Surviving in the cold: yeast mutants with extended hibernating lifespan are oxidant sensitive
Abstract
Metabolic activity generates oxidizing molecules throughout life, but it is still debated if the resulting damage of macromolecules is a causality, or consequence, of the aging process. This problem demands for studying growth- and longevity phenotypes separately. Here, we assayed a complete collection of haploid Saccharomyces cerevisiae knock-out strains for their capacity to endure long periods at low metabolic rates. Deletion of 93 genes, predominantly factors of primary metabolism, allowed yeast to survive for more than 58 months in the cold. The majority of these deletion strains were not resistant against oxidants or reductants, but many were hypersensitive. Hence, survival at low metabolic rates has limiting genetic components, and correlates with stress resistance inversely. Indeed, maintaining the energy consuming anti-oxidative machinery seems to be disadvantageous under coldroom conditions.
Calorie restriction (CR), the
practice of limiting caloric intake, retards aging phenotypes across species [1]. Furthermore, systematic exploration of the chronolo-gical
(survival in the stationary phase) [2] and replicative (number of mitoses per mother) [3] lifespan of S. cerevisiae identified several
metabolic genes and CR targets, such as the TOR pathway members, which lower
metabolic activity and cause yeast lifespan extension when deleted. High
metabolic turnover is a major source of free radicals and oxidative damage,
other important players in the aging process. Many long-living mutations confer
resistance against oxidants, and oxidatively damaged macromolecules are not
inherited to yeast daughters [4]. There are profound observations that support the
free radicals theory of aging. For instance, a recently identified yeast strain
lacking AFO1 is deficient in mitochondrial respiration, produces low
amounts of free radicals and exhibits a massive lifespan extension of + 60% in
median- and + 71% in maximum replicative
lifespan [5]. However, despite these intense investigations, it is
still unclear if the oxidative damage is indeed a cause, or simply a
consequence, of the aging process itself [6]. A primary argument for the latter is the fact that
genetic manipulations increasing the antioxidative capacity do generally not
increase lifespan, in fact, many oxidant-resistant mutants are short living [7,8].
Hence, it would be important to generate
data which allows distinguishing between growth rate, and long time survival.
We speculated that identifying genetic factors which limit survival under
conditions, at which the metabolic rate is naturally low, could bring us a step
forward in solving this question.
Yeast kept at cold temperatures has a
massively reduced growth- and metabolic rates; at 10°C the chronological
lifespan is prolonged [9]. We arrayed a complete, S288c derived, MATa
knock-out collection onto 106 yeast peptone dextrose (YPD) petridishes. The
plates were incubated at 30°C until giant colonies were formed, sealed and
stored light protected in a cold room at 4°C. For assaying colony survival,
plates were replicated onto fresh media and incubated at 30°C. After 12 months,
most spots were still forming new colonies (Figure 1A). Thus, compared to
higher temperature, yeast colonies kept at 4°C survive dramatically longer.
Next, viability was assayed after an incubation time of 58 months. Now, most
strains had lost their colony forming capacity. However a small fraction (2.3%)
was still alive and produced giant colonies within 2 days after replication.
These strains were exposed to a rigorous quality control and tested for
methionine auxotrophy, kanamycin resistance and colour shifts upon CuSO4
treatment. Suspicious colonies were further analyzed be determination of mating
capacity and auxotrophic markers. Finally, we verified the identity of all
strains by amplifying and sequencing genetic
barcodes. Ultimately, 93 gene deletions were confirmed; long-time survival in the cold is
obviously limited by genetic components (Supplementary Table 1). To pay a tribute to mammals
which can endure long winter periods at low metabolic rates, we propose the
term hibernating lifespan for this yeast phenotype.
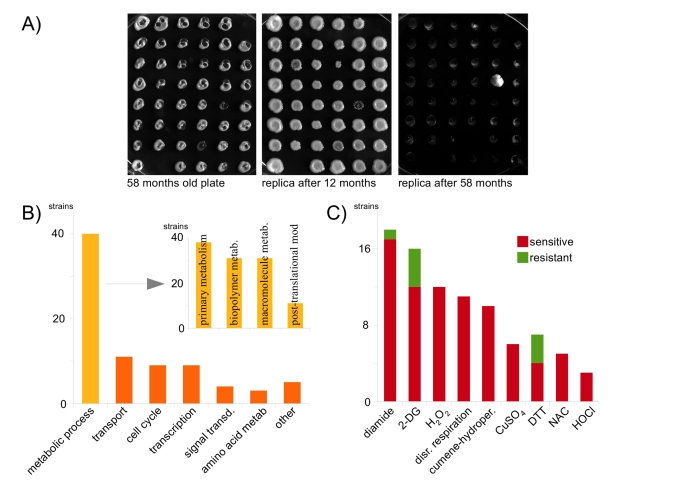
Figure 1. Oxidant-resistance is not a premise for long-time survival in the cold. (A)
106 48-position agar plates containing a systematic yeast knock-out
collection were incubated at 4°C and replicated after 12 and 58 months,
respectively. (B) GO analysis of surviving strains; the majority
groups to metabolic processes (C) Comprehensive phenotypic analysis
of mutants that survived for 58 months in the cold. Resistance to oxidants
or reductants is the exception.
First, we compared these results with aging
experiments performed at normal growth temperatures. No significant overlap
with the systematic lifespan analysis [2,3,10] was observed, only one gene (THI2) exhibited a
prolonged replicative lifespan. Thus, coldroom survival is neither a predictor
for chronological, nor replicative aging. Most of the identified genes (43.5%)
belong to the gene ontology (GO) term metabolic process,
followed by transport (12%) and cell cycle (9.8%) (Figure 1B). Metabolic
process genes were significantly enriched for terms primary metabolism,biopolymer metabolism, macromolecular metabolism and post-translational
protein modification (P < 0.05).
To gain insights into the role of oxidant
tolerances, we assayed the long-time survivors for potential phenotypes on
multiple oxidants, reductants and related stressors (Supplementary Table 1, Figure 1C). Salt
(NaCl) and polyamine (spermidine) tolerance was normal, and, compared to the
wild-type, only three of the mutants were resistant, four sensitive, against
the reductant dithiothreitol (DTT). Surprisingly, no strain was resistant to
N-acetylcysteine (NAC), CuSO4 and hypochloric acid, some were
sensitive (NAC: 5, CuSO4: 6, HOCl: 4). In addition, no
strain was resistant against the classic oxidants H2O2
and cumol-hydroperoxide, only one (ΔPUG1)
against diamide. Oxidant sensitivity, however, was common: 12 strains were
sensitive to H2O2,10 to cumol-hydroperoxide
and 17 to diamide. We further assayed the strains for potential deficits in
mitochondrial activity, since the respiratory chain is a primary source for the
production of free radicals under high metabolic rates. In agreement to the
oxidant phenotype, no strain was deficient for respiration; all grew on
non-fermentable carbon sources. However, for a quite significant number of
mutants [11], mitochondrial respiration was essential: they were unable to grow
after disruption of mitochondrial DNA by repeated ethidium bromide treatments.
We wondered if this phenotype might correlate with resistance against the
glycolytic inhibitor 2-deoxy-glucose (2-DG), whose toxicity increases with the
rate of glycolysis or glucose uptake [11,12]. 16 strains showed a 2-DG phenotype, among these
approx half of the strains for which respiration was essential, indicating that
the primary energy metabolism was often affected in these mutants.
Thus, long-time survival at low
temperatures has limiting genetic components that are, similar to mutations
which retard ageing phenotypes, pre-dominately found among primary metabolic
processes. However, oxidative stress resistance is not a premise for this phenotype.
Indeed, the random occurrence of oxidant-sensitivity is much lower in the yeast
knock-out collection [13]. It is evident that at low metabolic rates, less free
radicals are released by the respiratory chain. Consequently, a highly active
anti-oxidative system is not required; down-shutting of this energy consuming
system seems to be advantageous.
Does hibernating lifespan
resemble a classic aging phenotype? Cycles of death and growth allows bacterial
cultures to maintain viable cells for very long time, a phenotype termed GASP
(growth advantage in stationary phase). However, the longest surviving cultures
may be composed of individual cells that are replicatively short-living [14]. In yeast, chronological lifespan is determined by
monitoring the survival of a stationary cultures over time [15]. Also here, chronological ageing does not predict
replicative ageing of individual cells [16]. Nonetheless, important conserved mechanisms of
ageing were identified and understood in these experiments [2,15-17]. Similarly, hibernating lifespan may not be
regarded as classic ageing phenotype. However, the fact that this dataset
resembles a chronological ageing experiment performed at a very low
temperature, it will be highly valuable in defining the role and consequence of
free radicals and oxidative stress during ageing.
Acknowledgments
We are grateful to Sylvia Krobitsch and Ann
Ehrenofer-Murray for providing yeast strains, Cornelis Jakobs for support, Mona
Fechler for technical help and the Max Planck Society for funding.
Conflicts of Interest
The authors declare no competing interest.
References
-
1.
Fontana
L
The scientific basis of caloric restriction leading to longer life.
Curr Opin Gastroenterol.
2009;
25:
144
-150.
[PubMed]
.
-
2.
Powers
RW 3rd
, Kaeberlein
M
, Caldwell
SD
, Kennedy
BK
and Fields
S.
Extension of chronological life span in yeast by decreased TOR pathway signaling.
Genes Dev.
2006;
20:
174
-184.
[PubMed]
.
-
3.
Kaeberlein
M
, Powers
RW 3rd
, Steffen
KK
, Westman
EA
, Hu
D
, Dang
N
, Kerr
EO
, Kirkland
KT
, Fields
S
and Kennedy
BK.
Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients.
Science.
2005;
310:
1193
-1196.
[PubMed]
.
-
4.
Aguilaniu
H
, Gustafsson
L
, Rigoulet
M
and Nystrom
T.
Asymmetric inheritance of oxidatively damaged proteins during cytokinesis.
Science.
2003;
299:
1751
-1753.
[PubMed]
.
-
5.
Heeren
G
, Rinnerthaler
M
, Laun
P
, von
Seyerl P
, Kössler
S
, Klinger
H
, Hager
M
, Bogengruber
E
, Jarolim
S
, Simon-Nobbe
B
, Schüller
C
, Carmona-Gutierrez
D
and Breitenbach-Koller
L.
The mitochondrial ribosomal protein of the large subunit, Afo1p, determines cellular longevity through mitochondrial back-signaling via TOR1.
Aging.
2009;
1:
622
-636.
.
-
6.
Blagosklonny
MV
Aging: ROS or TOR.
Cell Cycle.
2008;
7:
3344
-3354.
[PubMed]
.
-
7.
Muller
FL
, Lustgarten
MS
, Jang
Y
, Richardson
A
and Van
Remmen H.
Trends in oxidative aging theories.
Free Radic Biol Med.
2007;
43:
477
-503.
[PubMed]
.
-
8.
Perez
VI
, Bokov
A
, Remmen
HV
, Mele
J
, Ran
Q
, Ikeno
Y
and Richardson
A.
Is the oxidative stress theory of aging dead.
Biochim Biophys Acta.
2009;
1790:
1005
-1014.
[PubMed]
.
-
9.
Muller
I
, Zimmermann
M
, Becker
D
and Flomer
M.
Calendar life span versus budding life span of Saccharomyces cerevisiae.
Mech Ageing Dev.
1980;
12:
47
-52.
[PubMed]
.
-
10.
Managbanag
JR
, Witten
TM
, Bonchev
D
, Fox
LA
, Tsuchiya
M
, Kennedy
BK
and Kaeberlein
M.
Shortest-path network analysis is a useful approach toward identifying genetic determinants of longevity.
PLoS One.
2008;
3:
e3802
[PubMed]
.
-
11.
Ralser
M
, Wamelink
MM
, Struys
EA
, Joppich
C
, Krobitsch
S
, Jakobs
C
and Lehrach
H.
A catabolic block does not sufficiently explain how 2-deoxy-D-glucose inhibits cell growth.
Proc Natl Acad Sci U S A.
2008;
105:
17807
-17811.
[PubMed]
.
-
12.
Wick
AN
, Drury
DR
, Nakada
HI
and Wolfe
JB.
Localization of the primary metabolic block produced by 2-deoxyglucose.
J Biol Chem.
1957;
224:
963
-969.
[PubMed]
.
-
13.
Thorpe
GW
, Fong
CS
, Alic
N
, Higgins
VJ
and Dawes
IW.
Cells have distinct mechanisms to maintain protection against different reactive oxygen species: oxidative-stress-response genes.
Proc Natl Acad Sci U S A.
2004;
101:
6564
-6569.
[PubMed]
.
-
14.
Finkel
SE
Long-term survival during stationary phase: evolution tion and the GASP phenotype.
Nat Rev Microbiol.
2006;
4:
113
-120.
[PubMed]
.
-
15.
Fabrizio
P
and Longo
VD.
The chronological life span of Saccharomyces cerevisiae.
Aging Cell.
2003;
2:
73
-81.
[PubMed]
.
-
16.
Laun
P
, Rinnerthaler
M
, Bogengruber
E
, Heeren
G
and Breitenbach
M.
Yeast as a model for chronological and reproductive aging - a comparison.
Exp Gerontol.
2006;
41:
1208
-1212.
[PubMed]
.
-
17.
MacLean
M
, Harris
N
and Piper
PW.
Chronological lifespan of stationary phase yeast cells; a model for investigating the factors that might influence the ageing of postmitotic tissues in higher organisms.
Yeast.
2001;
18:
499
-509.
[PubMed]
.