Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1
Abstract
Deficiency of the circadian clock protein BMAL1 leads to premature aging and increased levels of reactivate oxygen species in several tissues of mice. In order to investigate the role of oxidative stress in accelerated aging and development of age-related pathologies, we continuously administered the antioxidant N-acetyl-L-cysteine toBmal1-deficient mice through their entire lifespan by supplementing drinking water. We found that the life long treatment with antioxidant significantly increased average and maximal lifespan and reduced the rate of age-dependent weight loss and development of cataracts. At the same time, it had no effect on time of onset and severity of other age-related pathologies characteristic of Bmal1-/- mice, such as joint ossification, reduced hair regrowth and sarcopenia. We conclude that chronic oxidative stress affects longevity and contributes to the development of at least some age-associated pathology, although ROS-independent mechanisms may also play a role. Our bioinformatics analysis identified the presence of a conservative E box element in the promoter regions of several genes encoding major antioxidant enzymes. We speculate that BMAL1 controls antioxidant defense by regulating the expression of major antioxidant enzymes.
Introduction
The circadian clock is a universal time
keeping system that generates 24-hr rhythms in behavior and physiology. The
activity of the circadian system is important for synchronization of metabolic
processes within an organism and between an organism and its environment [1,2]. The
importance of this coordination for human health is supported by a number of
epidemiological studies demonstrating that
the risk of many diseases, including cardiovascular disease and cancer,
is significantly increased among shift workers; however, the exact mechanisms
linking circadian desynchronization and the development of various
pathological conditions remains largely unknown [3]. At the
molecular level the activity of the circadian clock is controlled by several
interlocked transcription/translation feedback loops formed by the core circadian
proteins [4,5]. Mice with
a targeted disruption of different circadian proteins lose rhythmic patterns of
behavior and develop multiple physiological abnormalities [3,6].
Recently, a connection between the circadian clock and aging has been
established. It is most prominently manifested in mice deficient in the BMAL1 protein.
During the normal course of their life, these animals develop multiple
pathological changes that are characteristic of premature aging [7]. This is in
sharp contrast to other circadian mutant mice models, such as Clock/Clock andPer2m/manimals, which accelerate their aging
program and develop phenotypes that are reminiscent of those in Bmal1-deficient
mice only after being exposed to a low dose of ionizing radiation [8,9].
BMAL1 is a basic helix-loop-helix
(bHLH)-PAS domain transcription factor and a key component of the circadian
clock [10]. Deficiency in the BMAL1 protein results in
disruption of rhythmicity in behavior and gene expression pattern [11]. BMAL1 is involved in the control of tissue
homeostasis by the direct regulation of reactive oxygen species (ROS);
accordingly, its deficiency is associated with the excessive production of ROS
resulting in chronic oxidative stress [7]. Many life-threatening diseases, including
cardiovascular disease, cancer and diabetes, have been linked to chronic
oxidative stress [12]. It has also been proposed that oxidative stress
plays an important role in the development of age-associated pathology [13,14]; however, many aspects regarding
its exact role in the process of aging are still under debate [15].
Previously we have shown that
age-related degenerative processes in several tissues of Bmal1-/- mice
are correlated with an age-dependent increase in the level of ROS [7]. If excessive production of ROS and increased
oxidative stress contribute to the early aging phenotype in Bmal1-/-
mice, then the reduction of oxidative stress by antioxidants might prevent
early aging or ameliorate its severity. This strategy has been previously
successfully used to delay ROS-initiated degenerative processes in nematode,
fly and mouse [16]. Among available antioxidants, a potent low
molecular weight (LMW) antioxidant N-acetyl-L-cysteine (NAC) was proved to be
efficient in mice; indeed, treatment with NAC significantly delayed
tumorigenesis in p53-/- mice [17] and ameliorate age-related pathological changes
induced by the deficiency of transcription factor FOXO [18]. Here we confirm the role of chronic oxidative
stress in early onset of aging in Bmal1-/- mice. We show that continuous
administration of NAC delays the onset of aging and extends the lifespan of Bmal1-deficient
mice. We speculate that BMAL1 controls antioxidant defense by regulating the expression of major
antioxidant enzymes.
Results
NAC slows age-dependent body weight loss in BMAL1-deficient
mice
To investigate the effect of antioxidants on
age-dependent weight loss in BMAL1-deficient mice, we started treating the
experimental animals from the time of their prenatal development by
supplementing drinking water of the breeders with NAC. To generate age-matched
control animals, similar breeding pairs, which were set up simultaneously,
received regular water. The prolonged administration of NAC had no effect on
the size of litters born or on the survival of pups during lactation. After
weaning, the animals in the experimental group received water supplemented with
NAC through their entire lifespan.
We started monitoring the body weight of wild type
(WT) and Bmal1-/- mice from 4 weeks of age. The administration of NAC
slows down age-dependent body weight gain in mice of both genotypes (Figure 1A,B). Thus, by the time the body weight of WT mice normally reaches its
maximum (30 weeks of age) and stabilizes, animals receiving NAC weigh ~18% less
than their littermates raised on regular water (Figure 1A). Similarly, until
reaching their maximal weight (at 18 weeks), Bmal1-deficient mice that
received NAC weigh less than the corresponding control animals drinking regular
water (23.6+
0.84 and 21.15+
0.55 g respectively, Figure 1B,
p<0.01). To test whether this effect could be attributed to taste
preferences, we measured daily levels of water consumption in both groups of WT
mice and in fact determined that mice that receive NAC drink significantly less
water (Figure 1C). To account for these differences in consumption, we compared
the effect of NAC on the relative weight of BMAL1-deficient animals (measured
at each time point as % of the body weight of WT mice of the same group (Figure 1D). As shown in Figure 1D, starting from 25 weeks of age, when Bmal1-/-
mice normally begin losing weight [7], animals
raised on NAC-supplemented water have significantly higher relative weight than
animals in the control group that received regular water. As a result, at 40
weeks of age Bmal1-/- mice in the control group lost on average 20% of
their maximal body weight; whereas the body weight of animals that received NAC
was reduced by 4%. Thus, treatment with the LMW antioxidant NAC significantly
delayed age-dependent weight loss in BMAL1-deficient mice.
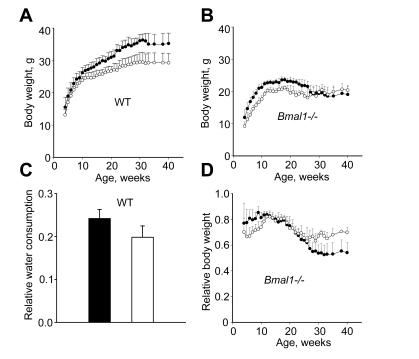
Figure 1. Continuous administration of NAC affects age-dependent changes in body weight.
Total body weight of male (A) WT and (B) Bmal1-/-
mice, closed circles - control mice raised on regular water; open circles -
mice raised on water supplemented with 40mM of NAC. (C) Relative
water consumption by WT mice receiving either regular (closed bar) or
NAC-supplemented (open bar) water. (D) Age-dependent changes in
relative body weight in Bmal1-/- mice measured at each time point as
the percentage of the body weight of WT mice of the same group.
Effect of NAC on development of the phenotype of
premature aging in Bmal1-/- mice.
Previously we have demonstrated that the deficiency of
BMAL1 is associated with an early onset of several phenotypes associated with
normal aging [7]. Among those
are reduced hair regrowth after shaving, development of cataracts, cornea
inflammation, sarcopenia and joint ossification. This prompted us to test whether
the administration of NAC affects the onset and/or severity of these changes.
One of the most striking age-dependent
changes related to deficiency in BMAL1 is the early onset of various eye
pathologies, such as cataracts and cornea inflammation [7]. At 30 weeks
of age, Bmal1-/- animals in both groups start showing various degrees of eye
pathologies with a slightly higher incidence in the control group (30% versus
15% in NAC-receiving animals). The difference between the two groups increased
with age; 80% of 45-week old control mice developed cataracts on one or both
eyes, whereas in the NAC-treated group only 25% of animals were affected (Figure 2A).
The comparison of the two groups
for the severity of other hallmarks of aging did not reveal any significant
differences. Thus, the administration of NAC did not improve reduced hair
regrowth characteristic of BMAL1-deficient mice: only 3 out of 10
NAC-treated mice de-monstrated partial or complete hair regrowth after shaving,
which was not different from controls (4 out of 10).
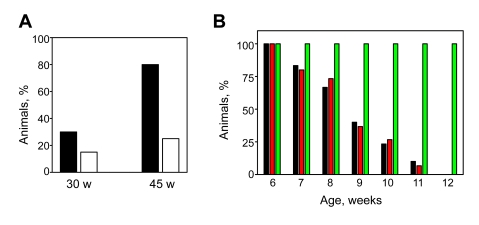
Figure 2. Effects of continuous administration of NAC on development of eye pathology and muscle strength. (A) Frequency of cataracts
in 30-week old and 45-week old Bmal1-/- mice raised on regular
(closed bars) or NAC-supplemented (open bars) water. Each eye was counted independently; the percentage of
cataracts was determined by dividing the number of cataracts by the total
number of eyes, if an animal was dead at the time of observation, then the
previous score for this animal was used. (B) Age-dependent
changes in muscular strength of WT (green bars) and Bmal1-/- mice
receiving regular (black bars) or NAC-supplemented (red bars) water.
Muscular strength was evaluated as the ability of animals of indicated age
to maintain their weight on the inverted grid. Each
animal was tested five times, if the animal did not fall down for 30 sec
the trial was counted as successful. The percentage of successful trials
was calculated and plotted. No difference was detected between NAC-treated
and control Bmal1-/- animals.
Treatment with NAC had no effect on the development of
joint ossification evaluated by changes in ankle joint flexibility and physical
performance. The latter was estimated by measuring righting reflex time (the
time required for mice to return to their normal position after being placed on
the back). Whereas young WT and Bmal1-/- mice normally take less than 1
sec, up to 3 sec was required for 30-week old Bmal1-/- mice, regardless
of NAC supplementation.
In order to estimate the effect of antioxidants on the
aging of muscles, we measured grip strength by monitoring the ability of mice
in both groups to maintain weight on the inverted grid. Performance of WT mice
in this task did not change during their lifespan, whereas in Bmal1-/-
mice it was gradually reduced with age (Figure 2B). However, administration of
NAC did not improve the age-related decrease in muscle strength. Thus,
administration of the LMW antioxidant NAC significantly delayed age-related development of cataracts, but had no effect on manifestation of other pathological changes in Bmal1-/-
mice associated with premature aging.
Continuous administration of NAC extends lifespan of Bmal1-/-
mice
Consistent with our previous report, Bmal1-deficient
mice in the control group had a very short average lifespan of 38+
11 weeks. As shown in Figure 3, continuous
administration of NAC extends the lifespan to 47+
12 weeks (p<0.05
Log-Rank Test). The survival curve for NAC-treated mice was significantly
shifted, with 90% survival at the age of 36 weeks (only 50% of animals survived
until this age in the control group). Treatment with the antioxidant also
significantly affects the maximum lifespan, extending it from 58 weeks in the
control group to 66 weeks in the NAC-treated group. All wild type mice in both
the control and NAC-treated groups survived until the termination of the
experiment (70 weeks). Thus, treatment with a dietary antioxidant increases the
average lifespan in Bmal1-deficient animals by about 24% and maximum
lifespan by 14%.
Genes encoding major antioxidant enzymes are potential
targets of the CLOCK/BMAL1 transcrip-tional complex
Our current and previous results led us to the
hypothesis that BMAL1 may be involved in the control of an organism's response
to oxidative stress and antioxidant defense. Antioxidant defense is controlled
by a complex system of LMW antioxidants and antioxidant enzymes [12]. As a
transcription factor working in complex with CLOCK or NPAS2, BMAL1 may regulate
the activity of major antioxidant enzymes (MAE) at the transcriptional level.
CLOCK/BMAL1 and NPAS2/ BMAL1 complexes specifically bind promoters containing
circadian E box in their regulatory regions.
Table 1. Position of the circadian E-box elements in the promoter regions (+/- 2000 nucleotides from major transcription starting site) of genes encoding major antioxidant enzymes.
SOD - Superoxide dismutase; CAT - catalase; GPX - glutathione peroxidase;
PRDX - peroxiredoxin; TXNRD - thioredoxin reductase; SESN - sestrin
| Homo Sapiens | Pan Troglodites | Macaca Mulatta | Mus Musculus | Rattus Norvegicus |
| SOD1 |
-886
|
-1055
|
-1044
|
-631, 768, 1787
|
-1661, -685, 743, 1749
|
| SOD2 |
none
|
n/a
|
n/a
|
none
|
none
|
| SOD3 |
-1673
|
n/a
|
n/a
|
none
|
none
|
| GPX1 |
-19
|
-23
|
-93
|
-1196, -908,
-54, 12
|
-82
|
| GPX2 |
974
|
n/a
|
n/a
|
1263
|
72
|
| GPX3 |
979
|
1068
|
1061
|
-570, 1268
|
-1507, 198, 921
|
| GPX4 |
-385
|
n/a
|
n/a
|
-1669
|
none
|
| GPX6 |
173
|
n/a
|
n/a
|
-617, -168, 1580
|
none
|
| CAT |
-1751
|
none
|
413
|
46, 1437
|
-969, 47, 1434
|
| PRDX1 |
372
|
none
|
407
|
218, 528
|
-1413, -1402, 198
|
| PRDX2 |
none
|
n/a
|
n/a
|
none
|
none
|
| PRDX3 |
-136
|
-142
|
-148
|
428
|
406, 1733
|
| PRDX4 |
none
|
n/a
|
n/a
|
none
|
none
|
| PRDX5 |
-836
|
n/a
|
n/a
|
-1019, -282
|
none
|
| PRDX6 |
-290, 991
|
-852, 474
|
-229, 1048
|
-159, -114, 264, 904
|
-185, -140, 248
|
| SESN1 |
345, 1089
|
464
|
-1549, -733
|
-292, -776, 1390
|
-759
|
| TXNRD1 |
-260
|
n/a
|
n/a
|
1510
|
-841, 170, 975
|
Two circadian E box elements have been identified: CACGTG and CACGTT [19]. To
test if any of the MAE genes can be directly regulated by the major circadian
transactivation complex, we performed in silico analysis of their
promoter regions for the presence of BMAL1-responsive elements. Nucleotide
sequences covering the region between -2000bp/+ and 2000bp (relative to the
position of the transcriptional start site) of the NCBI database were analyzed
using EditSeq and MegAlign software (DNASTAR, Inc.). The results of the
analysis summarized in Table 1 indicate that many of the MAE genes may in fact
be directly regulated by the CLOCK/BMAL1 transcriptional complex. Most
strikingly, the position of the BMAL1-responsive elements in the promoters of
several MAE genes such as Gpx1, Prdx1, Prdx6, and Sesn2 is conservative among
primates and rodents, indicating their potential functional significance.
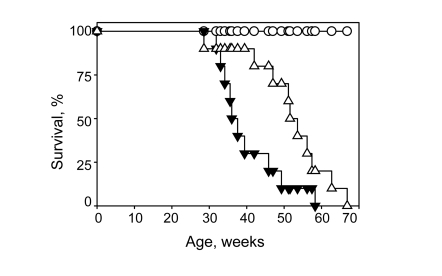
Figure 3. Continuous administration of NAC increases lifespan of Bmal1-/- mice. Kaplan-Meyer survival curves were obtained for WT mice
raised on regular (closed circles) or NAC-supplemented (open circles) water;
and Bmal1-/- mice raised on regular (closed triangles) or NAC-supplemented
(open triangles) water. NAC significantly increased lifespan of Bmal1-/- mice
(P =0.022, log-rank Mantel-Haenszel test).
Discussion
The free-radicals theory of aging
postulates that oxidative damage to biological macromolecules produced by ROS
and RNS play an important role in the aging process [13,14,20]. This theory is supported by the large amount of experimental data
demonstrating a direct correlation between the resistance to oxidative stress
and the lifespan in different organisms [16,21].
However, this theory was recently challenged by contradictory data obtained in
various mouse models demonstrating that although the overexpression of several
antioxidant enzymes makes mice more resistant to oxidative challenge, it fails
to increase their lifespan. Thus, the deficiency of superoxide dismutase
reduced the lifespan in mice, whereas the deficiency of other antioxidant
enzymes had no effect [22]. At the
same time, targeted overexpression of catalase in mitochondria results both in
reduced oxidative damage in tissues of transgenic mice and an increased
lifespan [23]. Such
conflicting data may arise from the fact that laboratory mice are normally
maintained under optimal husbandry conditions, their antioxidant defense is
well balanced and works efficiently in protecting from relatively low levels of
ROS, therefore overexpression of antioxidant enzymes has a marginal effect. At
the same time, the disruption of the antioxidant defense will have a more dramatic effect on the lifespan and may significantly
contribute to the development of age-associated pathologies.
ROS and RNS are produced in
the organisms either as side products of metabolic reaction or by a specific
group of enzymes. ROS and RNS serve as important mediators of intra- and extra-
cellular signaling and many physiological processes are regulated by specific
species [12]. An
excessive amount of ROS results in damage to biological macromolecules, which
is known as oxidative stress and is an essential contributor to the development
of such diseases as cancer, diabetes and cardiovascular diseases [12]. Therefore,
ROS and RNS levels are tightly controlled at both intra- and extra-cellular
levels by antioxidant systems. Previously we have demonstrated that accelerated
aging of Bmal1- deficient mice is associated with an age-dependent
increase in the level of ROS in different tissue [7]. The fact
that an increase in ROS concentration was detected in those tissues that
demonstrate pathological changes may suggest that the early onset of aging in Bmal1-deficient
mice is caused by excessive production and/or insufficient detoxification of
ROS. Here we show that continuous administration of antioxidant NAC can
significantly ameliorate the onset and severity of premature aging in Bmal1-deficient
mice. Thus, deregulation of ROS homeostasis in fact contributes significantly
to the premature aging phenotype initiated by the deficiency of the BMAL1
protein.
Noteworthy, treatment of Bmal1-/- mice with NAC
cannot completely prevent premature aging; growth retardation, reduced hair
regrowth, sarcopenia, and joint ossification were not affected by
administration of NAC. There are two possible explanations for the incomplete
rescue. First, NAC treatment may not be efficient enough due to tissue-specific
differences in its distribution, which may restrict the antioxidant effect to a
particular tissue. Second, BMAL1 may be involved in the control of aging
through both ROS- dependent and ROS-independent mechanisms. However, administration
of NAC attenuated the development of the most prominent age-related phenotype
of Bmal1-/- mice, development of cataracts, and even most importantly,
significantly extended the average and maximal lifespan of Bmal1-/-
mice.
BMAL1 is a transcription factor critical for circadian
function. In complex with its dimerization partners, CLOCK or NPAS2, BMAL1
controls the expression of several clock genes and multiple clock-controlled
genes (CCGs). Based on microarray data, the about 10% of all transcripts
display daily oscillations in expression, indicating that they may be
clock-regulated [24]. The
results of the bioinformatics analysis of the promoter regions of several genes
encoding major antioxidant enzymes reveal the presence of conservative
circadian E box elements, suggesting that at least some of the genes encoding
antioxidant enzymes can be CCGs. Importantly, potential targets of BMAL1
include antioxidant enzymes, which control different stages of ROS
detoxification. Among those are superoxide dismutase that converts superoxide
into hydrogen peroxide; catalase, peroxiredoxines and glutathione peroxidase
that reduce hydrogen peroxide and sestrins that are key regulators of oxidized
peroxiredoxins reduction. Therefore, by controlling different steps of the
process, the CLOCK/BMAL1 transcriptional complex may orchestrate the entire
chain of reduction/oxidation reactions, which are necessary for the efficient
detoxification of ROS.
The importance of circadian orchestration
of antioxidant defense is supported by the fact that the disruption of this
control results in oxidative stress leading to various pathological
developments. Supporting this hypothesis are epidemiological data on disease
spectra in shift workers. It is documented that disturbance of the circadian
system through shift work or frequent travel across time zones leads to increased
risk of cardiovascular diseases, diabetes and cancer. Although the molecular
pathways responsible for this link are mostly unknown [25-27], it is
well accepted that oxidative stress is one of the major causes in
pathophysiology of these diseases. We speculate that when BMAL1-dependent
circadian control of the antioxidant defense of an organism is disrupted by
shift work, it leads to oxidative stress and increases risk of disease.
In summary, we demonstrated that treatment with the
LMW antioxidant NAC delivered as a dietary supplement ameliorated the aging of
BMAL1 deficient mice. These results suggest that an increased level of ROS is
involved in the development of accelerating aging in this animal model. BMAL1
may control the ROS level through regulation of expression of major antioxidant
enzymes, some of which are potential transcriptional targets of the CLOCK/BMAL1
complex. While circadian control of ROS homeostasis is critical for aging, some
other oxidative stress independent mechanisms may also be involved.
Materials and Methods
Animals.
Bmal1-/- mice that
were originally obtained from Dr. Bradfield (University of Wisconsin) were
backcrossed to C57BL/6J mice for 12 generations. The colony was maintained as a
heterozygous intercross to obtain animals of all three genotypes. Mice were
genotyped by PCR as previously described [11]. All animals were maintained on a 12 h:12 h light:dark cycle in
standard plastic cages and lifespan was
determined by recording the age at spontaneous death.
Animals treated with the antioxidant received 40mM NAC in drinking water during
their entire life, starting from prenatal development (breeding pairs were
maintained on NAC); water bottles were changed once every three days. To
monitor body weight gain/loss, animals were weighed once a week. Mice were
observed daily for the general health status and to score mortality. Each group
was represented by 10 animals.
Hair regrowth assay.
Was performed on 30-week old mice as
previously described [7]. Dorsal segments of skin were shaved and animals were monitored
for hair regrowth for 3 months.
Estimation the muscle strength
. Animals were placed on a wire cage
top, which then was gently flipped over. Each animal was tested in five trials;
a trial, in which animal did not fall down for 30 sec was scored as successful
and the percentage of successful trials was calculated.
Righting reflex.
Mouse
was turned over onto its back and the time necessary to return back to a normal
position (i.e. to right itself onto all four feet) was measured. Each
measurement was performed five times for each mouse.
Detection of cataracts
. Eye opacity was evaluated and scored
under bright light by two independent experienced observers, who were blind to
treatment and genotype. Every eye was counted independently; therefore the
percentage was determined by dividing the number of cataracts by the total
number of affected eyes. If an animal was dead at the time of observation, the
previous score was added to the total number. Cataracts of different severity
were score equally. All animal studies were conducted in accordance with the
regulations of the Committees of Animal Care and Use at the Cleveland Clinic
Foundation, Cleveland State University and Roswell Park Cancer Institute.
Statistical analyses.
All statistical analyses were performed using
SigmaStat 3.5 software (Systat Software, Inc., CA). Lifespan curves were
calculated using Kaplan-Meier survival analysis; the statistical significance
of curves was assessed using log-rank Mantel-Haenszel tests. P values <0.05
were considered as significant; the median, mean, and maximum survivals were
calculated for each group.
ACKNOWLEDGEMENTS
We thank Dmitry Gudkov for the editorial help. This
work was supported by NIH grants CA102522 and GM075226 to M.P.A and AHA grant
0835155N to R.V.K.
Conflicts of Interest
The authors of this
manuscript have no conflict of interest to declare.
References
-
1.
Dardente
H
and Cermakian
N.
Molecular circadian rhythms in central and peripheral clocks in mammals.
Chronobiol Int.
2007;
24:
195
-213.
[PubMed]
.
-
2.
Gachon
F
, Nagoshi
E
, Brown
SA
, Ripperger
J
and Schibler
U.
The mammalian circadian timing system: from gene expression to physiology.
Chromosoma.
2004;
113:
103
-112.
[PubMed]
.
-
3.
Kondratov
RV
A role of the circadian system and circadian proteins in aging.
Ageing Res Rev.
2007;
6:
12
-27.
[PubMed]
.
-
4.
Harms
E
, Kivimae
S
, Young
MW
and Saez
L.
Posttranscriptional and posttranslational regulation of clock genes.
J Biol Rhythms.
2004;
19:
361
-373.
[PubMed]
.
-
5.
Hirayama
J
and Sassone-Corsi
P.
Structural and functional features of transcription factors controlling the circadian clock.
Curr Opin Genet Dev.
2005;
15:
548
-556.
[PubMed]
.
-
6.
Ko
CH
and Takahashi
JS.
Molecular components of the mammalian circadian clock.
Hum Mol Genet.
2006;
15 Spec No 2:
R271
-7.
[PubMed]
.
-
7.
Kondratov
RV
, Kondratova
AA
, Gorbacheva
VY
, Vykhovanets
OV
and Antoch
MP.
Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock.
Genes Dev.
2006;
20:
1868
-1873.
[PubMed]
.
-
8.
Antoch
MP
, Gorbacheva
VY
, Vykhovanets
O
, Toshkov
IA
, Kondratov
RV
, Kondratova
AA
, Lee
C
and Nikitin
AY.
Disruption of the circadian clock due to the Clock mutation has discrete effects on aging and carcinogenesis.
Cell Cycle.
2008;
7:
1197
-1204.
[PubMed]
.
-
9.
Lee
CC
Tumor suppression by the mammalian Period genes.
Cancer Causes Control.
2006;
17:
525
-530.
[PubMed]
.
-
10.
Gekakis
N
, Staknis
D
, Nguyen
HB
, Davis
FC
, Wilsbacher
LD
, King
DP
, Takahashi
JS
and Weitz
CJ.
Role of the CLOCK protein in the mammalian circadian mechanism.
Science.
1998;
280:
1564
-1569.
[PubMed]
.
-
11.
Bunger
MK
, Wilsbacher
LD
, Moran
SM
, Clendenin
C
, Radcliffe
LA
, Hogenesch
JB
, Simon
MC
, Takahashi
JS
and Bradfield
CA.
Mop3 is an essential component of the master circadian pacemaker in mammals.
Cell.
2000;
103:
1009
-1017.
[PubMed]
.
-
12.
Droge
W
Free radicals in the physiological control of cell function.
Physiol Rev.
2002;
82:
47
-95.
[PubMed]
.
-
13.
Barja
G
Endogenous oxidative stress: relationship to aging, longevity and caloric restriction.
Ageing Res Rev.
2002;
1:
397
-411.
[PubMed]
.
-
14.
Sohal
RS
and Weindruch
R.
Oxidative stress, caloric restriction, and aging.
Science.
1996;
273:
59
-63.
[PubMed]
.
-
15.
Blagosklonny
MV
Aging: ROS or TOR.
Cell Cycle.
2008;
7:
3344
-3354.
[PubMed]
.
-
16.
Bokov
A
, Chaudhuri
A
and Richardson
A.
The role of oxidative damage and stress in aging.
Mech Ageing Dev.
2004;
125:
811
-826.
[PubMed]
.
-
17.
Sablina
AA
, Budanov
AV
, Ilyinskaya
GV
, Agapova
LS
, Kravchenko
JE
and Chumakov
PM.
The antioxidant function of the p53 tumor suppressor.
Nat Med.
2005;
11:
1306
-1313.
[PubMed]
.
-
18.
Tothova
Z
, Kollipara
R
, Huntly
BJ
, Lee
BH
, Castrillon
DH
, Cullen
DE
, McDowell
EP
, Lazo-Kallanian
S
, Williams
IR
, Sears
C
, Armstrong
SA
, Passegue
E
, DePinho
RA
and Gilliland
DG.
FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress.
Cell.
2007;
128:
325
-339.
[PubMed]
.
-
19.
Hardin
PE
Transcription regulation within the circadian clock: the E-box and beyond.
J Biol Rhythms.
2004;
19:
348
-360.
[PubMed]
.
-
20.
Harman
D
Aging: a theory based on free radical and radiation chemistry.
J Gerontol.
1956;
11:
298
-300.
[PubMed]
.
-
21.
Liang
H
, Masoro
EJ
, Nelson
JF
, Strong
R
, McMahan
CA
and Richardson
A.
Genetic mouse models of extended lifespan.
Exp Gerontol.
2003;
38:
1353
-1364.
[PubMed]
.
-
22.
Perez
VI
, Bokov
A
, Van
Remmen H
, Mele
J
, Ran
Q
, Ikeno
Y
and Richardson
A.
Is the oxidative stress theory of aging dead.
Biochim Biophys Acta.
2009;
1790:
1005
-1014.
[PubMed]
.
-
23.
Schriner
SE
, Linford
NJ
, Martin
GM
, Treuting
P
, Ogburn
CE
, Emond
M
, Coskun
PE
, Ladiges
W
, Wolf
N
, Van
Remmen H
, Wallace
DC
and Rabinovitch
PS.
Extension of murine life span by overexpression of catalase targeted to mitochondria.
Science.
2005;
308:
1909 1911
[PubMed]
.
-
24.
Panda
S
, Antoch
MP
, Miller
BH
, Su
AI
, Schook
AB
, Straume
M
, Schultz
PG
, Kay
SA
, Takahashi
JS
and Hogenesch
JB.
Coordinated transcription of key pathways in the mouse by the circadian clock.
Cell.
2002;
109:
307
-320.
[PubMed]
.
-
25.
Curtis
AM
and Fitzgerald
GA.
Central and peripheral clocks in cardiovascular and metabolic function.
Ann Med.
2006;
38:
552
-9.
[PubMed]
.
-
26.
Fujino
Y
, Iso
H
, Tamakoshi
A
, Inaba
Y
, Koizumi
A
, Kubo
T
and Yoshimura
T.
A prospective cohort study of shift work and risk of ischemic heart disease in Japanese male workers.
Am J Epidemiol.
2006;
164:
128
-135.
[PubMed]
.
-
27.
Hardeland
R
, Coto-Montes
A
and Poeggeler
B.
Circadian rhythms, oxidative stress, and antioxidative defense mechanisms.
Chronobiol Int.
2003;
20:
921
-962.
[PubMed]
.