Abstract
Oxidative and hypertrophic stresses contribute to the pathogenesis of heart failure. Insulin-like growth factor-1 (IGF-1) is a peptide hormone with a complex post-transcriptional regulation, generating distinct isoforms. Locally acting IGF-1 isoform (mIGF-1) helps the heart to recover from toxic injury and from infarct. In the murine heart, moderate overexpression of the NAD+-dependent deacetylase SirT1 was reported to mitigate oxidative stress. SirT1 is known to promote lifespan extension and to protect from metabolic challenges. Circulating IGF-1 and SirT1 play antagonizing biological roles and share molecular targets in the heart, in turn affecting cardiomyocyte physiology. However, how different IGF-1 isoforms may impact SirT1 and affect cardiomyocyte function is unknown. Here we show that locally acting mIGF-1 increases SirT1 expression/activity, whereas circulating IGF-1 isoform does not affect it, in cultured HL-1 and neonatal cardiomyocytes. mIGF-1-induced SirT1 activity exerts protection against angiotensin II (Ang II)-triggered hypertrophy and against paraquat (PQ) and Ang II-induced oxidative stress. Conversely, circulating IGF-1 triggered itself oxidative stress and cardiomyocyte hypertrophy. Interestingly, potent cardio-protective genes (adiponectin, UCP-1 and MT-2) were increased specifically in mIGF-1-overexpressing cardiomyocytes, in a SirT1-dependent fashion. Thus, mIGF-1 protects cardiomyocytes from oxidative and hypertrophic stresses via SirT1 activity, and may represent a promising cardiac therapeutic.
Introduction
In
response to age-associated stresses or dysfunction such as pressure/volume
overload, myocardial infarction and cardiomyopathies, the heart undergoes
adaptation processes that lead to pathological hypertrophy [1]. One of the main
causes of cardiac dysfunction and cardiomyocytes loss is an imbalance between
the generation of reactive oxygen species (ROS) and the antioxidant defenses in
favor of the former [2]. Growing evidence demonstrate that oxidative stress and
hypertrophy are mechanistically linked to each other in the heart [3,4].
Several therapeutic strategies are now employed to counteract the deleterious
effects of cardiac hypertrophy and oxidative stress, making therefore the
analysis of specific cell signaling imperative to generate novel drugs. In this
scenario, the insulin like growth factor-1 (IGF-1) and Sirtuin -1 are novel
important mediators of cell survival, oxidative stress, regeneration, and
life-span regulation [5] in several tissues including the heart.
IGF-1 is a peptide hormone acting as a
growth and differentiation factor [6]. The pleiotropic functions of IGF-1 are
reflected in the intricate structure of the gene encoding it. The IGF-1 gene
spans more than 70 kb, contains two promoters and has six exons, giving rise to
multiple splicing variants. These splice variants all consist of the same
unvarying core flanked by varying termini. IGF-1 isoforms are classified
according to the N-terminal signal peptide (class 1 and 2) and to the
C-terminal extension peptides, Ea and Eb [6]. It is established that IGF-1 is
both a systemic growth factor produced primarily by liver and a local growth
factor functioning in an autocrine/paracrine manner in tissues such as heart
and skeletal muscle [6]. Post-transcriptionally, IGF-1 isoforms are cleaved to
give a mature 70 amino acid core hormone (identical for all isoforms) devoid of
both the signal peptide and the extension peptide. This mature hormone is
released into the bloodstream and has been implicated in the restriction of
life span [7]. Correspondingly, high levels of circulating IGF1 are associated
with increased mortality and cardiovascular diseases in the elderly [8]. When
expressed as transgenes in the cardiomyocytes, distinct IGF-1 isoforms result
in diverse phenotypes, ranging from protection from hypertrophy to its
exacerbation towards pathological states [6,9-11]. The role of IGF-1 in
cardiac oxidative stress is also debated: cardiomyocyte-specific IGF-1 overexpression
has been reported to protect from angiotensin II (Ang II)-mediated oxidative
stress [12] but, on the contrary, severe circulating IGF-1 deficiency, as in
hepatocyte-specific IGF-1 knock-out mice, antagonizes oxidative stress and cell
death in cardiomyocytes triggered by the potent oxidant agent paraquat (PQ)
[13].
The
mIGF-1 isoform comprises a Class 1 signal peptide and a C-terminal Ea extension
peptide [6]. It is highly expressed in neonatal tissues and in the adult liver,
but decreases during aging in extra-hepatic tissues, where its expression is
activated transiently in response to local damage [14]. Previous studies from
our laboratory showed that continuous expression of mIGF-1 throughout postnatal
life did not produce significant perturbations in normal heart physiology and,
in contrast to previous studies with other IGF-1 transgenes. [11] did not
progress to a pathological phenotype [15]. In response to injury however,
molecular analysis revealed that mIGF-1 curtails the inflammatory response,
enhances antioxidative cell-defense by upregulation of adiponectin, uncoupling
protein 1 (UCP1) and methallothionein 2 (MT-2), and induces cardiac tissue
restoration by increasing the number of proliferative cells at the border zone
of the infarcted heart [15]. Given the benefits of cardiac restricted mIGF-1
expression, we sought to elucidate the molecular targets of this isoform.
Sirtuin
1 (SirT1) belongs to the sirtuin family of nicotinamide adenine dinucleotide
NAD-dependent protein deacetylases, whose activation is considered beneficial
for metabolic, neurodegenerative and inflammatory diseases and to augment
longevity [5]. Moderate SirT1 activation in murine heart has been shown to
protect from oxidative stress and angiotensin II (Ang II)-induced cell death
[16,17]. Intriguingly, SirT1 expression is increased in the hypertrophic heart
of rodents and monkeys [16,18], although its functional relevance is unclear.
IGF-1 and SirT1 share downstream targets in cardiomyocytes, and this in turn may
affect cardiovascular function [19]. It has been reported that SirT1 is also
activated by the polyphenol resveratrol and by caloric restriction [20],
whereas its induction is counteracted by circulating IGF-1 [20]. Moreover, the
levels of circulating IGF-1 are lowered upon caloric restriction [21]. Hence,
SirT1 and IGF-1 apparently play opposite biological roles, although there is no
information on the impact of separate IGF-1 isoforms, acting locally or
systemically, on cardiac SirT1. In particular, in this study we sought to test
if the liver-produced and fully processed IGF-1 core protein isoform,
circulating in the blood stream, and the locally acting mIGF-1 isoform [14],
could display distinct effects in the protection from hypertrophic and
oxidative stress. We tested this in mouse HL-1 and primary neonatal
cardiomyocytes, using Ang II and PQ as hypertrophic and oxidative stressors.
We found that SirT1 and mIGF-1 co-regulate cardiomyocyte
survival and protection from damage. mIGF-1 overexpression protects HL-1
cardiac cells and neonatal mouse cardiomyocytes from the deleterious effects
induced by hypertrophic (Ang II) and oxidative (PQ) stressors in a
SirT1-dependent fashion. The beneficial activity of SirT1 is mediated by the
activation of protective molecules such as UCP1, adiponectin and MT2 and is
dependent on mIGF-1 expression. Interestingly, the circulating IGF-1 isoform
does not regulate SirT1 expression and activity and it is not beneficial during
hypertrophy and oxidative stress conditions. The in vitro system herein
described uncovers a novel signaling cross-talk that suggests potential
pharmacological targets to modulate cardiac protection.
Results
mIGF-1
increases SirT1 expression and catalytic activity in mouse cardiomyocytes
It has been reported
that SirT1 and IGF-1 share common downstream targets in cardiomyocytes [19], but antagonize each other's
activity [20,21] by mechanisms so far unexplored.
To elucidate the molecular interplay between the two molecules in cardiac
tissue, we examined if SirT1 expression is affected in the heart of mice
overexpressing the locally acting mIGF-1 isoform [15]. Analysis of nuclear extracts
prepared from whole heart lysates of mIGF-1 transgenic (Tg) and wild type (WT)
mice revealed increased SirT1 protein levels in mIGF-1 Tg mouse hearts compared
to wild type littermates (Figure 1A). To correlate the overexpression of SirT1
in Tg hearts with its deacetylase activity, we analysed the deacetylation
levels of the SirT1 targets, p53 [25] and histone H1 [26]. The increase in SirT1 expression
mediated by mIGF-1 correlated functionally with histone H1 and p53
deacetylation at Lys26 and Lys382 respectively (Figure 1A). To confirm a direct
effect of mIGF-1 on SirT1 expression, we overexpressed mouse mIGF-1 in HL-1
cardiomyocytes [22]. mIGF-1 was detected in the cell
medium already 24 hours after transient transfection (Figure 1B), and
correlated with increased SirT1 expression (Figure 1C). Interestingly, treatment
with 20 ng/ml of the circulating IGF-1, although induced comparable activation
of the IGF-1 receptor to that seen with transfected mIGF-1 (Figure 1D), and
moderately decreased SirT1 expression (Figure 1C), indicating that mIGF-1
activates differential downstream signaling compared to the circulating
peptide. Consistently with the results observed in whole heart lysates from mIGF-1
Tg mice, mIGF-1 overexpression in HL-1 cells promoted decreased deacetylation
of H1 and p53 at critical lysine residues (Figure 1C), whereas treatment with
circulating IGF-1 induced a moderate upregulation of acetylation levels of both
SirT1 targets. Unmodified acetylation levels of p53 and histone H1 were
observed in cells overexpressing a catalytic inactive SirT1 protein (H363Y) [20] (Figure 1C). These findings
demonstrate that the locally acting mIGF-1 isoform, but not the circulating
form, enhances SirT1 expression and activity in cardiomyocytes.
mIGF-1/SirT1
pathway inhibits Ang II-induced hypertrophic fetal gene expression program
Circulating IGF-1 and SirT1 have both been implicated
in the protection against cardiac hypertrophy [10,17],
although the role of IGF-1 and/or its isoforms in this process remains
controversial [6,9-11]. A
molecular hallmark of the progression to cardiac hypertrophy towards heart
failure is the re-activation of the ‘fetal' gene program in cardiomyocytes [27]. This
process involves an upregulation of genes encoding atrial and brain natriuretic
peptides (ANP and BNP), as well as fetal contractile protein isoforms such as α-myosin heavy chain 7 (MYH7) and α-skeletal actin
(ACTA-1) is observed. In parallel, cardiac hypertrophy correlates with
downregulation of adult α-myosin heavy chain (MYH6) and sarco/endoplasmic
reticulum calcium ATPase-2 (SERCA2) [27]. Since Ang
II is a potent hypertrophic agonist in cardiomyocyte, inducing re-activation of
the fetal gene program [28], we
investigated the role of mIGF-1-induced SirT1 expression on the Ang
II-dependent fetal gene activation in two different in vitro models: in
the HL1 cell line, resembling adult cardiomyocytes, and in mouse WT and Tg
neonatal cardiomyocytes.
When HL-1 cardiomyocytes were exposed for 24 hours to
Ang II at 1 μM, a supra-physiological but fairly used concentration
to elicit its signaling and hypertrophic effects in cardiomyocytes studies [29,30], the
hormone triggered an increase in the mRNA levels of
BNP (262±14%), ANP (265±6%), ACTA1 (189±10%) and MYH7 (164±9%) when compared to
untreated cells (CTL), and a decrease in SERCA2 (30±9%) and MYH6 (50±4%)
transcript levels when compared to CTL cells (Figure 2A). Over-expression of
locally acting mIGF-1 or SirT1, but not the catalytic mutant SirT1 H363Y, fully
prevented the activation of Ang II-induced fetal gene program (Figure 2A).
Importantly, overexpression of mIGF-1 and SirT1 H363Y together did not block
the changes in gene expression induced by Ang II, indicating that mIGF-1
protective effect is SirT1-dependent (Figure 2A). In contrast, exposure of HL-1
cells to the circulating form of IGF-1, similarly to Ang II, triggered to some
extent the activation of the fetal gene program (Figure 2A).
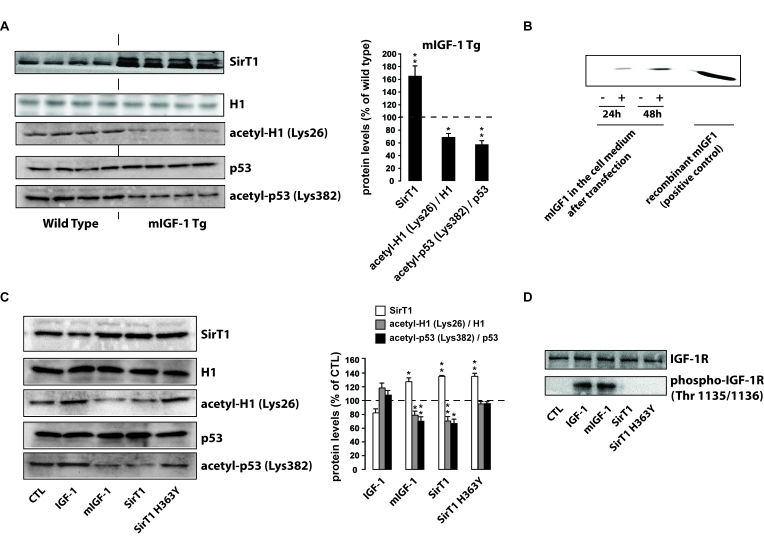
Figure 1. mIGF-1, but not IGF-1, increases SirT1 expression and activity in mouse cardiomyocytes. (A) Left
panel: representative Western blots of SirT1, histone H1, acetyl-H1
(Lys26), detected in nuclear extracts, and of p53 and acetyl-p53 (Lys382),
detected in whole tissue lysates, from wild type and mIGF-1 Tg mice. Four
animals of a total of 10 are shown; right panel: densitometric
quantification of SirT1, acetyl-H1(Lys26)/H1 and acetyl-p53(Lys382)/p53
levels in cardiomyocytes from mIGF-1 mice, expressed as % of those in wild
type cardiomyocytes. (B) representative Western Blot of mIGF-1
detected in the extracellular medium of HL-1 cardiomyocytes, transfected
with a plasmid carrying mouse mIGF-1 cDNA. (C) Left panel:
representative Western Blot of SirT1, histone H1, acetyl-H1 (Lys26)
detected in nuclear extracts, and of p53 and acetyl-p53 (Lys382) detected
in whole cell lysates, from HL-1 cardiomyocytes transfected with the
indicated constructs (SirT1 or SirT1 H363Y) or treated with 20 ng/ml IGF-1
for 24 hours; right panel: densitometric quantification of SirT1,
acetyl-H1(Lys26)/H1 and acetyl-p53(Lys382)/p53 levels in transfected or
treated cells, expressed as % of control (CTL). (D) Representative
Western blots of IGF-1 receptor (IGF-1R) or phospho-IGF-1R (on Thr
1135/1136) in HL-1 cardiomyocytes lysates. Results in (A) and (B)
are means ± SE of 3
independent experiments (**,***p versus unstimulated
control cells or untreated WT cardiomyocytes).
In a second in vitro model, 2
day-old WT and Tg hearts were excised and cardiomyocytes extracted. Cultured
cells were exposed for 24 hours to 1 μM Ang II and fetal
gene activation analysed by quantitative real-time PCR (qRT-PCR). As expected
Ang II treatment increased BNP (276±7%), ANP (306±27%), ACTA (178±15%) and MYH7
(161±16) transcript levels compared to untreated WT cells (Figure 2B). We observed
in parallel a decrease in SERCA2 (53±6%) and MYH6 (61±4%) mRNA levels compared
to WT untreated cardiomyocytes (Figure 2B). As in the HL-1 cell system, over-expression of locally acting
mIGF-1 fully
prevented the activation of the fetal gene program induced by Ang II (Figure 2B), indicating that mIGF-1 activates antagonist signaling to hypertrophy
during the early stages of cardiac development. These data correlates with
previous analyses in our laboratory. Although mIGF-1 is known to induce a
moderate physiological overgrowth in adult hearts [15], neonatal
mIGF-1 expressing hearts do not present increased ANP, BNP and ACTA-1
transcript levels (data not shown). Interestingly, treatment of wild
type cardiomyocytes with the circulating IGF-1 induced activation of
fetal-like gene expression pattern (Figure 2B).
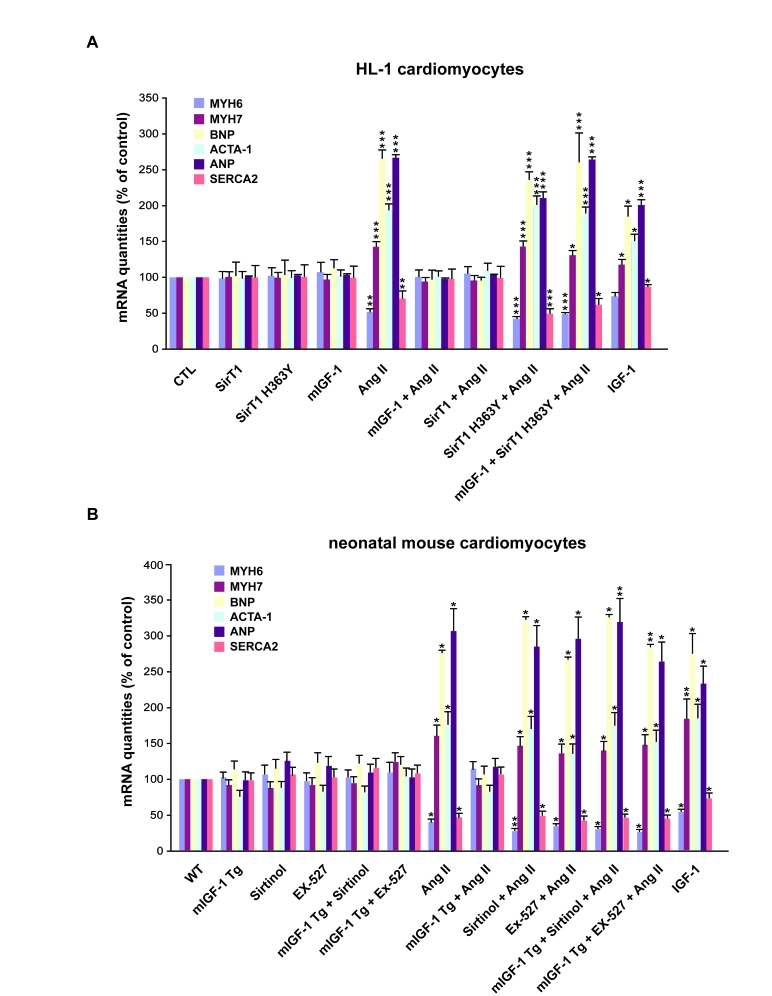
Figure 2. mIGF-1 prevents Ang II- and IGF-1-induced fetal gene program activation. (A) HL-1
cardiomyocytes were transfected with the indicated plasmids, or treated
with 20 ng/ml IGF-1 for 24 h, before exposure to Ang II (1 μM for 24 h).
Untransfected cells were used as control (CTL). (B) Neonatal mouse
cardiomyocytes from wild type (WT) or heart overexpressing mIGF-1 mice
(mIGF-1 Tg) were pre-incubated with sirtinol (100 μM) or EX-527 (1 μM), or
treated with 20 ng/ml IGF-1 for 24 h, prior to exposure to Ang II (1 μM for
24 h). Untreated WT cardiomyocytes were used as control. (A, B) The
expression levels of MYH6, MYH7, BNP, ACTA-1, ANP and SERCA2 mRNAs were
examined by qRT-PCR. Results are means ±
SE of 3 independent experiments (*,**,***p versus unstimulated
control cells).
To examine if the protective effects of mIGF-1 against
Ang II-induced fetal gene program in neonatal cardiomyocytes were dependent on
SirT1, WT and mIGF-1 Tg cardiomyocytes were treated with two SirT1 pharmacological
inhibitors, pan-sirtuin inhibitor (sirtinol, 100 μM) or a SirT1 specific inhibitor (EX-527, 1 μM) [31], prior to exposure to Ang II.
At these concentrations, both compounds did not affect cardiomyocyte viability and
blocked SirT1 activity, as assessed by increased acetylation levels of its downstream targets p53 (Lys382) and H1 (Lys26) (data not shown). Upon SirT1 blockade, Ang II
treatment induced a significant increase in fetal-like genes in both wild type
and mIGF-1 Tg car-diomyocytes, overcoming mIGF-1 protection (Figure 2B).
Taken
together, these data support the concept that the locally acting mIGF-1
isoform, but not the circulating liver-produced IGF-1, counteracts the
activation of the hypertrophic fetal gene program induced by Ang II in a
SirT1-dependent manner.
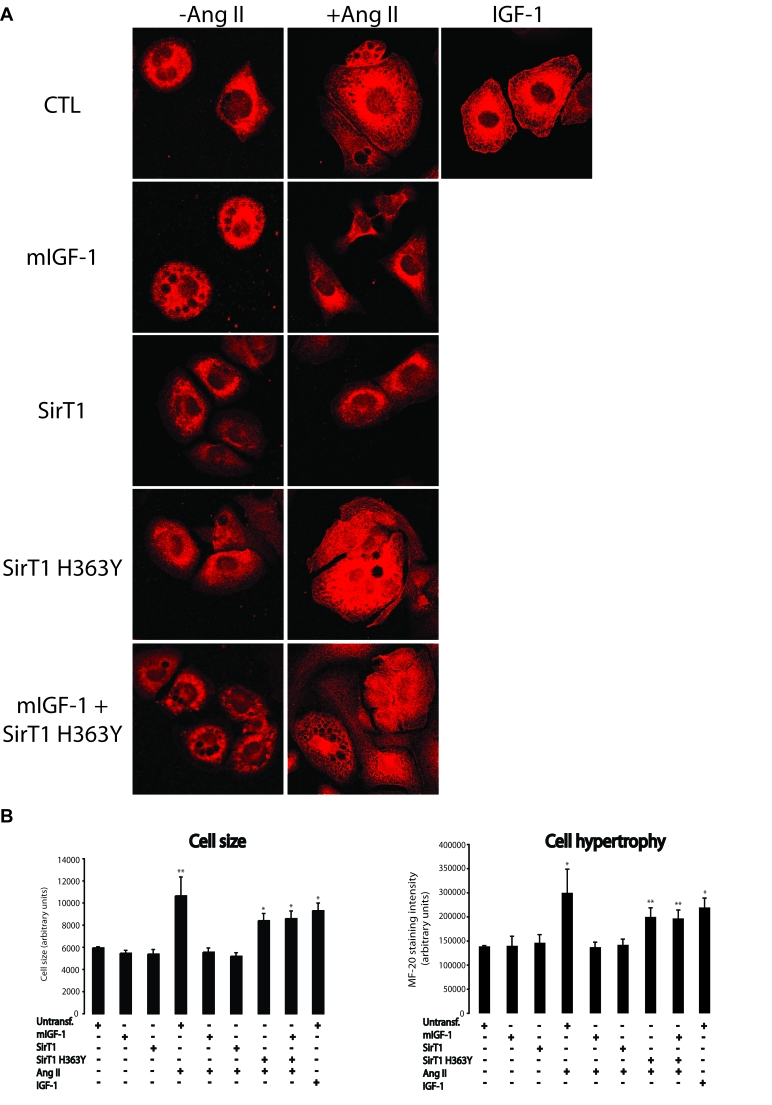
Figure 3. mIGF-1 prevents Ang II- and IGF-1-induced cell hypertrophy (MF-20 staining) in HL-1 cardiomyocytes. (A) HL-1 cardiomyocytes were
transfected or treated as in Legend of Figure 2A. Sarcomeric myosin was
stained with MF-20 antibody and images were acquired using a Leica confocal
microscope. (B) Cell size and cell hypertrophy quantified according
to MF-20 staining in HL-1 cardiomyocytes in the different experimental
conditions as in as in Legend of Figure 2A. Results are means ± SE of 3 independent experiments
(*,**p versus unstimulated
control cells). Bar: 25 μM.
mIGF-1/SirT1 pathway rescues cell hypertrophy
triggered by Ang II or IGF-1
Cardiomyocyte hypertrophy is typically characterized by
cell enlargement and increase in total sarcomeric myosin heavy chain. Here, we
sought to determine the impact of mIGF-1-induced SirT1 expression on cell
hypertrophy response by two complementary approaches, measure-ment of MF-20 (a
monoclonal antibody staining sarcomeric myosin heavy chain) immunoreactivity,
and radioactive [3H]-leucine incorporation into cellular proteins. Exposure of
HL-1 cardiomyocytes to Ang II led to an increase in cell size as assessed by
MF-20 staining intensity (Figure 3A and B). Surprisingly, about 30% of total HL-1 cells died when treated with this hormone (see
Figure 8), indicating that Ang II is both a pro-apoptotic and pro-hypertrophic
agonist at 1 μM concentration. When mIGF-1 or SirT1 were overexpressed in HL-1
cells, a full blockade of cell size increase induced by Ang II was observed
(Figure 3A and B), whereas the catalytic inactive SirT1 H363Y was unable to
prevent Ang II-triggered cell hypertrophy (Figure 3A and 3B). Interestingly,
the circulating IGF-1 isoform led to HL-1 cell hypertrophy (Figure 3A and 3B).
Similar results were obtained with [3H]-leucine incorporation experiments
(Figure 4A), confirming that mIGF-1 induced SirT1 activity prevents Ang II- and
IGF-1-induced cell hypertrophy in HL-1 cardiomyocytes.
![mIGF-1 prevents Ang II- and IGF-1-induced cell hypertrophy ([3H]-leucine incorporation) in HL-1 cardiomyocytes and in mouse neonatal primary cardiomyocytes](/article/100107/figure/F4/large)
Figure 4. mIGF-1 prevents Ang II- and IGF-1-induced cell hypertrophy ([3H]-leucine incorporation) in HL-1 cardiomyocytes and in mouse neonatal primary cardiomyocytes. (A) HL-1 cardiomyocytes were
transfected with the indicated plasmids, or treated with 20 ng/ml IGF-1 for
24 h, or exposed to Ang II (1 μM
for 24 h). Untransfected cells were used as control (CTL).
Together with Ang II, HL-1 cells were also incubated with 1μCi/ml of [3H]-labeled
leucine (24 h). (B)
Neonatal primary cardiomyocytes from wild type or mIGF-1 Tg mice were
treated with SirT1 inhibitors (sirtinol, 100 μM; EX-527, 1 μM),
or treated with 20 ng/ml IGF-1 for 24 h, or exposed to Ang II (1 μM for 24 h); concomitantly to Ang
II addition, cells were incubated with 1mCi/ml of [3H]-labeled
leucine (24
h).
(A, B) [3H]-leucine incorporation values were normalized to total
protein content and expressed as % of control. Results are means ± SE of 3 independent experiments
(**,***p versus unstimulated
control cells or untreated WT cardiomyocytes).
The effect of SirT1 in mIGF-1-dependent protection from
cell hypertrophy was investigated as well in neonatal primary cardiomyocytes
(Figure 5A-C and Figure 4B). mIGF-1 Tg cardiomyocytes were unresponsive to Ang
II-induced cell hypertrophy as indicated by MF-20 staining (Figure 5A and 5B),
while blocking SirT1 activity with sirtinol or EX-527 restored Ang II-induced
hypertrophy (Figure 5A and 5B), indicating that mIGF-1 inhibitory effect on Ang II- induced cell hypertrophy is
dependent on -SirT1 activity also in primary cardiomyocytes. In
addition, exposure of wild type neonatal cardiomyocytes to the circulating form
of IGF-1, triggered cell hypertrophy (Figure 5A and 5B). Similar findings were
observed with [3H]-leucine incorporation experiments (Figure 4B). Therefore,
using two different experimental approaches, we found that SirT1 activity
induced by mIGF-1, but not by liver-produced IGF-1 isoform, displays
antihypertrophic effects in mouse cardiomyocytes.
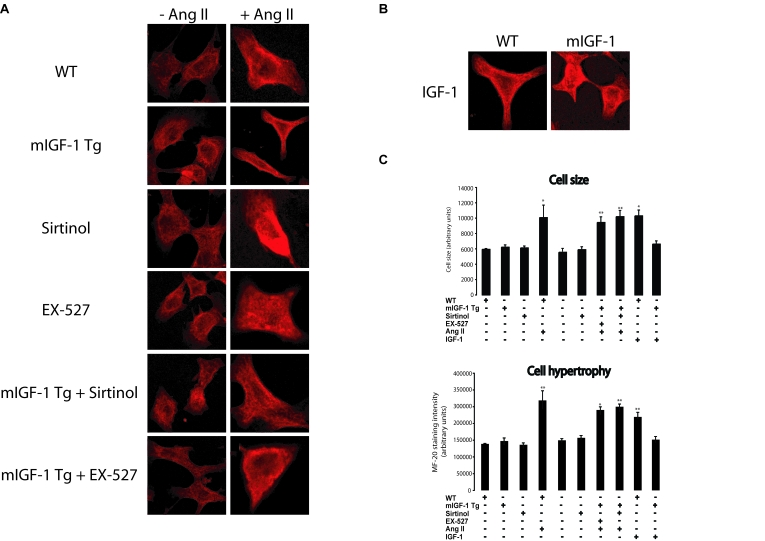
Figure 5. mIGF-1 prevents Ang II- and IGF-1-induced cell hypertrophy (MF-20 staining) in mouse neonatal primary cardiomyocytes. (A)
Neonatal primary cardiomyocytes from wild type or mIGF-1 Tg mice were
treated as in Legend of Figure 2B. (B) Cell size and hypertrophy
were quantified according to MF-20 staining in the different experimental
conditions as in as in Legend of Figure 2B. Results are means ± SE of 3 independent experiments
(*,**p versus unstimulated
control cells). Bar: 25 μM.
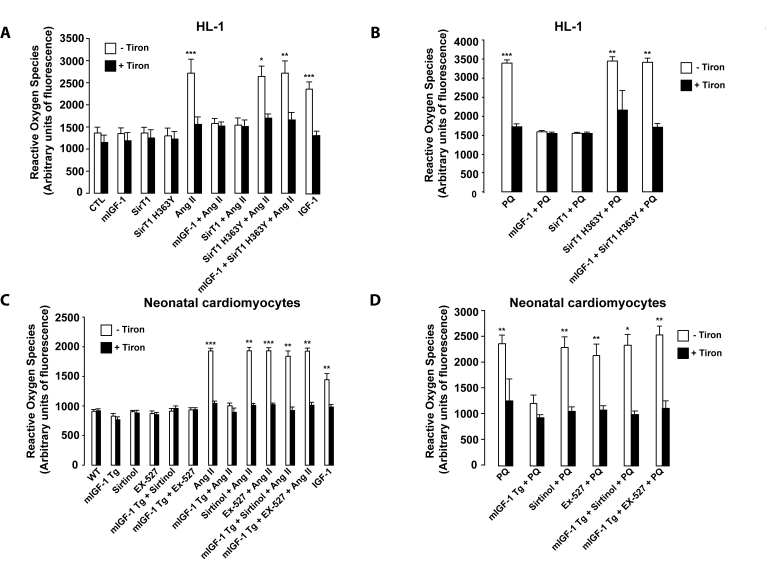
Figure 6. mIGF-1 prevents Ang II-, PQ- and IGF-1-induced increase in reactive oxygen species (ROS) generation in HL-1 cardiomyocytes and in mouse neonatal primary cardiomyocytes. (A,
B) HL-1 cardiomyocytes were transfected or treated as in Legend of
Figure 2A, except that Ang II (1 μM)
or PQ (100 μM) were added
for only 60 min. Untransfected cells were used as control (CTL). (C, D) Neonatal
primary cardiomyocytes from wild type or mIGF-1 Tg mice were treated as in
Legend of Figure 2A, except that Ang II (1 μM) or PQ (100 μM)
were added for only 60 min. (A-D) ROS production was monitored with
the fluorescent probe dichlorofluorescein diacetate (CM-DCFDA) and
fluorescence values were normalized to protein content. Results are means ± SE of 3 independent experiments
(*,**,***p versus unstimulated
control cells or untreated WT cardiomyocytes).
mIGF-1/SirT1
pathway prevents reactive oxygen species (ROS) generation, peroxidation
products and cell death triggered by oxidative stressors
ROS generation and oxidative stress
contribute to the progression of pathological
cardiac hypertrophy and heart failure. Indeed, oxidative stress and
hypertrophy are intimately linked in cardiac muscle[3]. It is increasingly appreciated that the Ang II hypertrophic
effects on cardiomyocytes are strictly dependent on the generation of ROS [32]. IGF-1 also triggers
ROS production, although it is controversial if this
growth factor antagonizes or favors oxidative stress in cardiomyocytes[12,13]. Since SirT1 overexpression has been reported to
protect the murine heart from PQ-induced oxidative stress [16], we
measured ROS content by dichlorofluorescein diacetate (CM-DCFDA) method in
mouse cardiomyocytes to shed light on the impact of IGF-1/SirT1 signaling on
oxidative stress generated by Ang II and by PQ. To this end, HL-1 or neonatal
cardiomyocytes were pretreated with
superoxide anion scavenger Tiron before exposure to Ang II or PQ for 1 hour (Figure 6A-D). In both cardiomyocytes models, Ang II and PQ triggered a significant
augmentation in intracellular ROS compared to untreated control cells, which was fully blocked by Tiron (Figure 6A-B, and
6C-D, for HL-1 and neonatal cardiomyocytes, respectively). mIGF-1 did not
induce ROS production and efficiently prevented ROS generation by Ang II and PQ
in both cardiomyocytes models (Figure 6A-D). Similarly, also SirT1
overexpression reversed ROS production in HL-1 cardiomyocytes (Figure 6A and
B). In addition, blocking SirT1 enzymatic activity, by overexpression of SirT1
H363Y in HL-1 cells or incubation with SirT1 inhibitors in neonatal
cardiomyocytes, abrogated the protective effects of mIGF-1 against Ang II- and
PQ-induced intracellular ROS generation (Figure 6A-D). In contrast to locally
acting mIGF-1 isoform, incubation of cardiomyocytes with the circulating IGF-1
isoform triggered a significant rise in ROS content, however less sustained than that generated by Ang II or by PQ (Figure 6A and C). Taken
together, these data clearly indicate that mIGF-1, but not IGF-1, shields mouse
cardiomyocytes from a rise of intracellular ROS generated by oxidative
stressors. To ascertain if mIGF-1 exerts a cardio-protective role against
oxidative stress as well in vivo, we injected peritoneally wild type and
mIGF-1 Tg mice with PQ, and we assessed lipid and protein peroxidation levels,
normally increasing upon ROS generation in cardiomyocytes [33]. Immunoblot
analyses of lipid peroxidation 4-hydroxy-2-nonenal (4-HNE) and malondialdehyde
(MDA) protein adducts in the heart showed that the levels of both protein
adducts were significantly increased in the heart of PQ-injected wild type
mice, whereas hearts of mIGF-1 Tg mice were to some extent protected from
forming these compounds upon PQ injection (Figure 7). These data indicate that
mIGF-1 protects the murine heart from oxidative stress as well in vivo.
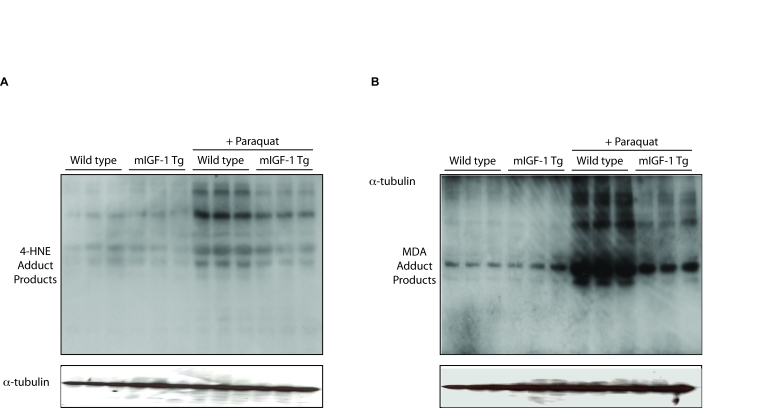
Figure 7. mIGF-1 protects the murine heart from PQ-induced oxidative stress. PQ was injected
intraperitoneally at a concentration of 30 mg/kg, while control animals
were injected with a saline solution. All mice were sacrificed 24 hours
after injections. The figure shows representative Western blots of
4-hydroxy-2-nonenal (4-HNE) adduct products (left panel) and of
malondialdehyde (MDA) adduct products (right panel) from wild type,
mIGF-1 Tg, wild type plus PQ and mIGF-1 mice plus PQ. Three animals of a
total of 10 are shown in both panels A and B.
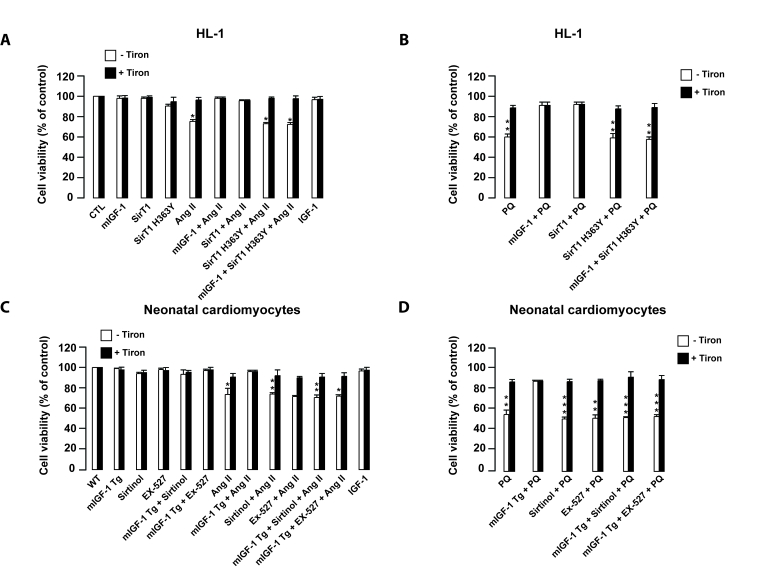
Figure 8. mIGF-1 prevents Ang II- and PQ-dependent cell death in HL-1 cardiomyocytes and in mouse neonatal primary cardiomyocytes. (A, B)
HL-1 cardiomyocytes were transfected or treated as in Legend of Figure 2A. (C, D)
Neonatal primary cardiomyocytes from wild type or mIGF-1 Tg mice were
treated as in Legend of Figure 2B. (A-D) Cell viability was
monitored with propidium iodide (PI) by flow cytometry and values were
normalized to protein content. Results are means ± SE of 3 independent experiments
(*,**,***p versus unstimulated
control cells or untreated wild type cardiomyocytes).
ROS-mediated oxidative stress may lead to
cardiomyocyte cell death [34]. Therefore,
we examined if ROS production induced by Ang II, PQ and IGF-1 contributed to
mouse cardiomyocyte cell necrosis and examined the role of mIGF-1/SirT1
signaling in this process. HL-1 or neonatal mouse cardiomyocytes were preincubated with Tiron before adding Ang II or PQ for
24 hours (Figure 8A-D). Consistently with ROS data, Ang II and PQ induced
necrosis in 30% and 50% of the total cell population respectively, as assessed
by flow cytometry with propidium iodide (PI) (Figure 8A-B, and 8C-D, for HL-1 and neonatal cardiomyocytes, respectively).
mIGF-1 had no effect on cardiomyocyte viability and efficiently prevented Ang II- and
PQ-induced necrosis (Figure 8A-D). Moreover, SirT1 overexpression protected
HL-1 cardiomyocytes from Ang II- and PQ-dependent cell necrosis (Figure 8A and
B). When SirT1 activity was inhibited by sirtinol or by SirT1 H363Y in both HL-1
cells and neonatal cardiomyocytes, no beneficial effect of mIGF-1 to Ang II-
and PQ-induced cell death was observed (Figure 8 A-D). Interestingly, despite
generating intracellular ROS, the circulating IGF-1 isoform did not impact cell
viability (Figure 8A and C). In summary, mIGF-1/SirT1 signaling protects
cardiomyocytes from cell death caused by sustained exposure to oxidative
stressors.
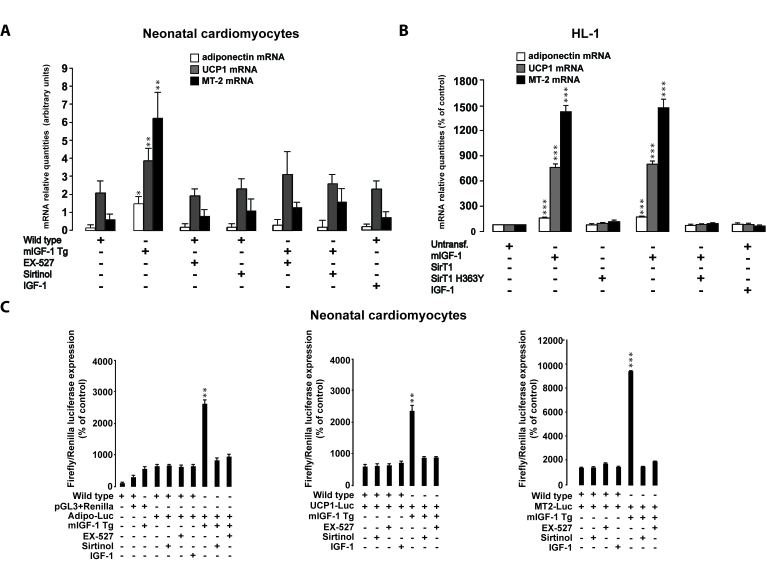
Figure 9. SirT1 is necessary for mIGF-1-dependent upregulation of anti-oxidant and hypertrophic genes adiponectin, UCP1 and MT-2. (A)
Neonatal primary cardiomyocytes from wild type or mIGF-1 Tg mice were
treated with sirtinol (100 μM)
or EX-527 (1 μM), or treated
with 20 ng/ml IGF-1 for 24 h. (B) HL-1 cardiomyocytes were
transfected with the indicated plasmids, or treated with 20 ng/ml IGF-1 for
24 h. Untransfected cells were used as control (CTL). (A, B)
The expression levels of adiponectin, UCP-1 and MT-2 mRNAs were examined by
Real Time-PCR. (C) Neonatal primary cardiomyocytes from wild type or
mIGF-1 Tg mice, and HL-1 cardiomyocytes, were transfected with 1 μg of
plasmids carrying Firefly luciferase under the control of promoters of
adiponectin (Adipo-Luc), UCP1 (UCP1-Luc) and MT-2 (MT-2-Luc) genes,
respectively, together with 1 μg of Renilla Luciferase plasmid. Neonatal
primary cardiomyocytes were also treated with different inhibitors or IGF-1
as described in (A). Dual luciferase assays were performed in
duplicate for each condition. (A-C) Results are means ± SE of 3 independent experiments
(*,**,***p versus untreated
cardiomyocytes).
Activation of cardio-protective genes by mIGF-1/SirT1
Next, we examined if the activation of
cardio-protective mediators/effectors by mIGF-1 is dependent on SirT1
signaling, mining our previous Affymetrix analysis of mRNA transcripts in the
heart of mIGF-1 Tg mice versus wild type littermates [15]. Among the
upregulated transcripts in the heart of mIGF-1 Tg mice, we focused on three
cardio-protective genes whose expression was significantly (2- to 4- fold)
increased: adiponectin, UCP-1 and MT-2 [35-37].
Increased cardiac
expression of UCP1, MT2 and adiponectin mRNA levels was confirmed in mIGF-1
transgenic hearts compared to WT (Figure 9A). In cardiomyocytes from mIGF-1 Tg
mice, inhibition of SirT1 activity lowered adiponectin, UCP-1 and MT-2 mRNAs to
wild type levels, indicating that their upregulation by mIGF-1 is tightly
dependent on SirT1 activity (Figure 9A). On the other hand, exposure of
neonatal cardiomyocytes to circulating IGF-1 did not alter adiponectin, UCP-1
and MT-2 mRNA levels (Figure 9A). Consistently, overexpression of mIGF-1 in
HL-1 cardiomyocytes led to significantly increased mRNA levels of adiponectin,
UCP-1 and MT-2, while IGF-1 had no effect (Figure 9B). SirT1 overexpression in
HL-1 cardiomyocytes also triggered an increase in these mRNAs (Figure 9B).
Conversely, overexpression of SirT1 H363Y did not affect mRNA expression of
these genes and blocked their upregulation by mIGF-1 (Figure 9B).
To elucidate if mIGF-1 could upregulate mRNA levels
through SirT1-dependent promoter activation, we transiently transfected
neonatal cardiomyocytes and HL1 cells with constructs carrying the minimal
promoter region of the three genes, driving the firefly luciferase expression
(see Table 2). The analysis showed that mIGF-1/SirT1 pathway activates the
expression of these genes, as indicated by a substantial increase in
luciferase activity (Figure 9C and Figure 10). Luciferase activation by mIGF-1
was tightly dependent on SirT1 function, since inhibition strategies
(overexpression of SirT1 H363Y in HL-1 cardiomyocytes and SirT1 inhibitors in
neonatal cardiomyocytes) blocked the increase in adiponectin, UCP-1 and MT-2-
promoter-driven luciferase activity (Figure 9C and Figure 10). In contrast to
mIGF-1 isoform, the circulating form of IGF-1 did not alter the promoter
activity of adiponectin, UCP-1 and MT-2 in mouse cardiomyocytes (Figure 9C and Figure 10). We conclude that mIGF-1, but not IGF-1, activates at least some
cardio-protective genes through SirT1-dependent activation of their promoters.
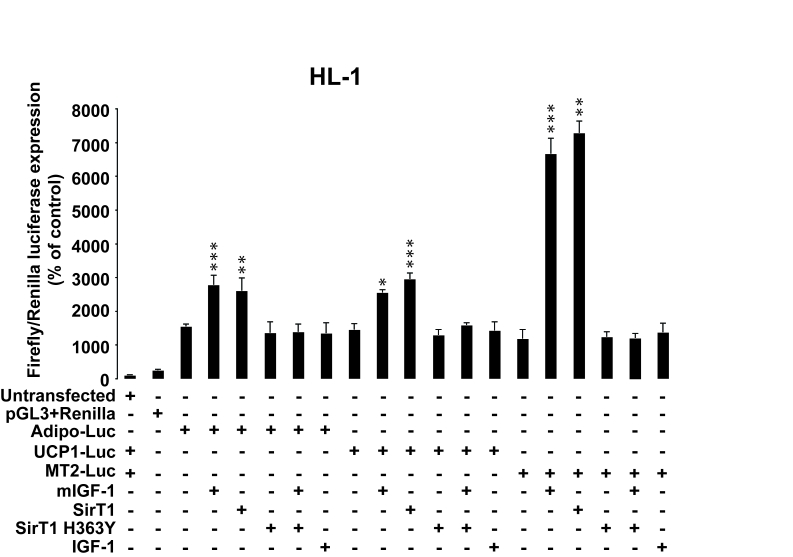
Figure 10. SirT1 is necessary for promoter-dependent mIGF-1-dependent upregulation of anti-oxidant and hypertrophic genes adiponectin, UCP1 and MT-2. HL-1
cardiomyocytes were transfected with the indicated plasmids, and/or treated
with 20 ng/ml IGF-1 for 24 h. HL-1 cardiomyocytes were also co-transfected
with 1 μg of plasmids carrying Firefly luciferase under the control of
promoters of adiponectin (Adipo-Luc), UCP1 (UCP1-Luc) and MT-2 (MT-2-Luc)
genes, respectively, together with 1 μg of Renilla Luciferase plasmid.
Untransfected cells were used as control. Dual luciferase
assays were performed in duplicate for each condition. Results are means ± SE of 3 independent experiments
(*,**,***p versus untransfected/unstimulated
control cells).
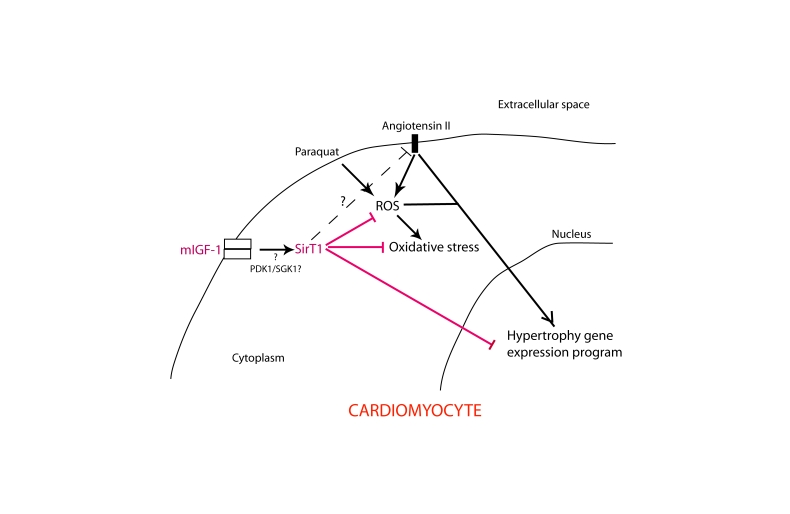
Figure 11. Simplified scheme illustrating the role of mIGF-1-induced SirT1 activity in protection against Ang II- and PQ-mediated oxidative stress and hypertrophy in cardiomyocytes.
Question point and dashed line indicate unanswered issues and hypothetical
signaling cross-talk, respectively.
Discussion
Hypertrophy and oxidative stress are intertwined processes in cardiomyocytes [3,4], contributing to heart disease progression [1,2]. In this study, we have identified a signaling pathway efficiently
protecting mouse cardiomyocytes from oxidative and hypertrophic stresses (Ang
II and PQ) that relies on the activation of NAD-dependent deacetylase SirT1 by
the locally acting mGF-1 isoform. We show that mIGF-1-dependent SirT1
activation reduces ROS levels and cell death triggered by Ang II and PQ, and
prevents Ang II-induced hypertrophic response (Figure 11). Our report is consistent with others showing
that SirT1 display cardio-protective effects against oxidative stress-dependent
cell death [16-18], and
that SirT1 may elicit protection from cell hypertrophy by restoring MYH7
expression [38].
Interestingly, in smooth muscle cells SirT1 inhibits the expression of Ang II
type 1 receptor [39], but if a
similar mechanism occurs in cardiomyocytes remains to be established (Figure 11). We demonstrated that SirT1 counteracts both Ang II-induced cardiomyocyte hypertrophy and ROS-dependent cell
death. The dual roles of Ang II as a prohypertrophic
and pro-apoptotic agent may rely on the cross-talk between the EGF receptor and
the different PI3K isoforms (a, b, gand δ) [40], leading in turn to hypertrophic growth or alternatively to cell
death.Recently, it has been shown that other sirtuins family members (SirT3
and SirT7), play an important protective role against cardiac pathology [41,42],
indicating that mIGF-1-mediated protective effects against oxidative and
hypertrophic stresses could be in part due to other members of this family.
However, our data showed that a specific SirT1 inhibitor (EX-527) or
overexpression of dominant negative SirT1 protein (H363Y) can reverse mIGF-1
protective effects, supporting a SirT1-specific mechanism.
Importantly,
our analysis showed that the locally produced mIGF-1 and the circulating IGF-1
have different roles in SirT1-mediated activity and cardiac protection.
Although circulating IGF-1 and mIGF-1
trigger phosphorylation of the same receptor(s) (Figure 1C), differences in
their respective signaling mechanisms
leading to changes in SirT1 expression/activity must rely downstream of IGF-1 receptor(s). In
this respect, it is important to stress that while circulating IGF-1 activates
typically PI3K/AKT/mTOR and MAPK pathways [43], locally
acting mIGF-1 does not activate these canonical pathways in cardiomyocytes,
impinging instead on PDK1 and SGK1 signaling [15]. Thus,
divergent signaling mechanisms could explain the apparently antagonistic roles
of the two IGF-1 isoforms in cardiomyocytes, with mIGF-1 able to prevent
circulating IGF-1-induced cell hypertrophy. We have previously reported that
mIGF-1 induced accelerated cardiac growth, related to higher expression levels
of ANP at 1 and 2 months, without any further significant change [15].
Interestingly, in the in vitro models herein described mIGF-1 did not
elicit increased hypertrophic markers and cell size in both neonatal and adult
(HL1) cardiomyocytes, indicating that the in vivo response is mainly due
to specific physiological signaling occurring during cardiac development.
Further work using in vivo and in vitro cardiac models is
necessary to shed light on the intermediate players between mIGF-1-dependent
signaling and SirT1 in cardiomyocytes (Figure 11). It would be important to
understand whether some specific effects of mIGF-1 could be recapitulated by
its N-terminal Class 1 signaling or C-terminal Ea extension peptides alone [6], which are
absent in cleaved circulating IGF-1. It would be of interest also to ascertain
if our data on mIGF-1/SirT1-dependent protection against oxidative and
hypertrophic challenges can be confirmed in an in vivo setting, where
circulating and autocrine/paracrine factors, absent in cultured cell systems,
may have an impact.
mIGF-1 Tg mice display activation in the
heart of genes involved in anti-apoptotic and anti-oxidant defenses [15]: among the most upregulated, we focused on adiponectin, UCP-1 and
MT-2 [15]. Although these proteins are functionally unrelated (adiponectin
is a hormone regulating metabolic
processes, UCP-1 is a mitochondrial protein allowing
protons to reenter the mitochondrial matrix short-circuiting the respiratory
chain, and MT-2 is a zinc-binding protein), remarkably they have been reported
independently to exert protection against hypertrophic and oxidative stresses
in the heart [35-37].
Strikingly, we found that the activation of these genes by mIGF-1 relies on
SirT1-dependent activation of their promoters, suggesting that at least some of
the mIGF-1 dependent transcriptional program in cardiomyocytes is mediated by
SirT1. Our data are in agreement with the finding that SirT1 upregulates
adiponectin [44], whereas to
our knowledge this is the first report about the SirT1-dependent regulation of
UCP-1 and MT-2 transcripts.
In
conclusion, there is increasing evidence that NAD-regulated enzymes such as
SirT1 finely interplay in the regulation of cardiomyocyte function [17,45], and
their role begins now to be appreciated. Consequently, research on the role of
IGF-1 isoforms in this "NAD world" is also in its infancy. This domain is
considered of clinical interest for the treatment of cardiovascular diseases [5,46], and the
mIGF-1/SirT1 pathway presented in this study may represent a promising
therapeutic target to fight cardiac hypertrophy and oxidative stress.
Materials and Methods
Animals.
Transgenic FVB mice carrying a rat mIGF-1 cDNA driven
by the mouse α-MyHC promoter were generated and maintained as
previously described [11].
Western
analyses.
Protein extraction from
whole cell or heart tissue preparation was performed in RIPA buffer (1% (w/w)
Nonidet P40, 1% (w/w) Sodiumdeoxycate, 0.1% (w/v) SDS, 150mM NaCl, 50mM HEPES
pH 7.0, 2mM EDTA pH 8.0, 100mM NaF, 10% glycerol, 1.5mM MgCl2, 100mM PMSF in
ETOH, 200mM sodium orthovanadate, 1 μg/ml aprotinin). For analyses of nuclear
proteins (SirT1, H1), nuclear fraction was isolated from cultured cells or
heart tissues according to the following procedure: cells or liquid
nitrogen-powderized heart tissues were dissolved in buffer A (10 mM HEPES, 1.5
mM MgCl2, 10 mM KCl, 0.5 mM DTT, 0.05% NP-40, 100mM PMSF in ETOH, 200mM sodium
orthovanadate, 1 μg/ml aprotinin, pH 7.9) and left on ice for 10 min.
After centrifugation, cytoplasmic fraction (supernatant) was kept aside and
frozen. Pellets were resuspended in buffer B (5 mM HEPES, 1.5 mM MgCl2, 0.2 mM
EDTA, 0.5 mM DTT, 26% glycerol (v/v), 100mM PMSF in ETOH, 200mM sodium
orthovanadate, 1 mg/ml aprotinin, pH 7.9) plus NaCl to give a final
concentration of 300mM NaCl. Lysates were then mechanically homogenized with a
Dounce homogenizer on ice; samples were left on ice for 30 min. After a final
centrifugation, supernatant (nuclear fraction) was collected for further
analysis. Protein concentration was determined using Bradford method (Biorad)
and 20 mg of protein lysates were separated in SDS polyacrylamide mini-gel
(Biorad system) and transferred onto a hybond ECL nitrocellulose membrane
(Amersham). Membranes were blocked with 5% milk, blotted with specific
antibodies o/n at 4oC, washed 3 times with washing buffer (TBS and 0,1%
Tween-20) for 30 min and blotted with specific secondary antibodies
(horseradish peroxidase-conjugated, 1:5000) with 5% milk for 1h at RT. The
membrane was incubated for 1 min using ECL reagent before exposure.
Cell cultures, transfections.
Cardiac
muscle cell line HL-1 was cultured as previously described, on
gelatin/fibronectin coated flasks or multi-wells plates [22]. For transient
plasmid transfection or co-transfection experiments, the lipid-based reagent
LipofectamineTM 2000 (Invitrogen) was used, according to manufacturer
instructions. For luciferase assays, 2 x 106 cells/well were transfected with 1
μg of luciferase reporter constructs and 1μg of pRL-TK (Renilla luciferase
construct from Promega). Luciferase assays were performed 48 hours after
transfection using a dual-luciferase reporter assay (Promega) and a
luminescence counter VictorTM Light 1420 (Perkin Elmer). Firefly luciferase
activity was normalized to renilla luciferase expression for each sample.
Preparation of primary neonatal cardiomyocytes culture.
One-day-old C57/Bl6 or mIGF-1
transgenic mice were sacrificed and hearts were excised. After scalpel
homogenization, ventricular cardiomyocytes were isolated following a series of
collagenase/pancreatin digestions (Collagenase type II, CSL2, Worthington/Pancreatin
4x NF, GIBCO) and cells were collected by centrifugation (8.000rpm for 5min).
Next, fibroblasts were removed from the culture after a 45 min pre-plating step
at 37°C in complete medium [DMEM/199 medium (5/1 ratio) supplemented with 10%
heat inactivated horse serum (Sigma), 5% heat inactivated fetal calf serum
(Sigma), 0.025 M HEPES, 0.002M L-glutamine (Sigma) and 1x penicillin/streptomycin
(Sigma)]. Alive cardiomyocytes were counted using Tryptan Blue solution
(Sigma). Cells were transferred on 1% gelatin (Sigma) -coated 12- or 96-well
plates.
Reactive
oxygen species (ROS) measurements.
The fluorescent probe dichlorofluorescein diacetate (CM-DCFDA, Sigma) was used
to monitor the intracellular generation of reactive oxygen species (ROS). HL-1
or neonatal mouse cardiomyocytes, grown on coated 96-wells plates were
transfected and/or treated for 60 minutes with Angiotensin II (1 mM, 60 min) or
paraquat (100 mM) as described, with or without 10 mM superoxide scavenger
Tiron. After washing with PBS, cells were incubated 20 min in the dark with 10
mM CM-DCFDA. Cells were washed again with PBS and fluorescence was detected at
excitation/emission wavelength of 485-535nm in a fluorimeter Fluoroskan Ascent
PL (Labsystems). Fluorescence values were normalized to protein content for
each well.
[3H]-leucine incorporation.
The cells (HL-1 or neonatal mouse cardiomyocytes)
were plated on 12-well-coated dishes at a density of 100 cells/mm2. Protein synthesis
was measured by [3H] Leucine (1 μCi/ml) incorporation
as described elsewhere [23].
MF-20 immunostaining and confocal microscopy.
Cells (HL-1 or neonatal mouse
cardiomyocytes) were plated on coated coverslips. Upon the indicated
treatment/transfection, cells were washed twice in PBS and fixed with 4%
paraphormaldheyde for 10 min ice. Blocking was performed in PBS calcium free
plus 10% goat serum, followed by 1 h incubation at RT with the MF-20 antibody
diluted 1/250 in PBS calcium free plus 1.5% goat serum, and by 45 min
incubation at RT with secondary Cy3 antibody (red) diluted 1/300 in PBS plus
1%BSA and 0.2% Triton-X. Coverslips were mounted on microscopy slides and
confocal images were acquired on a Leica TCS SP5 microscope. Cell size (total
area) and cell hypertrophy (total MF-20 staining intensity) were accurately
quantified using the Metamorph® imaging software (Molecular Devices).
Cell
viability assay.
Cell viability/cell
death was quantified by staining HL-1 cells or neonatal mouse cardiomyocytes
with propidium iodide (Invitrogen) following cell transfections and/or
treatments as indicated. Fluorescent intensity was analyzed using the BD
FACSCanTM System. All FACS data was analyzed with FlowJo (Tree Star, USA).
Real-Time
PCR.
Total RNA was isolated from
hearts using TRIzol (Invitrogen). Afterwards, the RNA was treated with DNaseI
enzyme (Promega) for 1h at 37oC and then cleaned by column purification
(Qiagen). The RNA concentration was determined with a spectrophotometer. After
RNA quality verification, 1-2 mg was used to prepare cDNA (Ready-To-Go,
T-Primed First-Strand Kit, Amersham Bioscience). Quantitative polymerase chain
reaction (PCR) was performed using the SYBR Green (SIGMA) in a Light-Cycler
(Roche). UbiC, Rn18S and GAPDH transcripts were used as internal controls, according
to the GeNorm method [24]. Primer sequences were designed with the Primer 3
software (http://frodo.wi.mit.edu/) and are listed in Table 1.
Statistical
analysis.
Results are expressed as
means ± S.E. Comparisons were made by using appropriated Student's t test.
Differences were considered as significant when P<0.05 (*), P<0.01 (**)
or P<0.001 (***).
Reagents,
antibodies and plasmids.
All
reagents, antibodies and plasmids not described elsewhere in the text are
listed below in Table 2.
Table 1. Primers sequences for real-time PCR.
| Mouse | Forward | Reverse |
| SirT1 |
5' AGTTCCAGCCGTCTCTGTGT
3'
|
5' CTCCACGAACAGCTTCACAA 3'
|
| UCP-1 |
5' GGGCCCTTGTAAACAACAAA
3'
|
5' GTCGGTCCTTCCTTGGTGTA 3'
|
| MYH6 |
5' GAGGACCAGGCCAATGAGTA 3'
|
5' GCTGGGTGTAGGAGAGCTTG
3'
|
| MYH7 |
5' TGCAGCAGTTCTTCAACCAC
3'
|
5' TCGAGGCTTCTGGAAGTTGT 3'
|
| Adiponectin |
5' GTTGCAAGCTCTCCTGTTCC
3'
|
5' TCTCCAGGAGTGCCATCTCT
3'
|
| Metallothionein-2 |
5' CCATATCCCTTGAGCCAGAA
3'
|
5' ATCGACGAGAGATCGGTTTG 3'
|
| Acta-1 |
5' GCATGCAGAAGGAGATCACA 3'
|
5' TTGTCGATTGTCGTCCTGAG 3'
|
| ANP |
5' CCTAAGCCCTTGTGGTGTGT
3'
|
5' CAGAGTGGGAGAGGCAAGAC 3'
|
| BNP |
5' CAGCTCTTGAAGGACCAAGG 3'
|
5' AGACCCAGGCAGAGTCAGAA 3'
|
| SERCA2a |
5' CTGTGGAGACCCTTGGTTGT
3'
|
5' CAGAGCACAGATGGTGGCTA
3'
|
| UbiC |
5' AGCCCAGTGTTACCACCAAG 3'
|
5' GCAAGAACTTTATTCAAAGTGCAA 3'
|
| GAPDH |
5' AACTTTGGCATTGTGGAAGG 3'
|
5' ACACATTGGGGGTAGGAACA 3'
|
| Rn18S |
5' CGCGGTTCTATTTTGTTGGT 3'
|
5' AGTCGGCATCGTTTATGGTC 3'
|
Table 2. Reagents and antibodies.
| Primary antibodies: |
| Protein targeted | Host | Clone | Provider | Catalogue number |
|
SirT1
|
mouse
|
B-7
|
Santa Cruz Biotechnology
|
sc-74465
|
|
H1
|
goat
|
N-16
|
Santa Cruz Biotechnology
|
sc-34464
|
|
acetyl-H1 (Lys26)
|
rabbit
|
-
|
Sigma
|
H-7789
|
|
Adiponectin
|
rabbit
|
-
|
Sigma
|
A6354
|
|
UCP-1
|
rabbit
| |
Sigma
|
U6382
|
|
p53
|
rabbit
|
-
|
Cell Signaling
|
#9282
|
|
acetyl-p53 (Lys382)
|
rabbit
|
-
|
Cell Signaling
|
#2525
|
|
MF-20
|
mouse
|
-
|
DSHB
|
from: Fischman, D.A.
|
|
IGF-1
|
goat
|
-
|
Sigma
|
12157
|
|
IGF-1 receptor
|
rabbit
|
-
|
Cell Signaling
|
#3027
|
|
phospho-IGF-1 receptor
(Tyr1135/1136)
|
rabbit
|
-
|
Cell Signaling
|
#3024
|
| Secondary antibodies: |
| Protein targeted | Host | Provider | Catalogue number |
|
HRP-conjugated anti-mouse
|
Goat
|
Amersham - GE Healthcare
|
NA9310V
|
|
HRP-conjugated anti-rabbit
|
Goat
|
Amersham - GE Healthcare
|
NA934V
|
|
HRP conjugated anti-Goat
|
Rabbit
|
Santa Cruz Biotechnology
|
sc-2020
|
|
Cy3-conjugated
|
Goat
|
Jackson ImmunoResearch
|
115-165-044
|
| Other reagents: |
| Name | Provider | Catalogue number |
|
Sirtinol
|
Sigma
|
S7942
|
|
EX-527
|
Tocris Biosciences
|
2780
|
|
Tiron
|
Sigma
|
89460
|
|
Lipofectamin
|
Invitrogen
|
1168
|
|
ECL reagent
|
Amersham - GE Healthcare
|
RPN2209
|
|
Trizol Reagent
|
Invitrogen
|
15596
|
|
Angiotensin II
|
Tocris Biosciences
|
1158
|
|
Paraquat
|
Sigma
|
313947
|
|
SYBR®Green dye
|
Sigma
|
QR0100
|
|
mouse recombinant IGF-1
|
Sigma
|
I8879
|
| Plasmids: |
| Insert | Plasmid | Source | References |
|
SirT1
|
pECE
|
Dr. Michael
Greenberg-Addgene
|
Science 2004
Mar 26; 303 (5666):2011-2015
|
|
SirT1 H363Y
|
pECE
|
Dr. Michael
Greenberg-Addgene
|
Science 2004
Mar 26; 303 (5666):2011-2015
|
|
Adiponectin
|
pGL3 basic
|
Dr. Bysani Chandrasekar
|
Mol.
Cell. Biol. 2005; 25(21): 9383-9391
|
|
UCP-1 promoter
|
pGL3 basic
|
Dr. Malcolm G. Parker
|
J. Biol. Chem. 2008; 283:
4200-4209
|
|
Metallothionein 2a promoter
|
pGL3 basic
|
Dr. Jean-Marc Vanacker
|
EMBO J 1999; 15: 4270-4279
|
|
mouse mIGF-1
|
pIGI-1Ea
|
Dr. Tommaso Nastasi
|
PCR cloned from mouse
genomic DNA into pIGI vector at restriction sites EcoRI/BamHI
|
Sources of funding
This work was supported by grants of the European Union (Heart Repair: LSHM-CT-2005-018630; EUMODIC: LSHG-CT-2006-037188) and of the Foundation Leducq (Transatlantic
Networks of Excellence Program: 04 CVD 03)to NR. MV is the recipient of an EIPOD (EMBL
Interdisciplinary POst-Doc) fellowship.
Acknowledgments
We
thank Paschalis Kratsios, Daniel Bilbao, Valeria Berno and Esfir Slonimisky for
technical help, and the members of the Rosenthal lab for insightful
discussions. We are grateful to Antonio Musaro for critical reading of the
manuscript.
Conflicts of Interest
The authors of this article report no conflict of interests.
References
-
1.
Frey
N
and Olson
EN.
Cardiac hypertrophy: the good, the bad, and the ugly.
Annu Rev Physiol.
2003;
65:
45
-79.
[PubMed]
.
-
2.
Giordano
FJ
Oxygen, oxidative stress, hypoxia, and heart failure.
J Clin Invest.
2005;
115:
500
-508.
[PubMed]
.
-
3.
Takimoto
E
and Kass
DA.
Role of oxidative stress in cardiac hypertrophy and remodeling.
Hypertension.
2007;
49:
241
-248.
[PubMed]
.
-
4.
Seddon
M
, Looi
YH
and Shah
AM.
Oxidative stress and redox signalling in cardiac hypertrophy and heart failure.
Heart.
2007;
93:
903
-907.
[PubMed]
.
-
5.
Lavu
S
, Boss
O
, Elliott
PJ
and Lambert
PD.
Sirtuins--novel therapeutic targets to treat age-associated diseases.
Nat Rev Drug Discov.
2008;
7:
841
-853.
[PubMed]
.
-
6.
Winn
N
, Paul
A
, Musaro
A
and Rosenthal
N.
Insulin-like growth factor isoforms in skeletal muscle aging, regeneration, and disease.
Cold Spring Harb Symp Quant Biol.
2002;
67:
507
-518.
[PubMed]
.
-
7.
Longo
VD
and Finch
CE.
Evolutionary medicine: from dwarf model systems to healthy centenarians.
Science.
2003;
299:
1342
-1346.
[PubMed]
.
-
8.
Andreassen
M
, Raymond
I
, Kistorp
C
, Hildebrandt
P
, Faber
J
and Kristensen
LO.
IGF1 as predictor of all cause mortality and cardiovascular disease in an elderly population.
Eur J Endocrinol.
2009;
160:
25
-31.
[PubMed]
.
-
9.
Reiss
K
, Cheng
W
, Ferber
A
, Kajstura
J
, Li
P
, Li
B
, Olivetti
G
, Homcy
CJ
, Baserga
R
and Anversa
P.
Overexpression of insulin-like growth factor-1 in the heart is coupled with myocyte proliferation in transgenic mice.
Proc Natl Acad Sci U S A.
1996;
93:
8630
-8635.
[PubMed]
.
-
10.
Li
Q
, Li
B
, Wang
X
, Leri
A
, Jana
KP
, Liu
Y
, Kajstura
J
, Baserga
R
and Anversa
P.
Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy.
J Clin Invest.
1997;
100:
1991
-1999.
[PubMed]
.
-
11.
Delaughter
MC
, Taffet
GE
, Fiorotto
ML
, Entman
ML
and Schwartz
RJ.
Local insulin-like growth factor I expression induces physiologic, then pathologic, cardiac hypertrophy in transgenic mice.
Faseb J.
1999;
13:
1923
-1929.
[PubMed]
.
-
12.
Kajstura
J
, Fiordaliso
F
, Andreoli
AM
, Li
B
, Chimenti
S
, Medow
MS
, Limana
F
, Nadal-Ginard
B
, Leri
A
and Anversa
P.
IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress.
Diabetes.
2001;
50:
1414
-1424.
[PubMed]
.
-
13.
Li
Q
, Yang
X
, Sreejayan
N
and Ren
J.
Insulin-like growth factor I deficiency prolongs survival and antagonizes paraquat-induced cardiomyocyte dysfunction: role of oxidative stress.
Rejuvenation Res.
2007;
10:
501
-512.
[PubMed]
.
-
14.
Hill
M
and Goldspink
G.
Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage.
J Physiol.
2003;
549:
409
-418.
[PubMed]
.
-
15.
Santini
MP
, Tsao
L
, Monassier
L
, Theodoropoulos
C
, Carter
J
, Lara-Pezzi
E
, Slonimsky
E
, Salimova
E
, Delafontaine
P
, Song
YH
, Bergmann
M
, Freund
C
, Suzuki
K
and Rosenthal
N.
Enhancing repair of the mammalian heart.
Circ Res.
2007;
100:
1732
-1740.
[PubMed]
.
-
16.
Alcendor
RR
, Gao
S
, Zhai
P
, Zablocki
D
, Holle
E
, Yu
X
, Tian
B
, Wagner
T
, Vatner
SF
and Sadoshima
J.
Sirt1 regulates aging and resistance to oxidative stress in the heart.
Circ Res.
2007;
100:
1512
-1521.
[PubMed]
.
-
17.
Pillai
JB
, Gupta
M
, Rajamohan
SB
, Lang
R
, Raman
J
and Gupta
MP.
Poly(ADP-ribose) polymerase-1-deficient mice are protected from angiotensin II-induced cardiac hypertrophy.
Am J Physiol Heart Circ Physiol.
2006;
291:
H1545
-1553.
[PubMed]
.
-
18.
Vahtola
E
, Louhelainen
M
, Merasto
S
, Martonen
E
, Penttinen
S
, Aahos
I
, Kyto
V
, Virtanen
I
and Mervaala
E.
Forkhead class O transcription factor 3a activation and Sirtuin1 overexpression in the hypertrophied myocardium of the diabetic Goto-Kakizaki rat.
J Hypertens.
2008;
26:
334
-344.
[PubMed]
.
-
19.
Ni
YG
, Wang
N
, Cao
DJ
, Sachan
N
, Morris
DJ
, Gerard
RD
, Kuro
OM
, Rothermel
BA
and Hill
JA.
FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases.
Proc Natl Acad Sci U S A.
2007;
104:
20517
-20522.
[PubMed]
.
-
20.
Cohen
HY
, Miller
C
, Bitterman
KJ
, Wall
NR
, Hekking
B
, Kessler
B
, Howitz
KT
, Gorospe
M
, de Cabo
R
and Sinclair
DA.
Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase.
Science.
2004;
305:
390
-392.
[PubMed]
.
-
21.
Huffman
DM
, Moellering
DR
, Grizzle
WE
, Stockard
CR
, Johnson
MS
and Nagy
TR.
Effect of exercise and calorie restriction on biomarkers of aging in mice.
Am J Physiol Regul Integr Comp Physiol.
2008;
294:
R1618
-1627.
[PubMed]
.
-
22.
Claycomb
WC
, Lanson
NA Jr
, Stallworth
BS
, Egeland
DB
, Delcarpio
JB
, Bahinski
A
and Izzo
NJ Jr.
HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte.
Proc Natl Acad Sci U S A.
1998;
95:
2979
-2984.
[PubMed]
.
-
23.
Calderone
A
, Thaik
CM
, Takahashi
N
, Chang
DL
and Colucci
WS.
Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts.
J Clin Invest.
1998;
101:
812
-818.
[PubMed]
.
-
24.
Vandesompele
J
, De
Preter K
, Pattyn
F
, Poppe
B
, Van
Roy N
, De
Paepe A
and Speleman
F.
Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes.
Genome Biol.
2002;
3:
RESEARCH0034
[PubMed]
.
-
25.
Luo
J
, Nikolaev
AY
, Imai
S
, Chen
D
, Su
F
, Shiloh
A
, Guarente
L
and Gu
W.
Negative control of p53 by Sir2alpha promotes cell survival under stress.
Cell.
2001;
107:
137
-148.
[PubMed]
.
-
26.
Vaquero
A
, Scher
M
, Lee
D
, Erdjument-Bromage
H
, Tempst
P
and Reinberg
D.
Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin.
Mol Cell.
2004;
16:
93
-105.
[PubMed]
.
-
27.
Chien
KR
, Knowlton
KU
, Zhu
H
and Chien
S.
Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response.
Faseb J.
1991;
5:
3037
-3046.
[PubMed]
.
-
28.
Sadoshima
J
, Xu
Y
, Slayter
HS
and Izumo
S.
Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro.
Cell.
1993;
75:
977
-984.
[PubMed]
.
-
29.
Touyz
R
, Fareh
J
, Thibault
G
, Tolloczko
B
, Larivière
R
and Schiffrin
EL.
Modulation of Ca2+ transients in neonatal and adult rat cardiomyocytes by angiotensin II and endothelin-1.
Am J Physiol.
1996;
270:
H857
-868.
[PubMed]
.
-
30.
Salas
M
, Vila-Petroff
MG
, Palomeque
J
, Aiello
EA
and Mattiazzi
A.
Positive inotropic and negative lusitropic effect of angiotensin II: intracellular mechanisms and second messengers.
J Mol Cell Cardiol.
2001;
33:
1957
-1971.
[PubMed]
.
-
31.
Yang
H
, Yang
T
, Baur
JA
, Perez
E
, Matsui
T
, Carmona
JJ
, Lamming
DW
, Souza-Pinto
NC
, Bohr
VA
, Rosenzweig
A
, de Cabo
R
, Sauve
AA
and Sinclair
DA.
Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival.
Cell.
2007;
130:
1095
-1107.
[PubMed]
.
-
32.
Hingtgen
SD
, Tian
X
, Yang
J
, Dunlay
SM
, Peek
AS
, Wu
Y
, Sharma
RV
, Engelhardt
JF
and Davisson
RL.
Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy.
Physiol Genomics.
2006;
26:
180
-191.
[PubMed]
.
-
33.
Boudina
S
, Sena
S
, Theobald
H
, Sheng
X
, Wright
JJ
, Hu
XX
, Aziz
S
, Johnson
JI
, Bugger
H
, Zaha
VG
and Abel
ED.
Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins.
Diabetes.
2007;
56:
2457
-2466.
[PubMed]
.
-
34.
Lee
Y
and Gustafsson
AB.
Role of apoptosis in cardiovascular disease.
Apoptosis.
2009;
14:
536
-548.
[PubMed]
.
-
35.
Hoerter
J
, Gonzalez-Barroso
MD
, Couplan
E
, Mateo
P
, Gelly
C
, Cassard-Doulcier
AM
, Diolez
P
and Bouillaud
F.
Mitochondrial uncoupling protein 1 expressed in the heart of transgenic mice protects against ischemic-reperfusion damage.
Circulation.
2004;
110:
528
-533.
[PubMed]
.
-
36.
Zhou
G
, Li
X
, Hein
DW
, Xiang
X
, Marshall
JP
, Prabhu
SD
and Cai
L.
Metallothionein suppresses angiotensin II-induced nicotinamide adenine dinucleotide phosphate oxidase activation, nitrosative stress, apoptosis, and pathological remodeling in the diabetic heart.
J Am Coll Cardiol.
2008;
52:
655
-666.
[PubMed]
.
-
37.
Shibata
R
, Ouchi
N
, Ito
M
, Kihara
S
, Shiojima
I
, Pimentel
DR
, Kumada
M
, Sato
K
, Schiekofer
S
, Ohashi
K
, Funahashi
T
, Colucci
WS
and Walsh
K.
Adiponectin-mediated modulation of hypertrophic signals in the heart.
Nat Med.
2004;
10:
1384
-1389.
[PubMed]
.
-
38.
Pillai
J
, Chen
M
, Rajamohan
SB
, Samant
S
, Pillai
VB
, Gupta
M
and Gupta
MP.
Activation of SIRT1, a class III histone deacetylase, contributes to fructose feeding-mediated induction of the alpha-myosin heavy chain expression.
Am J Physiol Heart Circ Physiol.
2008;
294:
H1388
-1397.
[PubMed]
.
-
39.
Miyazaki
R
, Ichiki
T
, Hashimoto
T
, Inanaga
K
, Imayama
I
, Sadoshima
J
and Sunagawa
K.
SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells.
Arterioscler Thromb Vasc Biol.
2008;
28:
1263
-1269.
[PubMed]
.
-
40.
Shah
B
and Catt
KJ.
A central role of EGF receptor transactivation in angiotensin II -induced cardiac hypertrophy.
Trends Pharmacol Sci.
2003;
24:
239
-244.
[PubMed]
.
-
41.
Rajamohan
S
, Pillai
VB
, Gupta
M
, Sundaresan
NR
, Konstatin
B
, Samant
S
, Hottiger
MO
and Gupta
MP.
SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly (ADP-ribose) polymerase 1.
Mol Cell Biol.
2009;
May 26
.
-
42.
Vakhrusheva
O
, Smolka
C
, Gajawada
P
, Kostin
S
, Boettger
T
, Kubin
T
, Braun
T
and Bober
E.
Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice.
Circ Res.
2008;
102:
703
-710.
[PubMed]
.
-
43.
Mourkioti
F
and Rosenthal
N.
IGF-1, inflammation and stem cells: interactions during muscle regeneration.
Trends Immunol.
2005;
26:
535
-542.
[PubMed]
.
-
44.
Qiao
L
and Shao
J.
SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex.
J Biol Chem.
2006;
281:
39915
-39924.
[PubMed]
.
-
45.
Pillai
J
, Isbatan
A
, Imai
S
and Gupta
MP.
Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity.
J Biol Chem.
2005;
280:
43121
-43130.
[PubMed]
.
-
46.
Borradaile
N
and Pickering
JG.
NAD(+), sirtuins, and cardiovascular disease.
Curr Pharm Des.
2009;
15:
110
-117.
[PubMed]
.