Introduction
The p53 gene encodes a
transcription factor that regulates apoptosis and metabolism and is mutated in
the majority of human cancers [1,2]. The p53 protein functions as a tetramer
with various protein domains mediating oligomerization,
DNA binding and transcriptional transactivation. Drosophila contains a
single p53 gene with a structure similar to humans [3-6] including two
promoters, and the major protein products are of similar size: 393 amino acid
residues for the human protein, Hp53, and 385 amino acid residues for the Drosophila
protein, Dmp53 (Drosophila protein diagrammed in Figure 1A). The central
DNA binding domain of Dmp53 protein
shows partial sequence conservation with Hp53 [3]. The other domains of Dmp53
show less obvious sequence similarity to Hp53, but appear conserved in
function. Similar to the N-terminal transcriptional activation domain of Hp53,
the N-terminus of Dmp53 contains a high proportion of acidic residues, and Dmp53
has been shown to bind to conserved p53 response elements and activate
transcription [3]. The C-terminus of Hp53 contains a basic region (9/26
residues) that can bind either DNA or RNA, and the C-terminus of Dmp53 is also
relatively basic (6/24 residues). Finally, the oligomerization domain is
located in the C-terminal portion of Hp53, and the corresponding region of
Dmp53 contains a conserved critical Gly "hinge" residue, and appears active in
oligimerization based on yeast two hybrid assays. The p53 message is
expressed at very low levels in adult tissues, with some enrichment indicated
for the eye, malphigian tubule (similar to mammalian kidney), and female germ
cells [7,8].
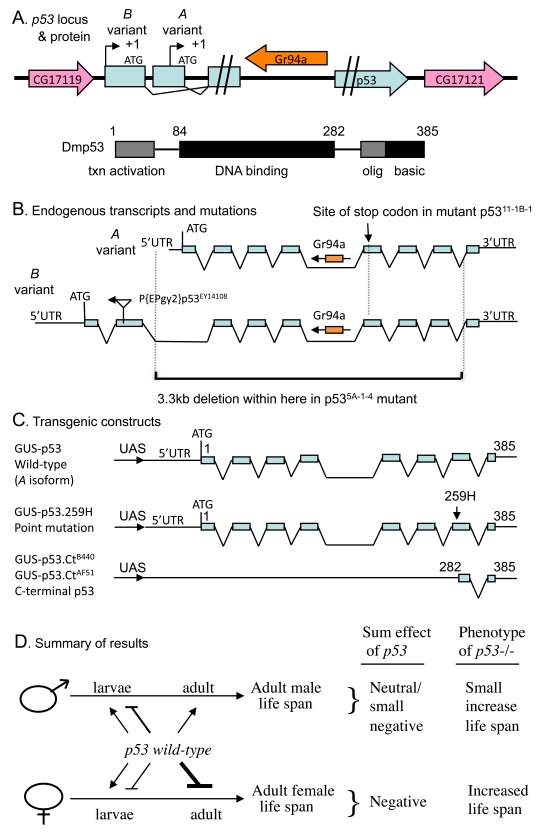
Figure 1. Summaryof Drosophila p53 locus, mutations, transgenes and life span effects. (A) Diagram of p53
locus and major protein product Dmp53. The p53 gene is indicated in
blue, including the two promoters, indicated by black arrows. The internal
intron/exon structure of p53 is omitted here for clarity, but is
shown below in (B). The pink arrows in indicate the genes that
flank p53 on the 5' and 3' side, genes CG17119 and CG17121,
respectively. The orange arrow indicates the gustatory receptor gene Gr94a,
located in the p53 intron. The 385 aa Dmp53 protein is diagrammed
using black and gray boxes, including the N-terminal transcriptional
activation domain, the central DNA binding domain, and the C-terminal
oligomerization domain and basic region. (B) Diagram of endogenous p53
transcripts and mutations. The intron/exon structure of the A and B variant
transcripts is indicated. The Gr94a gene is indicated in orange with an
arrow indicating orientation. The location of insertion of the P element P{EPgy2}p53EY14108
in the second exon of the B isoform is indicated by a triangle, with an
arrow indicating the orientation of the insert. The lower black bracket
indicates the breakpoints of the 3.3kb deletion in the p53[5A-1-4]
mutation. (C) Diagram of transgenic p53 constructs. (D)
Summary of p53 effects on adult life span. The effect on adult life
span of p53 wild type (A variant) over-expression during
larval development and in adults is diagrammed: Bars represent negative
effects of p53 wild-type on adult life span, while arrows represent
positive effects on adult life span; thickness of the lines indicates
relative strength of the effect. "Sum effect of p53" is the expected
summation of effects of p53 on adult life span, which is consistent
with the life span phenotype of p53 null mutation (p53-/-),
as indicated.
Mutant forms of p53 lacking
function of a particular domain can have powerful dose-dependent effects that
are often dependent upon the presence of wild-type p53 [3,9-11]. For example,
specific truncated forms of mouse p53 can cause enhanced cancer resistance and
accelerated aging phenotypes, generally interpreted as a state of p53
hyperactivation [12]. Based on studies in mammals it has been suggested that p53 may exhibit antagonistic
pleiotropy between life-cycle stages, in that it favors normal development,
fecundity and cancer resistance in young animals, but may
promote aging in old animals [9,13-15]. Recently p53 gene activity was
found to limit the life span of C. elegans hermaphrodites, and this
effect was dependent upon the activity of the insulin/IGF1-like signaling (IIS)
transcription factor gene Daf-16/FOXO [16]. In Drosophila,
several dominant p53 mutations and transgenes have been characterized,
that generally appear to antagonize p53 activity [3]. Nervous-tissue
expression of one of these dominant p53 transgenes (p53 point
mutation 259H) was found to inhibit IIS and extend life span in females [17,18]. However it remains unclear if and how p53 might normally affect
the life span of Drosophila males and females. Here the wild-type form
of p53, as well as mutant forms, were assayed for effects on Drosophila
life span, in both male and female flies.
| Strain # | Genotype | Group (notes) |
| 2 | w[1118] ; + ;
Df(3R)Exel6193, P{XP-U}Exel6193 /TM6B, Tb (BL7672) | - (Chromosomal Def
uncovers p53) |
| 3 | y[1] w[1118] ; + ;
p53[5A-1-4] (BL6815) | - (deletion of p53
gene) |
| 4 | y[1] w[1118] ; + ;
p53[11-1B-1] (BL6816) | M (pt mutant) |
| 5 | w[1118] ; p53[1] / TM6B,
Tb | M (pt mutant) |
| 6 | w[1118] ; + ; + | M (the same pt mutant
as line 4) |
| 7 | Oregon R ( + ; + ; +) | + |
|
8
| y[1] w[67c23];
P{EPgy2}p53[EY14108] (BL 20906) | + |
| 9 | w ; P{Switch}Actin 255B | (GeneSwitch Act-GS-255B
driver) |
| 16 | y[1]w[1118];
P{w[+mC]=UAS-p53.Ex}3/T(2;3)TSTL, CyO:TM6B, Tb | (UAS-p53 wild type) |
| 17 | w ; P{w[+mC]=GUS-p53}2.1 | (UAS-p53 wild type -
CDM26) |
| 18 | w;
P{w[+mC]=GUS-p53.Ct}AF51 | (C-terminal p53 - AF51) |
| 19 | w[1118]; +; P{w[+mC]=GUS-p53.Ct}B440/TM6B,
Tb | (C-terminal p53 - B440) |
| 20 | w[1118];
P{w[+mC]=GUS-p53.259H} | (p53 point mutation -
259H) |
Results
Transgenic manipulation of p53 in adult flies
Drosophila p53
transgenes were assayed for effects on life span both in adults and during
larval development (see below). The conditional transgenic system Geneswitch [19-21]
was used to over-express both wild-type and mutant forms of p53. With
the Geneswitch system transgene expression is triggered by feeding flies (or
larvae) the drug RU486/Mifepristone. A Geneswitch driver strain called
Act-GS-255B was used (Table 1, strain 9), where the tissue-general actin5C
promoter drives expression of the Geneswitch transcription factor. In the
presence of RU486, the Act- GS-255B driver produces expression of UAS-containing
target constructs in all the tissues of either larvae or adults [19,22]:
detailed characterization of the system using UAS-GFP reporter constructs
demonstrates that the Act-GS-255B driver produces abundant transgene expression
throughout all of the tissues of both adult flies and larvae, for both male and
female animals, with slightly less (but still abundant) expression in adult
males relative to females [22]. All of the flies examined in this study are the
progeny of a cross; for example "16-9" flies are the progeny of a cross of
males of strain 16 (containing the UAS-p53 wild-type transgene) with females of
strain 9 (containing the Act-GS-255B Geneswitch driver) to generate progeny
containing both constructs (strains summarized in Table 1); in all cases
crosses are indicated with the male parent genotype first, and the female
parent genotype second. The RU486 drug itself had no significant effect on
male or female life span when administered to adults (Figure 2A; statistical
analyses summarized in Supplementary Table 1). When wild-type p53 was
over-expressed specifically in adult flies, it had a negative effect (-16%) on
mean life span in females (cross 16-9: 95% bootstrap CI for the ratio of the
means [-21.11 - 11.61], log-rank p-value = 2.21 x10-6),
and a positive effect (+6%) on mean life span in males (cross 16-9: 95% bootstrap
CI [2.36 - 10.37], log-rank p-value = 6.97 x10-3)
(Figure 2B; Supplementary Table 1). Slightly larger changes were observed for
median life spans (Supplementary Table 1), and similar results were obtained
with multiple independent transgenic insertions of p53 wild-type (data
not shown). In contrast, adult-specific over-expression of the dominant mutantp53 (point mutation p53-259H) transgene did not have a negative effect
on female life span, and instead female life span tended to be increased (cross
20-9: +7%, 95% bootstrap CI [4.09 - 9.72], log-rank p-value = 4.05 x10-8) (Supplementary Figure 1B; Supplementary Table 1)
[22], and similar results were obtained with p53 dominant mutant
transgene p53-Ct[B440] (Supplementary Figure 1C; Supplementary Table 1).
Because these Drosophila p53 dominant mutation transgenes are
generally expected to antagonize the activity of wild-type p53, the data
are consistent with wild-type p53 having a negative effect on adult
female life span. The negative effect on life span of wild-type p53
over-expression in adult females and the lack of negative effect with dominant
mutant p53 transgenes was also confirmed using the FLP-out
conditional system [23] to cause transgene over-expression (data not shown).
Taken together, these data indicate that in adult flies, p53 inhibits
life span in females and favors life span in males.
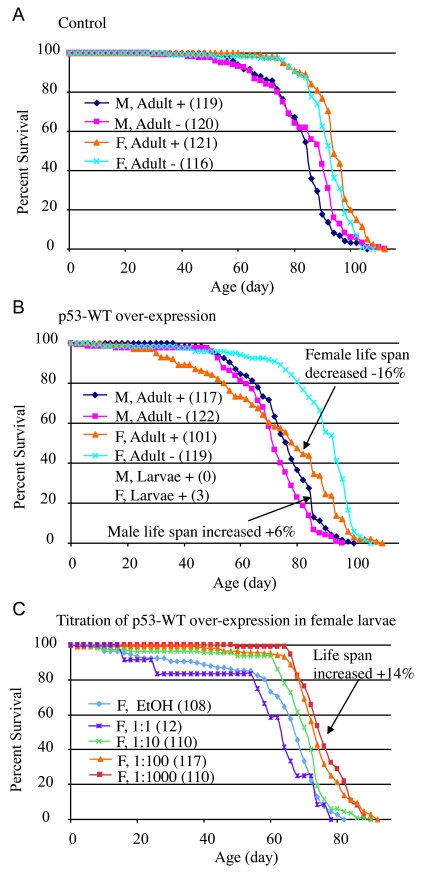
Figure 2. Conditional over-expression of wild-type p53 trans-genes using Geneswitch system.
All flies were the progeny of either Oregon R
control (A) or p53-WT transgenic strain (B, C) crossed
to the tissue-general Geneswitch driver Act-GS-255B. The flies were
cultured in the presence and absence of drug, as larvae or adults, as
indicated: M = males, F = females, + indicates culture in presence of
drug, - indicates culture in absence of drug. The number of flies in each
group are indicated in parentheses. (A, B) Blue diamonds
indicate male adults plus drug, pink squares indicate male adults minus
drug, orange triangles indicate female adult plus drug, turquoise x
indicates female adults minus drug. (A) Control flies, progeny of
Oregon R wild-type and Act-GS-255B. (B) p53 wild-type
transgene over-expression. Note male larvae plus drug produced no adult
flies, whereas female larvae plus drug produced only three escapers. (C)
Titration of p53 wild-type over-expression during female larval
development and effect on subsequent adult life span. EtOH indicates the
ethanol solvent for the drug alone (vector control, indi-cated with light
blue diamonds). Repeats of the titration experiments, including data for
males are presented in Supplementary Figure 1.
Transgenic
manipulation of p53 during development
A strikingly different set of results was obtained
when Drosophila p53 transgenes were expressed specifically during
larval development. When administered only during larval development, the drug
RU486 itself had no effect on subsequent adult female life span, and a small
negative effect on subsequent adult male life span (~-4%; Supplementary Table 1).
Over-expression of wild-type p53 at high levels during larval
development was toxic to both males and females, in that no male adults were
produced, and only three female adults (escapers) were obtained (Figure 2B).
Intriguingly, the three female escapers had unusually long life spans: 86 days, 92 days, and 96 days, respectively. To determine if this apparent life
span increase was significant, and to investigate the developmental effects of
wild-type p53 over-expression in greater detail, the over-expression was
modulated by titration of the RU486/Mifepristone drug, in replicated
experiments. Titration of wild-type p53 over-expression during
development again indicated toxicity at high levels of expression, with greater
toxicity evident for males (Supplementary Table 2). Strikingly, at lower
levels of induction, wild-type p53 produced both female and male adults
with increased mean and maximal life span (Figure 2C; Supplementary Figure 1E-F; Supplementary Table 2; female: +14%, 95% bootstrap CI [9.29 - 19.27]; log-rank
p-value ≈ 0; male: +15%, 95% bootstrap CI [10.54 - 19.30];
log-rank p-value = 4.97 x 10-7). These data demonstrate that
high-level expression of p53 can be toxic during development, whereas
moderate over-expression of p53 during development can cause increased
life span in the resulting male and female adults. Consistent with this
conclusion, expression of the dominant mutant transgenes during development
tended to decrease the life span of the resultant male and female adults
(Supplementary Figure 1A-D, Supplementary Table 1).
Effect of mutations in the endogenous p53 gene
To confirm the effects of p53 on Drosophila
life span, flies were examined that had a deletion or mutation of the
endogenous p53 gene (mutations diagrammed in Figure 1B; strains listed
in Table 1) [24]. Multiple trans-heterozygous p53 wild-type and mutant
allele combinations were assayed for life span simultaneously as a control for
genetic background effects and environmental effects (the "L" cohort, data
summarized in Supplementary Table 3, 4). This was done using two p53
wild-type strains (called the "+" group; strains 6 and 7), two strains
containing p53 null mutation (called the "-" group; strains 2 and 3),
and three strains containing p53 dominant mutations (called the "M" group; strains 4, 5 and 8), and crossing each strain to each
of the others in a "round-robin" approach. In this way each of the various p53
genotypes (+/+, -/-, +/-, +/M, -/M, M/M) represents the average of multiple
specific genetic backgrounds. This approach avoids the potential complication
of identifying p53 effects that might be specific to only one particular
genetic background, such as would be created by using a backcrossing strategy.
In flies with mutations of the endogenous p53
gene, the effect on life span should be the sum of the effects of p53 at
various life-cycle stages, both positive and negative (diagrammed in Figure 1D); and indeed, p53 mutations were found to have a significant effect
on life span in both sexes (ANOVA, p < 0.0001; Supplementary Table 5):
Null mutation (-/-) of the p53 gene increased mean female life span by
+13% (95% bootstrap CI [9.00 -17.28]; log-rank p-value ≈ 0) relative to wild-type (+/+) controls
(Figure 3A; Supplementary Figure 2A; Supplementary Table 4). In the heterozygous p53 mutant
genotype (-/+) average female life span was also increased relative to
wild-type controls by +11% (95% bootstrap CI [8.41 - 13.59]; log-rank p-value ≈ 0). In male flies null mutation (-/-) of the p53 gene
increased mean life span by +12% (95% bootstrap CI [4.92-14.50]; log-rank
p-value ≈ 0), whereas the effect of heterozygous mutation was
smaller, yielding mean life span increases of +5.5% (95% bootstrap CI [2.15 -
7.53]; log-rank p-value ≈ 0) (Figure 3B; Supplementary Figure 2B;
Supplementary Table 4). However, as seen below (Figure 4A, Supplementary
Figure 4), the life span increases in p53 mutant males were not
consistently observed when crosses were done in the opposite direction, and
therefore may not be biologically significant. Similar effects of p53
null (-/-) and heterozygous (+/-) genotypes were obtained when the experiments
were repeated using different culture conditions (richer food source and
presence of mates) that yield shorter overall life spans (the "W" cohort;
Supplementary Figure 3; Supplementary Table 6, Supplementary Table 7). Taken together, these
data with endogenous p53 gene mutations support the conclusion that, in
sum, p53 limits the life span of female flies, with smaller and more
variable effects in male flies.
Several Drosophila p53 dominant
mutations (M) were examined and found to have complex effects on adult life
span, depending upon the particular allele, and whether or not a wild-type copy
of p53 was present in the background (Figure 3; Supplementary Figure 2, Supplementary Figure 3). Some of the variability in life span across genotypes is expected to
result from differences in genetic
background. Indeed, the complexity of p53 dominant mutations and their
interactions with genetic background has recently been reviewed [25].
Strikingly, when the data for the various p53 genotypes in the L cohort
were grouped to control for genetic background effects, the dominant mutations
tended to increase life span in females (+/M, -/M, M/M), and to decrease life
span in males (+/M, M/M) (Figure 3; Supplementary Figure 2; Supplementary Table 4).
Since the Drosophila p53 dominant mutations are generally
expected to antagonize wild type p53 function, the increased life span
of +/M females relative to wild type (+/+) is consistent with the results
obtained above suggesting that, in sum, p53 limits the life span of
females. However, for the M/M genotype flies, a wild-type copy of the entire p53
gene is not present, and these genotypes produced the greatest increase in life span in females and the
greatest decrease in life span in males. Therefore, these data suggest that
the mutant forms of p53 may have sexually antagonistic effects on Drosophila
life span that are not necessarily dependent upon the presence of a wild-type p53.
Strikingly, these effects of dominant mutations on life span were highly
dependent upon environment, since in the W cohort the dominant mutations tended
to decrease life span in both males and females (Supplementary Figure 3;
Supplementary Table 7). It will be of interest in the future to determine what
is the mechanism for these opposite effects of dominant p53 mutations in
males versus fe-males, and to determine if the dramatic gene-by-environ-ment
effect of p53 dominant mutations in females is due to the presence of
mates, the richer food source, or both.
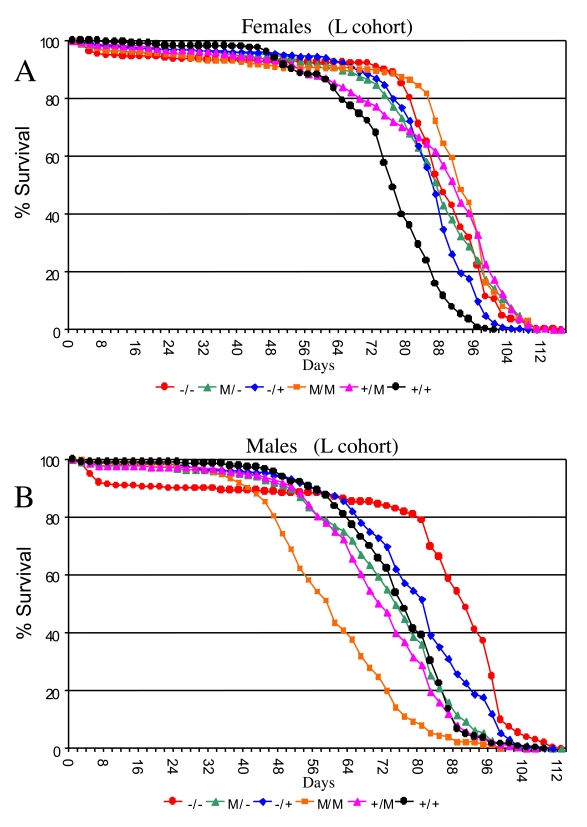
Figure 3. Effect of p53 mutations on life span. Cumulative survival
curves for L cohort. A key of p53 genotypes is presented below the graphs.
Males are indicated with solid symbols and females are indicated with open
symbols. (A) Females. (B) Males.
Controls for maternal effects and X chromosome
effects
In an effort to control for possible maternal effects
and X chromosome effects, several life span assays were repeated with
the crosses done in both directions simultaneously, i.e., varying which strain
serves as mother or father for the cross (Supplementary Figure 4). An
increase in life span of p53 null mutant (-/-) flies relative to
wild-type (+/+) controls was obtained in female progeny regardless of cross
direction (Supplementary Figure 4; Supplementary Table 8), thereby ruling out
a primary effect of maternal genotype. In males a consistent change in life
span was not observed, in that although the null mutants exhibited slight
differences in life span compared to controls, the direction of change differed
depending on the direction of the cross. Furthermore, while the survival curves
of many of the reverse cross pairs differed from one another in both sexes
(log-rank test, data not shown), in females there was strong concordance and
highly significant results from comparisons of survival curves in both cross
directions and relative to both controls, while this was not the case for males
(Supplementary Table 8). These results demonstrate that the increased life
span in females due to p53 mutation cannot be simply due to maternal or X
chromosome effects, and in conjunction with the above findings, these data
again suggest that p53 preferentially limits the life span of female
flies.
Sex-specific effects p53
on fly stress resistance
Drosophila p53 is required for normal resistance of
larval cells and tissues to certain kinds of stress, for example, ionizing
radiation and UV toxicity [26,27], and third-instar larvae that are null for p53
exhibit decreased survival when challenged with 4,000 Rads of ionizing
radiation [28]. To determine if p53 genotype might have sex-specific
effects on stress resistance in adult flies, male and female flies that were
either wild-type or mutant for p53 were subjected to two types of
life-shortening stress, ionizing radiation and 100% oxygen atmosphere, in
replicated experiments (Figure 4, Supplementary Table 9). Treatment with
90,000 Rads of gamma-irradiation on day 10 of adult age reduced adult life spans
by half, and p53 mutant female flies were again found to have greater
mean life span than wild-type controls (+/-: +18%, 95% bootstrap CI [13.13 -
23.36]; log-rank p-value = 0; -/-: +13%, 95% bootstrap CI [9.09 - 16.71];
log-rank p-value = 2.98 x10-4). In contrast, p53 mutations
were found to slightly reduce the survival of female flies subject to 100%
oxygen atmosphere (-/+: not significantly different than wild-type; -/-:
-4%, 95% bootstrap CI [-5.06 - -3.34]; log-rank
p-value = 1.28 x10-13). In males, p53 null mutants
subject to ionizing radiation had significantly reduced mean life span, whereas
heterozygotes fared slightly better than wild-type (+/-: +4%, 95% bootstrap CI
[1.80 - 6.00]; log-rank p-value = 2.02 x10-7;
-/-: -19%, 95% bootstrap CI [-20.68 - -17.06]; log-rank p-value ≈ 0). As with
females, p53 gene mutations tended to reduce male survival in response
to a 100% oxygen environment (+/-: -4%, 95% bootstrap CI
[-4.38 - -3.05]; log-rank
p-value = 4.44 x10-16; -/-: -15%, 95%
bootstrap CI [-16.13 - -14.10]; log-rank
p-value ≈ 0). Therefore, wild-type p53 tended to favor
the survival of both sexes under 100% oxygen stress conditions, yet was
detrimental to female life span in flies subject to ionizing radiation.
Therefore the results for adults subject to ionizing radiation were similar to
those observed during normal aging: normal p53 function increased
survival of males and decreased survival of females. The fact that p53
favored the survival of both sexes under the more severe life-shortening
condition of 100% oxygen stress may be indicative of a threshold effect on
survival that is sex-specific.
Discussion
In these experiments a combination of genetic and
transgenic approaches were used to study how p53 affects the life span
of male and female Drosophila. The conditional transgenic system
Geneswitch was employed to produce tissue-general expression of p53,
either during development or specifically in adults. Detailed characterization
of the Geneswitch driver strain ("Actin-GS-255B") using GFP reporter constructs
demonstrated that the system yields truly tissue-general expression during
larval development, as well as tissue-general expression in both male and
female adults [22]. The data indicate that Drosophila p53 has
effects on adult life span that are antagonistically pleiotropic between
developmental stages and sexes (summarized in Figure 1A). One advance of the
present study is that life span effects were identified using transgenes
encoding the full length, wild-type form of Drosophila p53 protein, as
well as ones encoding mutant forms. In adults, wild-type p53
over-expression limited life span in females and favored life span in males. In
contrast, during development, p53 over-expression acted in a
dose-dependent manner to either reduce or increase the subsequent longevity of
both male and female adults: high level expression during development was
detrimental, whereas moderate over-expression produced increased life span.
The dominant mutation transgenes generally produced the opposite effect of wild
type p53 transgenes, in both males and females. This indicates that the
opposing effects of p53 transgenes on male and female life span cannot
be simply due to some cryptic difference in
the efficiency of transgene expression in males versus females, or to
some differential toxicity of the encoded proteins in males versus females.
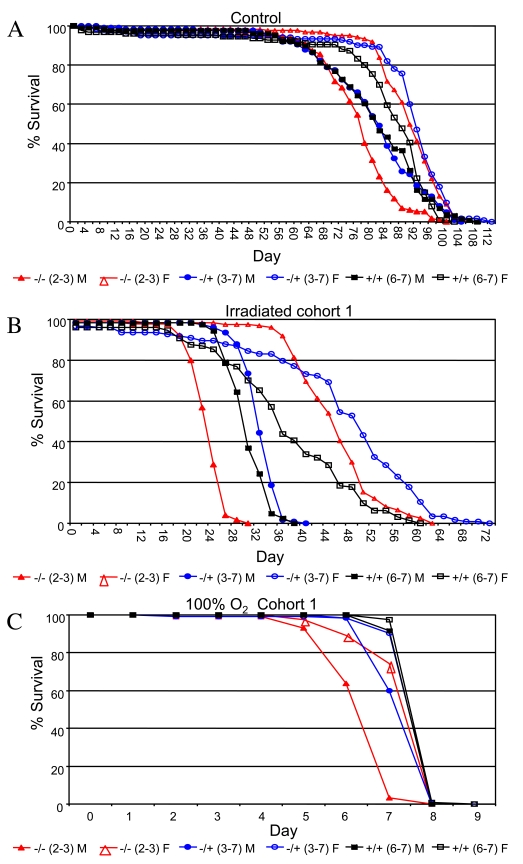
Figure 4. Survival curves for the indicated genotypes under stress conditions.
(A) Ionizing radiation. (B) 100% oxygen survival. A key of p53 genotypes
is presented below the graphs. Males are indicated with solid symbols and females
are indicated with open symbols. Survival curves for replicate experiments (cohort 2)
are presented in Supplementary Figure 5. Survival statistics for these and replicate
experiments are summarized in Supplementary Table 9.
Results consistent with the transgenic manipulations
were obtained from analysis of the endogenous p53 gene: Null mutation
of the endogenous p53 gene increased life span in females, and had
smaller, more variable effects on male life span. The effects of p53 on
adult fly survival under stress conditions were also sex-biased: wild-type p53
was found to favor the survival of both sexes under 100% oxygen stress
conditions, yet to be detrimental to female life span in flies subject to
ionizing radiation. In these experiments p53 expression and function is
being altered in all of the tissues of the animal simultaneously, and therefore
the effects observed are the sum of any possible tissue-specific effects of p53.
Indeed our results suggest that the positive and negative effects of p53
on life span observed here with tissue-general alterations are comprised of a
mix of both positive and negative tissue-specific effects, that combine to
result in the observed opposite effects in males versus females (J.S. and J.T.,
2009 Experimental Gerontology, in press).
The data presented here indicate that p53
null mutation increases life span in female flies, with smaller, more variable
increases observed for male flies. Helfand and coworkers have previously
reported that p53 null mutant male and female flies were sickly, with a
shortened life span, however, statistical analysis was not presented [17]. One
possibility is that the apparent reduction in life span and vigor previously
reported for p53 null flies may have resulted from inbreeding depression
in the homozygous mutant flies used in that study. In contrast, in the
experiments presented here, multiple trans-heterozygous p53 null mutant
genotypes were examined, so as to reduce possible inbreeding effects, and
thereby reveal the life span benefit of p53 null mutations. Helfand and
coworkers also analyzed the effect on life span of nervous system-specific
expression of two p53 dominant mutant transgenes, a C-terminal fragment
transgene (p53-Ct), and the point mutant (p53-259H). They found that nervous
system expression of p53-Ct throughout both development and adulthood increased
female life span by +58%, and increased male life span by +32% [17]. Because
the dominant mutations are generally expected to antagonize p53 activity, their
results are consistent with our conclusion that, in sum, p53 limits life
span in females, with smaller effect in males (summarized in Figure 1D). Using
the Elav-Geneswitch driver to restrict expression to the adult nervous system,
Helfand and coworkers found that the p53-Ct transgene increased female life
span by +18% to +26%, and the p53-259H transgene increased female life span by
+11% to +13%, again consistent with our finding that p53 limits the life
span of adult females. Indeed, using the tissue-general Act-GS-255B driver to
restrict transgene expression to adults, we also found that the p53-Ct and
p53-259H transgenes produced an increase in median life span in females
(Supplementary Figure 1A-D) [22]. For adult-specific expression in male
nervous system, Helfand and coworkers reported life span data for only two
assays, both using the p53-Ct transgene: using a high-calorie food condition,
male life span was reported to be increased by +13%, whereas using a low-calorie
food, male life span was unchanged, and results for normal food were not
presented [17]. That result might at first appear to be partly inconsistent
with our conclusion that p53 favors life span in adult males, however,
there are several possible explanations that might reconcile these results.
First, the previous experiment involved the p53-Ct transgene, encoding the p53
C-terminal fragment, and data from mammals suggests that certain dominant p53
mutants are capable of either antagonizing or promoting p53 activity, depending
upon the level of expression and the cellular context [11]. Second, the life
span increase was observed only under a high-calorie food condition, and our
data suggest sex-specific interactions between dominant p53 mutations
and diet/environment with regard to life span (Figure 3, Supplementary Figure 2). Under our conditions and using tissue-general expression, we found that
adult-specific expression of the dominant mutant p53 transgenes tended
to decrease male life span (Supplementary Figure 1, Supplementary Table 1), consistent with
our conclusion that p53 normally favors adult male life span. Finally,
the effects of tissue-general expression, as tested here, will be the sum of
all tissue-specific effects, be they positive or negative. Indeed our results
suggest that the positive and negative effects of p53 on life span
observed here with tissue-general alterations are comprised of a mix of both
positive and negative tissue-specific effects (J.S. and J.T., 2009 Experimental
Gerontology, in press), that combine to result in opposite effects in males
versus females (summarized in Figure 1D). Therefore, the previous results from
the Helfand group (with the possible exception of a single assay of males under
a high-calorie food condition), are generally consistent with the results
presented here.
One possible mechanism by which p53 might act
in adult flies to preferentially limit female life span is by stimulating IIS,
since IIS appears to preferentially limit life span in females of Drosophila
and other species [29,30]. Studies in mammals provide precedent for crosstalk
between p53 and the IIS pathway, including the target transcription
factor FOXO, in regulating both aging and cancer [31,32]. Consistent with
this idea, life span extension in Drosophila females produced by nervous
system-specific expression of the dominant mutant p53-259H transgene was
found to correlate with a reduction in IIS signaling [18]. In C. elegans,
mutation of the p53 homolog cep-1 increased life span of adult
hermaphrodites, and this increase required the function of the IIS target
transcription factor gene Daf-16/FOXO [16]. To definitively rule in (or
out) a role for IIS in Drosophila p53 life span effects will require
future assays in the presence and absence of the Foxo transcription factor.
Another possible mechanism by which p53 might
affect life span is by altering proliferation or causing apoptosis in
particular cell types. For example, ablation of germ-line cells in adult
animals by forced over-expression of the bam gene caused increased life
span in males and females [33]. However, while germ line ablation might be
attractive as a possible mechanism for the increased life span observed in p53-over-expressing
males, it is not consistent with the life span decrease observed in females.
Alternatively, over-expression of wild-type p53 specifically in adult
diploid cells using an escargot-GAL4 driver caused ablation of most stem
cells in the gut, and gut stem cell proliferation appears to be more rapid in
females than in males [34]. While this might be attractive as a possible
mechanism for the life span decrease observed in p53-over-expressing
females, it is not consistent with the life span increase observed in males;
indeed other experiments involving disruption of adult diploid cell function
caused an equally dramatic decrease in life span in both sexes [35]. It will
be of interest in the future to ask if p53 might be affecting life span
through highly sex-specific or sexually opposite effects on cell proliferation
and survival. Notably, over-expression of strong caspase inhibitors and other
apoptosis and senescence regulatory genes in adult flies did not yield
increased life span in either sex, and where negative effects on life span were
observed, such as with wingless and activated Ras, the negative
effects were similar in males and females [22]. Those results tend to suggest
that p53 may be acting through some other mechanisms, such as
alterations in metabolism or autophagy. Additional possible mechanisms by
which p53 might affect life span include sex-specific alterations in
behavior, such as food intake, or potentially costly activities such as
movement or aggression.
In these experiments Drosophila p53
was also found to have sex-specific effects on survival under stress
conditions. Wild-type p53 favored the survival of both sexes under 100%
oxygen stress, yet was detrimental to female life span in flies subject to
ionizing radiation. This may be indicative of a threshold effect on survival
that is sex-specific. Mechanistically the ability of p53 to either favor
survival or mortality may be related to p53's ability to regulate both
repair and apoptotic pathways [1,36-38], and perhaps the functional connection
between p53 and FOXO in response to oxidative stress [25]. In line with
our findings, C. elegans hermaphrodites that are long-lived due to p53
(cep-1) mutation did not demonstrate increased resistance to oxidative
(or UV) stress [16], however resistance to gamma irradiation was not examined.
Strikingly, in C. elegans hermaphrodites, p53 has recently been
found to increase life span in response to mild mitochondrial stress, and to
decrease life span in response to severe mitochondrial stress, consistent with
a threshold effect on survival [39] ; however effects in males have not been
reported. In mice, reduced p53 function results in resistance to
lethality caused by moderate gamma irradiation and increased sensitivity to
severe irradiation [40,41], again suggestive of a threshold effect, however any
potential sex-bias has not been reported. Finally, long-lived female Drosophila
that over-expressed dominant-mutant p53 in neurons exhibited increased
resistance to the oxidative stressor paraquat [17]; however effects in males
were not reported. Taken together the data are consistent with a model in whichp53 has a threshold effect on survival under stress, and the threshold
for the transition from favorable to detrimental depends upon the type of
stress and the sex of the animal. Such a threshold model is consistent with
extensive data from mammals and model systems demonstrating that p53 can
either favor oxidative stress resistance and cell survival, or favor oxidative
stress and cell death, depending upon the cellular and environmental context,
and the degree of activation of p53 [38]. In mammals, physiological
levels of p53 activity appear to maintain normal cellular redox status,
through sustained expression of antioxidant genes (e.g., Sesn1&2, GPX1,
AIF) and metabolic genes (e.g., SCO2, PGM, TIGAR). In contrast,
hypo-physiological levels of p53 activity can suppress expression of
antioxidant genes (e.g., Sesn1&2, GPX1) and cause increased
oxidative stress. Similarly, hyper-physiological levels of p53 activity
can induce pro-oxidant and apoptosis-promoting genes (e.g., NQO1, POX, BAX, PUMA,
p66shc), and/or cause an imbalance in expression of antioxidant genes
(e.g., MnSOD, PIG12, ALDH4, GPX), and again cause increased oxidative
stress [38].
Antagonistic pleiotropy of gene function between
younger and older animals is generally accepted as one of the most likely
genetic mechanisms underlying aging [42]; however, specific genes exhibiting
such pleiotropy have generally not been identified. One notable exception is
data from mammals that suggests p53 exhibits antagonistic pleiotropy
between developmental stages. At young ages p53 favors fecundity and
favors survival by acting as a tumor suppressor, yet at late ages it may limit
survival by promoting cell senescence, or through other mechanisms [13,43].
Increasing evidence suggests that genes can also exhibit antagonistic
plieotropy of function between the sexes, affecting a variety of traits
including reproductive fitness and life span [30,44-47]. The data presented
here suggest that Drosophila p53 exhibits a combination of both
developmental stage-specific and sex-specific antagonistic pleiotropy with
regard to life span. If this result were to translate to humans, it would have
implications for human aging related diseases such as cancer. Consistent with
our results using flies, the effects of human p53 and p53-interacting
genes such as MDM2 on cancer incidence and longevity are often
sex-biased [48], and p53 has recently been implicated in regulating
mammalian maternal fecundity [49]. Moreover, during mouse development, p53
null mutations cause a high frequency of neural tube defects and lethality that
preferentially affects female embryos [50,51], and interestingly, this sex
difference appears to result from the number of X chromosomes rather
than the presence or absence of the Y [52]. The sex-specific effects ofp53 may be related to recent observations that in humans the X-chromosome
dosage-compensation gene MOF can regulate p53 [53]; and notably
the MOF gene is conserved and also X-linked in flies. Taken
together the data support a sexual antagonistic pleiotropy model in which p53
function may be maintained by positive selection for fecundity and/or survival
benefit during development, in young animals, and under certain stress
conditions, despite acting at another stage of the life cycle and in the other
sex to limit adult life span (summarized in Figure 1D).
Methods
Drosophila culture.
Drosophila culture and life
span assays were performed as previously described [19]. Briefly, crosses were
conducted in 250 ml urine-specimen bottles (Genessee Scientific) containing 35
ml of medium. Adult flies were maintained in narrow polystyrene vials (Genesee
Scientific) containing 5 ml medium. Drosophila culture media contained cornmeal,
agar, dextrose, yeast, and propionic acid to inhibit bacterial growth and
tegosept to inhibit fungal growth [54]; except for the W cohort which were
cultured on an older recipe containing molasses rather than dextrose (food
recipes summarized in Supplementary Table 10). Flies were maintained at 25oC
and on a 12:12 dark/light cycle, and were removed to room temperature for less
than 1 hour every 2 days to provide fresh medium and remove and enumerate dead
flies. To estimate life expectancy, single-sex mortality vials were
established, with ~25 flies per vial (sample sizes were occasionally reduced
due to rare escapers) and 5 or 10 replicate vials (depending on the experiment)
per sex for every cohort. The L cohort deletion experiment used 10 replicate
vials per sex, the reverse-cross experiments used 5 vials per sex, the stress
experiments used 5 vials per sex, the Geneswitch experiments used 5 vials per
sex, and the drug-titration experiments used 5 vials per sex. Note that for
each line in the W cohort ~125 flies were maintained at ~25 flies per vial with
mates.
Drosophila
strains
.
All Drosophila strains and genotypes are listed in
Table 1, and several mutants and transgenes are diagrammed in Figure 1.
Wild-type (A-isoform) and dominant-mutant p53 transgene stocks
were obtained from Michael Brodsky [3] and Bloomington Drosophila Stock
Center. P{UAS-p53.Ex}, p53 wild-type. P{GUS-p53.Ct}AF51, C-terminal
fragment AA285-385, chromosome 2. P{GUS-p53.Ct}B440, C-terminal fragment
AA285-385, chromosome 3. P{GUS-p53.259H}, AA substitution, chromosome 3. The p53
mutant strains were obtained from Kent Golic and Bloomington Drosophila
Stock Center [55]. Df(3R)slo3 is deletion of entire p53 gene ("-").
Df(3R)Exel, P{XP-U}Exel is deletion of entire p53 gene ("-"). p53[5A-1-4]
is 3.3kb internal deletion ("-"), and it's structure was confirmed by PCR
amplification and sequencing (diagrammed in Figure 1B). p53[11-1B-1] is
a point mutation that introduces a stop codon at nucleotide residue 211, and is
predicted to yield a 70AA truncated protein ("M"). P{EPgy2}p53[EY14108] is a P
element insert mutation obtained from Bloomington Drosophila Stock
Center (BL 20906), and the insertion was mapped to the first exon of the p53
B-variant using inverse PCR (diagrammed in Figure 1B) [56]. Because the
p53[EY14108] mutation is predicted to produce an altered complement of p53
protein isoforms, it is grouped here with the dominant mutants ("M").
Geneswitch
conditional gene expression system
. Geneswitch strains and
protocols are as previously described [19-21]. The strain Act-GS-255B [19,22]
contains two inserts on the second chromosome of a construct in which the actin5C
promoter drives expression of the Geneswitch coding region. RU486
(Mifepristone, Sigma) was fed to adult flies or developing larvae by adjusting
the food to ~160ug/ml final concentration. A stock solution of 3.2mg/ml of
RU486 was prepared by dissolving drug in ethanol (100%). Control food received
ethanol solvent alone. In certain experiments RU486 concentrations were
titrated as indicated. All ages are expressed as days from eclosion at 25oC.
To generate flies containing both the Act-GS-255B driver and the
UAS-transgenes, virgins from the Act-GS-255B strain were crossed to males from
each transgenic strain and the Oregon R wild-type strain as a control. Certain
crosses were done in the opposite direction, as indicated in the "reverse
cross" experiments. The life span assay result for p53-259H transgene
over-expression in adult flies using Act-GS-255B driver has been previously
published [22], and is included here with additional statistical analysis for
comparison purposes (Supplementary Table 1).
Statistical analyses
. Initial cohort size was taken to be the number of flies in the vials
at the beginning of the second two-day interval. Deaths during the first
interval after transfer were considered to be due to injury during collection
and therefore were excluded from the calculations. Survivorship was scored
every other day and final cohort size was taken as summed deaths. The effect ofp53 deletion, mutation, and over-expression on Drosophila life
span was assayed in multiple trials for several lines. Life span summary statistics for each of the
experiments (data pooled across replicate vials) and detailed statistical
analyses are presented in the Supplementary Materials (Supplementary Table 1-9). A
non-parametric log-rank test was employed to compare the survival functions betweenp53 deficient or over-expression genotypes and controls [57]. To further
assess the effect of p53 on mean, median, and "maximal lifespan" (defined operationally here as the 90th
percentile of life span), 95% double
bootstrap-t confidence intervals for the ratio of the means (or ratio of the
percentiles) of the experimental and control samples were computed using a
custom Fortran script. Mixed effects models were fit to data from each sex
separately to ascertain the effects of mutation type (M) and genotype (G)
(fixed main effects) on life expectancy, with replicate vials (R) treated as a
random effect using the nlme package in R. Mixed-effects models allow
for a flexible representation of the covariance structure due to the grouping
of the data and enabled the variation induced in the survival response by
replicate vials to be characterized. As appropriate, the models were y = μ + M + R(M) + ε (where M = +/+, +/-, etc and G = 6-7, 2-6, etc was
treated as an "inner" grouping) and y = μ + G + R(G) + ε, where ε indicates the within vial error variance. Post-hoc
Tukey tests were performed to assess significant differences among means after
correcting for multiple testing. Analyses were performed using the R
statistical environment [58], unless otherwise noted.
We thank Michelle Arbeitman and Heidi
Scrable for helpful comments. This work was supported by a Senior Scholar
Award from the Ellison Medical Foundation to JT, and by grants from the
Department of Health and Human Services to ST (GM067243) and to JT (AG011833),
and by a pilot project award to JT from the USC ADRC (1P50 AG05142). ST is a
Royal Society-Wolfson Research Merit Award holder.
The authors of this
manuscript have no conflicts of interest to declare.