Circadian disruption induced by light-at-night accelerates aging and
promotes tumorigenesis in rats
Abstract
We evaluated
the effect of various light/dark regimens on the survival, life span and
tumorigenesis in rats. Two hundred eight male and 203 females LIO rats
were subdivided into 4 groups and kept at various light/dark regimens:
standard 12:12 light/dark (LD); natural lighting of the North-West of Russia (NL); constant light (LL), and constant darkness (DD) since the age of 25 days until
natural death. We found that exposure to NL and LL regimens accelerated
development of metabolic syndrome and spontaneous tumorigenesis, shortened
life span both in male and females rats as compared to the standard LD
regimen. We conclude that circadian disruption induced by light-at-night
accelerates aging and promotes tumorigenesis in rats. This observation
supports the conclusion of the International Agency Research on Cancer that
shift-work that involves circadian disruption is probably carcinogenic to
humans.
Introduction
The alternation of the day and night
circadian cycle is a most important regulator of a wide variety of
physiological rhythms in living organisms, including humans [1,2]. Due to the
introduction of electricity and artificial light about hundred years ago the
pattern and duration of human exposure to light has changed dramatically, and
thus light-at-night has become an increasing and essential part of modern
lifestyle. Light exposure at night seems to be associated with a number of both
serious behavioral and health problems, including excess of body mass index,
cardiovascular diseases, diabetes and cancer [3-14]. On the basis of "limited
evidence in humans for the carcinogenicity of shift-work that involves night
work", and "sufficient evidence in experimental animals for the carcinogenicity
of light during the daily dark period (biological night)" the International Agency for Research on
Cancer (IARC) Working Group concluded that "shift-work that involves circadian
disruption is probably carcinogenic to humans" (Group 2A) [15].
Erren and Pekarski [16] suggested that indigenous
populations in the Arctic region should be at lower risk of cancer. Cancer
incidence in the Sami living in the far north of Europe have reported a lower
risk than expected [17-19]. It is worth to note that mortality among Alaskan
native peoples (Eskimo, Indian and Aleut) from breast cancer has tripled since
1969 for unknown reason [20]. We believe that an increase in light pollution
could be one of causes of this phenomenon.
In the special issue of the International Journal of
Circumpolar Health (December 2008; 67:5), the data on cancer incidence in
circumpolar populations have been presented [21-25]. It was stressed that there
is no consistent pattern of the cancer risk level among circumpolar indigenous
people relative to European or North American populations [26]. The role of
genetic diversion and life style as well as methodological differences in
approaches to extract ethnic-specific data should be evaluated for solution of
the problem [26]. Analysis of the data on cancer risk presented in the "Cancer
in Five Continents" published by IARC has shown that there is a significant
positive correlation between geographical latitude and the incidence of breast,
colon and endometrial carcinomas and absence of the correlation in a case of
stomach and lung cancers [27].
According to the circadian disruption
hypothesis, light-at-night might disrupt the endogenous circadian rhythm, and
specifically suppress nocturnal production of pineal hormone melatonin and its
secretion in the blood [13-15]. However, a number of other mechanisms in
addition to melatonin suppression can be involved into the process of
development of pathologies at the constant illumination. Moreover, there are no
available data on effect of natural light/dark regimen at circumpolar region on
life span and tumorigenesis in rodents. The aim of this study was to evaluate
the effect of various light/dark regimens on some parameters of homeostasis
and of biological age, survival, life span and tumorigenesis in male and female
rats.
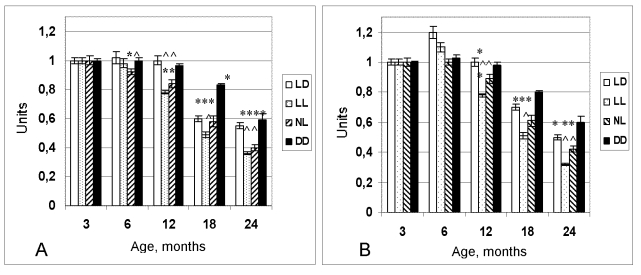
Figure 1. Dynamics of the
coefficient of homeostatic stability (CHS) in female (A) and male (B)
rats maintained at various light regimens.
^ The difference with the relevant parameter in the group LD is significant, р<0,05;
* The difference with the parameter at the age of 3 months in the same group is significant, р<0,05 (Wilcoxon-Mann-Whytney test).
Results
Effect of light/dark regimen on homeostatic parameters
in rats
Age-related body weight gain followed by its decrease
was observed in rats of all groups at any light/dark regimens. However maximal
weight of rats maintained at the LD or NL regimens was observed at the age of
15 months, whereas in the animals kept at the LL - at the 12th month. The
number of rats with abdominal obesity was increased in the LL and NL groups as
compared with the LD group (data are not shown). Food consumption widely has
been varying in all groups during the period of observation. There were periods
of an increase in food consumption and those of a decrease. In general, in
autumn and winter rats ate more lab food than in spring and summer. Male rats
from the LL and NL groups ate more food compared with the LD group at the age
of 18 and 21 months.
Monthly testing for glucosuria
showed that there were no such cases until the age of 16 months in all groups.
At the age of 16 months, 20% of rats from the group maintained at the LL
regimen had glucose in urine, whereas at the age of 24 months 60% of rats in
his group had glucosuria. In the NL group 40% of rats had glucosuria at the age
of 18 months. Both serum glucose level and that of serum C-peptide were much
higher in the LL and NL rats at the age of 18 and 24 months compared with the
LD rats.
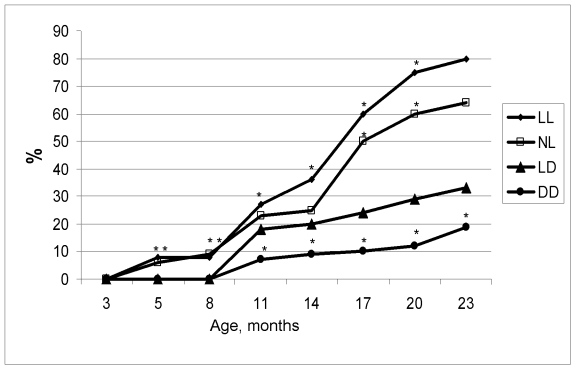
Figure 2.
Age-related dynamics of incidence of irregular estrous cycles in rats maintained
at various light/dark regimens. Ordinate, number of rats with irregular
estrous cycles (%).
The difference with the relevant parameter in the group LD is significant, р<0.05.
The level of the serum cholesterol and
β-lipoproteins was higher in young 3-months old rats and significantly
decreased at the age of 6 months. Hence age-related increase of serum
cholesterol and β-lipoproteins levels was observed to take place in rats
of all groups. It worthy of note, that the level of β-lipoproteins was
higher in the LL and NL rats compared with the LD rats at the age of 18 and 24
months.
At the age of 6 months the coefficient of homeostatic
stability (CHS) was practically same in all groups. Age-related decrease of the
CHS was observed in all groups as well. However most significant decline of its
value has been observed in the groups NL and LL. At the age from 12 to 24
months CHS in these groups was significantly lower in comparison to these in
the LD group (Figure 1). In females, age-related increase in the number of
rats with irregular estrous cycles was accelerated both in the NL and the LL
groups whereas was postponed in the DD group (Figure 2). Thus, constant and
natural illumination accelerated aging in rats evaluated by age-related
dynamics of CHS.
Effect of light/dark regimen on life span in rats
In male rats, the exposure to both NL and LL regimens
failed significantly influence the mean life span of all as well as the last of
10% survivors (Table 1).
At the same time, the rate of population
aging (parameter α in the Gompertz equation) was slightly increased in NL group and decreased in LL as compared
with the LD group. The survival curves for groups NL and LL were significantly
shifted to left in comparison to the survival curve for the group LD (Figure 3A). The log-rank test shows the significant difference in the distribution of
survivors between groups LD and NL (р= 0.001; χ2 = 10.3)
and between groups LD and LL (р= 0.01; χ2 = 6.7). In
ANOVA test the dependence of the life span on light regimen has been
significant (15.45%; F=15.32; p<0.001). Thus, both LL and NL regimens
accelerated mortality in male rats.
In female rats, the exposure
to both NL and DD regimens failed significantly influence the mean life span of
all as well as the last of 10% survivors, however the exposure to the LL
regimen significantly decreased the life span (Table 2). At the same time, the
rate of population aging was significantly increased by 2.1 times in the NL
group and, correspondingly, decreased the MRDT as compared with the LD group.
The survival curves for groups NL and DD were significantly shifted to left in
comparison to the survival curve for the group LD (Figure 3B). The log-rank
test shows the significant difference in the distribution of survivors between
groups LD and NL (р= 0.0000243; χ2 =22.2), between the
groups LD and LL (p=0.0000162; χ2 = 23.0) and between LD and DD
(p=0.0741; χ2 =3.2). In ANOVA test the dependence of the life
span on light regimen has been significant (15.45%; F=15.32; p<0.001). Thus,
both LL and NL regimens accelerated mortality in female rats.
Table 1. Effect of light regimen on survival and life span in male rats. Effect of light/dark regimen on spontaneous tumorigenesis in rats.
Notes: Difference with controls is significant: a, р<0.05. #, in
brackets 95% confidential intervals. MRDT, mortality rate doubling time.
|
Parameters
|
Light/dark regimen
|
|
LD
|
NL
|
LL
|
DD
|
|
Number of rats
|
57
|
50
|
50
|
51
|
|
Mean life span, days
|
644 ± 34.0
|
613 ± 32.9
|
580 ± 35.5
|
652 ± 32.5
|
|
Maximum life span, days,
|
1045
|
1046
|
1005
|
1017
|
|
Mean life span of last 10%
survivals,
Days
|
999 ± 11.5
|
972 ± 22.7
|
983 ± 13.8
|
987 ± 13.0
|
|
α x 103, days-1 |
6.06
(5.87; 6.47)
|
6.70a
(6.50; 6.97)
|
5.19a
(4.89; 5.57)
|
8.31a
(8.10; 8.54)
|
|
MRDT, days
|
112.4
(107.1; 118.1)#
|
103.4
(102.7; 111.6)
|
133.6a
(124.4; 141.7)
|
83.4a
(81.2; 85.5)
|
Table 2. Effect of light regimen on survival and life span in female rats.
Notes: Difference with controls is significant: a, р<0.05;
b, р<0.01; c, р<0.001. #, in brackets 95% confidential
intervals. MRDT, mortality rate doubling time.
|
Parameters
|
Light/dark regimen
|
|
LD
|
NL
|
LL
|
DD
|
|
Number of rats
|
40
|
48
|
54
|
61
|
|
Mean life span, days
|
706 ± 46.2
|
611 ± 29.5
|
526 ± 30.4b |
639 ± 30.1
|
|
Maximum life span, days,
|
1167
|
897
|
956
|
1266
|
|
Mean life span of last 10%
survials, days
|
1119 ± 16.7
|
830 ± 18.9
|
909 ± 19.1c |
1023 ± 56.0
|
|
α x 103, days-1 |
5.00
(4.73; 5.30)#
|
10.5
(10.3; 11.2)a |
5.21
(5.13; 5.35)
|
4.88
(4.86; 4.97)
|
|
MRDT, days
|
138.6
(130.8; 146.6)
|
65.8
(61.7; 67.0)a |
133.1
(129.6; 135.1)
|
142.0
(139.4; 142.5)
|
Effect of light/dark regimen on spontaneous tumorigenesis in rats
Pathomorphological
analysis shows that benign tumors were most frequent in all groups of males.
The significant part of them was represented by testicular Leydig cell tumors
(Table 3). Among malignant tumors lymphomas were most common however some
cases of hepatocellular carcinoma, soft tissues sarcomas and sporadic
carcinomas were detected.
The exposure to the LL regimen accelerated spontaneous tumors
development as compared to the LD group and not influenced their incidence in
male rats (Table 3; Figure 3C). The first tumor in the LL group was detected 5 months earlier,
and the first tumor in the DD group was observed 9 months later than the first
tumor in the LD group.
In the
female groups NL, the total incidence of tumors was significantly increase as
compared with the LD group mainly due to practically 2-times increase in
incidence of benign mammary tumors. It worthy of note that in the NL group 3
endometrial adenocarcinomas have been observed whereas no such type of malignancies
where revealed in the LD group. The light deprivation (group DD) significantly
inhibited the development of all tumors, mainly mammary neoplasia. The index
of tumor multiplicity (number of tumors per tumor-bearing rat) was maximal
(1.63) in the group LL and minimal (1.07) in the group DD (Table 4; Figure 3D).
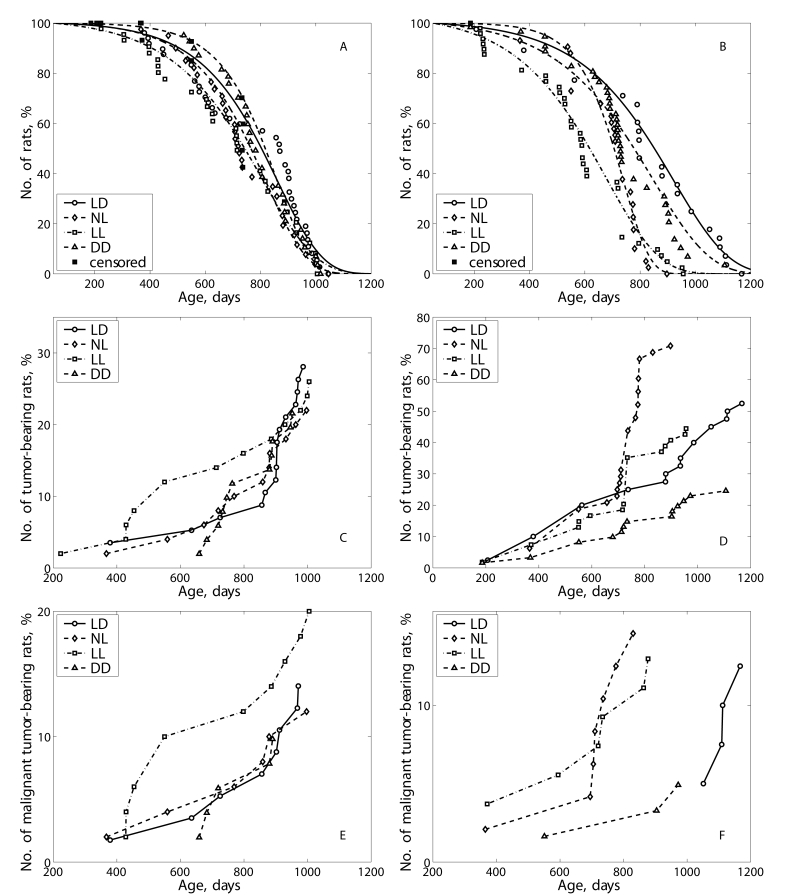
Figure 3. Effect of the exposure to various light regimens on survival and tumorigenesis in rats. (A) - survival, males; (B)- survival-
females; (C) - total tumor incidence, males; (D) - total
tumor incidence, females; (E) - malignant tumor incidence, males; (F)
- malignant tumorincidence, females.
Table 3. Effect of light regimen on tumorigenesis in male rats.
Notes: TBR - tumor-bearing rats.
| Parameters | Light/dark regimen |
| LD | NL | LL | DD |
|
Number of rats
|
57
|
50
|
50
|
51
|
|
Number of TBR (%)
|
17 ( 29.8 %)
|
11 (22%)
|
13 (26%)
|
11 (21.6%)
|
|
No. of tumors per TBR
|
1.35
|
1.18
|
1.08
|
1.36
|
|
Number of malignant TBR (%)
|
7 (12.3%)
|
6 (12%)
|
10 (20%)
|
5 (9.8%)
|
|
Total number of tumors
|
23
|
13
|
14
|
15
|
|
Time of the 1st
tumor detection, days
|
379
|
367
|
223
|
659
|
|
Mean life span of TBR, days
|
824 ± 49.0
|
782 ± 57.6
|
688 ± 73.2
|
805 ± 32,3
|
|
Mean life span of malignant
TBR, days
|
794 ± 72.4
|
738 ± 95.6
|
701 ± 76.0
|
766 ± 49.5
|
| Localization and type of
tumors |
|
Testes:
Leydigoma
hemangioma
|
7
|
6
|
4
|
6
|
|
1
|
-
|
-
|
-
|
|
Malignant lymphoma/
leukemia
|
3
|
4
|
6
|
3
|
|
Liver:
hepatocarcinoma
|
2
|
-
|
2
|
-
|
|
Skin:
papilloma
|
1
|
-
|
-
|
-
|
|
Soft
tisusues: fibroma
sarcoma malignant fibrous histiocytoma
|
-
|
-
|
-
|
1
|
|
1
|
2
|
-
|
1
|
|
2
|
-
|
-
|
-
|
|
Lung:
adenocarcinoma
light-cell carcinoma
|
-
|
-
|
1
|
-
|
|
1
|
-
|
-
|
-
|
|
Small
bowel: adenocarcinoma
|
-
|
-
|
1
|
-
|
|
Adrenal gland:
cortical adenoma
pheochromocytoma
malignant
pheochromocytoma
|
3
|
1
|
-
|
3
|
|
1
|
-
|
-
|
-
|
|
-
|
-
|
-
|
1
|
|
Urether:
fibroma
|
1
|
-
|
-
| |
|
Total:
benign
malignant
|
14
|
7
|
4
|
10
|
|
9
|
6
|
10
|
5
|
Discussion
Thus our data have shown that live-long
maintenance of male and female rats at the NL or LL regimens accelerated
age-related changes evaluated by the CHS, decreased life span and promoted
spontaneous tumorigenesis. These data and some additional results of this study
reported earlier are summarized in the Table 5 and supported this conclusion.
It was reported that risk of cancer is low in
indigenous populations in Arctic [17-19].
However there are data on significant increase in the breast carcinoma risk in
them since 1969 [20]. The cause of this phenomenon is
unknown. The one of the reason could be the increase in light pollution. Experiments
in female rodent presented significantly evidence that exposure to constant
illumination (24 hours per day) leads to disturbances in estrus function
(persistent estrus syndrome, anovulation) [31,36,37] and spontaneous tumor
development [4,34,36,38]. The evidence of promoting effect of exposure to
constant illumination on mammary carcinogenesis induced by chemical carcinogens
are discussed elsewhere [3,4,14]. This paper in the first time has shown that the exposure of male rats to
the constant illumination accelerated the development
spontaneous tumors. This paper firstly have shown that maintenance of female
rats to natural light conditions of the north (long "white night" and "polar
night" seasons) also leads to premature switching-off of reproductive function
and promotion of spontaneous carcinogenesis.
Table 4. Effect of light regimen on tumorigenesis in female rats.
Notes: TBR - tumor-bearing rats. Difference with the group LD is
significant: a, р<0.05; b, р<0.01; c, р<0.001.
| Parameters | Light/dark regimen |
| LD | NL | LL | DD |
|
Number of rats
|
40
|
48
|
54
|
61
|
|
Number of TBR (%)
|
21 (52.5%)
|
34 (70.8%)a |
24 (44.4%)
|
15 (24.6%)c |
|
No. of tumors per TBR rat
|
1.38
|
1.41
|
1.63
|
1.07
|
|
No. of mlgn. TBR rats (%)
|
5 (12.5%)
|
7 (14.6%)
|
7 (13.0%)
|
3 (4.9%)
|
|
Number of tumors
|
29
|
48
|
39
|
16
|
|
Time of the 1st
tumor detection, days
|
207
|
365
|
186
|
186
|
|
Mean life span of TBR, days
|
769 ± 63.0
|
683 ± 22.9b |
665 ± 40.3
|
720 ± 64.3
|
|
Mean life span of malignant
TBR, days
|
1098 ± 21.8
|
688 ± 56.8c |
647 ± 79.8c |
809 ± 130.5a |
| Localization and type of
tumors |
|
Mammary
gland: fibroma
fibroadenoma
adenocarcinoma
|
4
|
9
|
1
|
-
|
|
11
|
21
|
20
|
5
|
|
-
|
-
|
1
|
-
|
|
No. of rats with benign
mammary tumors
|
14
|
27a |
18
|
5c |
|
Utery:
polyp
fibroma
fibromyoma
adenocarcinoma
stromogenic sarcoma
|
4
|
1
|
4
|
5
|
|
1
|
-
|
1
|
-
|
|
-
|
2
|
1
|
-
|
|
-
|
3
|
1
|
-
|
|
-
|
-
|
-
|
1
|
|
Oviduct:
fibroma
|
-
|
3
|
-
|
-
|
|
Adrenal gland:
cortical adenoma
carcinoma
pheochromocytoma
|
1
|
1
|
3
|
1
|
|
-
|
-
|
-
|
1
|
|
2
|
-
|
-
|
-
|
|
Ovary:
fibroma
luteoma
hemangioma
carcinoma
|
1
|
-
|
-
|
-
|
|
-
|
1
|
-
|
-
|
|
-
|
-
|
-
|
1
|
|
-
|
1
|
-
|
-
|
|
Pituitary:
adenoma
|
-
|
-
|
1
|
-
|
|
Hematopoeitic tissue:
leukemia/lymphoma
|
3
|
3
|
4
|
-
|
|
Soft
tissues: fibroma
sarcoma
|
-
|
1
|
1
|
1
|
|
2
|
2
|
1
|
-
|
|
Lung:
adenocarcinoma
|
-
|
-
|
-
|
1
|
|
Colon: adenocarcinoma
|
-
|
1
|
-
|
-
|
|
Total:
benign
malignant
|
24
|
39
|
32
|
13
|
|
5
|
9
|
7
|
3
|
Table 5. Summary evaluation of effects of various light/dark regimen on biomarker of aging and homeostatic parameters in female and male rats [ 28-35].
Notes: ↑ - increases (acceleration); ↓ - decreases (slow down);
= - no effect, as compared with the parameter in the LD group;
M - male; F - female.
|
Parmeters
|
Sex
|
Ligh/dark regimen
|
|
NL
|
LL
|
DD
|
|
Body weight
|
Male
|
↑
|
=
|
=
|
|
Female
|
↓
|
↓
|
↓
|
|
Body weight gain
|
M & F
|
↓
|
↓
|
↑
|
|
Progressive growth period
|
Male
|
↑
|
↓
|
↑
|
|
Stable growth period
|
Male
|
↓
|
↓
|
↓
|
|
Presenile period
|
Male
|
↑
|
↑
|
↑
|
|
Senile period onset
|
Male
|
↑
|
↑
|
↓
|
|
Food consumption
|
M & F
|
↑
|
↑
|
↓
|
|
Water consumption
|
Male
|
=
|
=
|
=
|
|
Maturity onset
|
M & F
|
↑
|
↑
|
↓
|
|
Estrous function switching-off
|
Female
|
↑
|
↑
|
↓
|
|
Diuresis
|
Male
|
↓
|
↓
|
↓
|
|
Morbidity
|
M & F
|
↑
|
↑
|
↓
|
| Biochemical parameters in urine |
|
Glucose (age at appearance)
|
Male
|
↑
|
↑
|
↓
|
|
Ketones (age at appearance)
|
Male
|
↑
|
↑
|
↑
|
| Behavioral, cognitive and physical activity |
|
Locomotor activity
|
Male
|
↑
|
↑
|
↓
|
|
Psychoemotional feautures
|
Male
|
↑
|
↑
|
↓
|
|
Cognitive function
|
Male
|
↓
|
↓
|
=
|
|
Dynamic endurance
|
Male
|
↓
|
↓
|
↑
|
|
Static endurance
|
Male
|
↓
|
↓
|
↓
|
| Biochemical parameters in blood |
|
Glucose
|
M & F
|
↑
|
↑
|
=
|
|
Cholesterol
|
M & F
|
↑
|
↑
|
=
|
|
Β-lipoproteins
|
M & F
|
↑
|
↑
|
=
|
|
Total protein
|
M & F
|
↓
|
↓
|
↑
|
|
Urea
|
M & F
|
↑
|
↑
|
=
|
|
Creatinine
|
M & F
|
↑
|
↑
|
=
|
|
Sodium
|
M & F
|
=
|
=
|
=
|
|
Potassium
|
M & F
|
=
|
↓
|
↑
|
| Serum level of hormones |
|
Prolactin
|
M & F
|
↑
|
↑
|
↓
|
|
C-peptide
|
M & F
|
↑
|
↑
|
=
|
|
TSH
|
M & F
|
↓
|
↓
|
=
|
|
Т4
|
M & F
|
↑
|
↑
|
=
|
|
T3
|
M & F
|
↓
|
=
|
↑
|
|
Coefficient of homeo-static stability (CHS)
|
M & F
|
↓
|
↓
|
↑
|
| Antioxidant system |
|
Liver
|
M & F
|
↓
|
=
|
=
|
|
Kidney
|
M & F
|
=
|
↓
|
=
|
|
Heart
|
M & F
|
↓
|
↓
|
=
|
|
Skeletal muscles
|
M & F
|
↓
|
↓
|
=
|
|
Life span
|
Male
|
=
|
=
|
=
|
|
Female
|
↓
|
↓
|
↑
|
|
Spontaneous carcinogenesis
|
M & F
|
↑
|
↑
|
↓
|
The important finding of our experiments
was an observation of manifestations of metabolic syndrome in rats kept at the
NL and LL regimens. There is evidence of relationship between the pineal gland
and physiological regulation of carbohydrate and lipid metabolism. Thus, in
pinealectomized rats the decrease of tolerance to glucose, the increase in the
level of total lipids, free fatty acids, disturbances in the ratio of free and
bounded insulin were observed [39]. In patients with cardiac metabolic
syndrome, lower nocturnal peak and Δ melatonin (peak - lowest melatonin
level) were observed compared with normal healthy subjects [40]. Some
epidemiological studies show that night-shift workers, whose activity period is
chronically reversed, show an increased incidence of the metabolic syndrome
[41]. It was demonstrated impaired glucose metabolism in mice with clock genes Bmal1
or Clock mutations [42]. In homozygous mice with mutation in circadian clock
gene the metabolic syndrome characterized by obesity, hyperlipidemia,
hyperleptinemia, liver steatosis, hyperglycemia and hyperinsulinemia developed
[43]. In our experiment, the rats exposed to the disturbed light/dark regimen
developed the metabolic disorders which might be evaluated as a metabolic
syndrome: abdominal obesity, hyper-cholesterolemia, hyperglycemia,
hyperbetalipemia and glucosuria. It is worthy to note, that the life span was
shorter and the incidence of spontaneous tumors was higher in the rats exposed
to the LL or NL regimens compared the rats to be maintained at the standard LD
regimen. Chronic circadian disruption induced by chronic reversal in the
light/dark cycle was followed by the reduction by 11% in the mean life span in
cardiomyopathy-prone Syrian hamsters [44].
The insulin/insulin-like growth
factor-1 (IGF-1) signaling pathway plays a fundamental role in animal
physiology, influencing longevity, reproduction, and diapause in many species
[45]. Despite a large number of studies, the role of melatonin on glucose
metabolism is rather controversial [46-48].
Metabolic syndrome [49-51] characterized by obesity,
hypertriglyceridemia and hypercholesterolemia, by decrease in the level of high
density lipoproteins and blood fibrinolytic activity, by arterial hypertension,
by lowering of tolerance to glucose and by rise in insulin resistance. The
metabolic syndrome is a risk factor not only for cardiovascular diseases but
for cancer too [45,51,52]. The inhibition of pineal function due to exposure to
continuous light at night probably facilitates the metabolic syndrome
development.
Thus our results shown that the natural light/dark
regimen in Arctic as well as constant illumination acce-lerate the aging and
increase the tumor incidence in rodents. The significance of these findings for
human should be evaluated in well controlled population studies in humans.
Materials and Methods
Two hundred eight male and 203 female outbreed LIO
rats [53] were born during the first half of May, 2003. At the age of 25 days
they were randomly subdivides into 4 groups (males and females separately) and
kept at 4 different light/dark regimens: 1) standard alternating regimen (LD) -
12 hours light (750 lux): 12 hours dark; 2) natural light/dark regimen (NL) at
the latitude of Petrozavodsk (N 61º47'') - in winter minimal lighting was 4.5
hours (polar night), in summer - 24 hours light on ("white nights");
illumination at the level of cages varied from 50 to 200 lux in the morning to
1000 lx for bright sunny day and about 500 lx for cloudy or rainy day; 3)
constant light regimen (LL) - 24 hours light on (750 lux); 4) constant
darkness (DD) - only dim red light (0 - 0.5 lux) was switching-on for animal
service.
All animals were kept in the standard polypropylene
cages at the temperature 21-23 ºC and were given ad libitum standard
laboratory meal [54] and tap water. The study was carried out according to the
recommendations of the Committee on Animal Research of Petrozavodsk State
University about the humane treatment of animals.
All rats were weighted once a month and the amount of
food consumed was measured. Two hundred grams of food were given in each cage
after cleaning and 24 h later the food which was not eaten was collected
from each cage and weighted. The mean amount of food (grams) consumed per rat
for this day was calculated for each group. Every month rats were placed into
individual metabolic cages for urine collection. The concentration of glucose
in the urine was estimated with Ames test system for urine ("Bayer", Germany).
Once every 3 months, daily for 2 weeks
vaginal smears were cytologically examined in females to determine estrous
function. At the age of 3, 6, 12, 18 and 24 months 10 male rats from each
group were given guillotine after 24-hours fasting. Blood samples were taken in
each animal. The collected samples were centrifuged and the serum was stored at
-70 ºC for subsequent biochemical study. The serum level of free
triiodothyronine (T3), thyroxin (T4) and thyroid stimulating hormone (TSH) was
estimated by immunoenzymatic method (kits "Immulite"), level of C-peptide and
prolactin - by kits "BiochimMac", glucose - by enzymatic method, concentration
of β-liporoteins - by turbidimetric method, cholesterol - with kits "Vital
Diagnostics SPb", creatinine - with kits "Olvex Diagnosticum", urea - with kits
"Abris+". Concentration of potassium and sodium ions was estimated by
ionoselective method with ionometer ETs-59 (Russia).
Integral dynamics of
age-related changes of studied biochemical parameters was evaluated as a
Coefficient of Homeostatic Stability (CHS), which was estimated as a ratio of
total number of biochemical and endocrine parameters equal to relevant their
indices at the age 3 months to total number of parameters studied [55].
All other rats were allowed
to survive for natural death. All animals were autopsied. Tumors as well as the
tissues and organs with suspected tumor development were excised and fixed in
10% neutral formalin. After the routine histological processing the tissues
were embedded into paraffin. 5-7μm thin histological sections were stained
with hematoxylin and eosin and examined microscopically. Tumors were classified
according to the IARC recommendations [56,57].
Experimental results were statistically processed by the methods of
variation statistics with the use of STATGRAPH statistic program kit. The
significance of the discrepancies was defined according to the Student t-criterion,
Fischer exact method, χ2, non-parametric Wilcoxon-Mann-Whitney
and Friedman RM ANOVA on Ranks. Student-Newman-Keuls method was used for all
pairwise multiple comparisons. Coefficient of correlation was estimated by
Spearman method [58]. Differences in tumor incidence were evaluated by the
Mantel-Haenszel log-rank test.
Parameters of Gompertz model
were estimated using maximum likelihood method, non-linear optimization
procedure [59] and self-written code in 'Matlab'; confidence intervals for the
parameters were obtained using the bootstrap method [60].
For experimental group Cox
regression model [61] was used to estimate relative risk of death and tumor
development under the treatment compared to the control group: h(t, z) = h0(t)
exp(zβ), where h(t,z) and h0(t) denote the conditional hazard
and baseline hazard rates, respectively, β is the unknown parameter for
treatment group, and z takes values 0 and 1, being an indicator variable for
two samples − the control and treatment group. Semiparametric model of
heterogeneous mortality [62] was used to estimate the influence of the
treatment on frailty distribution and baseline hazard.
Acknowledgments
The work was supported by grants from the President of
the Russian Federation, grants of the Russian Foundation for Basic Research and
of the Russian Foundation for Humanistic Research.
Conflicts of Interest
The author of this manuscript
has no conflict of interests to declare.
References
-
1.
Arendt
J
Melatonin: characteristics, concerns, and prospects.
J Biol Rhythms.
2005;
20:
291
-303.
[PubMed]
.
-
2.
Bell-Pedersen
D
, Cassone
VM
and Earnest
DJ.
Circadian rhythms from multiple oscillators: lessons from diverse organisms.
Nat Rev Genet.
2005;
6:
544
-556.
[PubMed]
.
-
3.
Anisimov
VN
The light-dark regimen and cancer development.
Neuro Endocrinol Lett.
2002;
23 (Suppl):
28
-36.
[PubMed]
.
-
4.
Anisimov
VN
Light pollution, reproductive function and cancer risk.
Neuro Endocrinol Lett.
2006;
27:
35
-52.
[PubMed]
.
-
5.
Ha
M
and Park
J.
Shiftwork and metabolic risk factors of cardiovascular disease.
J Occup Health.
2005;
47:
89
-95.
[PubMed]
.
-
6.
Knutsson
A
Health disorders of shift workers.
Occupat Med.
2003;
53:
103
-108.
.
-
7.
Knutsson
A
and Boggild
H.
Shiftwork, risk factors and cardiovascular disease: review of disease mechanisms.
Rev Environ Health.
2000;
15:
359
-372.
[PubMed]
.
-
8.
Reiter
RJ
Potential biological consequences of excessive light exposure: melatonin suppression, DNA damage, cancer and neurodegenerative diseases.
Neuro Endocrinol Lett.
2002;
23(Suppl 2):
9
-13.
[PubMed]
.
-
9.
Schernhammer
ES
, Laden
F
and Speizer
FE.
Rotating night shifts and risk of breast cancer in women participating in the nurses' health study.
J Natl Cancer Inst.
2001;
93:
1563
-1568.
[PubMed]
.
-
10.
Schernhammer
ES
, Laden
F
and Spezer
FE.
Night-shift work and risk of colorectal cancer in the Nurses' Health Study'.
J Natl Cancer Inst.
2003;
95:
825
-828.
[PubMed]
.
-
11.
Steenland
K
and Fine
L.
Shift work, shift change, and risk of death from heart disease at work.
Am J Industr Med.
1996;
29:
278
-281.
.
-
12.
Stevens
RG
Circadian disruption and breast cancer. From melatonin to clock genes.
Epidemiology.
2005;
16:
254
-258.
[PubMed]
.
-
13.
Stevens
RG
Artificial lighting in the industrialized world: circadian disruption and breast cancer.
Cancer Causes Control.
2006;
17:
501
-507.
[PubMed]
.
-
14.
Stevens
RG
Light-at-night, circadian disruption and breast cancer: assessment of existing evidence.
Int J Epidemiol.
2009;
38:
963
-970.
[PubMed]
.
-
15.
Straif
K
, Baan
R
and Grosse
Y.
Carcinogenicity of shift-work, painting, and fire-fighting.
Lancet Oncol.
2007;
8:
1065
-1066.
[PubMed]
.
-
16.
Erren
TC
and Piekarski
C.
Does winter darkness in the Arctic protect against cancer.
Med Hypothesis.
1999;
53:
1
-5.
.
-
17.
Soininen
L
, Jarvinen
S
and Pukkala
E.
Cancer incidence among Sami in Northern Finland, 1979-1998.
Int J Cancer.
2002;
100:
342
-346.
[PubMed]
.
-
18.
Haldorsen
T
and Tynes
T.
Cancer in the Sami population of North Norway, 1970-1997.
Eur J Cancer Prev.
2005;
14:
63
-68.
[PubMed]
.
-
19.
Hassler
S
, Sjolander
P
, Gronberg
H
, Hohansson
R
and Damber
L.
Cancer in the Sami population of Sweden.
Eur J Epidemiol.
2008;
23:
80
.
-
20.
Kelly
JJ
, Lanier
AP
, Alberts
S
and Wiggins
CL.
Differences in cancer incidence among Indians in Alaska and New Mexico and U.S. Whites, 1993-2002.
Cancer Epidemiol Biomarkers Prev.
2006;
15:
1515
-1519.
[PubMed]
.
-
21.
Circumpolar
Inuit Cancer Review Working Group
Cancer among the circumpolar Inuit, 1988-2003. I. Background and methods.
Int J Circumpolar Health.
2008;
67:
396
-407.
[PubMed]
.
-
22.
Circumpolar
Inuit Cancer Review Working Group
Cancer among the circumpolar Inuit, 1988-2003. II. Patterns and trends.
Int J Circumpolar Health.
2008;
67:
408
-420.
[PubMed]
.
-
23.
Vaktskjold
A
, Ungurjanu
TN
and Klestsjinov
NM.
Cancer incidence in the Nenetskij Avtonomnyj Okrug, Arctic Russia.
Int J Circumpolar Health.
2008;
67:
433
-444.
[PubMed]
.
-
24.
Hassler
S
, Soininen
L
, Sjolander
P
and Pukkala
E.
Cancer among the Sami - A review on the Norvegian, Swedish and Finnish Sami populations.
Int J Circumpolar Health.
2008;
67:
421
-432.
[PubMed]
.
-
25.
Louchini
R
and Beaupre
M.
Cancer incidence ad mortality among Aboriginal people living on reserves and northern villages in Quebec, 1988-2004.
Int J Circumpolar Health.
2008;
67:
445
-451.
[PubMed]
.
-
26.
Young
TK
Cancer in circumpolar populations.
Int J Circumpolar Health.
2008;
67:
395
[PubMed]
.
-
27.
Anisimov
VN
, Baturin
DA
and Ailamazyan
EK.
Pineal gland, light and breast cancer.
Vopr Onkol.
2002;
48:
524
-535.
.
-
28.
Vinogradova
IA
Effect of various light regimen on indices of biological age and development of age-related pathology in rats.
Med Acad J.
2005;
2(Suppl. 6):
16
-18.
.
-
29.
Vinogradova
IA
Comparative study of effect of various light regimens on psychoemotional manifestations and locomotor activity in rats.
Vestn Novosibirsk Univ Ser Biol Clin Med.
2006;
4:
69
-77.
.
-
30.
Vinogradova
IA
Effect of light regimen on metabolic syndrome development during the aging in rats.
Adv Gerontol.
2007;
20:
70
-75.
[PubMed]
.
-
31.
Vinogradova
IA
and Chernova
IV.
Effect of light regimen on age-related dynamics of estrous function and blood prolactin level in rats.
Adv Gerontol.
2006;
19:
60
-65.
[PubMed]
.
-
32.
Vinogradova
IA
, Bukalev
AV
, Zabezhinski
MA
, Semenchenko
AV
and Anisimov
VN.
Effect of light regimen and melatonin on the homeostasis, life span and development of spontaneous tumors in female rats.
Adv Gerontol.
2007;
20:
40
-47.
[PubMed]
.
-
33.
Vinogradova
IA
, Bukalev
AV
, Zabezhinski
MA
, Semenchenko
AV
and Anisimov
VN.
Effect of light regimen and melatonin on the homeostasis, life span and development of spontaneous tumors in male rats.
Vopr Onkol.
2008;
54:
70
-77.
[PubMed]
.
-
34.
Vinogradova
IA
, Ilyukha
VA
, Fedorova
AS
, Khizhkin
EA
, Unzhakoc
AR
and Yunash
VD.
Age-related changes of physical efficience and some biochemical parameters of rats under the influence of light regimens and pineal preparations.
Adv Gerontol.
2007;
20:
66
-73.
.
-
35.
Ilyukha
VA
, Vinogradova
IA
, Fedorova
AS
and Velb
AN.
Effect of light regimens, pineal hormones and age on antioxidant system in rats.
Med Acad J.
2005;
3(Suppl.7):
18
-20.
.
-
36.
Lazarev
NI
, Ird
EA
and Smirnova
IO.
Moscow
Meditsina
Experimental Models of Endocrine Gynecological Diseases.
1976;
.
-
37.
Prata
Lima MF
, Baracat
EC
and Simones
MJ.
Effects of melatonin on the ovarian response to pinealectomy or continuous light in female rats: similarity with polycystic ovary syndrome.
Brazil J Med Biol Res.
2004;
37:
P987
-995.
.
-
38.
Baturin
DA
, Alimova
IN
and Anisimov
VN.
Effect of light regime and melatonin on the development of spontaneous mammary tumors in HER-2/neu transgenic mice is related to a downregulation of HER-2/neu gene expression.
Neuro Endocrinol Lett.
2001;
22:
439
-445.
.
-
39.
Ostroumova
MN
and Vasilieva
IA.
Effect of pineal extract on regulation of fat-carbohydrate metabolism.
Probl Endocrinol.
1976;
22:
66
-69.
.
-
40.
Altun
A
, Yaprak
M
, Aktoz
M
, Vardar
A
, Betul
U-A
and Ozbay
G.
Impaired nocturnal synthesis of melatonin in patients with cardiac syndrome X.
Neurosci Lett.
2002;
327:
143
-145.
[PubMed]
.
-
41.
Holmback
U
, Forslund
A
, Lowden
A
, Forslund
J
, Akerstedt
T
, Lennarnas
M
, Hambraueus
L
and Stridsberg
M.
Endocrine responses to nocturnal eating-possible implications for night work.
Eur J Nutr.
2003;
42:
75
-83.
[PubMed]
.
-
42.
Rudic
RD
, McNamara
P
and Curtis
AM.
BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose metabolism.
PloS Biol.
2004;
2:
e377
[PubMed]
.
-
43.
Turek
FW
, Joshu
C
and Kohsaka
A.
Obesity and metabolic syndrome in circadian clock mutant mice.
Science.
2005;
308:
1043
-1045.
[PubMed]
.
-
44.
Penev
PD
, Kolker
DE
, Zee
PC
and Turek
FW.
Chroninc circadian desynchronization decreases the survival of animals with cardiomyopathic heart diseases.
Am J Physiol.
1998;
275:
H2334
-2337.
[PubMed]
.
-
45.
Anisimov
VN
Insulin/IGF-1 signaling pathway driving aging and cancer as a target for pharmacological intervention.
Exp Gerontol.
2003;
38:
1041
-1049.
[PubMed]
.
-
46.
Bizot-Espiard
JG
, Double
A
and Cousin
B.
Lack of melatonin effects on insulin action in normal rats.
Horm Metabol Res.
1998;
30:
711
-716.
.
-
47.
Fabis
M
, Pruszynska
E
and Mackowiak
P.
In vivo and in situ action of melatonin on insulin secretion and some metabolic implications in the rat.
Pancreas.
2002;
25:
166
-169.
[PubMed]
.
-
48.
Picinato
MC
, Haber
EP
and Cipolla-Neto
J.
Melatonin inhibits insulin secretion and decreases PKA levels without interfering with glucose metabolism in rat pancreatic islets.
J Pineal Res.
2002;
33:
156
-160.
[PubMed]
.
-
49.
Mychka
VB
and Chazova
IE.
Metabolic syndrome: diagnostic and differential approach to treatment. Quality of Life.
Medicine.
2005;
10:
28
-33.
.
-
50.
Bechtold
M
, Palmer
J
, Valtos
J
, Iastello
C
and Sowers
J.
Metabolic syndrome in the elderly.
Curr Diab Rep.
2006;
6:
64
-71.
[PubMed]
.
-
51.
Dilman
VM
Chur
Harwood Academic Publ
Development, aging and disease. A new rationale for an intervention strategy.
1994;
.
-
52.
Luchsinger
J
A work in progress: The metabolic syndrome.
Sci Aging Knowl Environ.
2006;
10:
pe19
.
-
53.
Anisimov
VN
, Pliss
GB
and Iogannsen
MG.
Spontaneous tumors in outbreed LIO rats.
J Exp Clin Cancer Res.
1989;
8:
254
-262.
.
-
54.
Anisimov
VN
, Baturin
DA
and Popovich
IG.
Effect of exposure to light-at-night on life span and spontaneous carcinogenesis in female CBA mice.
Int J Cancer.
2004;
111:
475
-479.
[PubMed]
.
-
55.
Khavinson
VKh
and Morzov
VG.
Using of thymic peptides as geroprotectors.
Probl Aging Longevity (Kiev).
1991;
2:
123
-128.
.
-
56.
Gart
JJ
, Krewski
D
, Lee
PN
, Tarone
RE
and Wahrendorf
J.
Lyon
IARC Sci Publ
Statistical methods in cancer research.
1986;
.
-
57.
Turusov
VS
and Mohr
U.
Lyon
IARC
Pathology of tumours in laboratory animals. Vol. 1. Tumours of the rat.
1990;
.
-
58.
Goubler
EV
Leningrad
Meditsina
Computing methods of pathology analysis and recognition.
1978;
.
-
59.
Fletcher
R
New York
Wiley
Practical methods of optimization (2nd ed.).
1987;
.
-
60.
Davison
AC
and Hinkley
DV.
Cambridge
Cambridge University Press
Bootstrap methods and their application.
1997;
.
-
61.
Cox
DR
and Oakes
D.
London
Chapman & Hall
Analysis of Survival Data.
1996;
.
-
62.
Semenchenko
AV
, Anisimov
VN
and Yashin
AI.
Stressors and antistressors: How do they influence life span in HER-2/neu transgenic mice.
Exp Gerontol.
2004;
39:
1499
-1511.
[PubMed]
.