Introduction
Adult skeletal muscle is a highly
malleable tissue which can respond positively to pharmacological, environmental,
and mechanical stimuli with remarkable adaptations. A characteristic example of
adaptive muscle plasticity is mitochondrial biogenesis. Increased organelle
synthesis ultimately occurs as the result of the functional coordination
between nuclear, cytosolic, as well as mitochondrial domains [1]. During the
induction of organelle biogenesis, stress-sensitive signaling molecules, such as AMP-activated protein kinase
(AMPK) and p38 mitogen-activated protein kinase (MAPK), communicate with downstream
effectors including peroxisome proliferator-activated receptor γ
co-activator 1α (PGC-1α) that results in the transcriptional
upregulation of nuclear genes encoding mitochondrial proteins [2-4].
Newly-synthesized proteins which are destined for the organelle, such as
mitochondrial DNA (mtDNA) transcription factor A (Tfam) and apoptosis-inducing
factor (AIF), are directed to their specific mitochondrial sub-compartments by
the mitochondrial protein import machinery (PIM). The PIM is comprised of
translocase proteins of the outer mitochondrial membrane (TOM), as well as a
similar complex within the inner membrane (TIM proteins). The coordination of
these events, including the expression of mtDNA-encoded proteins by Tfam, leads
to mitochondrial biogenesis. This results in morphological as well as
functional alterations in the organelle, such as increased enzyme activity,
respiratory capacity, and reticular expansion throughout the myofibers.
Although all mitochondria
serve a similar function in providing ATP for the energy demands of the cell,
electron microscopy has revealed regional differences in the subcellular
location of muscle cell mitochondria [5,6]. Mitochondria that are clustered in
proximity to the sarcolemma are termed subsarcolemmal (SS) mitochondria, and
those embedded among the myofibrils are called intermyofibrillar (IMF)
mitochondria. Biochemical investigations have shown that isolated IMF
mitochondria contain lower levels of the phospholipid cardiolipin, but have
higher enzyme activities, respiratory and protein synthesis rates, as well as elevated
import rates of precursor proteins [7-11]. Furthermore, inherent differences in
reactive oxygen species (ROS) production, as well as apoptotic and autophagic
signaling have been previously documented [10,12]. In adults, the
mitochondrial subfractions differ in their adaptability to a common stimulus,
such as chronic muscle use [5,13,14], suggesting that their location within
the cell makes them differentially sensitive to a common intracellular signal.
Skeletal muscle in aged animals is
characterized by reductions in mass and the ability to develop force. This
condition, known as sarcopenia, is defined by increased fatigability and the
atrophy or loss of muscle fibers. Several mechanisms have been proposed to
cause age-related muscle fibre atrophy, including endocrine-mediated signaling
[15], diminished muscle progenitor cell activity [16], alterations in amino
acid metabolism [17], as well as apoptotic myocellular decay. An increased
incidence of apoptosis, as well as the expression of pro-apoptotic proteins
and mitochondrially-mediated cell death signaling, have been reported in aged
skeletal muscle [18-20]. Decrements in the oxidative capacity of aged skeletal
muscle is associated with the impairment of mitochondrial function, such as
reduced electron transport chain complex activity, ATP synthesis, and increased
ROS production [18,21,22]. Furthermore, some controversy exists regarding the
potential for adaptive plasticity of skeletal muscle in old, compared to young
animals. For example, Skorjanc et al. [23] demonstrated an unaltered
adaptability of skeletal muscle energy metabolism, including markers of
glycolysis and mitochondrial function, to chronic low-frequency electrical
stimulation-induced contractile activity in the aging rat. In contrast, other
studies of chronic muscle use have shown a loss of adaptive plasticity,
evidenced by a significantly slower rate of change in citrate synthase activity
and fatigue resistance as a result of aging [24]. In addition, when assessing
mitochondrial biogenesis, it is important to note whether the organelles have
been identified as SS or IMF subfractions, since these respective mitochondria
possess unique biochemical and functional properties which affect their
inherent malleability [25]. Thus, the purposes of this study were to
investigate the adaptive plasticity of skeletal muscle SS and IMF mitochondria
in old (36 months), compared to young (6 months) animals in response to period
of augmented organelle biogenesis. We employed chronic electrical stimulation
to evoke contractile activity of skeletal muscle in an effort to induce an
increase in mitochondrial volume. As observed with exercise training [26],
increased muscle use in response to chronic stimulation, an established
experimental model of endurance-type training, is a well-documented stimulus
for eliciting mitochondrial adaptations in skeletal muscle [1]. We hypothesized
that mitochondrial adaptive plasticity would be evoked in both young and old
animals, but that the extent of organelle remodeling would be attenuated in the
muscle of aged animals. Our results provide revealing insight into the reduced
adaptive potential of aging skeletal muscle.
Results
Contractile
activity-induced changes in skeletal muscle mass and contractile characteristics
are similar between young and old animals
Similar to our previous
reports [18,32], the skeletal muscle from the 36 mo old animals was
sarcopenic, evidenced by a significantly reduced TA muscle mass, lower maximal
force production, as well as slower rates of muscle contraction and relaxation
(Table 1). Chronic stimulation had no effect on multiple aspects of contractile
function, with the exception of the maximal force production per mg of TA
weight (TET/TAW) and the maximal rate of force development (+dF/dt), which were
both significantly reduced in young and old animals after chronic stimulation.
The TET/TAW was decreased by chronic contractile activity by approximately 20%
in both age groups, while the +dF/dt was reduced by 40-50% in the young and old
animals.
Table 1. Skeletal muscle characteristics, contractile properties, and SS and IMF mitochondrial yield.
Values are reported as means ± SE; n = number in parentheses.
TAW, TA weight; BW, body weight; TW, maximum twitch force; TET,
maximum tetanic force; TPT, time to peak twitch tension; 1/2 RT,
half relaxation time; +dF/dt, rate of force development; -dF/dt,
rate of relaxation; SS, subsarcolemmal; IMF, intermyofibrillar;
Fold, fold difference; ¶ P < 0.05, CON vs. STIM; * P < 0.05, 6 mo vs. 36 mo.
| | | | |
|
Muscle
characteristics
|
Contractile
properties
|
Protein yield
|
|
TAW
(mg)
|
TAW/
BW
(mg/g)
|
TW/
TAW
(mN/mg)
|
TET/
TAW
(mN/mg)
|
TPT
(msec)
|
1/2 RT
(msec)
|
+dF/dt
(N/s)
|
-dF/dt
(N/s)
|
SS
(mg/g)
|
IMF
(mg/g)
|
| | | | | | | | | | |
|
6
mo
CON
|
826
± 10
(8)
|
2.10
± 0.07
(8)
|
2.26
± 0.23
(8)
|
9.91
± 0.49
(8)
|
25.7
± 1.3
(8)
|
26.9
± 2.5
(8)
|
103
± 11.0
(7)
|
51.7
± 5.5
(8)
|
2.06
± 0.15
(21)
|
3.12
± 0.17
(22)
|
| | | | | | | | | | |
|
6
mo
STIM
|
826
± 77
(8)
|
2.18
± 0.12
(8)
|
1.96
± 0.23
(8)
|
7.95¶
± 0.49
(8)
|
25.0
± 0.88
(8)
|
25.8
± 4.4
(8)
|
62.2¶
± 12.1
(6)
|
56.0
± 7.8
(8)
|
2.56¶
± 0.17
(22)
|
4.5¶
± 0.19
(22)
|
|
Fold
6 mo STIM/CON
|
1.0
|
1.0
|
0.87
|
0.80
|
0.97
|
0.96
|
0.60
|
1.08
|
1.24
|
1.44
|
| | | | | | | | | | |
|
36
mo
CON
|
498*
± 29
(8)
|
1.02*
± 0.05
(8)
|
2.75
± 0.26
(7)
|
6.97*
± 0.88
(7)
|
28.4*
± 1.2
(6)
|
32.5*
± 1.39
(7)
|
48.1*
± 8.4
(5)
|
30.8*
± 1.3
(5)
|
2.71*
± 0.19
(17)
|
4.02*
± 0.32
(16)
|
| | | | | | | | | | |
|
36
mo
STIM
|
474
± 30
(8)
|
0.95
± 0.04
(8)
|
2.35
± 0.2
(8)
|
5.79¶
± 0.49
(8)
|
27.9
± 1.1
(8)
|
31.7
± 1.74
(8)
|
25.4¶
± 9.1
(6)
|
28.7
± 2.1
(6)
|
2.6
± 0.21
(17)
|
4.84¶
± 0.36
(17)
|
|
Fold
36 mo STIM/CON
|
0.95
|
0.93
|
0.85
|
0.83
|
0.98
|
0.98
|
0.53
|
0.93
|
0.96
|
1.2
|
| | | | | | | | | | |
|
Fold
36
mo/6 mo
|
0.60
|
0.49
|
1.22
|
0.70
|
1.11
|
1.21
|
0.47
|
0.60
|
1.32
|
1.29
|
| | | | | | | | | | |
Activity-induced
improvements in muscle performance are attenuated in aged animals
Subsequent to 7 days of
chronic contractile activity, we assessed the degree of fatigue resistance in
the STIM and the CON limbs of both the young and old animals. After 5 min of
acute, direct muscle stimulation, the force output of the TA muscle from the
CON limb of the young animals declined to 49% of initial tension (Figure 1A).
Skeletal muscle from the CON leg of old animals was significantly less fatigue
resistant, as force was reduced to 39% of initial, representing a 20%
difference between the age groups. In response to chronic contractile activity
in the young animals, force was maintained at 69% of initial tension, which
represented a 42% improvement (P < 0.05) compared to the CON limb. In the
old animals, the decline in force output was also significantly attenuated, to
50% of initial tension. The chronic stimulation-induced 28% increase in fatigue
resistance in the old animals was less than that observed in the young group.
However, the muscle performance evident in the old animals after STIM resembled
closely that documented in the CON limb of young animals.
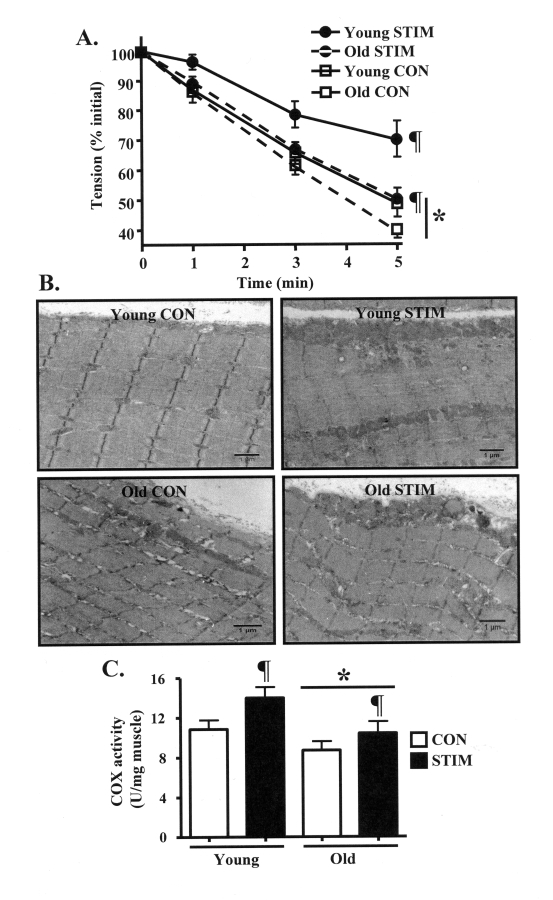
Figure 1. Chronic contractile activity-evoked increases in skeletal muscle endurance performance and mitochondrial content are reduced in old, compared to young animals. (A). Fatigue resistance during 5 min
of 1 Hz in situ stimulation of the control (CON, open squares) and
chronically stimulated (STIM, closed circles) tibialis anterior muscles
from young (solid lines) and old (dashed lines) animals (n = 7-8). (B)
Electron micrographs depicting skeletal muscle morphology and SS and
IMF mitochondrial volumes in young and old, control (CON, open bars) and
chronically stimulated (STIM, closed bars) extensor digitorum longus (EDL)
muscle sections. All images were taken at the same magnification. Scale bar
located at the lower right of each picture represents 1 μm. (C) COX
enzyme activity in EDL muscle homogenates (n = 9-13). Data represent
the mean ± SEM. * P < 0.05 vs. Young; ¶ P < 0.05 vs.
CON.
Chronic contractile
activity augments muscle SS and IMF mitochondrial content to a greater extent
in young animals
The physiologic assessments
of skeletal muscle function were accompanied by biochemical and molecular
assays of muscle and mitochondrial properties in young and old animals.
Skeletal muscle SS and IMF mitochondrial volume was first qualitatively
investigated using electron microscopy. In the CON muscle from young animals (Figure 1B, top left panel), the micrograph clearly shows a thick accumulation of SS
mitochondria positioned beneath the sarcolemmal membrane, as well as the
presence IMF mitochondria widely dispersed between the myofibrils. In contrast,
a lesser volume of SS and IMF mitochondria was apparent in the CON limb from
old animals (Figure 1B, bottom left). The adaptive response to chronic
contractile activity included robust increases in organelle content in both the
subsarcolemmal and intermyofibrillar regions of the muscle in both young and
old animals (Figure 1B, bottom panels). Next, we quantitatively investigated
muscle mitochondrial content by measuring cytochrome c oxidase (COX) activity,
an established biochemical indicator of mitochondrial volume [1]. Similar to
earlier reports [18], COX enzyme activity was 30% lower (P < 0.05) in the
muscle from old, compared to young animals (Figure 1C). Chronic stimulation
significantly elevated mitochondrial content in both young and old animals,
however the increase was greater (30%; P < 0.05) in the muscle from young
animals, compared to the 20% increase observed in the old animals. Furthermore,
chronic contractile activity also increased the yield of SS and IMF
mitochondria obtained during the mitochondrial isolation process to a greater
extent in the young, compared to the old animals (Table 1).
Chronic activity
increases the expression of mitochondrial biogenesis regulatory proteins in the
muscle from young and old animals
In an effort to understand the molecular
basis for the aging-associated attenuation of mitochondrial and muscle
plasticity in response to chronic contractile activity, we employed Western
blotting to measure the contents of 1) the critical mitochondrial biogenesis
regulatory proteins PGC-1α and Tfam, 2) molecules important for
mitochondrial and muscle function such as
AIF and HSP70, as well as 3) SIRT1, a protein involved in the aging process.
Chronic contractile activity significantly increased the expression of
PGC-1α, Tfam, AIF, and SIRT1 in the muscle of young animals by
approximately 50-65%, compared to the control limb (Figure 2A, B). Protein
expression was also increased by 40-50% in the muscle of old animals, however
the magnitude of this increase was lower compared to the young group. The
stress protein HSP70 was highly induced in response to chronic stimulation,
evidenced by the 3-fold and 11-fold increases in protein expression in the
muscles from old and young animals, respectively (Figure 2C).
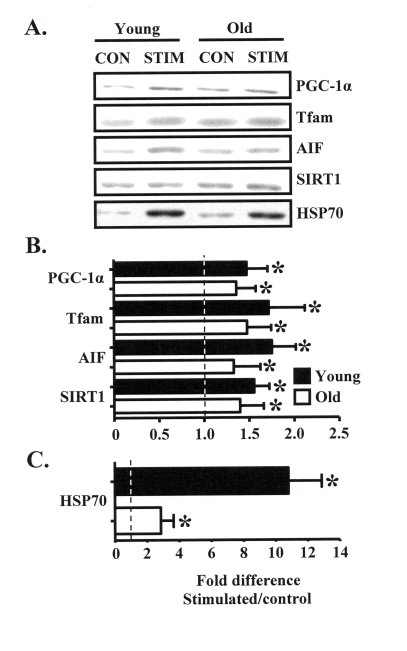
Figure 2. Chronic muscle use increases the expression of muscle and mitochondrial regulatory proteins in young and old animals. (A). Representative
Western blots and graphical summary (B) of the effects of chronic
contractile activity on the expression levels of proteins important for
mitochondrial biogenesis (PGC-1α, Tfam), apoptotic signaling (AIF,
HSP70), and aging (SIRT1), in muscles from young (closed bars) and old
(open bars) animals expressed as the fold increase in chronically
stimulated over control muscles (n = 6-11). (C) HSP70 protein
content is shown separately due to the difference in scale, compared to the
data in B. Data represent the mean ± SEM. * P < 0.05, stimulated
vs. control.
SS and IMF mitochondrial
protein import machinery components are increased after chronic stimulation in
young, but not old animals
Expansion of the mitochondrial reticulum
in response to organelle biogenesis-inducing stimuli requires the import of
nuclear-encoded mitochondrial proteins. We therefore investigated
contraction-evoked changes in the expression of mitochondrial protein import
machinery, including mtHSP70, Tim17, and Tim23 in SS and IMF mitochondrial
subfractions isolated from the control and chronically stimulated muscles of
young and old animals. In the young group, chronic contractile activity
augmented (P < 0.05) the expression of mtHSP70, Tim17, and Tim23 in SS
mitochondria by approximately 2-3-fold, and in IMF mitochondria by 1.5-2-fold (Figure 3A-C). In contrast, the protein expression of these components of the import
machinery did not increase within the mitochondria from old animals.
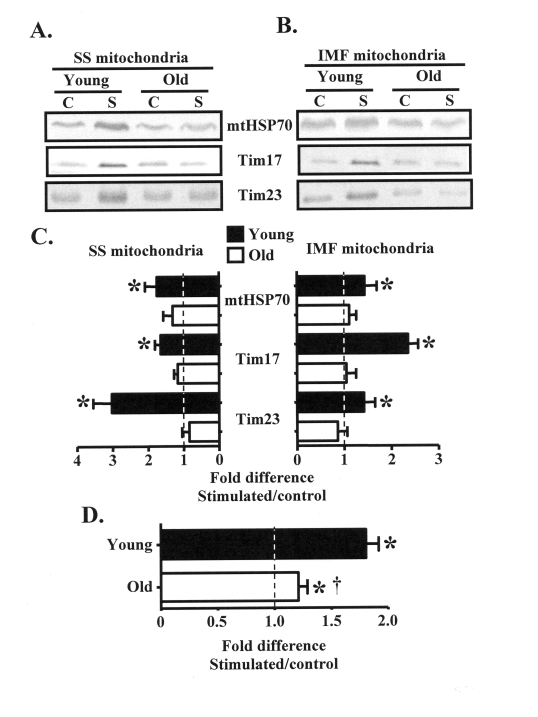
Figure 3. Subsarcolemmal (SS) and intermyofibrillar (IMF) mitochondrial protein import machinery components are increased in young, but not old animals in response to chronic contractile activity. (A). Representative
Western blots of mtHSP70, Tim17, and Tim23 proteins in SS and IMF (B) mitochondrial
subfractions isolated from the control (C) and chronically stimulated (S) limbs
of young and old animals. (C) Graphical summary of the data in
panels A and B expressed as the fold difference of the stimulated, over the
control legs (n = 7-9). (D) Pooled results of the protein
expression data in young, compared to old animals shown above in panel C,
and panel B of Figure 3 (n = 74-86). Data represent the mean ± SEM.
* P < 0.05, stimulated vs. control, † P < 0.05
vs. Young.
In an effort to summarize
the effect of chronic contractile activity on the expression of proteins
involved with mitochondrial plasticity, we pooled together results from Figures
2B and 3C. This analysis shows that chronic contractile activity significantly
increased the expression of multiple proteins in the young animals, on average,
by 1.8-fold above that found in the CON, non-stimulated muscle (Figure 3D).
Chronic contractile activity also increased (P < 0.05) the expression of
these proteins in the old animals by approximately 20% overall. However, the
extent of the adaptation was attenuated (P < 0.05) in the old, compared to
the young animals.
Mitochondrial protein
import is enhanced to a greater extent after chronic activity in muscle from
young animals
We next evaluated whether
impaired adaptations in mitochondrial protein import machinery components in
old animals would coincide with reduced functional rates of protein import into
the organelle. Thus, we assessed the import of the matrix protein ornithine
carbamoyltransferase (OCT) into isolated SS and IMF mitochondria harvested from
the control and chronically stimulated muscles of young and old animals.
Chronic stimulation significantly increased the import of OCT into the SS
subfraction by 1.8-fold in the young animals, and 1.3-fold in the old group
(Figs. 4A, B). Chronic contractile activity also resulted in a 30% induction (P
< 0.05) in OCT import into the IMF mitochondria isolated from the young
group, whereas there was no effect observed in the IMF subfraction from old
animals (Figure 4A, C). In contrast, the content of protein chaperones MSF-L
and HSP90, both involved in shuttling mitochondrial precursor proteins during
cytosolic transit to the organelle, was not affected by chronic contractile
activity (Figure 5D-E). However, the basal expression of these proteins was
40-100% higher (P < 0.05) in the skeletal muscle of old, compared to young
animals.
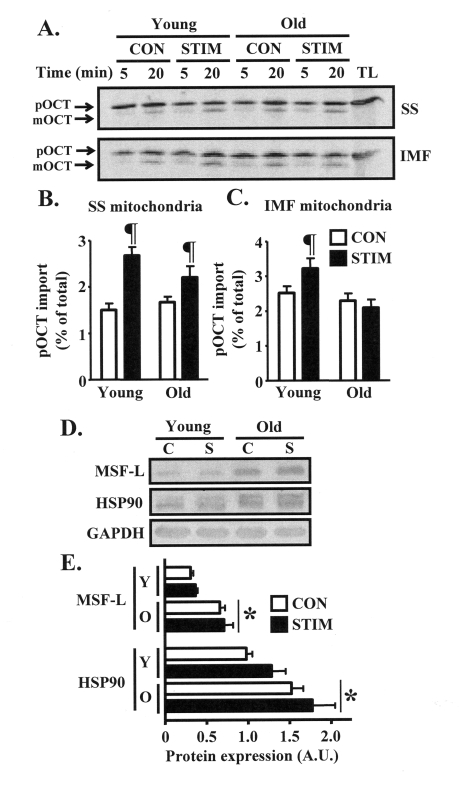
Figure 4. Mitochondrial import of the matrix protein ornithine carbamoyltransferase (OCT) is induced to a greater extent after chronic muscle use in young animals. (A) Representative
autoradiograms of precursor (pOCT) and mature (mOCT) OCT after
5 and 20 min of the import reaction timecourse in isolated SS
(top) and IMF (bottom) mitochondrial subfractions harvested from
the control (CON, open bars) and chronically stimulated (STIM, closed
bars) limbs of young and old animals (TL, translation lane without
mitochondria). (B) and (C) Graphical summaries of
the 20 min import data from repeated experiments shown in panel A
(n = 9-12). (D) Western blots of MSF-L and HSP90 in isolated
cytosolic fractions obtained from the control (C) and chronically
stimulated (S) legs of young and old animals. GAPDH was used to
confirm equal loading of protein. (E) Summary of repeated
experiments shown in panel D (n = 5-7). Data represent the
mean ± SEM. * P < 0.05 vs. Young; ¶ P < 0.05 vs. CON.
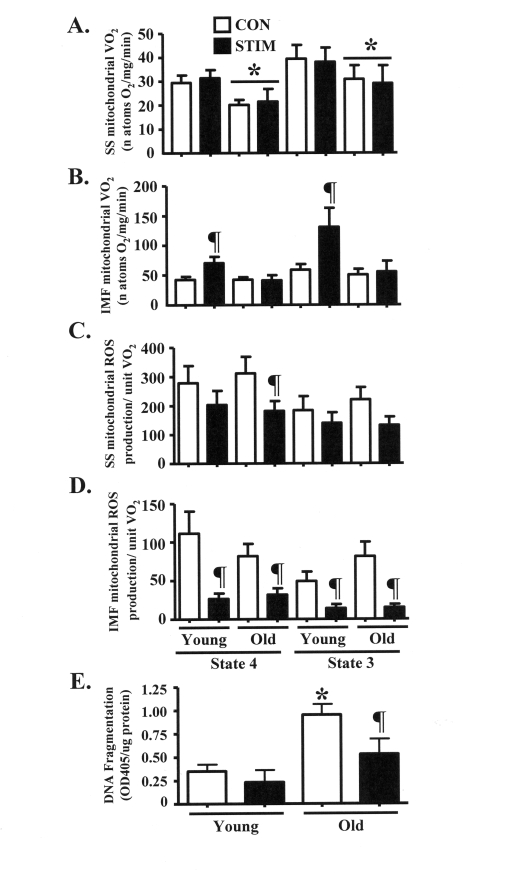
Figure 5. Chronic stimulation-induced adaptations in mitochondrial function and anti-apoptotic cell death signaling in young and old animals. (A). and (B) State 4 (2 μM rotenone and 10 mM succinate
as substrates) and state 3 (rotenone and succinate plus 0.44 mM ADP) rates
of oxygen consumption (VO2) in isolated subsarcolemmal (SS; A)
and intermyofibrillar (IMF; B) mitochondria from the control (CON,
open bars) and chronically stimulated (STIM, closed bars) limbs of young
and old animals (n = 6-10). (C) and (D) State 4 and
state 3 rates of reactive oxygen species (ROS) production per natom oxygen
consumed in SS (C) and IMF (D) mitochondria from the CON and
STIM limbs of young and old animals (n = 7-10). (E) Level of
fragmented DNA, in the form of mono- and oligonucleosomes, in
myonuclei-containing cytosolic extracts isolated from young and old animals
(n: STIM = 4, CON = 21). Data represent the mean ± SEM. * P <
0.05 vs. Young; ¶ P < 0.05 vs. CON).
Succinate-stimulated
mitochondrial oxygen consumption is increased in young, but not old animals
after chronic contractile activity
Previous assessments of glutamate-stimulated
state 4 and 3 mitochondrial oxygen consumption (VO2) through complex
I in our laboratory showed no difference between age groups [18]. Thus,
organelle function was further assessed by measuring rates of complex II-driven
VO2 in isolated SS and IMF mitochondrial subfractions. SS
mitochondrial VO2 was 20-30% lower (P < 0.05) in old animals during
both state 4 and state 3 VO2 driven in the presence of succinate (Figure 5A). Chronic stimulation not did
alter SS mitochondrial VO2 in either age group. Rates of VO2
were significantly increased by 70-100% in IMF mitochondria from young animals
in response to chronic contractile activity (Figure 5B). In contrast, the rate
of VO2 of the IMF mitochondrial subfraction isolated from the old
group was not affected by the treatment.
Chronic muscle use
evokes similar adaptations in mitochondrial reactive oxygen species (ROS)
production in young and old animals
ROS production is an
inherent metabolic byproduct of mitochondrial respiration within the organelle.
Therefore, we measured succinate-stimulated ROS production and expressed the
findings per unit of mitochondrial VO2. In young animals, chronic
contractile activity did not influence the rate of ROS production from the SS
mitochondrial subfraction (Figure 5C). However, in SS mitochondria isolated
from old animals, state 4 ROS production was reduced by 40% after chronic
stimulation (P < 0.05). In addition, state 3 ROS production tended to be
lower (40%; 0.05 < P < 0.1) after chronic contractile activity. In the
IMF mitochondria, chronic stimulation reduced ROS production by 60-80% (P <
0.05) in both the young and old groups (Figure 5D).
Chronic contractile
activity attenuates the elevated basal levels of myonuclear DNA fragmentation
in the skeletal muscle of old animals
We investigated a
downstream consequence of pro-apoptotic ROS signaling by assessing DNA
fragmentation in cytosolic extracts isolated from young and old animals. The
basal level of DNA fragmentation was approximately 3-fold greater (P < 0.05)
in muscle from old, compared to young animals (Figure 5E). Chronic contractile
activity had no influence on DNA fragmentation in young animals, however the
level of fragmented DNA was significantly reduced by 45% in the old animals.
Discussion
The intent of the present
study was to examine the adaptive potential of skeletal muscle mitochondria in
old animals in response to a potent stimulus for organelle expansion. To
rapidly evoke SS and IMF mitochondrial biogenesis, we used chronic electrical
stimulation-induced contractile activity of skeletal muscle, a well-established
treatment to augment mitochondrial content [1,33]. This model allows for the elimination
of any likely behavioural differences between young and old animals, and
presents a standardized, high intensity contractile stimulus to the muscle. Our
data illustrate that old animals retain the adaptive capacity for skeletal
muscle and mitochondrial plasticity, however the extent of this remodeling was
attenuated when compared to younger animals. Novel mechanistic insight for
these findings is provided by the blunted contractile activity-induced
elevations in mitochondrial biogenesis regulatory proteins, as well as the
reduced potential for mitochondrial protein import. Notably however, molecular
markers indicative of mitochondrially-mediated cell death signaling displayed
similar, or greater improvements in the muscle from aged, compared to young
animals in response to chronic contractile activity.
Skeletal muscle from
healthy adult animals is highly responsive to stimuli such as chronic
contractile activity [1,34]. In an effort to further our understanding of the
aging-associated alterations in skeletal muscle biology, we compared young
adult to senescent animals, which present with a high degree of sarcopenia.
Indeed, aging-evoked muscle pathology was evidenced by a 40-50% lower muscle
mass, as well as significant reductions in maximal force-producing capacity and
slower rates of contraction. While chronic contractile activity induced only
modest changes in the skeletal muscle contractile properties of young and old
animals, which were similar between the age groups, this treatment resulted in
significant adaptations in muscle fatigue resistance. Moreover, chronic
contractile activity effectively rescued the aging-induced decline in muscle
performance, resulting in a younger phenotype in the old animals. However, the
magnitude of the increase was greater in the young, compared to the old group,
indicative of an attenuated adaptive plasticity of pathways involved in
oxidative metabolism in aging muscle. This was certainly related in large
measure to the greater increase in overall mitochondrial content in young
animals, which was confirmed by assessments of multiple indices of
mitochondrial volume, including COX enzyme activity, as well as electron
microscopy and yield of SS and IMF mitochondrial subfractions. It is well known
that the content of mitochondria is closely correlated with muscular endurance
performance [35]. Thus, chronic stimulation evoked adaptive plasticity in the
performance of aging muscle, which was based, in part, on increased SS and IMF
mitochondrial volume. The greater increase in organelle biogenesis observed in
young animals suggests that the molecular mechanisms driving mitochondrial
synthesis in response to chronic muscle use are less responsive in old animals.
During the process of mitochondrial
biogenesis, a number of proteins have been demonstrated to play important roles
in the proper assembly and function on the organelle. These factors include the
critical nuclear and mitochondrial genome transcriptional regulatory proteins
PGC-1α and Tfam, as well as the anti-apoptotic stress molecule HSP70, and
the mitochondria-localized AIF [25,36,37]. Collectively, the content of these
proteins were augmented in aging muscle in response to chronic contractile
activity, however the increase was lower than that observed in the younger
animals. We conclude that the reduced plasticity of mitochondria in aged muscle
is partly due to the blunted expression of these factors in response to chronic
contractile activity. This phenomenon is likely the result of diminished
upstream contraction-induced signaling to mitochondrial biogenesis, as
recently described in aged, compared to young animals [32]. It has been
previously shown that decreased levels of PGC-1α and Tfam depress
mitochondrial biogenesis [38,39]. Further, AIF is a critical component for the
maintenance of normal mitochondrial cristae structure and oxidative
phosphorylation [37,40]. Mice deficient in AIF exhibit fragmented organelles
of punctuate morphology [37]. While there are conflicting reports regarding the
role of the SIRT1 longevity factor in skeletal muscle mitochondrial biogenesis
[41-43], as well as its expression in response to chronic muscle use [41,44], our data show, for the first time, that SIRT1 content is increased in both
young and senescent animals coincident with the chronic contractile
activity-evoked upregulation of mitochondrial content. This finding suggests
that chronic muscle use may represent an effective component of a treatment
regimen for aging-associated pathology, in part through enhanced SIRT1
expression, given its putative pro-survival function [45,46].
Post-transcriptional and
-translational processing of nuclear-encoded mitochondrial gene products are
essential for mitochondrial adaptations. The majority of mitochondrial proteins
are encoded in the nucleus, and must be targeted and translocated to the
mitochondrial subcompartment. The PIM, consisting of the TOM and TIM assembly
complexes, is responsible for ushering these proteins and assembling them into
a functional organelle. The components of this pathway and the mechanisms
regulating this process remain poorly understood in skeletal muscle. Our
previous work has demonstrated that specific PIM components, including HSP60,
CPN10, Tom20, and Tom34 are highly inducible by chronic muscle use in adult
muscle [29,47,48]. Data from the present study show that the expression of
Tim23, Tim17, and mtHSP70 are induced with chronic stimulation in SS and IMF
mitochondria isolated from the muscle of young, but not old animals. Thus, the
diminished plasticity of mitochondria from aged muscle is associated with a
collective attenuation in the adaptive response of proteins critical for
organelle remodeling.
The PIM constituents that
were examined here are responsible for targeting proteins that are destined for
the mitochondrial inner membrane, intermembrane space, and matrix. We have
previously demonstrated that the import rate of matrix-localized molecules,
including Tfam and MDH, is increased during conditions of chronic contractile
activity-induced mitochondrial biogenesis [29,49]. Our results support these
earlier findings, as import of the matrix protein OCT was augmented in response
to chronic muscle use in adult animals. In contrast, in aged muscle contractile
activity did not affect OCT import into IMF mitochondria, while the magnitude
of the increase in the SS subfraction was significantly lower, compared to the
increase observed in the young animals. In the absence of any change in protein
import machinery components, including auxiliary factors such as the cytosolic
chaperones MSF-L and HSP90, the modest increase in OCT import into SS
mitochondria from aged muscle may be attributed to potential alterations in
other PIM components, such as HSP60, CPN10, Tim50, or Tim21 [50]. Thus, it
seems reasonable to suggest that the attenuated protein import response in
aged, compared to young muscle, as well as the muted adaptive plasticity of
multiple protein factors involved in organelle synthesis, including the PIM
components, reveals a mechanistic basis for the reduced level of mitochondrial
biogenesis and muscle performance documented in old animals. Assessments of the
insertion of discrete proteins into other mitochondrial compartments, such as
the inner and outer membranes, as well as the assembly of multi-subunit enzyme
complexes (e.g. COX, TOM), remain fertile areas of future investigations into
the plasticity of muscle biological chemistry.
The decrement in the
adaptive potential of aged muscle was also manifest by the functional
evaluation of SS and IMF mitochondrial respiration in the presence of
succinate. Whereas both state 4 and state 3 respiration were significantly
elevated in the IMF subfraction from young animals, mitochondria from muscle of
old animals did not adapt to chronic contractile activity. Farrar et al. [51] have
previously shown that state 3 mitochondrial respiration was increased in SS and
IMF subfractions isolated from young and old animals after a period of chronic
muscle use. Notably, the training-induced increase in mitochondrial respiration
was similar, or greater in the organelles isolated from the aged muscle.
However, the authors employed a regimen of exercise training to evoke
mitochondrial adaptations in animals that were only ~24 months of age. This
represents a considerable difference in experimental design compared to the
present study. These data suggest that the reduced adaptive plasticity of
muscle in this model of organismal aging occurs between 24 and 36 months of
age.
Excessive ROS production within the
mitochondria acts as an early signal to initiate the mitochondrially-mediated
cell death pathway, leading ultimately to myonuclear decay and apoptosis [52].
In adult animals, chronic muscle use reduces apoptogenic mitochondrial
signaling in skeletal muscle, while muscle disuse has the opposite effect [14,53,54]. Our data illustrate that in IMF mitochondria, complex II-driven ROS
production was decreased to a similar extent in organelles isolated from
chronically stimulated young and aged muscle. This adaptation represents a
significant reduction in pro-apoptotic signaling throughout the myofiber, in
light of the fact that the IMF subfraction accounts for approximately 80% of
the total mitochondrial volume in the cell [5]. Indeed, when we assessed the
level of DNA fragmentation, the terminal step and hallmark indicator of
apoptosis, we found that chronic contractile activity exerted a more powerful
influence in reducing DNA fragmentation in aged, compared to young muscle. This
adaptive response may be related to potential chronic stimulation-induced
alterations in antioxidant and/or antiapoptotic signaling in the mitochondrial,
cytoplasmic, or nuclear domains of aged muscle. Our data demonstrate a chronic
stimulation-evoked increase in the antiapoptotic stress protein HSP70 in aged
animals, and it is known that the apoptosis repressor with a caspase
recruitment domain is also inducible in skeletal muscle in response to chronic
contractile activity [14]. It is evident that skeletal muscle from older
animals is more receptive to reductions in DNA catabolism which may be due, in
part, to the high level of DNA fragmentation already apparent under basal
conditions. We have shown previously that the muscle of young animals possesses
a resistance to alterations in DNA fragmentation even under conditions of
aggressive proapoptotic signaling evoked by chronic muscle disuse (i.e.
denervation; [53]). Thus, chronic contractile activity elicits a robust
antiapoptotic adaptive response in aged muscle, and suggests a heightened
molecular plasticity in defense of the myonuclear decay and myofiber loss
associated with the sarcopenia of aging.
In summary, the present
study demonstrates that the adaptive plasticity of skeletal muscle and
mitochondria is attenuated in aged, compared to young animals under conditions of
chronic contractile activity-induced organelle biogenesis. Our data reveal
novel insight into the molecular processes that are in part responsible for
this decrement, including lesser elevations in important mitochondrial
biogenesis regulatory factors, reduced signaling kinase activation [32], as
well as decreased functional rates of SS and IMF mitochondrial protein import
and ATP provision [32]. Despite this attenuated response, chronic contractile
activity resulted in beneficial functional adaptations in a number of muscle
and mitochondrial parameters in aged animals. This finding has obvious
relevance for the development of potential pharmacological and/or lifestyle
therapeutics, such as chronic physical activity, for aging-associated diseases including
sarcopenia and diabetes.
Methods
Animals.
Experiments were conducted after approval by the York
University Animal Care Committee in accordance with Canadian Council of Animal
Care guidelines. Male Fischer 344 Brown Norway rats were obtained from the National
Institute of Aging (Bethesda, MD) and divided into 6 mo (young) and 36 mo
(senescent) groups. Animals were housed individually and given food and water
ad libitum.
Chronic contractile
activity.
The procedure as outlined
previously [9] was followed for implantation of electrodes and chronic
low-frequency electrical stimulation of animals. Briefly, rats were
anaesthetized, and under aseptic conditions, an internal stimulation unit
encased in silicone [27] was secured to the interior of the abdominal musculature
in the intraperitoneal cavity. Platinum electrode wires were passed
subcutaneously and two stimulating electrodes were sutured unilaterally
flanking the common peroneal nerve of the left hindlimb. Stimulation was
adjusted at the time of electrode implantation to result in palpable
contractions of the tibialis anterior (TA) and extensor digitorum longus (EDL)
muscles. After a 1-week recovery period, the TA and EDL muscles were
chronically stimulated (STIM; 10 Hz, 0.1 ms duration) 3 h/day for 7 days. The
contralateral limb was used as a non-stimulated internal control (CON) in all
animals. After the stimulation period, animals were anaesthetized and the in
situ stimulation protocol was performed.
In situ acute stimulation
. Approximately 21 hours after the last bout of
chronic stimulation, the animals were anesthetized, and the chronically
stimulated, as well as the contralateral control TA muscles from young and old
animals were exposed and prepared for in situ direct muscle stimulation, as
detailed earlier [28]. The distal tendon of each TA muscle was isolated, and a
hooked pin was affixed to the tendon. The pin of one limb was attached to a
strain gauge, while the other leg was misted with saline and wrapped in plastic
to prevent dehydration. Intramuscular stimulating electrodes were placed in the
belly of the muscle, parallel to the fibers. The experimental protocol involved
stimulation with 100 ms trains at 100 Hz to determine maximal tetanic tension
produced by the muscle. This was followed by a stimulation period of 5 min at a
frequency of 1 Hz (0.1 ms duration) to evaluate muscle performance during
fatigue-inducing conditions. Force and pressure signals were sampled online
(Powerlab 4/SP, ADInstruments, Colorado Springs, CO) and stored for analysis
using Chart 5 software. Immediately upon the cessation of contractions, the TA
muscle of the acutely stimulated limb was quickly harvested, weighed, and
placed in ice-cold mitochondrial isolation buffer 1. The EDL muscle was
sectioned, with one portion freeze-clamped with aluminum tongs pre-cooled in
liquid nitrogen, and stored at -70 °C for use in subsequent and cytochrome c
oxidase (COX) enzyme activity measurements and Western blotting analyses, while
the other portion was prepared for serial sectioning and electron microscopy.
Acute stimulation and sampling of the TA and EDL muscles from the contralateral
limb followed. Animals were then sacrificed by exsanguination after a medial
thoractomy.
Isolation of
mitochondrial and cytosolic fractions.
The TA muscles were briefly minced, and the SS and IMF mitochondria were
fractionated by mechanical disruption, differential centrifugation, and 0.025
ml/g tissue protease digestion as described previously in detail [10].
Cytosolic extracts were prepared concurrently during this process as outlined
earlier [29]. Mitochondria were resuspended (100 mM KCl, 10 mM MOPS, 0.2% BSA)
and an aliquot of the suspension was taken for measurements of protein content
[30], and the yield was expressed as mg/g muscle wet weight.
Mitochondrial
respiration.
Samples of isolated SS
and IMF mitochondrial subfractions were incubated with 250 μl of VO2
buffer (250 mM sucrose, 50 mM KCl, 25 mM Tris-HCl, and 10 mM K2HPO4,
pH 7.4) at 30 °C in a water-jacketed respiratory chamber with continuous
stirring. Respiration rates (n atoms O2•min-1•mg-1)
driven by complex II in the mitochondrial electron transport chain were
evaluated in the presence of 2 μM rotenone and 10 mM succinate (state 4
respiration), or rotenone and succinate plus 0.44 mM ADP (state 3 respiration)
using the Mitocell S200 Micro Respirometry System (Strathkelvin Instruments,
Motherwell, UK). The addition of NADH during state 3 measurements had no effect
on the respiration rate (data not shown), indicating excellent mitochondrial
membrane integrity.
Mitochondrial
ROS production
. ROS were measured as
described previously [14]. Briefly, SS and IMF mitochondria (50 μg) from
young and old animals were incubated with VO2 buffer in a 96-well
plate. ROS production was assessed at 37 °C for 30 min during state 4 and state
3 respiration by adding 2 μM rotenone and 10 mM succinate, or rotenone and
succinate plus 0.44 mM ADP, respectively, immediately prior to the addition of
50 μM dichlorodihydrofluorescein diacetate. The fluorescence emission
between 480-520 nm measured with a multi-detection micro-plate reader (Synergy
HT, Biotek Instruments Inc., Winooski, VT) is directly related to ROS
production. Data were recorded and interpreted using KC4 (v 3.0) software. ROS
production measured in absolute fluorescence units was linear over the entire
measurement period. ROS levels were expressed per natom of O2
consumed, measured during the mitochondrial respiration assay.
DNA
isolation and in vitro transcription.
The plasmid containing the full-length cDNA encoding precursor ornithine
carbamoyltransferase (pOCT) was isolated from bacteria using an alkaline lysis
method. The cDNA resulting from this preparation was linearized with Sac I at
37°C for 2 hours. Plasmid DNA was extracted with phenol and precipitated in
ethanol overnight at -80°C. DNA, at a final concentration of 0.8
μg/μl, was transcribed with SP6 RNA polymerase, ribonucleoside
triphosphate substrates and the cap analog m7G(5')ppp(5')G at 40°C for 90 min.
The pOCT mRNA was extracted with phenol and precipitated in ethanol at -80°C
overnight. mRNA was resuspended in sterile distilled water and adjusted to a
final concentration of 2.8 μg/μl. Aliquots were stored at -20°C for
in vitro translation assays.
In vitro translation and mitochondrial
protein import.
The pOCT mRNA was
translated and labeled with the use of a rabbit reticulocyte lysate system in
the presence of [35S]-methionine. Freshly isolated SS and IMF
mitochondria and the translated radiolabeled precursor proteins were equilibrated
separately at 30°C for 10 min. The translated precursor proteins were added to
the mitochondrial samples and incubated at 30°C to initiate the protein import
reaction. Equal aliquots of the import reaction were withdrawn at 0, 5, and 20
min to determine basal pOCT import rates in control and chronically active
muscle from young and aged animals. Final import reactions consisted of 25
μg of mitochondria and 12 μl of the lysate containing the
radiolabeled precursor protein. Mitochondria were then recovered by
centrifugation through a 20% sucrose cushion for 15 min at 4°C. Pellets were
resuspended, lysed and then separated using 8% SDS-PAGE. After electrophoresis,
gels were boiled for 5 min in 5% TCA, rinsed for 30 seconds in distilled water,
followed by rinsing in 10 mM TRIS (5 min) and 1 M sodium salicylate (30 min).
Gels were subsequently dried for ~ 1 hour at 80°C and exposed overnight to a
Kodak Phosphor screen. Total intensities were quantified (Quantity One,
Bio-Rad). Import was expressed as the percent of processed mature protein
(mOCT) per minute, relative to the total protein available.
DNA fragmentation.
Aliquots of cytosolic extracts [29] from young and
old animals were prepared for spectrophotometric detection of DNA fragments, in
the form of mono- and oligonucleosomes, as per the manufacturers instructions
(Cell Death Detection ELISAPLUS, Roche Applied Science, Laval, PQ).
Electron microscopy.
EDL muscles from the CON and STIM legs of young and
old animals were excised and cut at mid-belly to obtain 2-3 mm serial sections.
Muscle samples were incubated on ice for 1 hour in 3.0% glutaraldehyde buffered
with 0.1 M sodium cacodylate. Sections were then washed three times in 0.1 M
sodium cacodylate buffer before being post-fixed for 1 hour in 1% osmium
tetroxide in 0.1 M sodium cacodylate at room temperature. Muscle sections were
then dehydrated by washes with 30%, 50%, 80% and 100% ethanol, then in
ethanol-propylene oxide for 1 hour, and followed by 100% propylene oxide for 1
hour. Subsequently, muscle sections were left overnight in a propylene
oxide-epon resin mixture in a glass dessicator. Groups of muscle fibers were
then dissected from the sections, embedded in fresh resin and incubated at 60°C
for 48 hours. Ultrathin sections (60 nm) were cut, collected on copper grids,
and stained with uranyl acetate and lead citrate. Electron micrographs were
obtained using a Philips EM201 electron microscope.
Cytochrome c oxidase
(COX) enzyme activity.
COX activity
of the EDL muscles from CON and STIM limbs was evaluated as described
previously [31]. Enzyme activity was determined spectrophotometrically at 30 °C
as the maximal rate of oxidation of fully reduced cytochrome c, measured by the
change in absorbance at 550 nm.
Western blotting.
Frozen EDL sections from CON and STIM limbs of young
and old animals were pulverized to a fine powder with a stainless steel mortar
that was cooled to the temperature of liquid nitrogen. The protein extraction
was performed as previously described [31]. Proteins extracted from the muscle
homogenates, isolated mitochondria, or cytosolic samples were resolved by
SDS-PAGE (10-12% polyacrylamide) and subsequently electroblotted to
nitrocellulose membranes (Amersham, Baie D'Urfé, PQ). After transfer, membranes
were blocked (1 h) with a 5% skim milk in 1 X TBST [Tris-buffered saline-Tween
20: 25 mM Tris•HCl (pH 7.5), 1 mM NaCl, and 0.1% Tween 20] solution. Blots were
then incubated in blocking solution with antibody directed against PGC-1α
(Calbiochem, 516-557), Tfam, apoptosis-inducing factor (AIF; Santa Cruz,
sc-9416), sirtuin 1 (SIRT1; Sigma, S5313), heat shock protein 70 (HSP70;
Stressgen, SPA-810), mitochondrial HSP70 (mtHSP70; Stressgen, SPS-825), the
translocase of the inner mitochondrial membrane 17 (Tim17; Santa Cruz,
sc-13293), Tim23 (BD Bioscience, 611222), mitochondrial import-stimulating
factor (MSF-L; gifted by Dr. K. Mihara, Kyushu University), HSP90 (Stressgen,
SPA-845), and glyceraldehyde-3 phosphate dehydrogenase (GAPDH; Abcam, ab8245)
overnight at 4 °C. After 3 X 5 min washes with TBST, blots were incubated at
room temperature (1 h) with the appropriate secondary antibody coupled to
horseradish peroxidase. Blots were then washed again 3 X 5 min with TBST,
followed by visualization with enhanced chemiluminescence. Films (Hyperfilm,
Amersham) were then scanned and analyzed using SigmaScan Pro 5 software (Jandel
Scientific, San Rafael, CA).
Statistics.
The data were analyzed using paired and unpaired
Student's t-tests and analysis of variance (ANOVA) procedures, as appropriate.
Bonferroni's post hoc test was used to test significant differences revealed by
the ANOVA. Statistically significant distinctions between groups represented in
the graphs depicted as fold differences are computed using the raw data sets prior
to conversion to the fold difference values. Significance was accepted at P
< 0.05.
Acknowledgments
We thank Keir J. Menzies
for his technical assistance during this study. This work was supported by the
Canadian Institutes of Health Research. During the course of this
investigation, Vladimir Ljubicic was a Doctoral Research Award scholar of the
Heart and Stroke Foundation of Canada, and Giulia Uguccioni was a recipient of
a scholarship from the Heart and Stroke Foundation of Ontario. David A. Hood is
the Canada Research Chair in Cell Physiology.
Conflicts of Interest
The authors declare no
conflict of interests.
References
-
1.
Hood
DA
Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle.
J Appl Physiol.
2001;
90:
1137
-1157.
[PubMed]
.
-
2.
Akimoto
T
, Pohnert
SC
, Li
P
, Zhang
M
, Gumbs
C
, Rosenberg
PB
, Williams
RS
and Yan
Z.
Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway.
J Biol Chem.
2005;
280:
19587
-19593.
[PubMed]
.
-
3.
Wright
DC
, Geiger
PC
, Han
DH
, Jones
TE
and Holloszy
JO.
Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation.
J Biol Chem.
2007;
282:
18793
-18799.
[PubMed]
.
-
4.
Jäger
S
, Handschin
C
, St-Pierre
J
and Spiegelman
BM.
AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha.
Proc Natl Acad Sci U S A.
2007;
104:
12017
-12022.
[PubMed]
.
-
5.
Hoppeler
H
Exercise-induced ultrastructural changes in skeletal muscle.
Int J Sports Med.
1986;
7:
187
-204.
[PubMed]
.
-
6.
Ogata
T
and Yamasaki
Y.
Scanning electron-microscopic studies on the three-dimensional structure of mitochondria in the mammalian red, white and intermediate muscle fibers.
Cell Tissue Res.
1985;
241:
251
-256.
[PubMed]
.
-
7.
Takahashi
M
and Hood
DA.
Protein import into subsarcolemmal and intermyofibrillar skeletal muscle mitochondria. Differential import regulation in distinct subcellular regions.
J Biol Chem.
1996;
271:
27285
-27291.
[PubMed]
.
-
8.
Cogswell
AM
, Stevens
RJ
and Hood
DA.
Properties of skeletal muscle mitochondria isolated from subsarcolemmal and intermyofibrillar regions.
Am J Physiol.
1993;
264:
C383
-389.
[PubMed]
.
-
9.
Ljubicic
V
, Adhihetty
PJ
and Hood
DA.
Role of UCP3 in state 4 respiration during contractile activity-induced mitochondrial biogenesis.
J Appl Physiol.
2004;
97:
976
-983.
[PubMed]
.
-
10.
Adhihetty
PJ
, Ljubicic
V
, Menzies
KJ
and Hood
DA.
Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli.
Am J Physiol Cell Physiol.
2005;
289:
C994
-C1001.
[PubMed]
.
-
11.
Connor
MK
, Bezborodova
O
, Escobar
CP
and Hood
DA.
Effect of contractile activity on protein turnover in skeletal muscle mitochondrial subfractions.
J Appl Physiol.
2000;
88:
1601
-1606.
[PubMed]
.
-
12.
O'Leary
MF
and Hood
DA.
Denervation-induced oxidative stress and autophagy signaling in muscle.
Autophagy.
2009;
5:
230
-231.
[PubMed]
.
-
13.
Krieger
DA
, Tate
CA
, McMillin-Wood
J
and Booth
FW.
Populations of rat skeletal muscle mitochondria after exercise and immobilization.
J Appl Physiol.
1980;
48:
23
-28.
[PubMed]
.
-
14.
Adhihetty
PJ
, Ljubicic
V
and Hood
DA.
Effect of chronic contractile activity on SS and IMF mitochondrial apoptotic susceptibility in skeletal muscle.
Am J Physiol Endocrinol Metab.
2007;
292:
E748
-755.
[PubMed]
.
-
15.
Solomon
AM
and Bouloux
PM.
Modifying muscle mass - the endocrine perspective.
J Endocrinol.
2006;
191:
349
-360.
[PubMed]
.
-
16.
Brack
AS
, Bildsoe
H
and Hughes
SM.
Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy.
J Cell Sci.
2005;
118:
4813
-4821.
[PubMed]
.
-
17.
Nair
KS
Aging muscle.
Am J Clin Nutr.
2005;
81:
953
-963.
[PubMed]
.
-
18.
Chabi
B
, Ljubicic
V
, Menzies
KJ
, Huang
JH
, Saleem
A
and Hood
DA.
Mitochondrial function and apoptotic susceptibility in aging skeletal muscle.
Aging Cell.
2008;
7:
2
-12.
[PubMed]
.
-
19.
Dirks
A
and Leeuwenburgh
C.
Apoptosis in skeletal muscle with aging.
Am J Physiol Regul Integr Comp Physiol.
2002;
282:
R519
-527.
[PubMed]
.
-
20.
Dirks
AJ
and Leeuwenburgh
C.
Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12.
Free Radic Biol Med.
2004;
36:
27
-39.
[PubMed]
.
-
21.
Choksi
KB
, Nuss
JE
, Deford
JH
and Papaconstantinou
J.
Age-related alterations in oxidatively damaged proteins of mouse skeletal muscle mitochondrial electron transport chain complexes.
Free Radic Biol Med.
2008;
45:
826
-838.
[PubMed]
.
-
22.
Drew
B
, Phaneuf
S
, Dirks
A
, Selman
C
, Gredilla
R
, Lezza
A
, Barja
G
and Leeuwenburgh
C.
Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart.
Am J Physiol Regul Integr Comp Physiol.
2003;
284:
R474
-480.
[PubMed]
.
-
23.
Skorjanc
D
, Traub
I
and Pette
D.
Identical responses of fast muscle to sustained activity by low-frequency stimulation in young and aging rats.
J Appl Physiol.
1998;
85:
437
-441.
[PubMed]
.
-
24.
Walters
TJ
, Sweeney
HL
and Farrar
RP.
Influence of electrical stimulation on a fast-twitch muscle in aging rats.
J Appl Physiol.
1991;
71:
1921
-1928.
[PubMed]
.
-
25.
Ljubicic
V
, Joseph
AM
, Saleem
A
, Uguccioni
G
, Collu-Marchese
M
, Lai
RY
, Nguyen
LM
and Hood
DA.
Transcriptional and post-transcriptional regulation of mitochondrial biogenesis in skeletal muscle: Effects of exercise and aging.
Biochem Biophys Acta.
2009;
In press
.
-
26.
Holloszy
JO
Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle.
J Biol Chem.
1967;
242:
2278
-2282.
[PubMed]
.
-
27.
Jarvis
JC
and Salmons
S.
A family of neuromuscular stimulators with optical transcutaneous control.
J Med Eng Technol.
1991;
15:
53
-57.
[PubMed]
.
-
28.
Takahashi
M
and Hood
DA.
Chronic stimulation-induced changes in mitochondria and performance in rat skeletal muscle.
J Appl Physiol.
1993;
74:
934
-941.
[PubMed]
.
-
29.
Takahashi
M
, Chesley
A
, Freyssenet
D
and Hood
DA.
Contractile activity-induced adaptations in the mitochondrial protein import system.
Am J Physiol.
1998;
274:
C1380
-1387.
[PubMed]
.
-
30.
Bradford
MM
A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.
Anal Biochem.
1976;
72:
248
-254.
[PubMed]
.
-
31.
Ljubicic
V
and Hood
DA.
Kinase-specific responsiveness to incremental contractile activity in skeletal muscle with low and high mitochondrial content.
Am J Physiol Endocrinol Metab.
2008;
295:
E195
-204.
[PubMed]
.
-
32.
Ljubicic
V
and Hood
DA.
Diminished contraction-induced intracellular signaling towards mitochondrial biogenesis in aged skeletal muscle.
Aging Cell.
2009;
8:
394
-404.
[PubMed]
.
-
33.
Ljubicic
V
, Adhihetty
PJ
and Hood
DA.
Application of animal models: chronic electrical stimulation-induced contractile activity.
Can J Appl Physiol.
2005;
30:
625
-643.
[PubMed]
.
-
34.
Hood
DA
, Irrcher
I
, Ljubicic
V
and Joseph
AM.
Coordination of metabolic plasticity in skeletal muscle.
J Exp Biol.
2006;
209:
2265
-2275.
[PubMed]
.
-
35.
Irrcher
I
, Adhihetty
PJ
, Joseph
AM
, Ljubicic
V
and Hood
DA.
Regulation of mitochondrial biogenesis in muscle by endurance exercise.
Sports Med.
2003;
33:
783
-793.
[PubMed]
.
-
36.
Kavazis
AN
, McClung
JM
, Hood
DA
and Powers
SK.
Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli.
Am J Physiol Heart Circ Physiol.
2008;
294:
H928
-935.
[PubMed]
.
-
37.
Cheung
EC
, Joza
N
, Steenaart
NA
, McClellan
KA
, Neuspiel
M
, McNamara
S
, MacLaurin
JG
, Rippstein
P
, Park
DS
, Shore
GC
, McBride
HM
, Penninger
JM
and Slack
RS.
Dissociating the dual roles of apoptosis-inducing factor in maintaining mitochondrial structure and apoptosis.
EMBO J.
2006;
25:
4061
-4073.
[PubMed]
.
-
38.
Leone
TC
, Lehman
JJ
, Finck
BN
, Schaeffer
PJ
, Wende
AR
, Boudina
S
, Courtois
M
, Wozniak
DF
, Sambandam
N
, Bernal-Mizrachi
C
, Chen
Z
, Holloszy
JO
, Medeiros
DM
, Schmidt
RE
, Saffitz
JE
, Abel
ED
, Semenkovich
CF
and Kelly
DP.
PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis.
PLoS Biol.
2005;
3:
e101
[PubMed]
.
-
39.
Scarpulla
RC
Transcriptional paradigms in mammalian mitochondrial biogenesis and function.
Physiol Rev.
2008;
88:
611
-638.
[PubMed]
.
-
40.
Vahsen
N
, Candé
C
, Brière
JJ
, Bénit
P
, Joza
N
, Larochette
N
, Mastroberardino
PG
, Pequignot
MO
, Casares
N
, Lazar
V
, Feraud
O
, Debili
N
, Wissing
S
, Engelhardt
S
, Madeo
F
, Piacentini
M
, Penninger
JM
, Schägger
H
, Rustin
P
and Kroemer
G.
AIF deficiency compromises oxidative phosphorylation.
EMBO J.
2004;
23:
4679
-4689.
[PubMed]
.
-
41.
Gurd
B
, Yoshida
Y
, Lally
J
, Holloway
G
and Bonen
A.
The deacetylase enzyme SIRT1 is not associated with oxidative capacity in rat heart and skeletal muscle and its overexpression reduces mitochondrial biogenesis.
J Physiol.
2009;
587:
1817
-1828.
[PubMed]
.
-
42.
Gerhart-Hines
Z
, Rodgers
JT
, Bare
O
, Lerin
C
, Kim
SH
, Mostoslavsky
R
, Alt
FW
, Wu
Z
and Puigserver
P.
Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha.
EMBO J.
2007;
26:
1913
-1923.
[PubMed]
.
-
43.
Lagouge
M
, Argmann
C
, Gerhart-Hines
Z
, Meziane
H
, Lerin
C
, Daussin
F
, Messadeq
N
, Milne
J
, Lambert
P
, Elliott
P
, Geny
B
, Laakso
M
, Puigserver
P
and Auwerx
J.
Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha.
Cell.
2006;
127:
1109
-1122.
[PubMed]
.
-
44.
Suwa
M
, Nakano
H
, Radak
Z
and Kumagai
S.
Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor gamma coactivator-1alpha protein expressions in rat skeletal muscle.
Metabolism.
2008;
57:
986
-998.
[PubMed]
.
-
45.
Westerheide
SD
, Anckar
J
, Stevens
SM Jr
, Sistonen
L
and Morimoto
RI.
Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1.
Science.
2009;
323:
1063
-1066.
[PubMed]
.
-
46.
Oberdoerffer
P
, Michan
S
, McVay
M
, Mostoslavsky
R
, Vann
J
, Park
SK
, Hartlerode
A
, Stegmuller
J
, Hafner
A
, Loerch
P
, Wright
SM
, Mills
KD
, Bonni
A
, Yankner
BA
, Scully
R
, Prolla
TA
, Alt
FW
and Sinclair
DA.
SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging.
Cell.
2008;
135:
907
-918.
[PubMed]
.
-
47.
Ornatsky
OI
, Connor
MK
and Hood
DA.
Expression of stress proteins and mitochondrial chaperonins in chronically stimulated skeletal muscle.
Biochem J.
1995;
311:
119
-123.
[PubMed]
.
-
48.
Joseph
AM
, Rungi
AA
, Robinson
BH
and Hood
DA.
Compensatory responses of protein import and transcription factor expression in mitochondrial DNA defects.
Am J Physiol Cell Physiol.
2004;
286:
C867
-875.
[PubMed]
.
-
49.
Gordon
JW
, Rungi
AA
, Inagaki
H
and Hood
DA.
Effects of contractile activity on mitochondrial transcription factor A expression in skeletal muscle.
J Appl Physiol.
2001;
90:
389
-396.
[PubMed]
.
-
50.
Bolender
N
, Sickmann
A
, Wagner
R
, Meisinger
C
and Pfanner
N.
Multiple pathways for sorting mitochondrial precursor proteins.
EMBO Rep.
2008;
9:
42
-49.
[PubMed]
.
-
51.
Farrar
RP
, Martin
TP
and Ardies
CM.
The interaction of aging and endurance exercise upon the mitochondrial function of skeletal muscle.
J Gerontol.
1981;
36:
642
-647.
[PubMed]
.
-
52.
Adhihetty
PJ
, O'Leary
MF
and Hood
DA.
Mitochondria in skeletal muscle: adaptable rheostats of apoptotic susceptibility.
Exerc Sport Sci Rev.
2008;
36:
116
-121.
[PubMed]
.
-
53.
Adhihetty
PJ
, O'Leary
MF
, Chabi
B
, Wicks
KL
and Hood
DA.
Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle.
J Appl Physiol.
2007;
102:
1143
-1151.
[PubMed]
.
-
54.
Alway
SE
and Siu
PM.
Nuclear apoptosis contributes to sarcopenia.
Exerc Sport Sci Rev.
2008;
36:
51
-57.
[PubMed]
.