Introduction
Chemotherapy can extend survival in
patients diagnosed with a wide range of malignancies. However, side effects caused
by toxicity to normal cells and tissues limit chemotherapy dose
density and intensity, which may compromise efficacy. For instance, the
cardiotoxicity and nephrotoxicity associated with the widely prescribed
anti-cancer drugs, doxorubicin and cisplatin respectively limit their full
therapeutic potential [1,4]. Thus, reduction of undesired toxicity by selective
protection of normal cells without compromising the killing of malignant cells
represents a promising strategy to enhance cancer treatment.
Calorie restriction (CR) is an
effective and reproducible intervention for increasing life span, reducing
oxidative damage, enhancing stress resistance and delaying/preventing aging and age-associated
diseases such as cancer in various species, including mammals (mice, rats, and
non- human primates) [5-8]. Recently, a
fasting-based intervention capable of differentially protecting normal and
cancer cells against high-dose chemotherapy in cell culture and in
neuroblastoma-bearing mice was reported [9]. In the neuroblastoma xenograft model, mice were allowed to consume only
water for 48 hours prior to etoposide treatment. Whereas high dose etoposide
led to 50% lethality in ad libitum fed mice, fasting protected against
the chemotoxicity without compromising the killing of neuroblastoma cells [9].
Table 1. Toxicity side effect survey.
* Grade: 0 no symptom, 1 to 4 from mild, moderate, severe and life threatening (requires medical assistance)
** Fatigue: unusual tiredness which is not relieved by either a good night of sleep or rest.
*** Weakness: lack of strength, vigor or firmness
| Toxicity Side Effect Survey |
| General symptoms | Grade* |
| Fatigue
** |
0
|
1
|
2
|
3
|
4
|
| 4
Being extreme Fatigue |
| Weakness
*** |
0
|
1
|
2
|
3
|
4
|
| 4
Being Extreme Weakness |
| Hair
Loss |
0
|
1
|
2
|
3
|
4
|
| 4
Being Maximum Hair Loss |
| Body
Temperature | | 36.5°C /97.7° | 37.0°C /98.6° | 37.5°C /99.5° | 38.0°C /100.4° | 38.5°C /101.3° | 39.0°C /102.2° | 39.5°C /103.1° | 40.0°C /104° | 40.5°C /104.9° | 41.0°C /105.8° |
| Head
Aches |
0
|
1
|
2
|
3
|
4
|
| 4
Being the Worst Headache |
| Gastrointestinal Side Effects |
| Appetite |
0
|
1
|
2
|
3
|
4
|
| 4
Being Strong Appetite |
| Nausea |
0
|
1
|
2
|
3
|
4
|
| 4
Being Unbearable Nausea |
| Vomiting |
0
|
Mild
|
Moderate
|
Severe
|
|
< 2
times/Day
|
3-5
times/ Day
|
>5
times/Day
|
| Diarrhea |
0
|
Mild
|
Moderate
|
Severe
|
|
< 2
times/Day
|
3-5
times/ Day
|
>5
times/Day
|
| Abdominal
Cramps |
0
|
1
|
2
|
3
|
4
|
| 4
Being Extreme Abdominal Cramps |
| Mouth
Sores |
0
|
1
|
2
|
3
|
4
|
| 4
Being Extremely Painful |
| Dry
Mouth |
0
|
1
|
2
|
3
|
4
|
| 4
Extreme Dryness |
| CNS AND PNS Side Effects |
| Short
memory impairment |
0
|
1
|
2
|
3
|
4
|
| 4
Being High Impairment |
| Numbness |
0
|
1
|
2
|
3
|
4
|
| 4
Being Maximum |
| Tingling |
0
|
1
|
2
|
3
|
4
|
| 4
Being Maximum |
| Neuropathy-motor |
0
|
1
|
2
|
3
|
4
|
| 4
Being = Paralysis |
Previous human studies have shown that alternate day
dietary restriction and short-term fasting (5 days) are well tolerated and safe
[10-12]. In fact, children ranging from 6 months to 15 years of age were
able to complete 14 to 40 hours of fasting in a clinical study carried out at
the Children's hospital of Philadelphia
[13]. Furthermore, alternate day calorie
restriction caused clinical improvements and reduced markers of inflammation
and oxidative stress in obese asthmatic patients [12,14].
Here, we report 10 cases of
patients diagnosed with various types of cancer, who have voluntarily fasted
prior to and following chemotherapy. The results presented here, which are based on self-assessed health
outcomes (Table 1) and laboratory reports, suggest
that fasting is safe and raise the possibility that it can
reduce chemotherapy-associated side effects.
However, only a randomized controlled clinical trial can establish its
efficacy.
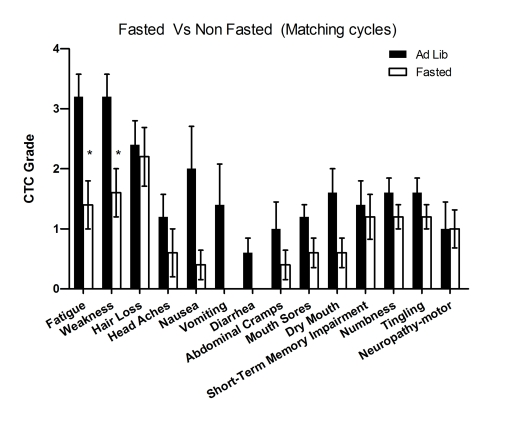
Figure 1. Self-reported side-effects after chemotherapy with or without fasting. Data represent
average of CTCAE grade from matching fasting and non-fasting cycles (Ad Lib). 6 patients received
either chemotherapy-alone or chemo-fasting treatments. Self-reported side
effects from the closest two cycles were compared one another. Statistic
analysis was performed only from matching cycles. Data presented as
standard error of the mean (SEM). P value was calculated with unpaired, two
tail t test. (*, P<0.05).
Results
Ten cancer patients receiving
chemotherapy, 7 females and 3 males with a median age of 61 years (range 44-78
yrs), are presented in this case series report. Four suffered from breast
cancer, two from prostate cancer, and one each from ovarian, uterine, non small
cell carcinoma of the lung, and esophageal adenocarcinoma. All patientsvoluntarily fasted for a total of 48 to 140 hours
prior to and/or 5 to 56 hours following chemotherapy administered by their
treating oncologists (Table 2, Table 3).
Table 2. Additional data from patients, including scheme of chemotherapy cycles, fasting regimens and tumor response.
* also utilized low glycemic diet for 24 hours prior to fast.
** also utilized liquid diet for 24 hours after fast.
n/a = not applicable, due to chemotherapy being administered in the adjuvant setting.
| Cycle# | Fast(hours) | Chemotherapy | Tumor
Response |
| Case
1 |
1
|
140
pre
40
post
|
Docetaxel
75mg/m2 +
Cyclophosphamide
600mg/m2 |
n/a
|
|
4
|
120
pre
24
post
|
Docetaxel
75mg/m2 +
Cyclophosphamide
600mg/m2 |
n/a
|
| Case
2 |
4
|
72
pre
51
post
|
Docetaxel
64.6mg/m2 + carboplatin 485mg
+
5FU 2415.7 mg/m2 |
---
|
|
5
|
48
pre
56
post
|
Docetaxel
79 mg/m2 + carboplatin 470mg
+
5FU 2415.7 mg/m2 |
Stable
disease on CT/PET
|
|
6
|
48
pre
56
post
|
Docetaxel
79 mg/m2 + carboplatin 470mg
+
5FU 2415.7 mg/m2 |
Improvement
on CT/PET. Refer to text.
|
|
7
|
48
pre
56
post
|
Docetaxel
79 mg/m2 + carboplatin 470mg
+
5FU 2415.7 mg/m2 |
---
|
|
8
|
48
pre
56
post
|
Docetaxel
79 mg/m2 + carboplatin 470mg
+
5FU 2415.7 mg/m2 |
Progression
of Disease on CT/PET
|
| Case
3 |
5-
12
|
60-66
pre
24
post
|
Docetaxel
75 mg/m2 |
See
PSA Graph
|
| Case
4 |
6
|
48
pre
24
post
|
Docetaxel
75mg/m2 + carboplatin 540mg
|
Stable
disease CT/PET refer to text
|
| Case
5 |
2
|
36
pre
|
Carboplatin
480 mg + Paclitaxel 280 mg
|
---
|
|
3-4
|
60
pre
|
Carboplatin
480 mg + Paclitaxel 280 mg
|
87%
decline in CA 125, Reduction in lymph nodes on CT
|
|
5-6
|
60
pre
24post
|
Carboplatin
480 mg + Paclitaxel 280 mg
| |
| Case
6 |
3
|
62
pre
24post
|
Gemcitabine
720 mg/m2 (day1)+
GMZ
720 mg/m2 Docetaxel 80 mg/m2 (Day8)
|
---
|
|
4
|
62
pre
24post
|
Gemcitabine
720 mg/m2 (day1)+
GMZ
720 mg/m2 Docetaxel 80 mg/m2 (Day8)
|
---
|
|
5-6
|
62
pre
24post
|
Gemcitabine
900 mg/m2 (day1)+
GMZ
900 mg/m2 Docetaxel 100 mg/m2 (Day8)
|
Stable
disease on PET scan,
No
new MTS.
|
| Case 7 |
1
|
65
pre
8
post
|
Docetaxel
60 mg/m2 |
See
PSA Graph
|
|
2-8
|
65
pre
25post*^
|
Docetaxel
75 mg/m2 |
See
PSA Graph
|
| Case
8 |
1-4
|
64
pre
24
post**
|
Docetaxel
75 mg/m2 + Cyclophosphamide 600 mg/m2 |
n/a
|
| Case
9 |
1
|
48
pre
|
Doxorubicin
110 mg +
Cyclophosphamide
1100 mg
|
n/a
|
|
2-4
|
61
pre
4
post
|
Doxorubicin
110 mg +
Cyclophosphamide
1100 mg
|
n/a
|
| Case
10 |
1
|
60
pre
|
Docetaxel
75 mg/m2 + Carboplatin 400mg
|
n/a
|
|
2
|
48
pre
|
Docetaxel
75 mg/m2 + carboplatin 400mg
|
n/a
|
|
3
|
40
pre
24post
|
Docetaxel
75 mg/m2 + carboplatin 400mg
|
n/a
|
|
4
|
48
pre
24post
|
Docetaxel
75 mg/m2 + carboplatin 400mg
|
n/a
|
|
5
|
36
pre
24post
|
Docetaxel
75 mg/m2 + carboplatin 400mg
|
n/a
|
|
6
|
20
pre
20post
|
Docetaxel
75 mg/m2 + carboplatin 400mg
|
n/a
|
Table 3. Additional demographical and clinical information of patients.
| Gender | Age | Primary Neoplasia | Stage at Diagnosis |
| Case 1 |
Female
|
51
|
Breast
|
IIA
|
| Case 2 |
Male
|
68
|
Esophagus
|
IVB
|
| Case 3 |
Male
|
74
|
Prostate
|
II
|
| Case 4 |
Female
|
61
|
Lung (NSCLC)
|
IV
|
| Case 5 |
Female
|
74
|
Uterus
|
IV
|
| Case 6 |
Female
|
44
|
Ovary
|
IA
|
| Case 7 |
Male
|
66
|
Prostate
|
IV/DI
|
| Case 8 |
Female
|
51
|
Breast
|
IIA
|
| Case 9 |
Female
|
48
|
Breast
|
IIA
|
| Case 10 |
Female
|
78
|
Breast
|
IIA
|
Case 1
This is a 51-year-old Caucasian woman
diagnosed with stage IIA breast cancer receiving adjuvant chemo-therapy
consisting of docetaxel (TAX) and cyclophosphamide(CTX). She fasted prior to her first
chemotherapy administration. The fasting regimen consisted of a complete
caloric deprivation for 140 hours prior and 40 hours after chemotherapy (180
hours total), during which she consumed only water and vitamins. The patient completed
this prolonged fasting without major inconvenience and lost 7 pounds,
which were recovered by the end of the treatment (Figure 2H). After the
fasting-chemotherapy cycle, the patient experienced mild fatigue, dry mouth and
hiccups (Figure 2I); nevertheless she was able to carry out her daily
activities (working up to 12 hours a day). By contrast,
in the subsequent second and third treatment, she received chemotherapy accompanied by a regular diet and complained of moderate to severe fatigue,
weakness, nausea, abdominal cramps and diarrhea (Figure 2I). This time the
side effects forced her to withdraw from her regular work schedule. For the
forth cycle, she opted to fast again, although with a different regimen which
consisted of fasting 120 hours prior to and 24 hours post
chemotherapy. Notably, her self-reported side effects were lower despite the expected
cumulative toxicity from previous cycles. Total white
blood cell (WBC) and absolute neutrophil counts (ANC) were slightly better at
nadir when chemotherapy was preceded by fasting (Figure 2A, C; Supplementary Table 1). Furthermore, platelets level decreased by 7-19% during cycles 2 and 3 (ad libitum
diet) but did not drop during the first and forthcycles (fasting), (Figure 2D). After the forthchemotherapy cycle combined with
144-hour fast her ANC, WBC, and platelet counts reached their highest level since
the start of chemotherapy 80 days earlier (Figure 2A, C and D).
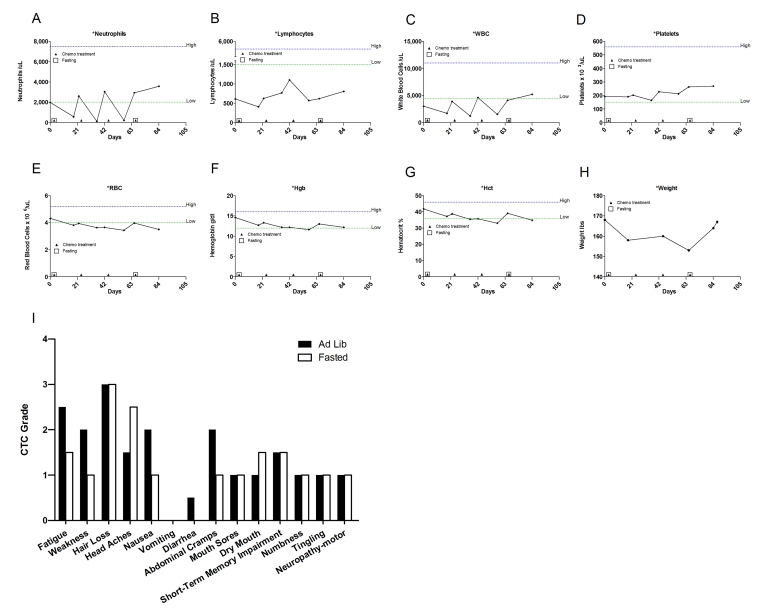
Figure 2. Laboratory values of blood cell counts for case 1. (A)
Neutrophils; (B) Lymphocytes; (C) White blood cells, WBC; (D)
Platelets; (E) Red blood cells, RBC (F) Hemoglobin, Hgb; (G)
Hematocrit, Hct; (H) Body weight. Filled triangle indicates day of
chemotherapy; open square indicates fasting. Normal ranges of laboratory
values are indicate by dash lines; (I) Self-reported side-effects after
chemotherapy for case 1. Data represent the average of 2 cycles of
chemo-alone vs the average of 2 cycles of chemo-fasting treatments.
Case 2
This is a 68-year-old Caucasian male
diagnosed in February 2008 with esophageal adenocarcinomametastasic to
the left adrenal gland. The initial
treatment consisted of 5-fluorouracil (5-FU) combined
with cisplatin(CDDP) concurrent with radiation for the first two cycles. Throughout these first two cycles, the
patient experienced multiple
side effects including
severe weakness, fatigue, mucositis, vomits and grade 2-3 peripheral neuropathy
(Figure 3). During the third cycle, 5-FU
administration was interrupted due
to severe nausea and refractory vomiting (Figure 3). In spite of the aggressive
approach with chemotherapy and radiation, his disease progressed with new
metastases to the right adrenal gland, lung nodules, left sacrum, and coracoid
process documented by computed tomography - positron emission tomography
(CT-PET) performed in August 2008. These prompted
a change in his chemotherapy regimen for the fourth cycle to carboplatin (CBDCA) in combination with TAX and 5-FU (96 hour infusion)
(Table 2). During the fourth cycle, the patient incorporated a 72-hour fast
prior to chemotherapy and continued the fast for 51 hours afterward, consuming
only water. The rationale for the 51 hour post-chemotherapy fasting was to
cover the period of continuous infusion of 5-FU. The patient lost approximately 7
pounds, of which 4 were regained during the first few days after resuming ad
libitum diet (data not shown). Although a combination of three
chemotherapeutic agents were used during this cycle, self-reported sideeffects were
consistently less severe compared to cycles in which calories were consumed ad
lib (Figure 3). Prior to his fifth cycle the patient opted to fast again. Instead of receiving the 5-FU infusion for
96 hours, as he did previously, the same
dose of the drug was administered within
48 hours, and the fasting regimen was also modified to 48 hours prior and 56
hours post chemotherapy delivery. Self-reported side effects were again less
severe than those in association with ad libitum diet and the
restaging CT-PET scan indicated objective tumor response, with decreased
standard uptake values (SUV) in the esophageal mass,the adrenal
gland metastases, and the lung nodule. From
the sixth to eight cycle, the
patient fasted prior to and following chemotherapy treatments
(Table 2). Fasting was well tolerated in all cycles and chemotherapy-dependent
side effects were reduced except for mild diarrhea and abdominal cramps that
were developed during the seventh cycle (Figure 3).
Ultimately, the patient's disease progressed and the patient died in February
2009.
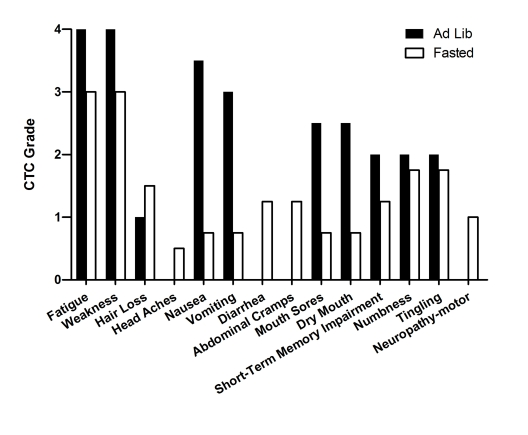
Figure 3. Self-reported side-effects after chemotherapy for case 2. Data represent
the average of 3 cycles of chemo-alone vs the average of 5 cycles of
chemo-fasting treatments.
Case 3
This is a 74-year-old Caucasian man who was diagnosed in July 2000 with stage II prostate
adeno-carcinoma, Gleason score 7 and
baseline PSA level of 5.8 ng/ml. He achieved an undetectable PSA nadir after radical prostatectomy performed in September of 2000, but
experienced biochemical recurrence inJanuary 2003 when
PSA rose to 1.4 ng/ml. Leuprolide acetate together
with bicalutamide
and finasteride
were prescribed. However, administration of these drugs had to be suspended in
April 2004 due to severe side effects related to testosterone
deprivation. Additional therapies including triptorelin pamoate, nilutamide, thalidomide, CTX and ketoconazole failed to
control the disease. In 2007 the patient's PSA level reached 9 ng/ml and new
metastases were detected on bone scan. Despite that TAX at 25mg/m2 was
administered on weekly basis, the PSA level continued to increase, reaching
40.6 ng/ml (data not shown). Bevacizumab was added to the treatment and only
then did the PSA drop significantly (data not shown). Throughout the cycles
with chemotherapy the patient experienced significant side effects including
fatigue, weakness, metallic taste, dizziness,
forgetfulness, short-term memory impairment and peripheral neuropathy (Figure 4I). After discontinuing the
chemotherapy, his PSA rose rapidly. TAX was resumed at 75mg/m2 every 21 days, and was
complemented with granulocytic colony stimulating factor (G-CSF). Once again
the patient suffered significant side effects (Figure 4I). In June 2008, chemotherapy was halted. The
patient was enrolled in a phase III clinical trial with abiraterone acetate, a drug that can selectively block CYP17, a microsomal enzyme thatcatalyzes
a series of reactions critical to nongonadal androgenbiosynthesis
[15]. During the trial, the patient's PSA levels increased to 20.9ng/dl (Figure 4H), prompting resumption of chemotherapy and G-CSF. This time the patient opted to fast prior to
chemotherapy. His fasting
schedule consisted
of 60 hours prior to and 24 post drug administration (Table 2). Upon restarting chemotherapy with fasting the PSA level dropped, and
notably, the patient reported considerably lower side effects than in previous
cycles in which he consumed calories ad-lib (Figure 4I). He also experienced reduced myelosuppression (Figure4A-G). During the last three cycles, in addition to fasting, the patient applied testosterone (cream, 1%) for
five days prior to chemotherapy. As a
consequence the PSA level along with the testosterone level increased dramatically. Nonetheless, 3 cycles of chemotherapy combined
with fasting reduced PSA from 34.2 to 6.43 ng/ml (Figure 4H). These results imply that the cytotoxic activity of TAX to cancer
cells was not blocked by fasting.
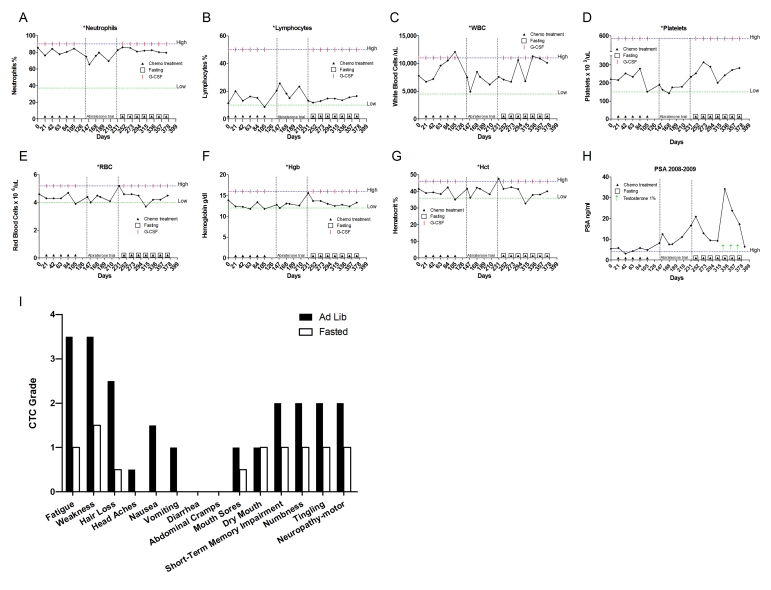
Figure 4. Laboratory values of blood cell counts for case 3. (A)
Neutrophils; (B) Lymphocytes; (C) White blood cells, WBC; (D)
Platelets; (E) Red blood cells, RBC (F) Hemoglobin, Hgb; (G) Hematocrit, Hct; (H) Prostate specific
antigen (PSA) level. The patient was enrolled in abiraterone acetate (CYP17 inhibitor) trial for 90
days indicated by vertical dash lines. The patient also received G-CSF
(Neulasta) on the day of chemotherapy except during the treatment with
abiraterone acetate. Filled triangle indicates day of chemotherapy; open
square indicates fasting, arrow indicates testosterone application (cream 1%).
Normal ranges of laboratory values are indicated by horizontal dash lines; (I)
Self-reported side-effects after chemotherapy for case 3. Data represent
the average of 5 cycles of chemo-alone vs the average of 7 cycles of
chemo-fasting treatments.
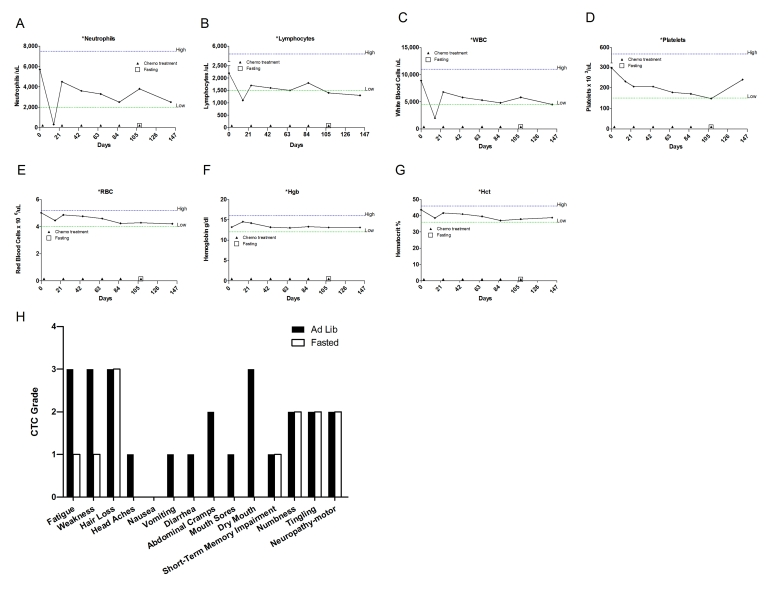
Figure 5. Laboratory values of blood cell counts for case 4. (A)
Neutrophils; (B) Lymphocytes; (C) White blood cells, WBC; (D)
Platelets; (E) Red blood cells, RBC (F) Hemoglobin, Hgb; (G)
Hematocrit, Hct; Filled triangle indicates day of chemotherapy; open
square indicates fasting. Normal ranges of laboratory values are indicated
by dash lines; (H) Self-reported side-effects after chemotherapy for
case 4. Data represent the average of 5 cycles of chemo-alone vs 1
cycle of chemo-fasting treatment.
Case 4
This is a 61-year-old Caucasian
female who was diagnosed in June
2008 with poorly differentiated non-small cell lung carcinoma (NSCLC). A
staging PET scan documented a hypermetabolic lung mass, multiple mediastinal
and left perihilar lymph nodes, and widespread metastatic disease to the bones,
liver, spleen, and pancreas. The initial treatment
commenced with the administration of TAX 75 mg/m2 and CBDCA 540mg every 21 days. Although she
was on a regular diet, during the first 5 cycles she lost an average of 4
pounds after each
treatment, most likely due to chemotherapy-induced
anorexia. The patient reported that she did return to her
original weight but only after three weeks of the drug administration, just
before a new cycle. Additional side effects included severe muscle spasms, peripheral neuropathy, significant
fatigue, mucositis, easy bruising and bowel discomfort (Figure 5H). During the sixthcycle,which consisted of the same drugs and dosages, the patient fasted
for 48-hours-prior and 24-hours-post chemotherapy. She lost approximately 6
pounds during the fasting period, which she recovered within 10 days (data
not shown). Besides mild fatigue and weakness, the patient did not complain of
any other side effect which was experienced during the five previous cycles (Figure 5H). Cumulative side effects such as peripheral
neuropathy, hair loss and cognitive impairment were not reversed. By contrast
self-reported acute toxic side effects were consistently reduced when
chemotherapy was administered in association with fasting (Figure 5H). In the sixth and last cycle, the patient reported that her strength returnedmore quickly after the chemotherapy so that she was able to walk 3 miles three days after the
drug administration, whereas in previous cycles (ad libitum diet) she had experienced severe
weakness and fatigue which limited any physical activity. No significant
differences were observed in the patient's blood analysis (Figure 5A-G). The last PET scan performed on February 2009
showed stable disease in the main mass (lungs) and decreased uptake in the
spleen and liver when compared to her baseline study.
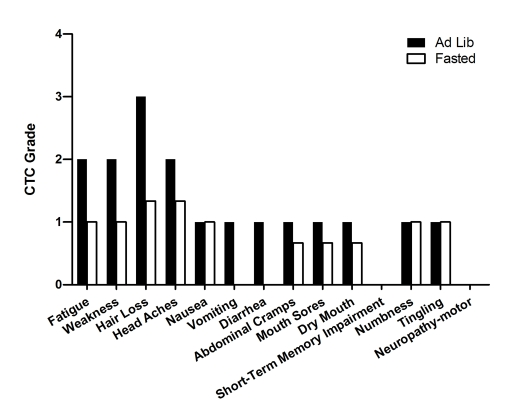
Figure 6. Self-reported side-effects after chemotherapy for case 5. Data represent
1 cycle of chemotherapy-alone (first cycle) vs the average of 5
cycles of chemo-fasting treatments.
Case 5
This is a 74 year-old woman diagnosed in 2008 with
stage IV uterine papillary serous carcinoma. Surgery (Total Abdominal
Hysterectomy-Bilateral Salpingo-Oopherectomy, TAH-BSO, with lymph node
dissection) followed by adjuvant chemotherapy were recommended. Due to
significant enlargement of the right ureter, a right nephrectomy was also
performed. Post-operatively, six cycles of CBDCA (480mg) and paclitaxel (280mg)
were administered every 21-days. During the first treatment the patient
maintained her regular diet and experienced fatigue, weakness, hair loss,
headache and gastrointestinal discomfort (Figure 6). By contrast, during cycles
2-6, the patient fasted before and after chemotherapy, and reported a reduction
in the severity of chemotherapy-associated side effects (Table 2; Figure 6). Fasting did
not appear to interfere with chemotherapy efficacy, as indicated by the 87%
reduction in the tumor marker CA-125 after the forthcycle (data not
shown).
Case 6
This is a 44-year-old Caucasian female diagnosed with a
right ovarian mass (10x12 cm.) in July 2007. Surgery (TAH-BSO) revealed stage
IA carcinosarcoma of the ovary with no lymph node involvement. Adjuvant
treatment consisted of six cycles of ifosfamide and CDDP, administered from July to November of 2007. She remained free of disease until an MRI
revealed multiple new pulmonary nodules in August 2008. Consequently chemotherapy with taxol,
carboplatin and bevacizumab was initiated. By November, however, a CT scan
showed progression of the cancer. Treatment was changed to gemcitabine plus TAX complemented with G-CSF
(Neulasta) (Table 2 and Supplementary Table 2). After the first dose of gemcitabine (900 mg/m2), the patient
experienced prolonged neutropenia (Figure 7A) and thrombocytopenia (Figure 7D), which forced a delay of day 8 dosing. During the second cycle the
patient received a reduced dose of gemcitabine (720 mg/m2), but again developed prolonged
neutropenia and thrombocytopenia, causing dose delays. For the third and subsequent cycles, the patient fasted
for 62 hours prior to and 24 hours after chemotherapy. The patient not only did
not find hardship on carrying out the fasting but also showed a faster recovery
of her blood cell counts, allowing the completion of the chemotherapy regimen
(gemcitabine 720mg/m2 on day 1 plus gemcitabine 720mg/m2
and TAX 80mg/m2 on day 8). During the fifth cycle, she fasted under the same regimen and received
a full dose of gemcitabine (900mg/m2) and TAX (Table 2 and Supplementary Table 2).
Her complete blood count showed consistent improvement during the cycles in
which chemotherapy was combined with fasting. A trend in which nadirs were
slightly less pronounced and the zeniths were considerably higher in ANC, lymphocyte and WBC counts was observed (Figure 7A, B, C, respectively; Supplementary Table 2). During the first
and second cycle (ad
libitum diet) gemcitabinealone induced prolonged thrombocytopenia, which took 11 and 12 days to
recover, respectively (Figure 7D;
Supplementary Table 2) but following the first combined fasting-gemcitabine treatment (thirdand
subsequent cycles), the duration of thrombocytopenia was significantly shorter
(Figure 7D; Supplementary Table 2).
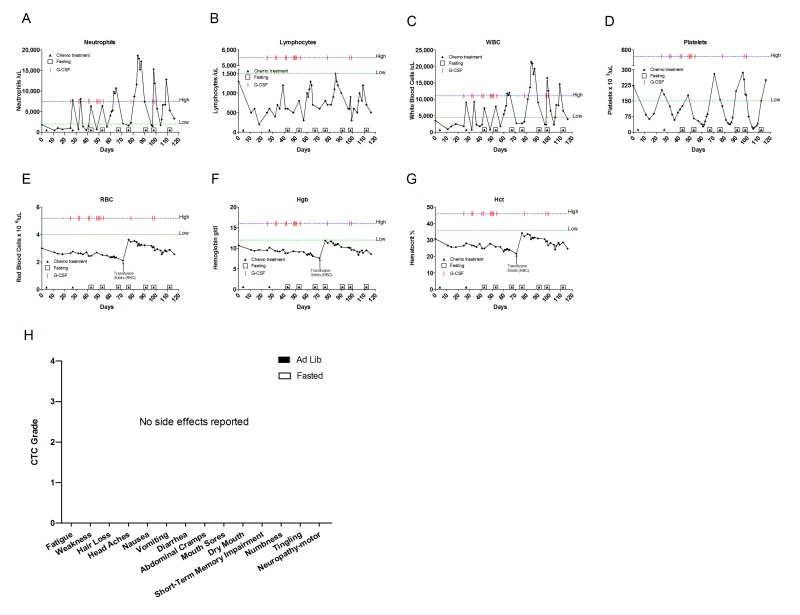
Figure 7. Laboratory values of blood cell counts for case 6. (A) Neutrophils;
(B) Lymphocytes; (C) White blood cells, WBC; (D)
Platelets; (E) Red blood cells, RBC (F) Hemoglobin, Hgb; (G)
Hematocrit, Hct; Filled triangle indicates day of chemotherapy; open square
indicates fasting. Normal ranges of laboratory values are indicated by dash
lines. The patient received red blood cell transfusion (3 units) on day 71
and also received G-CSF (Neulasta) as indicated.

Figure 8. Laboratory values of blood cell counts for case 7. (A)
Neutrophils; (B) Lymphocytes; (C) White blood cells, WBC; (D)
Platelets; (E) Red blood cells, RBC (F) Hemoglobin, Hgb; (G)
Hematocrit, Hct; (H) Prostate specific antigen (PSA) level. Filled
triangle indicates day of chemotherapy; open square indicates fasting,
arrow indicates abiraterone administration. Normal ranges of laboratory
values are indicate by dash lines. The patient also received G-CSF
(Neulasta) as indicated; (I) Self-reported side-effects after
chemotherapy for case 7. Data represent the average of 8 cycles of
chemo-fasting treatments.
Case 7
This is a 66-year-old Caucasian male who was diagnosed
in July 1998 with prostate adenocarcinoma, Gleason score 8. A Prosta Scint
study performed in the same year displayed positive uptake of the radiotracer
in the right iliac nodes, consistent with stage D1 disease. The patient was
treated with leuprolide, bicalutamide and
finasteride. In December 2000, the
diseases progressed. He started on a second cycle with leuprolide acetate and also
received High Dose Rate (HDR) brachytherapy and external beam radiation with
Intensity Modulated Radiation Therapy (IMRT) to the prostate and pelvis. In
April 2008, a Combidex scan revealed a 3 x 5 cm pelvic mass and left
hydronephrosis prompting initiation of TAX chemotherapy supplemented with G-CSF. The patient received 60-75 mg/m2 of TAX for
8 cycles. Throughout this period the patient fasted for 60-66 prior to and 8-24 hours following chemotherapy(Table 2). Side
effects from fasting included grade one
lightheadedness (accordingly CTCAE 3.0) and a drop in blood
pressure, none of which interfered with his routine. Chemotherapy-associated
self-reported side effects included grade one sensory neuropathy (Figure 8I). The
patient's ANC, WBC,
platelet and lymphocyte levels remained in the normal range throughout
treatment, although he did develop anemia (Figure8A-G). PSA levels consistently decreased, suggesting that fasting did not interfere with
the therapeutic benefit of the chemo-treatment (Figure 8H).
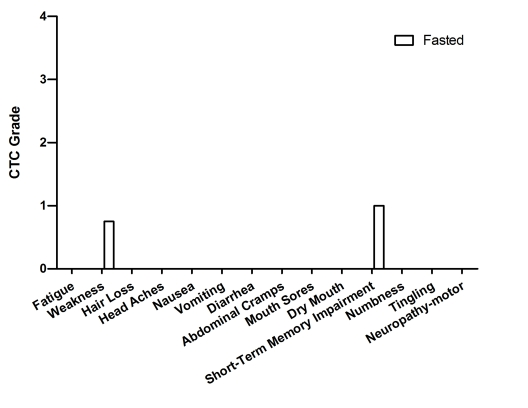
Figure 9. Self-reported side-effects after chemotherapy for case 8. Data represent
the average of 4 cycles of chemo-fasting treatments.
Case 8
This is a 53-year-old Caucasian female who was
diagnosed with stage IIA breast cancer (HER2+) in 2008. After a lumpectomy
procedure, she received 4 cycles of adjuvant chemotherapy with TAX (75mg/m2)
and CTX (600mg/m2) every 21 days. For all 4 cycles the patient
fasted 64 hours prior to and 24 hours post chemotherapy administration (Table 2).
Self-reported side effects included mild weakness and short-term memory
impairment (Figure 9).
Case 9
This is a 48 year-old Caucasian female diagnosed with
breast cancer. Her adjuvant chemotherapy consisted of 4 cycles of doxorubicin
(DXR, 110mg/dose) combined with CTX (1100mg/dose) followed by weekly paclitaxel
and trastuzumab for 12 weeks. Prior to her first chemotherapy treatment, the
patient fasted for 48 hours and reported no adverse effects. During the second
and subsequent cycles the patient fasted for 60 hours prior to the chemotherapy
followed by 5 hours post drug administration (Table 2). She reported no difficulties
in completing the fasting. Although she experienced alopecia and mild weakness,
the patient did not suffer from other commonly reported side effects associated
with these chemotherapy drugs (Figure 10).
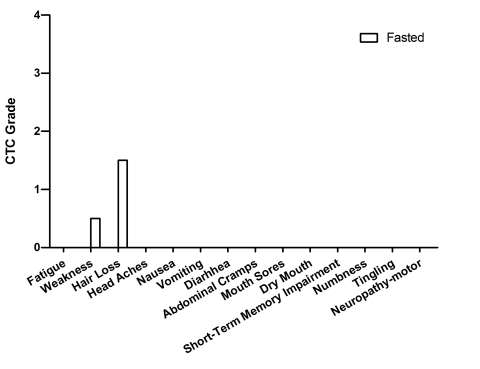
Figure 10. Self-reported side-effects after chemotherapy for case 9. Data represent
the average of 4 cycles of chemo-fasting treatments.
Case 10
This is a 78 year-old Caucasian
female diagnosed with HER2 positive breast cancer. After mastectomy, six cycles
of adjuvant chemotherapy were prescribed with CBDCA 400 mg (AUC= 6), TAX
(75mg/m2) complemented with G-CSF (Neulasta), followed by 6 months of
trastuzumab (Table 2). Throughout the treatmen the patient fasted
prior and after chemotherapy administration. Although the patient adopted fasting
regimens of variable length, no severe side effects were reported (Figure 11H; Table 2). Her WBC, ANC, platelet and lymphocyte
counts remained within normal levels (Figure11A-D) throughout the treatment, but
she developed anemia (Figure11E-G).
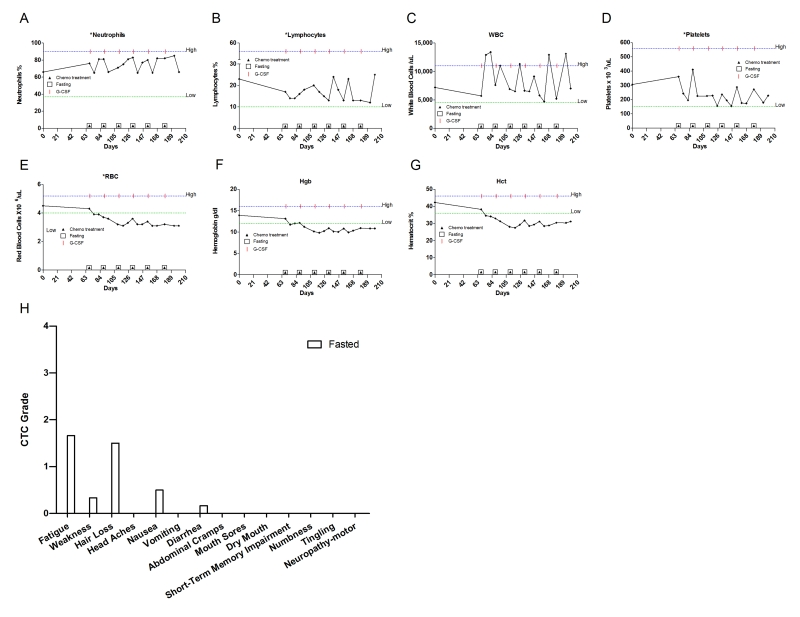
Figure 11. Laboratory values of blood cell counts for case 10. (A)
Neutrophils; (B) Lymphocytes; (C) White blood cells, WBC; (D)
Platelets; (E) Red blood cells, RBC (F) Hemoglobin, Hgb; (G)
Hematocrit, Hct. Filled triangle indicates day of chemotherapy; open square
indicates fasting. Normal ranges of laboratory values are indicated by dash
lines. The patient also received G-CSF (Neulasta) as indicated. (H) Self-reported
side-effects after chemotherapy for case 10. Data represent the average of
6 cycles of chemo-fasting treatments.
Discussion
Dietary recommendations during cancer
treatment are based on the prevention or reversal of nutrient deficiencies to
preserve lean body mass and minimize nutrition-related side effects, such as
decreased appetite, nausea, taste changes, or bowel changes [16].
Consequently, for cancer patients who
have been weakened by prior chemotherapy cycles or are emaciated, many
oncologists could consider a fasting-based
strategy to be potentially harmful. Nevertheless studies in cell culture and animal models indicate
that fasting may actually reduce chemotherapy side effects by selectively
protecting normal cells [9]. Following the publication of this pre-clinical work,several patients, diagnosed with a wide variety of cancers, elected to
undertake fasting prior to chemotherapy and shared their experiences with us.
In this heterogeneous group of men and women fasting was safely repeated in
multiple cycles for up to 180 hours prior and/or following chemotherapy. Minor
complaints that arose during fasting
included dizziness, hunger, and headaches at a level that did not interfere with daily activities. Weight lost
during fasting was rapidly recovered in most of the patients and did not lead
to any detectable harm.
We obtained self-reported assessments of toxicity from
all 10 patients who incorporated fasting with their chemotherapy treatments.
Since many of the chemotoxicities are cumulative, we evaluated serial data
including all the combined fasting- and non-fasting (ad libitum diet)
associated chemotherapy cycles (Supplementary Figure 1). Toxicity was graded
utilizing a questionnaire based on the Common Terminology Criteria for Adverse
Events of National Cancer Institute, version 3.0 (Table 1). Although the lack of
prospective collection of toxicity data and grading are a significant
limitation, this series provide an early insight into the feasibility and
potential benefit of combining fasting with chemotherapy. Fewer and less severe
chemotherapy-induced toxicity was reported by all the patients, even though fasting cycles were often carried out in the later
portion of the therapy (Supplementary Figure 1). Nausea, vomiting, diarrhea, abdominal cramps, and
mucositis were virtually absent from the reports of all 10 patients in the
cycles in which fasting was undertaken prior to and/or following chemotherapy;
whereas at least one of these symptoms was reported by 5 out of the 6 patients
during cycles in which they ate ad libitum (Supplementary Figure 1). The four
patients that fasted throughout their treatments reported low severity for the
majority of the side effects, in contrast to the typical experience of cancer
patients receiving the same chemotherapy regimens (Figures 8I, 9, 10, 11H). For the 6 patients who received chemotherapy with or without
fasting, we compared the severity of the self-reported side effects in the 2
closest fasting/non-fasting (ad libitum diet) cycles in which the
patient received the same chemotherapy drugs at the same dose. There was a
general and substantial reduction in the self-reported side effects in
combination with fasting (Figure 1). Symptoms such as fatigue and weakness were
reported to be significantly reduced (p< 0.001 and p< 0.00193,
respectively), whereas vomiting and diarrhea were virtually absent in
combination with fasting (Figure 1). In addition, there was no side effect
whose average severity was reported to be increased during fasting-chemotherapy
cycles (Figure 1 and Supplementary Figure 1).
Challenging conditions such as fasting or
severe CR stimulate organisms to suppress growth and reproduction, and divert
the energy towards cellular maintenance and repair to maximize the chance of
survival [17,19]. In simple organisms such as yeast, resistance to
oxidants and chemotherapy drugs can be increased by up to 10-fold in response
to fasting/starvetion and up to 1,000-fold in those cells lacking homologs of
Ras, AKT and S6 kinase [9]. Nevertheless, such protection and oxidative stress
resistance is completely reversed by the expression of oncogene-like genes [9,18]. In
mammals, the mechanism(s) responsible for the protective effect of fasting against
chemotherapy induced-toxic side effects is not completely understood. It may
involve reduction in anabolic and mitogenic hormones and growth factors such as
insulin and insuline-like growth factor 1 (IGF-1) as well as up-regulation of
several stress resistance proteins[20-25]. In fact, mice with liver specific IGF-I
gene-deletion (LID) which have ~80% reduction of circulating IGF-I and mice
with genetic disruptions in the IGF-I receptor (heterozygous knockout IGF-IR
+/-) or its downstream elements have been shown to be more resistant against
multiple chemotherapy agents and oxidative stress, respectively [26,27].
Alternatively, fasting-dependent DSR may be, in part, mediated by cell cycle arrest
in normal cells whereas transformed cells continue to proliferate, remaining
vulnerable to anticancer drugs [25,28]. Although mutations driving cancer progression are
heterogeneous across tumor types, the majority of the oncogenic mutations
render cancer cells independent of growth signals [28,29], which we hypothesize
prevents cancer cells from responding to the fasting-induced switch to a
protected mode [9]. Therefore, DSR would have the potential to be
applied independently of the cancer type. Although this has not been yet
demonstrated, the remarkable effects of fasting on the down-regulation of a
number of growth factors and signal transduction pathways targeted by
anti-cancer drugs, including IGF-I and the TOR/S6 kinase pathways, raises the possibility
that it could enhance the efficacy of cancer treatment drugs and may even be as
effective as some of them.
In summary, in this small and
heterogeneous group of cancer patients, fasting was well-tolerated and was
associated with a self-reported reduction in multiple chemotherapy-induced side
effects. Although bias could affect the estimation of the side effects by the
patients, the case reports presented here are in agreement with the results
obtained in animal studies and provide preliminary data indicating that
fasting is feasible, safe and has the potential to differentially protect
normal and cancer cells against chemotherapy in humans. Nevertheless, only a
clinical trial, such as the randomized controlled clinical trial currently
carried out at the USC Norris Cancer Cen-ter, can establish whether fasting
protects normal cells and increases the therapeutic index of chemotherapies.
We thank the patients, nurses and oncologists at a
number of clinics for devoting a considerable amount of time to collecting the
information contained in this case series. We
thank Dr. Charles Loprinzi and Dr. Roxana Dronca for valuable comments and
suggestions. This study was sponsored in
part by the Bakewell Foundation.
The authors of this
manuscript have no conflict of interest to declare.