, cardiac and uterine smooth muscles.
Cell Struct Funct.
2002;
27:
375
-382.
[PubMed]
.
-
5.
Harman
D
Aging: a theory based on free radical and radiation chemistry.
J Gerontol.
1956;
11:
298
-300.
[PubMed]
.
-
6.
Chen
QM
Replicative senescence and oxidant-induced premature senescence. Beyond the control of cell cycle check-points.
Ann N Y Acad Sci.
2000;
908:
111
-125.
[PubMed]
.
-
7.
Lundberg
AS
, Hahn
WC
, Gupta
P
and Weinberg
RA.
Genes involved in senescence and immortalization.
Curr Opin Cell Biol.
2000;
12:
705
-709.
[PubMed]
.
-
8.
Dimri
GP
, Lee
X
, Basile
G
, Acosta
M
, Scott
G
, Roskelley
C
, Medrano
EE
, Linskens
M
, Rubelj
I
and Pereira-Smith
O.
A biomarker that identifies senescent human cells in culture and in aging skin in vivo.
Proc Natl Acad Sci U S A.
1995;
92:
9363
-9367.
[PubMed]
.
-
9.
Black
EJ
, Clark
W
and Gillespie
DA.
Transient deactivation of ERK signalling is sufficient for stable entry into G0 in primary avian fibroblasts.
Curr Biol.
2000;
10:
1119
-1122.
[PubMed]
.
-
10.
Sherr
CJ
and DePinho
RA.
Cellular senescence: mitotic clock or culture shock.
Cell.
2000;
102:
407
-410.
[PubMed]
.
-
11.
Wynford-Thomas
D
Cellular senescence and cancer.
J Pathol.
1999;
187:
100
-111.
[PubMed]
.
-
12.
Kim
NW
, Piatyszek
MA
, Prowse
KR
, Harley
CB
, West
MD
, Ho
PL
, Coviello
GM
, Wright
WE
, Weinrich
SL
and Shay
JW.
Specific association of human telomerase activity with immortal cells and cancer.
Science.
1994;
266:
2011
-2015.
[PubMed]
.
-
13.
Lee
SW
, Reimer
CL
, Oh
P
, Campbel
lDB
and Schnitzer
JE.
Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells.
Oncogene.
1998;
16:
1391
-1397.
[PubMed]
.
-
14.
Chen
Q
and Ames
BN.
Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells.
Proc Natl Acad Sci U S A.
1994;
91:
4130
-4134.
[PubMed]
.
-
15.
Frippiat
C
, Chen
QM
, Zdanov
S
, Magalhaes
JP
, Remacle
J
and Toussaint
O.
Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts.
J Biol Chem.
2001;
276:
2531
-2537.
[PubMed]
.
-
16.
Chen
QM
, Bartholomew
JC
, Campisi
J
, Acosta
M
, Reagan
JD
and Ames
BN.
Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication.
Biochem J.
1998;
332 ( Pt 1):
43
-50.
[PubMed]
.
-
17.
Galbiati
F
, Volonte
D
, Liu
J
, Capozza
F
, Frank
PG
, Zhu
L
, Pestell
RG
and Lisanti
MP.
Caveolin-1 Expression Negatively Regulates Cell Cycle Progression by Inducing G(0)/G(1) Arrest via a p53/p21(WAF1/Cip1)-dependent Mechanism.
Mol Biol Cell.
2001;
12:
2229
-2244.
[PubMed]
.
-
18.
Bartholomew
JN
, Volonte
D
and Galbiati
F.
Caveolin-1 regulates the antagonistic pleiotropic properties of cellular senescence through a novel Mdm2/p53-mediated pathway.
Cancer Res.
2009;
In Press:
.
-
19.
Dasari
A
, Bartholomew
JN
, Volonte
D
and Galbiati
F.
Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements.
Cancer Res.
2006;
66:
10805
-10814.
[PubMed]
.
-
20.
Park
WY
, Park
JS
, Cho
KA
, Kim
DI
, Ko
YG
, Seo
JS
and Park
SC.
Up-regulation of caveolin attenuates epidermal growth factor signaling in senescent cells.
J Biol Chem.
2000;
275:
20847
-20852.
[PubMed]
.
-
21.
Cho
KA
, Ryu
SJ
, Oh
YS
, Park
JH
, Lee
JW
, Kim
HP
, Kim
KT
, Jang
IS
and Park
SC.
Morphological adjustment of senescent cells by modulating caveolin-1 status.
J Biol Chem.
2004;
279:
42270
-42278.
[PubMed]
.
-
22.
Cho
KA
, Ryu
SJ
, Park
JS
, Jang
IS
, Ahn
JS
, Kim
KT
and Park
SC.
Senescent phenotype can be reversed by reduction of caveolin status.
J Biol Chem.
2003;
278:
27789
-27795.
[PubMed]
.
-
23.
MacNee
W
Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease.
Proc Am Thorac Soc.
2005;
2:
50
-60.
[PubMed]
.
-
24.
Macnee
W
and Rahman
I.
Oxidants and antioxidants as therapeutic targets in chronic obstructive pulmonary disease.
Am J Respir Crit Care Med.
1999;
160:
S58
-65.
[PubMed]
.
-
25.
Snider
GL
Emphysema: the first two centuries--and beyond. A historical overview, with suggestions for future research: Part 2.
Am Rev Respir Dis.
1992;
146:
1615
-1622.
[PubMed]
.
-
26.
Nyunoya
T
, Monick
MM
, Klingelhutz
A
, Yarovinsky
TO
, Cagley
JR
and Hunninghake
GW.
Cigarette smoke induces cellular senescence.
Am J Respir Cell Mol Biol.
2006;
35:
681
-688.
[PubMed]
.
-
27.
Holz
O
, Zuhlke
I
, Jaksztat
E
, Muller
KC
, Welker
L
, Nakashima
M
, Diemel
KD
, Branscheid
D
, Magnussen
H
and Jorres
RA.
Lung fibroblasts from patients with emphysema show a reduced proliferation rate in culture.
Eur Respir J.
2004;
24:
575
-579.
[PubMed]
.
-
28.
Nobukuni
S
, Watanabe
K
, Inoue
J
, Wen
FQ
, Tamaru
N
and Yoshida
M.
Cigarette smoke inhibits the growth of lung fibroblasts from patients with pulmonary emphysema.
Respirology.
2002;
7:
217
-223.
[PubMed]
.
-
29.
Muller
KC
, Welker
L
, Paasch
K
, Feindt
B
, Erpenbeck
VJ
, Hohlfeld
JM
, Krug
N
, Nakashima
M
, Branscheid
D
, Magnussen
H
, Jorres
RA
and Holz
O.
Lung fibroblasts from patients with emphysema show markers of senescence in vitro.
Respir Res.
2006;
7:
32
[PubMed]
.
-
30.
Campisi
J
Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors.
Cell.
2005;
120:
513
-522.
[PubMed]
.
-
31.
Boren
E
and Gershwin
ME.
Inflamm-aging: autoimmunity, and the immune-risk phenotype.
Autoimmun Rev.
2004;
3:
401
-406.
[PubMed]
.
-
32.
Chung
HY
, Kim
HJ
, Kim
KW
, Choi
JS
and Yu
BP.
Molecular inflammation hypothesis of aging based on the anti-aging mechanism of calorie restriction.
Microsc Res Tech.
2002;
59:
264
-272.
[PubMed]
.
-
33.
Volonte
D
, Kahkonen
B
, Shapiro
S
, Di
Y
and Galbiati
F.
Caveolin-1 expression is required for the development of pulmonary emphysema through activation of the ATM-p53-p21 pathway.
J Biol Chem.
2009;
284:
5462
-5466.
[PubMed]
.
-
34.
Shackelford
RE
, Innes
CL
, Sieber
SO
, Heinloth
AN
, Leadon
SA
and Paules
RS.
The Ataxia telangiectasia gene product is required for oxidative stress-induced G1 and G2 checkpoint function in human fibroblasts.
J Biol Chem.
2001;
276:
21951
-21959.
[PubMed]
.
-
35.
Drab
M
, Verkade
P
, Elger
M
, Kasper
M
, Lohn
M
, Lauterbach
B
, Menne
J
, Lindschau
C
, Mende
F
, Luft
FC
, Schedl
A
, Haller
H
and Kurzchalia
TV.
Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice.
Science.
2001;
293:
2449
-2452.
[PubMed]
.
-
36.
Razani
B
, Engelman
JA
, Wang
XB
, Schubert
W
, Zhang
XL
, Marks
CB
, Macaluso
F
, Russell
RG
, Li
M
, Pestell
RG
, Di Vizio
D
and Hou
H Jr.
, Knietz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable, but show evidence of hyper-proliferative and vascular abnormalities.
J Biol Chem.
2001;
.