Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment
Abstract
Of all the major organ systems in the body, the ovaries of females are the first to exhibit impaired function with advancing age. Until recently, traditional thinking was that female mammals are provided with a non-renewable pool of oocyte-containing follicles at birth that are depleted during postnatal life to exhaustion, driving ovarian failure. However, a growing body of evidence, including the isolation of germline stem cells (GSC) from adult mouse ovaries that produce developmentally-competent oocytes, has challenged this belief. In addition, rare germline stem-like cells capable of generating oocytes in vitro that undergo parthenogenesis to form blastocyst-like structures have recently been identified in postmenopausal human ovaries. Here we show that the germline-specific meiosis-commitment genes,Stimulated by retinoic acid gene 8 (Stra8) and Deleted in azoospermia-like (Dazl), are highly expressed in aged mouse ovaries. However, histological and marker analyses fail to demonstrate the presence of oocytes, supporting that Stra8 and Dazl are expressed in premeiotic germ cells that do not undergo further differentiation. Through the use of aged germline-specific GFP-expressing transgenic mice, we further show that these germ cells can generate GFP-positive oocytes that co-express the primordial oocyte marker NOBOX and form follicles when grafted into young adult wild-type female hosts. Thus, aged mouse ovaries possess a rare population of premeiotic germ cells that retain the capacity to form oocytes if exposed to a young host environment.
Introduction
In humans and laboratory rodent models
(rats and mice), the ovaries exhibit age-related dysfunction relatively early
in life, with failure noted long before aging-associated changes in other
organs are manifest. In humans, this loss of ovarian function drives the
menopause and its associated increased risk for development of diverse health
complications, many of which are tied to disrupted ovarian hormone production [1].
Endocrine function of the ovaries is carried out primarily by structures termed
follicles, which are composed of a centralized germ cell arrested in meiosis
(oocyte) surrounded by one or more layers of supporting somatic cells [2].
Traditional thinking has been that female mammals are provided with a
non-renewable pool of oocyte-containing follicles at birth that are
continuously depleted during postnatal life to the point of exhaustion [3].
However, a growing body of evidence (reviewed in [4]), including the recent
purification and in-vitro propagation of premeiotic germ cells from neonatal
and young adult mouse ovaries that can generate developmentally-competent
oocytes in transplanted host females [5], has challenged this belief, thus
offering new avenues to consider in the context of deciphering the role that
adult stem cells may play in ovarian function and aging in females [6].
For example, findings from gene mutant mice show that
p16INK4a and p19ARF, two senescence-associated proteins
that contribute to stem cell failure during aging of the hematopoietic, neural
and cardiac systems [7-9], do not play a comparable role in restraining
oogenesis in adult females [10]. However, another cell cycle-regulatory protein
termed CABLES1 [cyclin-dependent kinase (CDK)-5 and ABL enzyme substrate 1] was
identified as serving this function in the mouse female germline, uncovering a
cell lineage-specificity with respect to the role that cell cycle modulators
play in controlling somatic versus germline stem/progenitor cell activity [10]. Other studies have shown that
postmenopausal
human ovaries devoid of oocytes possess rare stem-like cells with germline characteristics
[11]. When maintained in vitro under defined conditions, these cells spontaneously
generate oocytes (or oocyte-like cells) that can undergo parthenogenetic
develop-ment to form preimplantation embryo-like structures [12]. Although
these reports indicate that aged ovarian tissue retains at least some degree of
germline cell function, it is unclear whether these cells contribute to
oogenesis under physiological conditions and, if they do, why these cells
would then fail to maintain the follicle reserve with advancing age. Herein we
used mice as a model to further test whether changes in premeiotic germ cell
function might be an important variable to at least consider in the context of
understanding the mechanisms involved in ovarian aging in mammals.
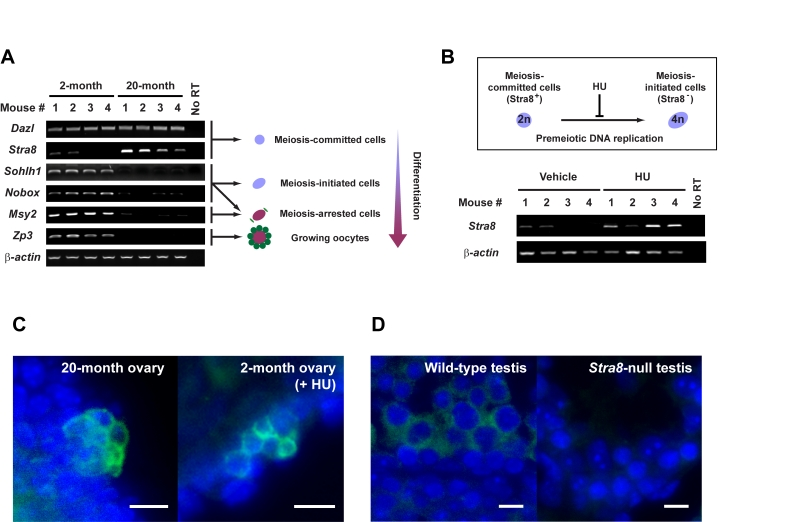
Figure 1. Premeiotic germ cells persist in aged atrophic mouse ovaries.
(A) Analysis of germline marker gene expression in ovaries of young adult
(2-month) and aged (20-month) female mice. Results from all 4 mice per age
group are shown (β-actin, housekeeping
gene used as a sample loading control). (B) In-vivo blockade of
premeiotic DNA replication by HU in ovaries of young adult mice results in
enhanced levels of Stra8 expression, consistent with premeiotic germ
cell accumulation. (C) Immunofluorescence analysis of STRA8
expression (green, cytoplasm) in ovaries of aged or HU-treated young
adult female mice. (D) Control immunofluorescence analysis of STRA8
expression (green, cytoplasm) in testes of young adult wild-type or Stra8-null
male mice (a representative cross-section of seminiferous tubule is shown
for each.). C, D: scale bar = 10 μm;
DAPI counterstain, blue (nucleus).
Results
Premeiotic germ cells are present in aged atrophic mouse ovaries
To
first determine if aged ovaries lacking oocytes possess premeiotic germ cells
or stem cells with germline potential, we screened ovaries from young adult
(2-month) and aged (20-month) C57BL/6 female mice for germline-specific gene
expression. Genes associated with meiotic competence - namely Deleted in
azoospermia-like (Dazl) and Stimulated by retinoic acid gene 8
(Stra8) - were consistently detected in aged ovaries (Figure 1A).
Complete oocyte depletion from ovaries of mice at these advanced ages was confirmed
by both histological (data not shown) and gene marker analysis. Specifically
for the latter, genes marking primordial oocyte formation (Sohlh1, Nobox)
and diplotene-stage
meiotically-arrested oocytes (Msy2) were variably (and very minimally)
or not expressed; similarly, expression of a gene that marks growing oocytes (Zp3)
was not detected in aged ovary tissue (Figure 1A). In contrast and as expected,
young adult mouse ovaries which contain both premeiotic germ cells [5,13] and
oocytes expressed all genes tested (Figure 1A). Cells expressing STRA8 protein,
which heralds commitment of germ cells to meiotic entry by initiating
premeiotic DNA synthesis [14], were localized to cells in the surface
epithelium of aged ovaries, often detected in small cell clusters (Figure 1C).
A similar pattern of STRA8 expression was observed in young adult mouse ovaries
after in-vivo blockade of premeiotic DNA replication using hydroxyurea (HU) (Figure 1B and 1C). Thus, a rare population of premeiotic germ cells exists in atrophic
ovaries of aged mice, but these cells are apparently unable to transition into
oocytes.
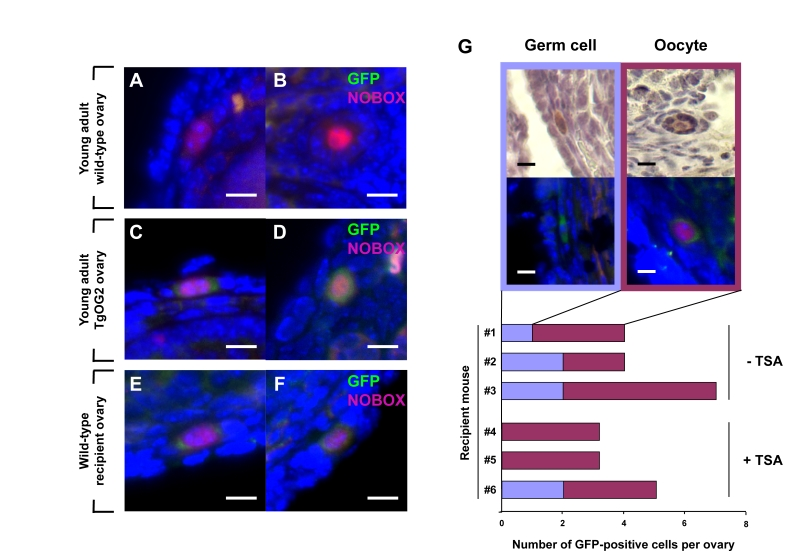
Figure 2. Young adult female mice support oocyte formation from germ cells in aged mouse ovaries.
(A-F) Dual immunofluorescence analysis of GFP (green) and
NOBOX (red, nucleus) expression in ovaries of young adult wild-type
female mice (A, B), young adult TgOG2 female mice (C, D), and
young adult wild-type recipient mice 6 weeks after proximal intrabursal
grafting of aged TgOG2 ovarian tissue (E, F) (scale bar = 10 μm; DAPI, blue, nucleus). (G)
Immunohistochemical detection of GFP (brown; upper), or dual
immunofluorescence analysis of GFP (green) and NOBOX (red, nucleus)
expression (middle; DAPI, blue, nucleus), along with numbers of
non-follicle-enclosed GFP-positive germ cells and follicle-enclosed
GFP-positive oocytes, in ovaries of wild-type young adult recipients
treated with vehicle or TSA (recipient mouse #1-#3 or #4-#6, respectively)
after proximal intrabursal grafting of aged TgOG2 ovarian tissue (scale bar
= 10 μm).
Dormant
germ cells derived from aged ovaries can be re-activated to form oocytes
To
test if these quiescent germ cells in aged mouse ovaries still possess the
ability to generate oocytes and form follicles, we grafted ovarian tissue
harvested from aged female germline-specific GFP-expressing mice (ΔPE-Oct4-Gfp or TgOG2 transgenic) into the ovarian bursal
sacs of young adult wild-type female recipients. In brief, the bursal sac
surrounding a wild-type host ovary was opened, and one-half of the host ovary
was removed prior to inserting one-half of an ovary from an aged TgOG2 female
in its place. The tissue was then allowed to settle back into the peritoneal
cavity and the incision was closed. The remaining half of each aged TgOG2 ovary
not transplanted was fixed immediately and processed for pre-grafting GFP
expression analysis. Six weeks later, the mice were given a single
intraperitoneal injection of vehicle or trichostatin-A (TSA), the latter of
which enhances oogenesis in young adult and middle-age female mice [15,16].
Ovaries were collected 24 hours later for serial section immunohistochemical
analysis of GFP-expressing cells. These experiments revealed an absence of
GFP-positive germ cells in the aged ovarian tissue before grafting. However, a
small number of GFP-positive germ cells, most of which were enclosed within
somatic cells as immature follicles and co-expressed the primordial oocyte
marker NOBOX [17], were detected after transplantation into a young host
environment (Figure 2A-F). These germ cells and follicles were consistently observed
in wild-type recipient ovaries close to the graft interface with aged
transgenic donor ovary tissue, and the
frequency of their detection was unaltered by TSA exposure
prior to collection (Figure 2G).
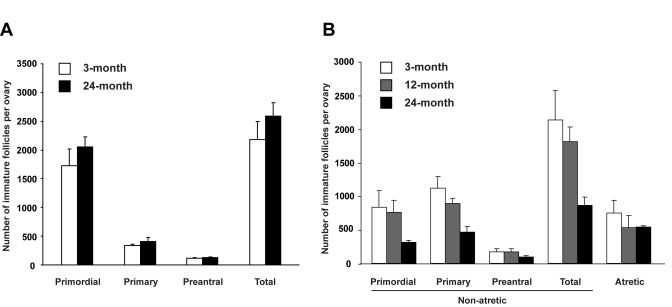
Figure 3. Assessment of the influence of age on the ovarian follicle reserve. (A) Immature
follicle numbers in ovaries of young adult (2-month-old) female mice 7
weeks after parabiotic joining with either young adult (3-month-old) or
aged (24-month-old) female mice (mean ± SEM, n = 5 mice per group). (B)
Immature follicle numbers in young adult (2-month-old) mouse ovaries 3
weeks after grafting under the kidney capsules of young adult
(3-month-old), middle-aged (12-month-old) or aged (24-month-old) female
mice (mean ± SEM, n = 4 mice per group).
Negative
impact of age on maintenance of the follicle reserve
We
next tested whether age-related systemic changes underlie the inability of aged
ovaries to support oocyte formation from these rare premeiotic germ cells. To
accomplish this, young adult female mice were parabiotically joined with young
adult or aged female mice to examine if circulating factors from aged animals
negatively affect the size of follicle reserve in ovaries of young adult
females. Seven weeks after joining, comparable numbers of immature follicles
were detected in ovaries of young adult mice joined with young adult or aged parabionts
(Figure 3A). However, when young adult mouse ovaries were exposed directly to
an aged systemic environment by grafting under the kidney capsules of
24-month-old female mice, immature follicle numbers in these ovaries were
reduced within 3 weeks to less than 50% of those in age-matched ovaries grafted
into young adult (3-month-old) or middle-aged (12-month-old) female recipients
(Figure 3B). The change occurring between 12-24 months of age that triggers
rapid deterioration of the follicle reserve may involve impaired oocyte renewal
rather than accelerated loss, since the incidence of oocyte death (follicle
atresia) was similar among groups (Figure 3B). This latter observation also
indicates that loss of follicles from young ovaries grafted into aged recipients
was not due to ischemia associated with reduced vascularization of the grafted
tissue, since such an outcome would have been associated with elevated cell
death.
Discussion
Since
initial claims that female mammals possess GSC and the ability to produce new
oocytes and follicles during adult life [13], the existence of premeiotic germ
cells and their potential roles in ovarian biology have been extensively
debated (reviewed in [4,18]. However, skepticism surrounding this line work
was greatly minimized by a recent study reporting on the purification of GSC
(or at least their mitotically active progeny) from neonatal and young adult
mouse ovaries [5,19]. These germ cells could not only be established and
propagated in vitro for months, but were also shown to generate developmentally
competent eggs that yielded viable offspring after transplantation into
chemotherapy-conditioned adult female hosts [5]. By monitoring expression of Stra8,
which is widely accepted as a germline-specific gene required for meiotic
competency and commitment in mammals [14,20], herein we identified premeiotic
germ cells in ovaries of aged mice that appear arrested in their ability to
develop into oocytes.
Interestingly,
despite evidence for ongoing oocyte production in ovaries of young adult mice [13,15], (reviewed in [4]), STRA8-positive cells are rarely detected in young adult
mouse ovaries [16]. This may reflect a quick transition of premeiotic germ
cells, once committed by inducing Stra8 expression, into oocytes during
young adulthood. Further, in young adult female mice
the levels of detectable Stra8 expression vary from ovary to ovary ([16];
present study), which is probably due
to collection of ovaries from females without regard to stage of the
reproductive cycle at the time of collection [16]. Indeed, past studies have
shown that primordial follicle renewal in young adult female mice occurs only
during metestrus and diestrus [15,21], and Stra8 expression is more
frequently detected in ovaries with lower than average oocyte counts presumably
on the verge of estrous cycle-related
replenishment [16]. In further support of this, STRA8-positive cells were
consistently detected in young adult mouse ovaries after HU-mediated blockade
of premeiotic DNA replication, which is an essential step for meiotic entry in
mammalian germ cells. Thus, one would expect an accumulation of premeiotic
(viz. Stra8-expressing) germ cells in the ovaries after HU treatment,
similar to that reported to occur in the testes of HU-treated males [22]. Along
these same lines, we previously demonstrated that immature follicles are
rapidly regenerated in young adult mouse ovaries after acute oocyte loss induced
by doxorubicin (DXR) exposure [15]. In the present study, we found a strong
positive correlation between ovarian Stra8 expression and regeneration
of follicles following DXR treatment in young adult female mice (Figure 4).
Similar to that observed in HU-treated young adult ovaries as well as in aged
ovaries, STRA8-immunopositive cells were localized to the ovarian surface
epithelium after DXR exposure, and were found at a time coincident with oocyte
regeneration (Figure 4).
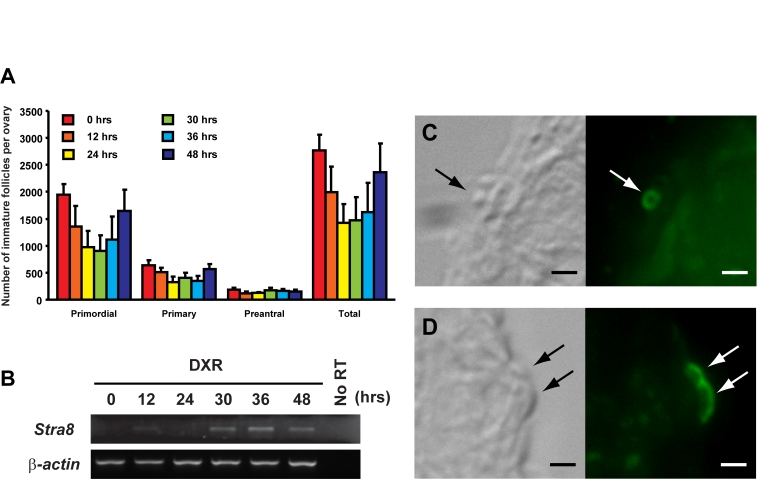
Figure 4. Induction of ovarian Stra8 expression in adult female mice is correlated with oocyte renewal. (A) Number of non-atretic
immature follicles in ovaries of 2-month-old mice at the indicated times
following a single intraperitoneal injection of DXR (mean ± SEM, n = 4 mice
per group). (B) RT-PCR analysis of Stra8 expression in
contralateral ovaries of 2-month-old mice at the indicated times following
DXR injection (β-actin, house-keeping
gene used as a sample loading control). (C, D) Examples of STRA8-immunopositive
cells (green) in the surface epithelial layer of ovaries of mice 30
hours after DXR injection. Scale bar = 5 μm.
It
is important to keep in mind, however, that the identification of premeiotic
germ cells in aged ovary tissue - along with the demonstration that these cells
retain the capacity to make oocytes if provided with a young host environment -
should not be taken as evidence per se that these cells play a key role in the
normal biology of the ovary or in the loss of its function with age.
Nevertheless, these observations at least open the possibility that ovarian
failure in mammals reflects not just atretic depletion of the oocyte pool to
the point of exhaustion but also a progressive loss of oocyte input back into
the ovarian reserve due to impaired function of premeiotic germ cells that are
capable of generating new oocytes. Whatever the case, it bears mention that
atrophied testes of aged male mice which no longer produce sperm are known to
retain quiescent GSC capable of driving spermatogenesis in a young host
environment following transplantation [23,24]. Thus, our new findings
presented herein, along with recent data raising serious questions over the
long-held belief that the mammalian ovary is endowed with a non-renewable
oocyte pool at birth [4,5,13,15,16], provide an impetus to further explore
if aging-related gonadal failure might occur through similar stem cell
failure-based mechanisms in males and females.
Methods
Animals and reagents.
Wild-type C57BL/6 female mice were
obtained from Charles River Laboratories (Wilmington, MA) or the National
Institute on Aging (Bethesda, MD). Transgenic mice with GFP expression driven
by a modified Oct-4 promoter (ΔPE-Oct4-Gfp or TgOG2 mice) to convey germline
specificity of the transgene [4,25-28] were obtained from J.R. Mann through
K.J. MacLaughlin (University of Pennsylvania, Kennett Square, PA). For blockade
of premeiotic DNA replication, 2-month-old wild-type female mice were given
intraperitoneal injections of HU (Sigma Chemical Co., St. Louis, MO) (500 mg/kg
body weight, in saline; 100 μl total volume per injection) every 12 h for 2-3
days [22]. Doxorubicin (Sigma) was administered as a single intraperitoneal
injection (5 mg/kg body weight, in saline; 100 μl total volume). Trichostatin-A
(Sigma) was also administered as a single intraperitoneal injection (10 mg/kg
body weight, in DMSO; 100 μl total volume). The institutional animal care and
use committee of Massachusetts General Hospital reviewed and approved all
animal procedures described herein.
RT-PCR analysis.
Total RNA was extracted from each
whole ovary sample followed by DNase treatment to eliminate contaminating
genomic DNA, and 1 μg was reverse transcribed (Superscript II RT; Invitrogen,
Carlsbad, CA) using oligo-dT primers. Amplification via 26-45 cycles of PCR was then performed using
platinum Taq polymerase (Invitrogen) and Buffer-D (Epicentre, Madison,
WI). For each sample, mRNA encoded by the β-actin gene was
amplified and used as a sample loading control for standardization. All PCR
products were subcloned and sequenced for confirmation. Forward and reverse
primers used were as follows, with GenBank Accession Number, size of amplified
cDNA product and region of coding sequence amplified indicated:
| β-actin
(Accession No. X03672; 439-bp product, nucleotides 4-443)
|
| 5'-GATGACGATATCGCTGCGCTG-3'
|
| 5'-GTACGACCAGAGGCATACAGG-3'
|
| Dazl
(Accession No. NM_010021; 317-bp product, nucleotides 230-547)
|
| 5'-GTGTGTCGAAGGGCTATGGAT-3'
|
| 5'-ACAGGCAGCTGATATCCAGTG-3'
|
| Msy2 (Accession
No. NP_058571; 637-bp product, nucleotides 676-1313)
|
| 5'-CCACCACCCTTCTTCTATCGA-3'
|
| 5'-GGTGATGCCTCGGAACAATA-3'
|
| Nobox (Accession
No. AY061761; 378-bp product, nucleotides 1088-1466)
|
| 5'-CCCTTCAGTCACAGTTTCCGT-3'
|
| 5'-GTCTCTACTCTAGTGCCTTCG-3'
|
| Sohlh1 (Accession
No. NP_001001714; 234-bp product, nucleotides 288-522)
|
| 5'-GATGTCTGTGTACTTCCTCC-3'
|
| 5'-CTGGCTCACTGAATGACAAC-3'
|
| Stra8
(Accession No. NP_033318; 631-bp product, nucleotides 429-1060)
|
| 5'-GCCAGAATGTATTCCGAGAA-3'
|
| 5'-CTCACTCTTGTCCAGGAAAC-3'
|
| Zp3 (Accession
No. M20026; 182-bp product, nucleotides 50-232)
|
| 5'-CCGAGCTGTGCAATTCCCAGA-3'
|
| 5'-AACCCTCTGAGCCAAGGGTGA-3'
|
Oocyte (follicle) counts.
Ovaries were fixed in a solution containing 0.34 N glacial
acetic acid, 10% formalin and 28% ethanol, and embedded in paraffin. Serial
sections were cut (8 μm), aligned in order on glass slides, and stained with
hematoxylin and picric methyl blue. The number of non-atretic and atretic
immature (primordial, primary, and preantral) follicles per ovary was then
determined as detailed previously [29,30].
Immunodetection of STRA8, GFP and NOBOX.
Ovaries were fixed in 4% neutral-buffered paraformaldehyde at room
temperature for 3-4 hours and embedded in paraffin. Tissue sections (6 μm) were
cut and mounted on glass microscope slides. Sections were de-waxed in xylenes,
re-hydrated in a graded ethanol series, and then boiled in 10 mM sodium citrate
for antigen retrieval [15,16,31]. A chicken polyclonal anti-STRA8
antibody was generated in chickens (Aves Labs, Tigard, OR) using the synthetic
peptide, QEQEESLDKLLKLKAS, which corresponds to amino acids 76-91 of the mouse
STRA8 coding sequence. For fluorescence visualization, a biotin-conjugated
goat-anti-chicken IgY (B-1005; Aves Labs) and a streptavidin-conjugated Alexa
Fluor-488 (S11223; Molecular Probes, Eugene, OR) were used. For chromogenic
visualization of GFP expression, a mouse monoclonal antibody against GFP
(sc-9996; Santa Cruz Biotechnology, Santa Cruz, CA) was used in conjunction
with the MOM Kit (PK2200; Vector Labs, Burlingame, CA) for antigen detection [15,28]. For dual
immunofluorescence analysis of GFP and NOBOX expression [15], GFP detection was
first performed using a mouse monoclonal antibody against GFP along with the
MOM Kit (see above) and a streptavidin-conjugated Alexa Fluor-488 probe
(Molecular Probes) followed by NOBOX staining using a rabbit polyclonal
anti-NOBOX antibody (ab41521; Abcam, Cambridge, MA) with Alexa Fluor-568
conjugated goat anti-rabbit IgG (A11011; Invitrogen). Sections were mounted with DAPI
(Vectashield; Vector Labs), and images were captured using a Nikon ECLIPSE
TE2000-S microscope equipped with an EXFO X-Cite 120 fluorescence illuminator.
Positive and negative controls, consisting of ovarian sections from young adult
TgOG2 and wild-type females, respectively, were always included with the
experimental tissues on each slide.
Parabiosis.
Each
parabiont (young adult wild-type female mouse joined with either a young or
aged wild-type female mouse) was anesthetized and an incision was made from the
olecranon to the knee joint of each mouse. The olecranon and knee joint were
attached by a single 5-0 Polyglactin 910 suture and tie, and the dorsal and
ventral skin flaps were approximated by staples and suture [32]. Seven weeks
after surgery, ovarian tissues were collected and processed for follicle
counts.
Ovarian grafting.
For kidney capsule transplants, young (3-month-old), middle-age
(12-month-old) or aged (24-month-old) wild-type female mice were anaesthetized
(Avertin, 200 mg/kg, intraperitoneal) to expose the left kidney in each mouse
through a dorso-lateral incision. For each recipient animal, a small space was
made under the kidney capsule, and ovaries collected from young adult
(2-month-old) wild-type donor mice were placed into the space. At the same
time, both host ovaries were removed. The kidney was then allowed to settle
back into the peritoneal cavity and the incision was closed. Three weeks after
surgery, grafted ovaries were removed from host kidneys and processed for
follicle counts. For grafting into ovarian bursal sacs, similar surgical
procedures were followed with the exception that the left ovary instead of the
kidney of each recipient mouse was exposed. The bursal sac surrounding the
ovary was opened, and one-half of the wild-type host ovary was removed prior to
inserting one-half of an ovary from an aged TgOG2 female in its place,
essentially as described [13]. The tissue was then allowed to settle back into
the peritoneal cavity and the incision was closed. To help facilitate oogenesis
some of the wild-type recipient female mice were given a single intraperitoneal
injection of TSA 6 weeks after surgery [15,16]. Twenty-four hours later, the
grafted ovaries were removed and serially-sectioned for analysis. The remaining
half of each aged TgOG2 ovary not transplanted was fixed immediately and
processed for pre-grafting GFP expression analysis.
Data
presentation and analysis.
All experiments were independently replicated at least
3 times (see figure legends for details), using different mice in each
experiment. Where appropriate, assignment of mice to each experimental group
was made randomly. Quantitative data from experimental replicates were pooled
and presented as the mean ± SEM. Representative outcomes from the RT-PCR and
immunodetection analyses are provided for qualitative assessment.
Acknowledgments
We
thank D.C. Page for testes from Stra8 gene knockout mice, J.R. Mann and
K.J. MacLaughlin for TgOG2 transgenic mice, and A. Wagers for teaching us how
to perform parabiosis in mice. This work was supported by NIH MERIT Award
R37-AG012279, the Rubin Shulsky Philanthropic Fund, the Henry and Vivian
Rosenberg Philanthropic Fund, the Sea Breeze Foundation, and Vincent Memorial
Research Funds.
Conflicts of Interest
The
authors of this manuscript have no conflict of interests to declare.
References
-
1.
Buckler
H
The menopause transition: endocrine changes and clinical symptoms.
J Br Menopause Soc.
2005;
11:
61
-65.
[PubMed]
.
-
2.
Hirshfield
AN
Development of follicles in the mammalian ovary.
Int Rev Cytol.
1991;
124:
43
-101.
[PubMed]
.
-
3.
Zuckerman
S
The number of oocytes in the mature ovary.
Recent Prog Horm Res.
1951;
6:
63
-109.
.
-
4.
Tilly
JL
, Niikura
Y
and Rueda
BR.
The current status of evidence forand against postnatal oogenesis in mammals: a case of ovarianoptimism versus pessimism.
Biol Reprod.
2009;
80:
2
-12.
[PubMed]
.
-
5.
Zou
K
, Yuan
Z
, Yang
Z
, Luo
H
, Sun
K
, Zhou
L
, Xiang
J
, Shi
L
, Yu
Q
, Zhang
Y
, Hou
R
and Wu
J.
Production of offspring from a germline stem cell line derived from neonatal ovaries.
Nat Cell Biol.
2009;
11:
631
-636.
[PubMed]
.
-
6.
Tilly
JL
and Telfer
EE.
Purification of germline stem cells from adult mammalian ovaries: a step closer towards control of the female biological clock.
Mol Hum Reprod.
2009;
15:
393
-398.
[PubMed]
.
-
7.
Torella
D
, Rota
M
, Nurzynska
D
, Musso
E
, Monsen
A
, Shiraishi
I
, Zias
E
, Walsh
K
, Rosenzweig
A
, Sussman
MA
, Urbanek
K
, Nadal-Ginard
B
, Kajstura
J
, Anversa
P
and Leri
A.
Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression.
Circ Res.
2004;
94:
514
-524.
[PubMed]
.
-
8.
Janzen
V
, Forkert
R
, Fleming
HE
, Saito
Y
, Waring
MT
, Dombkowski
DM
, Cheng
T
, DePinho
RA
, Sharpless
NE
and Scadden
DT.
Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a.
Nature.
2006;
443:
421
-426.
[PubMed]
.
-
9.
Molofsky
AV
, Slutsky
SG
, Joseph
NM
, He
S
, Pardal
R
, Krishnamurthy
J
, Sharpless
NE
and Morrison
SJ.
Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing.
Nature.
2006;
443:
448
-452.
[PubMed]
.
-
10.
Lee
HJ
, Sakamoto
H
, Luo
H
, Skaznik-Wikiel
ME
, Friel
AM
, Niikura
T
, Tilly
JC
, Niikura
Y
, Klein
R
, Styer
AK
, Zukerberg
LR
, Tilly
JL
and Rueda
BR.
Loss of CABLES1, a cyclin-dependent kinase-interacting protein that inhibits cell cycle progression, results in germline expansion at the expense of oocyte quality in adult female mice.
Cell Cycle.
2007;
6:
2678
-2684.
[PubMed]
.
-
11.
Virant-Klun
I
, Zech
N
, Rožman
P
, Vogler
A
, Cvjetičanin
B
, Klemenc
P
, Maličev
E
and Meden-Vrtovec
H.
Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes.
Differentiation.
2008;
76:
843
-856.
[PubMed]
.
-
12.
Virant-Klun
I
, Rožman
P
, Cvjetičanin
B
, Vrtacnik-Bokal
E
, Novakovic
S
and Ruelicke
T.
Parthenogenetic embryo-like structures in the human ovarian surface epithelium cell culture in postmenopausal women with no naturally present follicles and oocytes.
Stem Cells Dev.
2009;
18:
137
-150.
[PubMed]
.
-
13.
Johnson
J
, Canning
J
, Kaneko
T
, Pru
JK
and Tilly
JL.
Germline stem cells and follicular renewal in the postnatal mammalian ovary.
Nature.
2004;
428:
145
-150.
[PubMed]
.
-
14.
Baltus
AE
, Menke
DB
, Hu
YC
, Goodheart
ML
, Carpenter
AE
, de Rooij
DG
and Page
DC.
In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication.
Nat Genet.
2006;
38:
1430
-1434.
[PubMed]
.
-
15.
Johnson
J
, Bagley
J
, Skaznik-Wikiel
M
, Lee
HJ
, Adams
GB
, Niikura
Y
, Tschudy
KS
, Tilly
JC
, Cortes
ML
, Forkert
R
, Spitzer
T
, Iacomini
J
, Scadden
DT
and Tilly
JL.
Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood.
Cell.
2005;
122:
303
-315.
[PubMed]
.
-
16.
Wang
N
and Tilly
JL.
Epigenetic status determines germ cell meiotic commitment in embryonic and postnatal mammalian gonads.
Cell Cycle.
2010;
In press
.
-
17.
Rajkovic
A
, Pangas
SA
, Ballow
D
, Suzumori
N
and Matzuk
MM.
NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression.
Science.
2004;
305:
1157
-1159.
[PubMed]
.
-
18.
Powell
K
Going against the grain.
PLoS Biol.
2007;
5:
e338
[PubMed]
.
-
19.
Normile
D
Study suggests a renewable source of eggs and stirs more controversy.
Science.
2009;
324:
320
[PubMed]
.
-
20.
Bowles
J
and Koopman
P.
Retinoic acid, meiosis and germ cell fate in mammals.
Development.
2007;
134:
3401
-3411.
[PubMed]
.
-
21.
Allen
E
Ovogenesis during sexual maturity.
Am J Anat.
1923;
31:
439
-482.
.
-
22.
Chandley
AC
, Hotta
Y
and Stern
H.
Biochemical analysis of meiosis in the male mouse. I. Separation of DNA labeling of specific spermatogenic stages.
Chromosoma.
1977;
62:
243
-253.
[PubMed]
.
-
23.
Ryu
BY
, Orwig
KE
, Oatley
JM
, Avarbock
MR
and Brinster
RL.
Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal.
Stem Cells.
2006;
24:
1505
-1511.
[PubMed]
.
-
24.
Zhang
X
, Ebata
KT
, Robaire
B
and Nagano
MC.
Aging of male germ line stem cells in mice.
Biol Reprod.
2006;
74:
119
-124.
[PubMed]
.
-
25.
Yeom
YI
, Fuhrmann
G
, Ovitt
CE
, Brehm
A
, Ohbo
K
, Gross
M
, Hübner
K
and Schöler
HR.
Germline regulator element of Oct-4 specific for the totipotent cycle of embryonal cells.
Development.
1996;
122:
881
-894.
[PubMed]
.
-
26.
Yoshimizu
T
, Sugiyama
N
, De
Felice M
, Yeom
YI
, Ohbo
K
, Masuko
K
, Obinata
M
, Abe
K
, Schöler
HR
and Matsui
Y.
Germline-specific expression of the Oct-4/green fluorescent protein (GFP) transgene in mice.
Dev Growth Differ.
1999;
41:
675
-684.
[PubMed]
.
-
27.
Szabó
PE
, Hübner
K
, Schöler
H
and Mann
J R.
Allele-specific expression of imprinted genes in mouse migratory primordial germ cells.
Mech Dev.
2002;
115:
157
-160.
[PubMed]
.
-
28.
Lee
HJ
, Selesniemi
K
, Niikura
Y
, Niikura
T
, Klein
R
, Dombkowski
DM
and Tilly
JL.
Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure.
J Clin Oncol.
2007;
25:
3198
-3204.
[PubMed]
.
-
29.
Jones
EC
and Krohn
PL.
The relationships between age, numbers of oocytes and fertility in virgin and multiparous mice.
J Endocrinol.
1961;
21:
469
-495.
[PubMed]
.
-
30.
Skaznik-Wikiel
M
, Tilly
JC
, Lee
HJ
, Niikura
Y
, Kaneko-Tarui
T
, Johnson
J
and Tilly
JL.
Serious doubts over "Eggs forever?".
Differentiation.
2007;
75:
93
-99.
[PubMed]
.
-
31.
Matikainen
T
, Perez
GI
, Jurisicova
A
, Pru
JK
, Schlezinger
JJ
, Ryu
HY
, Laine
J
, Sakai
T
, Korsmeyer
SJ
, Casper
RF
, Sherr
DH
and Tilly
JL.
Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals.
Nat Genet.
2001;
28:
355
-360.
[PubMed]
.
-
32.
Eggan
K
, Jurga
S
, Gosden
R
, Min
IM
and Wagers
AJ.
Ovulated oocytes in adult mice derive from non-circulating germ cells.
Nature.
2006;
441:
1109
-1114.
[PubMed]
.