I. Circadian rhythms,
well-being, and life span
Organisms on earth evolved to restrict their activity to the night or day, being
nocturnal or diurnal, respectively. By developing an endogenous circadian
(circa - about; dies - day) clock, which can be entrained to external stimuli, primarily light, animals and plants ensure that physiological processes are performed at the appropriate, optimal time of day or night [1]. Adaptation to external conditions through clock entrainment imparts a survival advantage, as the organism can predict environmental changes [1-3]. The clock core machinery is self sustained, so that in the absence of external cues, e.g., in constant darkness, the endogenous rhythms free-run, generating
cycles of approximately but not exactly 24 hours.
In mammals, the circadian clock influences nearly all aspects of physiology and behavior, such as sleep-wake cycles, cardiovascular activity, endocrine system, body temperature, renal activity, physiology of the gastrointestinal tract, and hepatic metabolism [1,2]. Epidemiological studies indicate that myocardial infarction, pulmonary edema, hypertensive crises, and asthma and allergic rhinitis attacks, all peak at certain times during the day [4-6]. Disruption of circadian coordination in humans or animals is manifested by hormone imbalance, some aspects of disease, and reduced life span [2,7-12].For instance, psychological and sleep disorders [2] and cardiovascular diseases [13,14] can be associated with irregular or dysfunctional circadian clock. Disruption of circadian coordination can also accelerate cancer proneness and malignant growth in animals and humans, suggesting that the circadian clock controls tumor progression [8-10]. In addition, symptoms seen in jet lagged travelers, e.g., fatigue, disorientation, and insomnia, or in shift workers, e.g., altered hormone profiles and morbidity, result from the constant need to extend wakefulness or to repeatedly invert the normal sleep-wake cycle [10,15,16]. Also, chronic reversal of the external light-dark cycle at weekly intervals resulted in a significant decrease in the survival time of cardiomyopathic hamsters [7]. Importantly, circadian rhythms change with normal aging in animals and humans, including a shift in the phase and decrease in amplitude [15-18]. By using a more direct approach, it was shown thatlongevity was diminished in golden hamsters carrying a 20 h-period mutation, tau, raised in 24 h light-dark cycles [19]. On the contrary, life span was extended in aged animals given fetal suprachiasmatic implants that restore higher amplitude rhythms [19-21]. Altogether, it seems that circadian disruption is associated with multiple negative manifestations, whereas resetting of circadian rhythms could lead to increased longevity. These findings, although largely correlative, point to a critical role for the circadian clock in maintaining normal peripheral physiology.
II. The circadian clock
A. The location of the mammalian circadian clock.
In mammals, the central circadian clock is located in the suprachiasmatic nuclei (SCN), a distinct bilateral group
of cells located in the anterior hypothalamus in the brain. Similar clock oscillators have been found
in many peripheral tissues, such as the liver, intestine, heart, adipose tissue, retina and in various
regions of the brain [2,22-24].
The SCN clock is composed of multiple, intracellular circadian oscillators, which, when synchronized,
generate coordinated circadian outputs that regulate overt rhythms [25-28].
SCN oscillation is not exactly 24 h and it is necessaryto entrain the circadian pacemaker each day to the external
light-dark cycle to prevent drifting (or free-running) out of phase. Light perceived primarily by melanopsin-expressing
retinal ganglion cells transmit signals to the SCN via the retinohypothalamic tract (RHT)
[2,29,30].
As a result, vasoactive intestinal polypeptide (VIP), an intrinsic SCN factor, acutely activates and synchronizes
SCN neurons [31,32]. Synchronization among SCN neurons leads
to the sending of signals to peripheral oscillators to prevent the dampening of circadian rhythms in these tissues.
The SCN accomplishes this task via neuronal connections or circulating humoral factors [33]
although the mechanisms are not fully understood (Figure 1). Several humoral factors expressed cyclically by the SCN,
such as transforming growth factor α (TGFα) [34], prokineticin 2 (PK2) [35],
and cardiotrophin-like cytokine (CLC) [36], have been shown to affect peripheral clocks.
Their intracerebroventricular injection inhibits nocturnal locomotor activity, an SCN output.
Complete electrical destruction of SCN neurons abolishes overall circadian rhythmicity in SCN-controlled tissues,
because of the loss of synchrony among individual cells in the periphery and damping of the rhythm at the population level
[37]. However, at the cellular level each cell oscillates, but with
a different phase [37,38].
The fraction of cyclically expressed transcripts in each peripheral tissue ranges between 5-20% of the total
population and the vast majority of these genes are tissue-specific [24,39-47].
These findings emphasize the circadian control over a large portion of the transcriptomes in peripheral tissues.
Considering the circadian gene expression in peripheral tissues, it is difficult to determine whether the SCN
clock drives these rhythmic patterns directly or indirectly by driving rhythmic feeding, activity, and/or body temperature,
which, in turn, contribute to rhythms in gene expression in the periphery.
It has been shown that for a peripheral tissue, such as the liver, signals both from the SCN clock or the local
endogenous clock may control rhythmic gene expression [48,49].
B. The biological clock at the
molecular level.
Genetic analysis of mutations
affecting the clock in Neurospora, Drosophila, Cyanobacteria, Arabidopsis,
and, recently, the mouse have paved the way for the identification of clock
genes. In mammals, the clock is an intracellular mechanism sharing the same
molecular components in SCN neurons and peripheral cells [3]. Generation of
circadian rhythms is dependent on the concerted co-expression of specific clock
genes. Transcriptional-translational feedback loops
lie at the very heart of the core clock mechanism. Many clock gene
products function as transcription factors, which
possess PAS (PER, ARNT, SIM) and basic helix-loop-helix (bHLH) domains
involved in protein-protein and protein-DNA interactions, respectively. These
factors ultimately activate or repress their own
expression and, thus, constitute self-sustained transcriptional feedback loops. Changes in concentration, subcellular localization, post-transcriptional microRNA
regulation, posttranslational modifications (phosphorylation, acetylation,
deacetylation, SUMOylation), and delays between transcription and
translation are crucial in order to achieve a 24-h cycle [1,2,50-52].
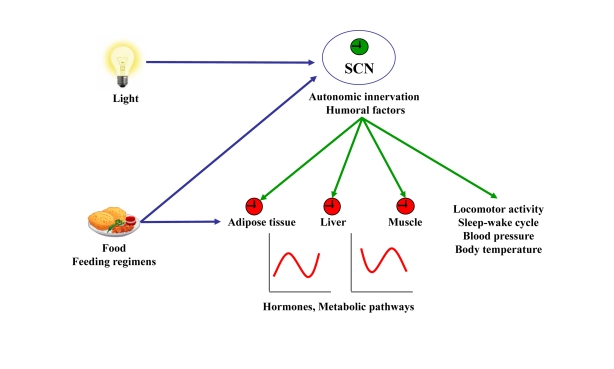
Figure 1. Resetting signals of the central and peripheral clocks.
The SCN resets peripheral oscillators via
humoral factors or autonomic innervation leading to circadian hormone
expression and secretion and rhythmic activity of metabolic pathways. In
addition, the SCN dictates rhythms of locomotor activity, sleep-wake cycle,
blood pressure, and body temperature. Light, food, and feeding regimens
affect either the central clock in the SCN or peripheral clocks. Input to
central or peripheral clocks are in blue. Outputs from the central clock to
the periphery are in green.
In the mouse, the first clock gene identified, encodes the
transcription factor CLOCK (Circadian Locomotor
Output Cycles Kaput) [53], which dimerizes with BMAL1 (brain and muscle ARNT-like protein 1) to activate
transcription. CLOCK and BMAL1, two bHLH-PAS
transcription factors, are capable of activating transcription upon binding to
E-box (5'- CACGTG -3') and E-box-like promoter sequences [2]. BMAL1 can also
dimerize with other CLOCK homologs, such as neuronal PAS domain protein
2 (NPAS2), to activate transcription and sustain
rhythmicity [54,55]. Amongst the regulatory targets of CLOCK:BMAL1 are
the three Period genes (Per1, Per2, and Per3),
which encode PAS domain factors, and two Cryptochrome genes (Cry1
and Cry2). PERs and CRYs function as negative regulators, blocking
CLOCK:BMAL1-mediated transcriptional activation [2,56] (Figure 2A).Thus, CLOCK:BMAL1
heterodimers bind to E-box sequences and mediate transcription of a large number of genes including those of
the negative feedback loop Pers and Crys. When PERs and CRYs are
produced in the cytoplasm, they
oligomerize after reaching an appropriate concentrationand translocate to the nucleus to inhibit
CLOCK:BMAL1-mediated transcription. All the aforementioned clock genes
exhibit a 24-h oscillation in SCN cells and peripheral tissues, except for Clock
that has been shown not to oscillate in the SCN [50]. Recent studies have
demonstrated that CLOCK has intrinsic histone acetyltransferase activity,
suggesting that rhythmic activation of chromatin remodeling may underlie the
clock transcriptional network [57,58]. Indeed, cyclic histone acetylation and
methylation have been observed on the promoters of several clock genes [58-63].
In addition, CLOCK also acetylates several proteins of the core clock
apparatus, thus, enabling cycles of acetylation and deacetylation, the latter
activity involving SIRT1 will be discussed below (Figure 2).
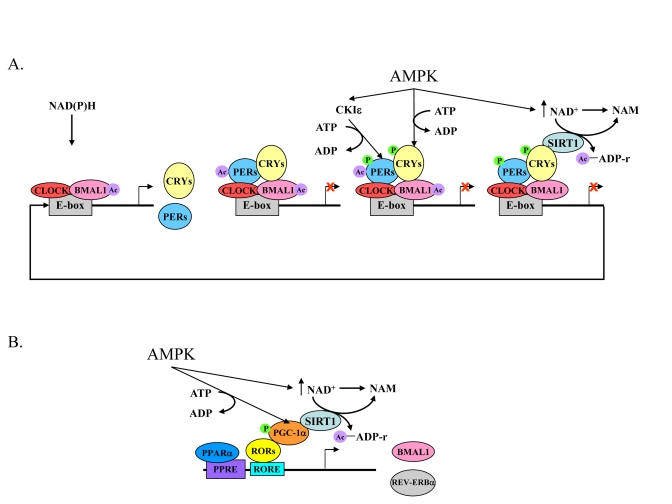
Figure 2. The core mechanism of the mammalian circadian clock and its link to energy metabolism.
(A) High NAD(P)H levels promote
CLOCK:BMAL1 binding to E-box sequences leading to the acetylation of BMAL1
and expression of Pers, Crys, and other clock-controlled genes. The
negative feedback loop, PERs:CRYs, binds to CLOCK:BMAL1 and consequently
PERs are acetylated. Activated AMPK leads to a rise in NAD+ levels,
phosphorylation of CRYs, and phosphorylation of CKI?, which then phosphorylates
the PERs. As a result of increased NAD+ levels, SIRT1 deacetylates PERs and BMAL1.
This and the destabilization of phosphorylated PERs and CRYs relieves PERs:CRYs
repression and another cycle starts. (B) Expression of Bmal1 and Rev-erbα
genes are controlled by PPARα and binding of RORs to RORE sequences. RORs
need a co-activator, PGC-1α, which is phosphorylated by activated AMPK. In
parallel, AMPK activation leads to an increase in NAD+ levels, which, in turn
activate SIRT1. SIRT1 activation leads to PGC-1α deacetylation and activation.
Acetyl adenosine diphosphate ribose (Ac-ADP-r) and nicotinamide (NAM) are released
after deacetylation by SIRT1.
Several other players appear
to be important to sustain clock function. Casein kinase I
epsilon (CKIε) phosphorylates the PER proteins and, thereby, enhances
their instability and degradation [50,64-66]. CKIε also phosphorylates and
partially activates the transcription factor BMAL1 [67]. Bmal1
expression is nega�tively regulated by the transcription factor reverse
erythroblastosis virus α (REV-ERBα)
[68], which recruits histone
deacetylase (HDAC) complexes [69]. Bmal1 expression is positively regulated by retinoic acid receptor-related orphan
receptor α (RORα) and RORγ [70]via the ROR response element (RORE) [71]. Thus, Bmal1 oscillation is driven by a rhythmic change in
RORE occupancy by RORs and REV-ERBα. This alternating occupancy occurs due
to the robust rhythmic levels of REV-ERBα, a result of direct
transcriptional activation of the Rev-erbα gene by the heterodimer
CLOCK:BMAL1 [68]. Indeed, mice
deficient in RORα or REV-ERBα have impaired circadian rhythms of
locomotor activity and clock gene expression [68,70] (Figure 2B).
III. The biological clock and energy homeostasis
A. Circadian rhythms and metabolism
The circadian clock has been reported to regulate
metabolism and energy homeostasis in peripheral tissues [72,73]. This is
achieved by mediating the expression and/or activity of certain metabolic
enzymes and transport systems [74,75] involved in metabolic pathways [76-80].
In addition, a large number of nuclear receptors involved in lipid and glucose
metabolism has been found to exhibit circadian expression [81]. Many hormones
involved in metabolism, such as insulin [76], glucagon [82], adiponectin [83],
corticosterone [84], leptin, and ghrelin [85,86], have been shown to exhibit
circadian oscillation. Leptin, an adipocyte-derived circulating hormone, acts
at specific receptors in the hypothalamus to suppress appetite and increase
catabolism. Leptin exhibits striking circadian patterns in both gene expression
and protein secretion, with peaks during the sleep phase in humans [87].
Neither feeding time nor adrenalectomy affected the rhythmicity of leptin
release. However, ablation of the SCN has been shown to eliminate leptin
circadian rhythmicity in rodents, suggesting that the central circadian clock
regulates leptin expression [88]. Receptors for leptin and ghrelin are present
on SCN cells [89-91], so it is possible that these hormones bind directly to
SCN neurons, similarly to their effect on the orexigenic neuropeptide Y (NPY)
and agouti-related protein (AgRP) neurons. Indeed, exogenous leptin was
reported to phase-advance rhythms of neuronal firing in rat SCN slices [92].Activation of ventromedial arcuate nucleus (vmARC) neurons by systemic
administration of the ghrelin mimetic growth hormone-releasing peptide 6 combined
with SCN tracing showed that vmARC neurons transmit feeding-related signals to
the SCN [90]. This injection induced Fos in the vmARC and resulted in
attenuation of light-induced phase delay in mice and light-induced Fos
expression in the SCN in rats [93]. Administration of ghrelin in vitro
to SCN slices or SCN explants from Per2::luc transgenic mice caused
phase shifts in Per2::luc reporter gene expression. However, intra-peritoneal
injection of ghrelin to wild type mice caused phase shifts only after 30 h of
food deprivation, but not when the mice were fed ad libitum [94]. Thus,
it seems that ghrelin and leptin may affect the SCN directly or through their
effect on the ARC, which is then relayed to the SCN.
Experiments using cultured cells have suggested that the cellular redox state is
capable of influencing rhythms [95]. CLOCKand itshomolog
NPAS2 can bind efficiently to BMAL1 andconsequently to E-box sequences in the
presence of reduced nicotinamide adenine dinucleotides (NADH and NADPH)
(Figure 2A). On the other hand, the oxidized forms of the nicotinamide adenine
dinucleotides (NAD+ and NADP+) inhibit DNA binding of
CLOCK:BMAL1 or NPAS2:BMAL1 [95,96].
As the NAD(P)+/NAD(P)Hredox equilibrium depends on the metabolicstate of
the cell, this ratio could dictate the binding of CLOCK/NPAS2:BMAL1 to E-boxes and
result in phase-shifting of cyclic gene expression [74,95,96].
NAD+ is also required for the activation of SIRT1, a deacetylase involved in
clock modulation, as will be discussed below.
B. The circadian clock and crucial metabolic factors are tightly linked
BMAL1
: Circadian clocks have been shown to be present in several fat tissues, including inguinal white adipose tissue, epididymal white adipose tissue, and brown adipose tissue [45,97,98]. Recent transcriptome studies revealed rhythmic expression of clock and adipokine genes, such as resistin, adiponectin, and visfatin, in visceral fat tissue [83]. Recent molecular studies established the involvement of BMAL1 activity in the control of adipogenesis and lipid metabolism in mature adipocytes. Embryonic fibroblasts from Bmal1-/- knockout mice failed to differentiate into adipocytes. Loss of BMAL1 expression led to a significant decrease in adipogenesis and gene expression of several key adipogenic/lipogenic factors. Furthermore, over-expression of BMAL1 in adipocytes increased lipid synthesis activity. Thus, BMAL1, a master regulator of circadian rhythms, plays important roles in the regulation of adipose differentiation and lipogenesis in mature adipocytes [99].
REV-ERBα
: Another important candidate to link the circadian clock with lipid metabolism is REV-ERBα. This pro-adipogenic transcription factor, whose levels increase dramatically during adipocyte differentiation [100], exhibits striking diurnal variations in expression in murine adipose tissue [101] and rat liver [102]. During adipocyte differentiation, REV-ERBα acts downstream of the differentiation factor peroxisome proliferator receptor-γ (PPARγ) by facilitating gene expression of PPARγ target genes [103,104]. Ectopic REV-ERBα expression in 3T3L1 pre-adipocytes promotes their differentiation into mature adipocytes [103]. In addition to its role in lipid metabolism and adipocyte differentiation, REV-ERBα is a component of the core clock apparatus, as mentioned above (Figure 2B). It acts as a negative regulator of Bmal1 expression, and its encoding gene, Rev-erbα, is directly activated by the CLOCK:BMAL1 heterodimer [68].
PPARα
: Peroxisome proliferator-activated receptor α (PPARα) is a member of the nuclear receptor family that plays a unique role at the intersection of circadian and lipid metabolic pathways. The CLOCK:BMAL heterodimer mediates the expression of PPARα, which subsequently binds to the peroxisome-proliferator response element (PPRE) and activates transcription of Bmal1 [105-107] (Figure 2B). PPARα also regulates transcription of genes involved in lipid and glucose metabolism upon binding of endogenous free fatty acids [108,109]. Thus the circadian rhythmicity of PPARα provides an example of a reciprocal link between circadian and lipid metabolic processes.
PPARγ coactivator (PGC-1α)
: PGC-1α, a transcriptional co-activator that regulates energy metabolism, is rhythmically expressed in the liver and skeletal muscle of mice. PGC-1α stimulates the expression of Bmal1 and Rev-erbα, through co-activation of the ROR family of orphan nuclear receptors [110,111] (Figure 2B). Mice lacking PGC-1α show abnormal diurnal rhythms of activity, body temperature, and metabolic rate, due to aberrant expression of clock genes and those involved in energy metabolism. Analyses of PGC-1α-deficient fibroblasts and mice with liver-specific knockdown of PGC-1α indicate that it is required for cell-autonomous clock function [110].
AMP-activated protein kinase (AMPK)
: AMPK could be another important link that integrates the circadian clock with metabolism. AMPK is a sensor of the energy status within cells, which upon activation acts to restore energy balance [112,113]. This is done in part by modulating NAD+ levels and SIRT1 activity [114,115]. AMPK has been found to directly phosphorylate Ser-389 of CKIε in Rat-1 fibroblasts, resulting in increased CKIε activity and degradation of mPER2 (Figure 2A). mPER2 degradation led to a phase advance in the circadian expression pattern of clock genes [116]. AMPK has also been shown to phosphorylate and destabilize mCRY1 in mouse fibroblasts, leading to altered circadian rhythms [117] (Figure 2A). In addition, the expression profile of clock-related genes, such as Per1 and Cry2 in skeletal muscle, as well as the diurnal shift in energy utilization, is impaired in AMPKγ3 subunit knockout mice in response to 5-amino-4-imidazole-carboxamide riboside (AICAR), an AMPK activator [118]. In addition to its intracellular role, AMPK is involved in whole body energy metabolism by regulating the response to feeding in the hypothalamus [112]. In this brain area, AMPK activation is inhibited by leptin and insulin, hormones which suppress feeding, whereas it is activated under starvation by ghrelin, a hormone primarily produced by the stomach that leads to increased food intake [119-122].
SIRT1
: Another protein recently found to link metabolism
with the circadian clock is SIRT1. This is the mammalian ortholog of yeast
Sir2, an NAD+-dependent histone deacetylase involved in
transcriptional silencing and genome stability in yeast [123,124]. Sir2 or its
ortholog enzymes are involved in life span extension and the response to
caloric restriction in yeast, Caenorhabditis
elegans, Drosophila [123,125], and mice [115,126]. The dependence on NAD+ as a cofactor for catalysis is thought
to link SIRT1 activity to the energy state of the cell [127]. Non-histone
substrates of SIRT1, as found in C2C12 myotubes, include regulatory molecules
that modulate energy metabolism, such as PPARγ and PGC-1α [114], key
factors that regulate the core molecular clock (Figure 2). Recent studies showthat SIRT1 interacts directly with CLOCK and
deacetylates BMAL1 and PER2 in cultured fibroblasts [128,129] (Figure 2A). It
seems that after binding to E-box, CLOCK and CBP/p300 acetylate histones H3 and
H4 [57] and BMAL1 [130]. BMAL1 acetylation potentiates its binding by the
repressive PER/CRY complex [130] and, as a result, PER2 is acetylated [128].
When acetylated, PER2 [128] and possibly BMAL1 [129] are more stable. SIRT1
then becomes activated and deacetylates BMAL1, PER2, and histones [131].
Deacetylated PER2 is further phosphorylated and degraded and a new cycle begins
(Figure 2A). It has also been shown that the CLOCK:BMAL1 heterodimer regulates
the circadian expression of NAMPT (nicotinamide phosphoribosyl-transferase), a
rate-limiting enzyme in the NAD+ salvage pathway. SIRT1 is recruited
to the Nampt promoter and contributes to the circadian synthesis of its
own coenzyme [132]. Most recently, it has been shown that AMPK enhances SIRT1
activity by increasing cellular NAD+ levels, resulting in the
deacetylation and modulation of the activity of downstream SIRT1 targets [114].
Thus, the levels of NAD+ together with the cycling of SIRT1 can
determine the activity and robustness of clock gene transcription at least in
cultured cells.
C. Clock mutations and metabolic disorders
The most compelling linkage between metabolic
disorders and the circadian clock is demonstrated by the phenotypes of clock
gene mutants and knockouts. Homozygous C57BL/6J ClockΔ19 mice,
with a truncated exon 18 and deleted exon 19 of the Clock gene, have a
greatly attenuated diurnal feeding rhythm, are hyperphagicand
obese, and develop a metabolic syndrome of hyperleptinemia,hyperlipidemia,
hepatic steatosis, and hyperglycemia [133]. Loss of circadian rhythms in ClockΔ19 mutant
mice was accompanied by attenuated expression of hypothalamic peptides associated
with energy balance, such as ghrelin and orexin [133]. Insulin administration
caused significantlygreater hypoglycemia in ClockΔ19 mutant
mice than in wildtype mice [134]. Increased insulin sensitivity was
also seen in ClockΔ19 mutant mice of the BALB/c/CBA
background that preserve rhythmicity in melatonin production [135]. In ClockΔ19 on an
Jcl:ICR background, serum levels of triglyceride and free fatty acids were
significantly lower than in wild type control mice, whereas total cholesterol
and glucose, insulin, and leptin levels did not differ [136]. Unlike C57BL/6J
ClockΔ19 mutant
mice, Jcl:ICR ClockΔ19 mutantmice were not obese, had low or
normal fasting plasmaglucose, low plasma free fatty acids, and
normal plasma leptin. However, in Jcl:ICR
ClockΔ19 mutant mice, high
fat diet amplified the diurnal variation in glucose tolerance and insulin
sensitivity, and obesity was attenuated through impaired dietary fat absorption
[136]. Although the effects on metabolism were variable due to strain
differences, the overall picture is that disruption of the clock gene leads to
disruption of metabolic pathways.
Bmal1-/- knockout mice, similarly to C57BL/6J ClockΔ19mutant
mice, exhibited suppressed diurnal variations in glucose and triglycerides as
well as abolished gluconeogenesis. Liver-specific deletion of Bmal1
showed a direct effect of the liver clock on glucose metabolism, as exhibited
by hypoglycemia during fasting, exaggerated glucose clearance, and loss of
rhythmic expression of hepatic glucose regulatory genes [137]. Although
recovery from insulin-induced hypoglycemia was impaired in C57BL/6J ClockΔ19 mutant
and Bmal1-/- knockout mice, the counter-regulatory responses
of corticosterone and glucagon were retained [134].
Mutation in another central clock gene, Per2 (mPer2-/-
mice), exhibited no glucocorticoid rhythm even though the corticosterone
response to hypoglycemia was intact. In addition, the diurnal feeding rhythm
was absent in these mice. Although food consumption was similar during the
light and dark periods on high fat diet, mPer2-/- mice
developed significant obesity [138].
IV. Effect of feeding regimens on circadian rhythms
In addition to light, feeding regimens have been reported to affect the clocks in the SCN and/or the periphery.
A. Restricted feeding (RF)
RF limits the time and duration of food availability with no calorie reduction [3,74,139].Animals, which receive food ad libitum everyday at the same time for only a few hours, adjust to the feeding period within a few days [49] and can consume their daily food intake during that limited time [140,141]. Restricting food to a particular time of day has profound effects on the behavior and physiology of animals. Many physiological activities that are normally dictated by the master clock in the SCN are altered by RF, such as hepatic P450 activity, body temperature, locomotor activity, and heart rate [142-145]. 2-4 h before the meal, the animals display food anticipatory activity (FAA), which is typifiedby an increase in locomotor activity, body temperature, corticosterone secretion,gastrointestinal motility, and activity of digestive enzymes [140,146-148], all are known output systems of the biological clock. RF is dominant over the SCN and drives rhythms in arrhythmic and clock mutant mice and animals with lesioned SCN, regardless of the lighting conditions [142,143,148-151]. In most incidents, RF affects the core clock apparatus in peripheral tissues, such as liver (Figure 3), kidney, heart, and pancreas, with no effect on the central pacemaker in the SCN [3,74,139,143,150,152,153], causing uncoupling from the central pacemaker in the SCN. This suggests that nutritional regulation of clock oscillators in peripheral tissues may play a direct role in coordinating metabolic oscillations [154]. As soon as food availability returns to normal, the SCN clock, whosephase remains unaffected,resets the peripheral oscillators [152]. The location of this food-entrainable oscillator (FEO) has been elusive. Lesions in the dorsomedial hypothalamic nucleus (DMH) [155-158], the brain stem parabrachial nuclei (PBN) [156,159], and the core and shell regions of nucleus accumbens [160,161] revealed that these brain regions may be involved in FEO output, but they cannot fully account for the oscillation [162]. Neither vagal signals nor leptin are critical for the entrainment [163,164].CLOCK [165] or BMAL1 [166] and other clock genes [167] have been shown not to be necessary for food anticipatory activity. However, it has recently been demonstrated that mPer2 mutant mice did not exhibit wheel-running food anticipation [168,169]. Recently, the FEO was suggested to be localized, in part, in ghrelin-secreting cells in the stomach [170]. Clearly, the localization and nature of the FEO and the effect of RF on circadian rhythms warrants further study.
The effect of RF on ageing and longevity has never been studied. Interestingly, the survival of Glasgow osteosarcoma-inoculated mice was prolonged under an RF regimen during the light period compared to those under the dark period or those fed ad libitum [171]. Also, RF modified the expression of genes involved in carcinogenesis and tumor progression, such as c-myc and p53 [172]. It remains to be determined whether RF feeding affects life span.
B. Calorie restriction (CR)
CR, sometimes denoted dietary restriction (DR), refers
to a dietary regimen low in calories without malnutrition, that restricts the
daily amount of calories derived from carbohydrates, fats, or proteins
usually to 60-75% of ad libitum-fed animals.
CR extends the life span of diverse species, such as C. elegans, Drososphila,
rodents [125,173,174], and recently monkeys [175]. CR in mice, rats,
and monkeys prevents or delays the onset of major age-related diseases, such as
cancer, diabetes, kidney disease, and cataracts [173,176]. In humans,
long-termed CR results in sustained beneficial effects on major risk factors
for atherosclerosis, type 2 diabetes, and inflammation [177]. The reduction of
energy intake is considered to be the critical beneficial factor in the CR
regimen [173]. Theories on how CR modulates aging and longevity abound, but the
exact mechanism is still unclear [178]. For a longtime,
the most prevalent explanation was related to the widely acceptable theory on
aging, the Free Radical Theory. This theory attributes the aging process to the
continuousaccumulation of oxidative damage
in macromolecules generated by reactive oxygen species (ROS) produced in the
mitochondria [179]. A later variation of this theory, the Oxidative
Stress Theory, attributes the oxidative damage to the imbalance between
preoxidant and antioxidant components, and CR was suggested to increase the
resistance to oxidative stress [180]. Recently, this explanation was put into
question, at least for rodents, as increasing oxidative stress by several
genetic alterations increased aging-related diseases, such as cancer, but did
not diminish life span [181-183]. ROS, primarily H2O2,
have recently been suggested to promote aging as activators of the TOR (target
of rapamycin) pathway [184]. This
signaling pathway acts as a sensor of the nutritional and energetic state in
the cell and transmits anabolic signals to regulate cell size, growth, and
metabolism. Mammalian TOR (mTOR) could play an important role in the regulation
of life span, as indicated by findings showing that CR attenuated mTOR
signaling in several tissues in mice [185], and mice
deficient of ribosomal protein S61 kinase 1, a central component in mTOR signaling, or mice treated with rapamycin, an inhibitor of the mTORC1 component, exhibited
increased life span [186,187].
Interestingly, mTOR has also been recently linked to the circadian clock as a
light-activated signaling cascade in the SCN of mice [188].
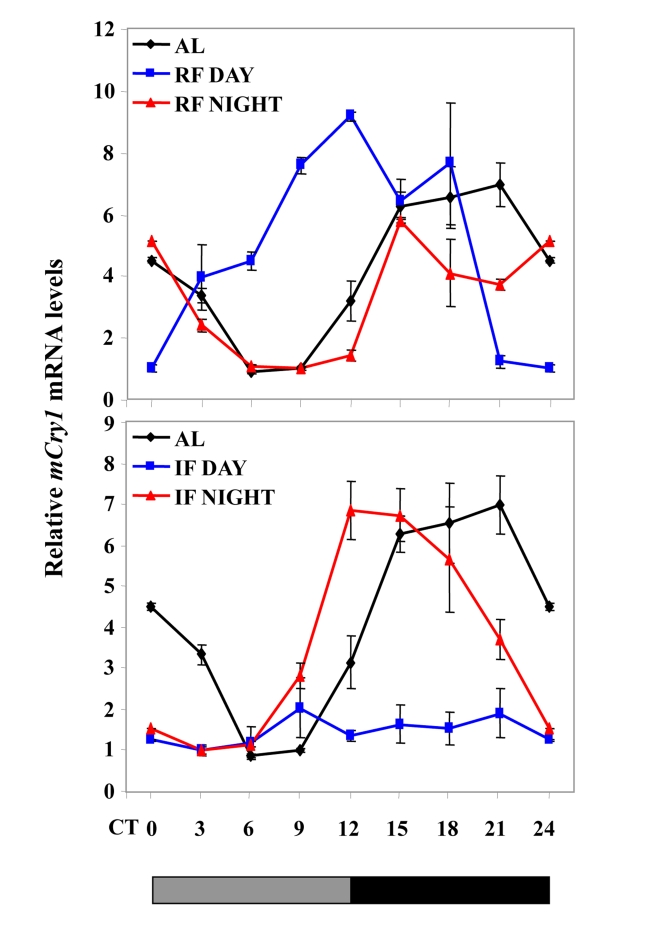
Figure 3. Effect of night vs. day RF and night vs. day IF on clock gene expression. Expression
of a representative clock gene mCry1 was measured in the liver of
C57BL mice during
ad libitum (AL), day and night RF, and day and night IF.Total
RNA extracted from liver tissue collected every 3 h around the circadian
cycle (mean ± SEM; n=3 for each time-point and each mouse group) was
reverse transcribed and analyzed by quantitative real time PCR. Clock gene levels
were normalized using Gapdh as the reference gene. The grey and
black bars designate the subjective light and dark cycles, respectively.
CR-fed animals resemble RF-treated animals, as they
usually consume all or most of their food within a short period of time. While
anticipating for food, calorically restricted animals show a rise in body
temperature [189]. Thus, due
to the temporal component of food intake, it is possible that CR, similarly to
RF, synchronizes peripheral clocks and influences clock-controlled output
systems, such as the anticipatory behavior and body temperature. As opposed to
RF, CR entrains the clock in the SCN [190-192]. Under
light-dark conditions and daytime feeding, calorically restricted mice showed
strong FAA but with a phase advance of the nocturnal pattern of activity, a
direct output of the SCN clock. When mice were transferred to dark-dark
conditions, i.e. under free-running conditions, and fed ad libitum, the
onset of the nocturnal period of locomotor activity occurred significantly
earlier (1.3 h) in the calorically restricted than in ad libitum-fed
animals, indicating an SCN effect. The period, however, did not differ between
calorically restricted and ad libitum-fed mice [190]. Also,
when SCN clock gene expression was tested, slight changes in gene expression
were observed [191,192]. Overall, these results suggest that CR during the
daytime affects the temporal organization of the SCN clockwork and circadian outputs in mice under light-dark cycle. In
addition, CR affects photic responses of the circadian system, as measured by
light pulses, suggesting that energy metabolism modulates gating of photic
inputs in mammals [192]. It is noteworthy that
microarray data comparing gene expression in seven different tissues under CR
identified circadian rhythms among the three most over-expressed biological
processes, with Per2 being the most up-regulated gene [193]. Collectively,
these findings suggest that synchronization of peripheral oscillators
during CR could be achieved directly due to the temporal feeding, as has been
reported for RF [143,152,153], or by synchronizing the SCN [190-192], which, in turn, sends humoral
or neuronal signals to entrain the peripheral tissues [194,195]. It is not known whether there
is dominancy or harmony between the central pacemaker and peripheral
oscillators under CR.
C. Intermittent fasting (IF)
During
IF, also denoted alternate day fasting (ADF), food is available ad libitum
every other day. IF-treated mice eat on the day they have access to food
approximately twice as much as those having continuous access to food [196-198]. Similarly to
calorically restricted animals, IF-fed animals exhibit increased life span in
comparison with the ad libitum-fed control, even if there is little or
no overall decrease in calories [199,200]. IF-fed
animals also exhibit improved glucose metabolism, cardio-protection,
neuro-protection [196,201-205], and increased
resistance to cancer [197,200]. IF may also
decrease the risk for cardiovascular diseases in humans [206].The IF-induced beneficial effects are thought to
occur independently of the overall caloric intake, but the underlying
mechanisms are still unknown. One suggested mechanism is stimulation of
cellular stress pathways induced by the IF regimen [196,207,208]. Brain-derived
neurotrophic factor (BDNF), normally involved in brain development and
plasticity, is elevated in IF animals, and is causally linked to the protective
effect of the IF regimen against neuronal damage inflicted by the neurotoxin
kainic acid [209]. It must be
noted, however, that BDNF could not be linked to the neuro-protective effects
in the brain of calorically restricted rats [210,211], but increased
levels of another neurotrophic factor, glial cell line-derived factor (GDNF),
were correlated with neuro-protection of a calorically restricted primate model
of Parkinson's disease [212]. Interestingly,
BDNF is also a component of the hypothalamic melanocortin pathway that controls
food intake and body weight in adult mice [213], and it
has been implicated in the regulation of energy metabolism [214]. Heterozygousknockout BDNF (BDNF+/-) mice exhibit metabolic abnormalities,
hyperphagia, obesity, and insulin resistance that could be significantly
reversed by IF, indicating that BDNF is indeed involved in the beneficial
effects induced by IF [214]. Interestingly,the BDNF+/- mice resemble circadian Clock mutant
mice [133] in metabolic
abnormalities. In addition, BDNF and its cognate receptor TrkB were suggested
to play a role in circadian modulation of the SCN pacemaker sensitivity to
light [215,216]. These
data point to the possibility that IF could affect the SCN and, as a result,
peripheral clocks, at least via elevating BDNF levels.
Recently, we have shown that, under an IF protocol,when food was introduced during the light period,
mice exhibited almost arrhythmicity in clock gene expression in the liver.
Unlike daytime feeding, nighttime feeding yielded rhythms similar to those
generated during ad libitum feeding [198] (Figure 3). Furthermore, rhythms were maintained when
daytime IF occurred under disruptive light, suggesting that SCN signals were
involved in inducing the arrhythmic state in the periphery [198]. Thus, the fact that IF can affect circadian rhythms
differently depending on the timing of food availability and light conditions
suggests that this regimen affects the SCN clock, similarly to CR. We assume
that SCN resetting by IF and CR could be involved in the health benefits
conferred by these regimens [195].
The effects of
IF are in contrast to those of restricted feeding (RF) that dictates peripheral
rhythms in arrhythmic and mutant mice and animals with lesioned SCN regardless
of the lighting conditions [142,143,148-151]
(Figure 3). It, thus, appears that IF is not as dominant as RF in dictating
peripheral rhythms. Never-theless, this feeding regimen exhibits some
similarities with RF, as reflected by the anticipatory feeding behavior that
preceded food availability and restoration of circadian rhythms under
disruptive light conditions, due most likely to the effect on the food
entrainable oscillator (FEO) [198]. Thus, under
daytime IF, clock gene expression in the periphery would be controlled by the
SCN, which responds to both light-dark cycle and IF, as well as directly by the
temporal feeding via the FEO. Co-activation of both the FEO and the SCN
would yield rhythms at two opposite phases leading to overall arrhythmicity. In
contrast, under nighttime IF, normal rhythms are generated, as both the FEO and
the SCN work in synchrony to dictate peripheral rhythms [198].
V. The circadian
clock as a possible mediator in CR- or IF-induced increased longevity
A.
Long-lived, spontaneously calorically restricted αMUPA mice
αMUPA mice carry as a transgene, the urokinase-type
plasminogen activator (uPA) [217], an extracellular fibrinolytic serine protease
implicated in tissue remodelling [218] and brain
development and plasticity [219-224]. αMUPA mice
spontaneously eat less (20-30%) compared to their wild type (WT) FVB/N control
mice when fed ad libitum, indicating that their appetite is genetically
suppressed. The mechanism linking transgenic uPA to reduced hunger is not yet
clear. It could be related to uPA over-expression in the brain stem, as was
found in two transgenic lines showing reduced food intake [225]. The transgenic
effect is likely to be developmental, similarly to the remodelling effect
recently detected in αMUPA developing incisor teeth [226]. αMUPA
mice live longer (median, 16%; 10th decile, 15%) than WT mice [227], thus resembling
calorically restricted animals in showing an inverse relation betweenfood intake and life span.αMUPA
mice exhibit additional similarities with calorically restricted mice, such as
reduced body weight, reduced levels of serum IGF-1 or glucose, enhanced
capacity to conduct apoptosis, and reduced incidence of tumors[225,227-230].
B.
αMUPA mice, circadian rhythms, and aging
Recent
data show that αMUPA mice exhibit higher amplitude in the circadian
expression of several clock genes in the liver compared with FVB/N WT mice.
This change
coincides with higher amplitude rhythms of food intake and body temperature [194].
Since circadian patterns of food intake and body temperature constituteclock-controlled output systems, it is
conceivable that their alteration in the transgenic
mice stems from the higher amplitude of clock gene expression in the periphery,
and possibly also in the central biological clock in the SCN. Higher amplitude
of circadian rhythms have been previously associated with young age [15] and
extended life span [19].
Support for a linkage between circadian rhythms and attenuation of aging in
αMUPA mice is provided by comparing young vs. old mice. When tested for
circadian food intake, an SCN output system, 18-month-old WT control mice
exhibit a 4-6-h shift in circadian food intake compared to 5-month-old mice [195].
This behaviour is consistent with literature data showing that aging can alter
the amplitude and/or phase of circadian rhythms [15,16,18].
In contrast to WT mice, adult and young αMUPA mice show similar circadian
food intake, indicating that at least some aspects of circadian behavior
maintain a youthful pattern at an old age in these mice. At an old age,
αMUPA mice maintain a young and healthier
appearance, they look lean, and their fur is shiny, whereas WT mice are
sluggish and they look old (Figure 4). In addition, αMUPA
mice do not become obese throughout their life-time, whereas about one third of
the WT mice show severe obesity (Figure 4). The major difference in body weight
between αMUPA and WT mice stems from the fact that the quantitative
difference in food intake between αMUPA and WT mice is maintained at the
old age.
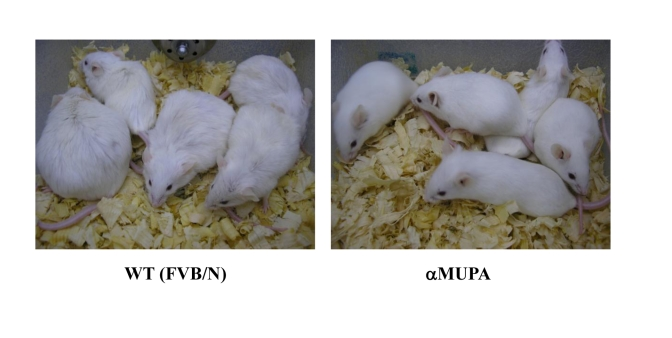
Figure 4. 18-month-old αMUPA and FVB/N WT mice. αMUPA
mice maintain a youthful and healthy appearance, whereas WT mice look old.
C.
αMUPA mice reveal effectsof feeding regimens
on circadian rhythms
It is difficult
to eliminate the effect of temporal food consumption in calorically restricted
animals, as mice consume their food within a few hours. αMUPA mice spontaneously
consume reduced calories (20-30% reduction) compared with WT mice under
different feeding regimens, i.e. AL, RF and IF, suggesting that these mice can
be utilized as a model for CR in the absence of the imposed temporal food
consumption under ad libitum feeding, and a model for imposed temporal CR under
RF or IF conditions. Therefore,
the transgenic mouse model αMUPA [225] has recently
been used to investigate the contribution of calorie reduction per se vs.
timed feeding to clock adaptation [49]. Under light-dark
conditions and ad libitum feeding, αMUPA mice show high amplitude,
appropriately reset circadian rhythms in peripheral clock genes [194] (Figure 5). This finding
could reflect the effect of the reduced calorie intake on the SCN in αMUPA
mice, as has been previously reported for calorically restricted animals [190-192].
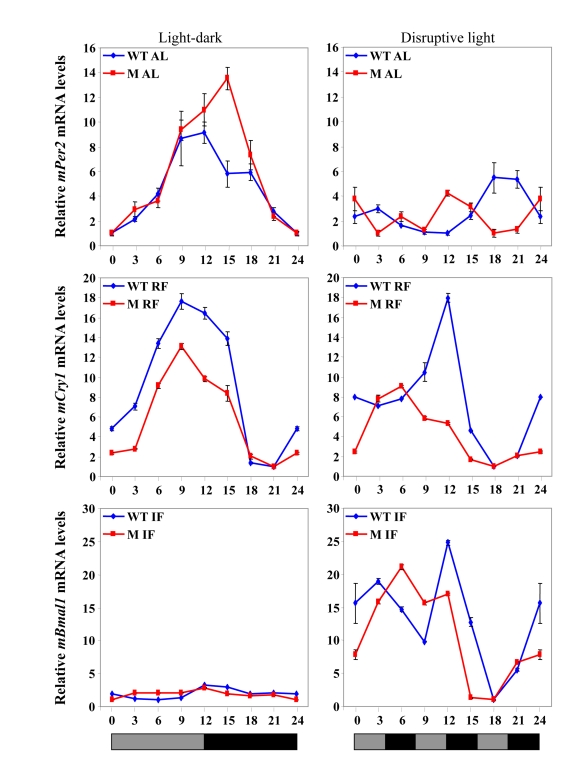
Figure 5. Clock gene expression in the liver under various feeding and lighting conditions in αMUPA (M) and WT mice. Expression levels
of the following clock genes are presented under light-dark or disruptive
light conditions:mPer2during ad
libitum (AL) feeding, mCry1 under RF, and Bmal1 under IF.Total
RNA extracted from liver tissue collected every 3 h around the circadian
cycle (mean ± SEM; n=3 for each time-point and each mouse group) was
reverse transcribed and analyzed by quantitative real time PCR. Clock gene levels
were normalized using Gapdh as the reference gene.
Under light-dark
conditions, RF advanced the expression phase of all clock genes in the liver in
a similar manner in both WT mice and αMUPA mice, and in some cases
increased the amplitude [49](Figure 5). These findings
were in concert with previous results in the literature, as is mentioned
above. Nevertheless, an effect of the SCN on clock-controlled output systems
under RF could be seen when the phases of peripheral clock gene expression in
WT mice vs. αMUPA mice were compared under arrhythmicity imposed by
disruptive light. Whereas the pattern of clock gene expression did not change
in WT mice, a phase shift was seen in several genes in αMUPA mice (Figure 5), suggesting an SCN effect. Again, this effect of the SCN could be
attributed to the reduced calorie intake of αMUPA mice. Altogether,
these findings suggest that both the reduced calories and the light-dark cycle
work in synchrony on the central biological clock of αMUPA mice to
generate rhythms in the periphery. However, it
seems that in WT mice, as has been found for other mouse strains, RF is
dominant over the SCN in dictating rhythms in the periphery regardless of the
lighting conditions. In αMUPA mice, RF dictates the phase of clock gene
expression under light-dark; but under disruptive light conditions, as the SCN
is under the influence of calorie restriction, it becomes dominant in dictating
the phase of clock gene expression.
Unlike RF, the
effect of IF on circadian rhythms in αMUPA mice was similar to that of WT
mice under light-dark or disruptive light conditions, and it resembled also
that on C57BL mice (Figure 3, Figure 5).
Thus, in all mice tested, daytime IF caused arrhythmia in clock gene expression
in the liver under light-dark, whereas rhythmicity was restored under
disruptive light [198] (Figure 3).
These observations suggest that IF, similarly to CR, may affect the SCN clock.
This effect could possibly be mediated through a metabolic state generated by
the day of fast during IF regardless of the calories consumed, as discussed
earlier.
Altogether, the
findings in αMUPA mice suggest that reduced calories affect the SCN so it
becomes dominant over RF in the periphery only under disruptive light
conditions. In addition, IF affects peripheral rhythms depending on the timing
of food availability and light conditions, but regardless of the total daily
calorie consumption, suggesting that this regimen induces a metabolic state
that affects the SCN.
D. Temporal vs. quantitative food consumption and
circadian rhythms
Previous
publications have dealt with the issue of timed feeding and life span,
reporting that calorically restricted mice showed increased longevity whether
fed twice a day at daytime, once a day at daytime or nighttime, several times a
day at nighttime [231,232],
or three meals a week [233]. In
these studies, the low-calorie feeding was practically timed and confined to
the day or night, similarly to MUPA, or introduced in large intervals and
continued throughout life time allowing appropriate adaptation. As a result,
timed feeding was suggested to lead to high amplitude circadian rhythms and
increased life span [232,234].
However, others rejected any contribution of timed feeding to CR-induced
longevity [174,231].
The
uncoupling of timed meals from reduced calories could practically be achieved
only with animals, such as αMUPA, that spontaneously eat less. The results
obtained with αMUPA indicate that temporal and quantitative aspects of
food intake can be separately controlled. The timing of food intake is
controlled by the central biological clock, whereas a separate mechanism
appears to dictate the amount of food or calorie intake, that, in turn, could
entrain the SCN clock, as has experimentally been shown for calorically
restricted animals [191,192].
The
results achieved with IF suggest that IF can be beneficial when food is given
during the activity period of the animal, as explained above. Indeed, neuro-
and cardio-protection alongside increased fatty acid oxidation and improved
stress resistance have been induced after weeks of IF treatment when food was
introduced at the beginning of the activity period [205,235-237].
It
is noteworthy that cardio- and neuro-protection and life span extension
were also seen when food was introduced during the day, but after many months
of IF treatment [196,199],
so that the animals could adjust after such a prolonged treatment. In
light of these findings, we assume that the effect of IF on the SCN through a
metabolic change, as mentioned above, alongside the timed feeding might affect
the SCN to yield better-reset rhythms.
E.
Differences between αMUPA mice and calorically restricted rodents
Although
αMUPA mice exhibit reduced calorie intake and body fat, they show
remarkable differences in energy metabolism compared with CR-treated animals.
In particular, calorically restricted animals exhibit high levels of ghrelin [238,239],
but low levels of leptin [240,241]and insulin [173],
indicating an overall state of hunger. It is noteworthy that
leptin-deficient animals are long-lived under CR feeding regimen, suggesting
that leptin is not necessary for the CR-mediated benefits [242,243].
In addition, CR-treated mice exhibit high expression levels of PGC-1α and
no change in PPARγ levels in the liver [244,245].
All these findings are in sharp contrast with those found in αMUPA mice
that have low levels of ghrelin and high levels of leptin and insulin,
suggesting that αMUPA mice eat less because their metabolism is of
satiated rather than hungry animals (unpublished data). Nevertheless, one
aspect found to be common to both αMUPA mice and calorically restricted
animals are the low SIRT1 expression levels in the liver [246] and
high levels in the brain [247]. It
is noteworthy that the information regarding SIRT1 levels in the hypothalamus
of calorically restricted animals is still lacking, and data for peripheral
tissues is sometimes contradictory [124,246].
Results obtained with SIRT1-null micehave
recently suggested that this enzyme could be required for the in vivo
response to CR [248], and
transgenic mice over-expressing SIRT1 show a phenotype resembling calorically
restricted animals [249]. We
assume that, in calorically restricted mice, SIRT1 activity could be elevated
in the brain, possibly in the hypothalamus and SCN, through AMPK activation, as
AMPK can be activated in the hypothalamus under starvation conditions and high
ghrelin [112,121].
In αMUPA mice, that show low AMPK levels in the hypothalamus, the high
leptin levels could lead to SIRT1 elevation, as leptin is required for the
increase in SIRT1 protein levels in the hypothalamus under starvation [250].
Thus, although stimulated through different pathways in αMUPA and
calorically restricted mice, SIRT1 could act as a common factor modulating the
SCN clock and, as a result, longevity.
αMUPA
mice share also some similarities with those of Lou/C rats, both obesity-resistant long-lived rodents.
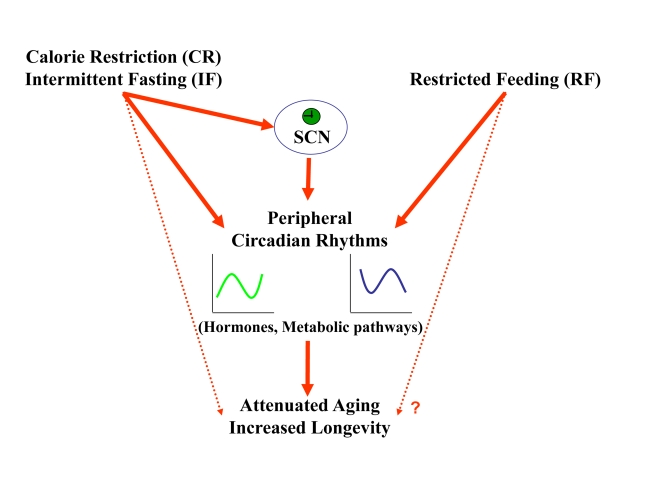
Figure 6. A schematic model describing the effect of feeding regimens on longevity through peripheral and SCN clock resetting. CR and IF reset
circadian rhythms in the periphery and the SCN. The synchronized, robust
circadian rhythms could be the mediator though which these feeding regimens
lead to aging attenuation and life span extension. RF resets circadian
rhythms only in the periphery, but its effect on life span is not known.
However,
there are some fundamental differences in Lou/C rats compared with αMUPA
mice, such as increased levels of PGC-1α and SIRT1 in the liver,
increased levels of ghrelin, and reduced levels of leptin and insulin in the
serum, although with some improved sensitivity for the latter two hormones [251,252].Overall, it seems that αMUPA mice are metabolically different from
Lou/C rats.
F. Role of
circadian rhythms in CR on health and longevity
The
capacity of CR to reset the SCN clock, as previously reported [191,192],and the pronounced circadian rhythms seen in the long-lived
αMUPA mice, pose the biological clock as a possible major factor
determining longevity of calorically restricted mice [194,195].
The beneficial effect induced by CR on health and longevity can be achieved by
appropriately resetting and synchronising a variety of hormonal, biochemical,
and physiological functions. In turn, some of these functions can feedback to
the biological clock in the periphery [143,153]
and the SCN [191,192]
and help sustain the rhythms. Indeed, the redox state affects the dimerization of
the two clock proteins CLOCK and BMAL1 in vitro [96]. As
SIRT1 has been linked to life span and suggested to mediate CR-induced effects [115,126,253],
it could be a candidate that modulates the clock of calorically restricted
animals, as discussed above. Clock resetting could
lead to robust circadian rhythms that are associated with young ages and
extended life span [15,16,19,195].
VI.
Conclusions
RF
entrains peripheral clocks due to temporal food consumption, whereas CR and IF
appear to synchronize the central pacemaker in the SCN, suggesting a role for a
metabolicstate imposed by low calories in
central clock entrainment. In αMUPA mice, reduced calories alone were
found not to be sufficient to sustain rhythms, unless feeding was spontaneously
timed at night, or timed at day through a restricted feeding protocol. Therefore,
it appears that when reduced calories are timed, as always occurs during CR and
IF regimens, clock adjustment can influence a wide variety of output systems,
so that cellular and physiological systems perform in a more synchronised and
appropriately reset manner. We assume that SIRT1 could be a key mediator in
clock synchronization at least under CR. Robust circadian rhythms can ensure a
better tissue and body homeostasis, and could constitute an important mediator
in aging attenuation and longevity extension (Figure 6).
Conflicts of Interest
The authors of this
manuscript have no conflict of interest to declare.
References
-
1.
Panda
S
, Hogenesch
JB
and Kay
SA.
Circadian rhythms from flies to human.
Nature.
2002;
417:
329
-335.
[PubMed]
.
-
2.
Reppert
SM
and Weaver
DR.
Coordination of circadian timing in mammals.
Nature.
2002;
418:
935
-941.
[PubMed]
.
-
3.
Schibler
U
, Ripperger
J
and Brown
SA.
Peripheral circadian oscillators in mammals: time and food.
J Biol Rhythms.
2003;
18:
250
-260.
[PubMed]
.
-
4.
Maron
BJ
, Kogan
J
, Proschan
MA
, Hecht
GM
and Roberts
WC.
Circadian variability in the occurrence of sudden cardiac death in patients with hypertrophic cardiomyopathy.
J Am Coll Cardiol.
1994;
23:
1405
-1409.
[PubMed]
.
-
5.
Staels
B
When the Clock stops ticking, metabolic syndrome explodes.
Nat Med.
2006;
12:
54
-55.
[PubMed]
.
-
6.
Burioka
N
, Fukuoka
Y
, Takata
M
, Endo
M
, Miyata
M
, Chikumi
H
, Tomita
K
, Kodani
M
, Touge
H
, Takeda
K
, Sumikawa
T
, Yamaguchi
K
, Ueda
Y
, Nakazaki
H
, Suyama
H
, Yamasaki
A
, Sano
H
, Igishi
T
and Shimizu
E.
Circadian rhythms in the CNS and peripheral clock disorders: function of clock genes: influence of medication for bronchial asthma on circadian gene.
J Pharmacol Sci.
2007;
103:
144
-149.
[PubMed]
.
-
7.
Penev
PD
, Kolker
DE
, Zee
PC
and Turek
FW.
Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease.
Am J Physiol.
1998;
275:
H2334
-H2337.
[PubMed]
.
-
8.
Fu
L
, Pelicano
H
, Liu
J
, Huang
P
and Lee
C.
The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo.
Cell.
2002;
111:
41
-50.
[PubMed]
.
-
9.
Filipski
E
, King
VM
, Li
X
, Granda
TG
, Mormont
MC
, Claustrat
B
, Hastings
MH
and Levi
F.
Disruption of circadian coordination accelerates malignant growth in mice.
Pathol Biol.
2003;
51:
216
-219.
[PubMed]
.
-
10.
Davis
S
and Mirick
DK.
Circadian disruption, shift work and the risk of cancer: a summary of the evidence and studies in Seattle.
Cancer Causes Control.
2006;
17:
539
-545.
[PubMed]
.
-
11.
Kondratov
RV
, Kondratova
AA
, Gorbacheva
VY
, Vykhovanets
OV
and Antoch
MP.
Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock.
Genes Dev.
2006;
20:
1868
-1873.
[PubMed]
.
-
12.
Kondratov
RV
A role of the circadian system and circadian proteins in aging.
Ageing Res Rev.
2007;
6:
12
-27.
[PubMed]
.
-
13.
Montagnana
M
, Salvagno
GL
and Lippi
G.
Circadian variation within hemostasis: an underrecognized link between biology and disease.
Semin Thromb Hemost.
2009;
35:
23
-33.
[PubMed]
.
-
14.
Anea
CB
, Zhang
M
, Stepp
DW
, Simkins
GB
, Reed
G
, Fulton
DJ
and Rudic
RD.
Vascular disease in mice with a dysfunctional circadian clock.
Circulation.
2009;
119:
1510
-1517.
[PubMed]
.
-
15.
Hofman
MA
and Swaab
DF.
Living by the clock: the circadian pacemaker in older people.
Ageing Res Rev.
2006;
5:
33
-51.
[PubMed]
.
-
16.
Gibson
EM
, Williams
WP 3rd
and Kriegsfeld
LJ.
Aging in the circadian system: considerations for health, disease prevention and longevity.
Exp Gerontol.
2009;
44:
51
-56.
[PubMed]
.
-
17.
Scarbrough
K
, Losee-Olson
S
, Wallen
EP
and Turek
FW.
Aging and photoperiod affect entrainment and quantitative aspects of locomotor behavior in Syrian hamsters.
Am J Physiol.
1997;
272:
R1219
-R1225.
[PubMed]
.
-
18.
Yamazaki
S
, Straume
M
, Tei
H
, Sakaki
Y
, Menaker
M
and Block
GD.
Effects of aging on central and peripheral mammalian clocks.
Proc Natl Acad Sci U S A.
2002;
99:
10801
-10806.
[PubMed]
.
-
19.
Hurd
MW
and Ralph
MR.
The significance of circadian organization for longevity in the golden hamster.
J Biol Rhythms.
1998;
13:
430
-436.
[PubMed]
.
-
20.
Hurd
MW
, Zimmer
KA
, Lehman
MN
and Ralph
MR.
Circadian locomotor rhythms in aged hamsters following suprachiasmatic transplant.
Am J Physiol.
1995;
269:
R958
-968.
[PubMed]
.
-
21.
Li
H
and Satinoff
E.
Fetal tissue containing the suprachiasmatic nucleus restores multiple circadian rhythms in old rats.
Am J Physiol.
1998;
275:
R1735
-1744.
[PubMed]
.
-
22.
Lee
C
, Etchegaray
JP
, Cagampang
FR
, Loudon
AS
and Reppert
SM.
Posttranslational mechanisms regulate the mammalian circadian clock.
Cell.
2001;
107:
855
-867.
[PubMed]
.
-
23.
Froy
O
and Chapnik
N.
Circadian oscillation of innate immunity components in mouse small intestine.
Mol Immunol.
2007;
44:
1954
-1960.
[PubMed]
.
-
24.
Young
ME
The circadian clock within the heart: potential influence on myocardial gene expression, metabolism, and function.
Am J Physiol Heart Circ Physiol.
2006;
290:
H1
-H16.
[PubMed]
.
-
25.
Welsh
DK
, Logothetis
DE
, Meister
M
and Reppert
SM.
Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms.
Neuron.
1995;
14:
697
-706.
[PubMed]
.
-
26.
Liu
C
, Weaver
DR
, Strogatz
SH
and Reppert
SM.
Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei.
Cell.
1997;
91:
855
-860.
[PubMed]
.
-
27.
Herzog
ED
, Takahashi
JS
and Block
GD.
Clock controls circadian period in isolated suprachiasmatic nucleus neurons.
Nat Neurosci.
1998;
1:
708
-713.
[PubMed]
.
-
28.
Reppert
SM
and Weaver
DR.
Molecular analysis of mammalian circadian rhythms.
Annu Rev Physiol.
2001;
63:
647
-676.
[PubMed]
.
-
29.
Gooley
JJ
, Lu
J
, Chou
TC
, Scammell
TE
and Saper
CB.
Melanopsin in cells of origin of the retinohypothalamic tract.
Nat Neurosci.
2001;
4:
1165
[PubMed]
.
-
30.
Lucas
RJ
, Freedman
MS
, Lupi
D
, Munoz
M
, David-Gray
ZK
and Foster
RG.
Identifying the photoreceptive inputs to the mammalian circadian system using transgenic and retinally degenerate mice.
Behav Brain Res.
2001;
125:
97
-102.
[PubMed]
.
-
31.
Harmar
AJ
, Marston
HM
, Shen
S
, Spratt
C
, West
KM
, Sheward
WJ
, Morrison
CF
, Dorin
JR
, Piggins
HD
, Reubi
JC
, Kelly
JS
, Maywood
ES
and Hastings
MH.
The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei.
Cell.
2002;
109:
497
-508.
[PubMed]
.
-
32.
Maywood
ES
, Reddy
AB
, Wong
GK
, O'Neill
JS
, O'Brien
JA
, McMahon
DG
, Harmar
AJ
, Okamura
H
and Hastings
MH.
Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling.
Curr Biol.
2006;
16:
599
-605.
[PubMed]
.
-
33.
Le
Minh N
, Damiola
F
, Tronche
F
, Schutz
G
and Schibler
U.
Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators.
EMBO J.
2001;
20:
7128
-7136.
[PubMed]
.
-
34.
Kramer
A
, Yang
FC
, Snodgrass
P
, Li
X
, Scammell
TE
, Davis
FC
and Weitz
CJ.
Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling.
Science.
2001;
294:
2511
-2515.
[PubMed]
.
-
35.
Cheng
MY
, Bullock
CM
, Li
C
, Lee
AG
, Bermak
JC
, Belluzzi
J
, Weaver
DR
, Leslie
FM
and Zhou
QY.
Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus.
Nature.
2002;
417:
405
-410.
[PubMed]
.
-
36.
Kraves
S
and Weitz
CJ.
A role for cardiotrophin-like cytokine in the circadian control of mammalian locomotor activity.
Nat Neurosci.
2006;
9:
212
-219.
[PubMed]
.
-
37.
Yoo
SH
, Yamazaki
S
, Lowrey
PL
, Shimomura
K
, Ko
CH
, Buhr
ED
, Siepka
SM
, Hong
HK
, Oh
WJ
, Yoo
OJ
, Menaker
M
and Takahashi
JS.
PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues.
Proc Natl Acad Sci U S A.
2004;
101:
5339
-5346.
[PubMed]
.
-
38.
Welsh
DK
, Yoo
SH
, Liu
AC
, Takahashi
JS
and Kay
SA.
Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression.
Curr Biol.
2004;
14:
2289
-2295.
[PubMed]
.
-
39.
Kornmann
B
, Preitner
N
, Rifat
D
, Fleury-Olela
F
and Schibler
U.
Analysis of circadian liver gene expression by ADDER, a highly sensitive method for the display of differentially expressed mRNAs.
Nucleic Acids Res.
2001;
29:
E51
[PubMed]
.
-
40.
Akhtar
RA
, Reddy
AB
, Maywood
ES
, Clayton
JD
, King
VM
, Smith
AG
, Gant
TW
, Hastings
MH
and Kyriacou
CP.
Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus.
Curr Biol.
2002;
12:
540
-550.
[PubMed]
.
-
41.
Duffield
GE
, Best
JD
, Meurers
BH
, Bittner
A
, Loros
JJ
and Dunlap
JC.
Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells.
Curr Biol.
2002;
12:
551
-557.
[PubMed]
.
-
42.
Panda
S
, Antoch
MP
, Miller
BH
, Su
AI
, Schook
AB
, Straume
M
, Schultz
PG
, Kay
SA
, Takahashi
JS
and Hogenesch
JB.
Coordinated transcription of key pathways in the mouse by the circadian clock.
Cell.
2002;
109:
307
-320.
[PubMed]
.
-
43.
Storch
KF
, Lipan
O
, Leykin
I
, Viswanathan
N
, Davis
FC
, Wong
WH
and Weitz
CJ.
Extensive and divergent circadian gene expression in liver and heart.
Nature.
2002;
417:
78
-83.
[PubMed]
.
-
44.
Kita
Y
, Shiozawa
M
, Jin
W
, Majewski
RR
, Besharse
JC
, Greene
AS
and Jacob
HJ.
Implications of circadian gene expression in kidney, liver and the effects of fasting on pharmacogenomic studies.
Pharmacogenetics.
2002;
12:
55
-65.
[PubMed]
.
-
45.
Zvonic
S
, Ptitsyn
AA
, Conrad
SA
, Scott
LK
, Floyd
ZE
, Kilroy
G
, Wu
X
, Goh
BC
, Mynatt
RL
and Gimble
JM.
Characterization of peripheral circadian clocks in adipose tissues.
Diabetes.
2006;
55:
962
-970.
[PubMed]
.
-
46.
Reddy
AB
, Karp
NA
, Maywood
ES
, Sage
EA
, Deery
M
, O'Neill
JS
, Wong
GK
, Chesham
J
, Odell
M
, Lilley
KS
, Kyriacou
CP
and Hastings
MH.
Circadian orchestration of the hepatic proteome.
Curr Biol.
2006;
16:
1107
-1115.
[PubMed]
.
-
47.
McCarthy
JJ
, Andrews
JL
, McDearmon
EL
, Campbell
KS
, Barber
BK
, Miller
BH
, Walker
JR
, Hogenesch
JB
, Takahashi
JS
and Esser
KA.
Identification of the circadian transcriptome in adult mouse skeletal muscle.
Physiol Genomics.
2007;
31:
86
-95.
[PubMed]
.
-
48.
Kornmann
B
, Schaad
O
, Bujard
H
, Takahashi
JS
and Schibler
U.
System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock.
PLoS Biol.
2007;
5:
e34
[PubMed]
.
-
49.
Froy
O
, Chapnik
N
and Miskin
R.
The suprachiasmatic nuclei are involved in determining circadian rhythms during restricted feeding.
Neuroscience.
2008;
155:
1152
-1159.
[PubMed]
.
-
50.
Dunlap
JC
Molecular bases for circadian clocks.
Cell.
1999;
96:
271
-290.
[PubMed]
.
-
51.
Cardone
L
, Hirayama
J
, Giordano
F
, Tamaru
T
, Palvimo
JJ
and Sassone-Corsi
P.
Circadian clock control by SUMOylation of BMAL1.
Science.
2005;
309:
1390
-1394.
[PubMed]
.
-
52.
Nagel
R
, Clijsters
L
and Agami
R.
The miRNA-192/194 cluster regulates the Period gene family and the circadian clock.
Febs J.
2009;
276:
5447
-5455.
[PubMed]
.
-
53.
Vitaterna
MH
, King
DP
, Chang
AM
, Kornhauser
JM
, Lowrey
PL
, McDonald
JD
, Dove
WF
, Pinto
LH
, Turek
FW
and Takahashi
JS.
Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior.
Science.
1994;
264:
719
-725.
[PubMed]
.
-
54.
Asher
G
and Schibler
U.
A CLOCK-less clock.
Trends Cell Biol.
2006;
16:
547
-549.
[PubMed]
.
-
55.
Debruyne
JP
, Noton
E
, Lambert
CM
, Maywood
ES
, Weaver
DR
and Reppert
SM.
A clock shock: mouse CLOCK is not required for circadian oscillator function.
Neuron.
2006;
50:
465
-477.
[PubMed]
.
-
56.
Froy
O
, Chang
DC
and Reppert
SM.
Redox potential: differential roles in dCRY and mCRY1 functions.
Curr Biol.
2002;
12:
147
-152.
[PubMed]
.
-
57.
Doi
M
, Hirayama
J
and Sassone-Corsi
P.
Circadian regulator CLOCK is a histone acetyltransferase.
Cell.
2006;
125:
497
-508.
[PubMed]
.
-
58.
Nakahata
Y
, Grimaldi
B
, Sahar
S
, Hirayama
J
and Sassone-Corsi
P.
Signaling to the circadian clock: plasticity by chromatin remodeling.
Curr Opin Cell Biol.
2007;
19:
230
-237.
[PubMed]
.
-
59.
Etchegaray
JP
, Lee
C
, Wade
PA
and Reppert
SM.
Rhythmic histone acetylation underlies transcription in the mammalian circadian clock.
Nature.
2003;
421:
177
-182.
[PubMed]
.
-
60.
Curtis
AM
, Seo
SB
, Westgate
EJ
, Rudic
RD
, Smyth
EM
, Chakravarti
D
, FitzGerald
GA
and McNamara
P.
Histone acetyltransferase-dependent chromatin remodeling and the vascular clock.
J Biol Chem.
2004;
279:
7091
-7097.
[PubMed]
.
-
61.
Naruse
Y
, Oh-hashi
K
, Iijima
N
, Naruse
M
, Yoshioka
H
and Tanaka
M.
Circadian and light-induced transcription of clock gene Per1 depends on histone acetylation and deacetylation.
Mol Cell Biol.
2004;
24:
6278
-6287.
[PubMed]
.
-
62.
Ripperger
JA
and Schibler
U.
Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions.
Nat Genet.
2006;
38:
369
-374.
[PubMed]
.
-
63.
Etchegaray
JP
, Yang
X
, DeBruyne
JP
, Peters
AH
, Weaver
DR
, Jenuwein
T
and Reppert
SM.
The polycomb group protein EZH2 is required for mammalian circadian clock function.
J Biol Chem.
2006;
281:
21209
-21215.
[PubMed]
.
-
64.
Whitmore
D
, Cermakian
N
, Crosio
C
, Foulkes
NS
, Pando
MP
, Travnickova
Z
and Sassone-Corsi
P.
A clockwork organ.
Biol Chem.
2000;
381:
793
-800.
[PubMed]
.
-
65.
Eide
EJ
and Virshup
DM.
Casein kinase I: another cog in the circadian clockworks.
Chronobiol Int.
2001;
18:
389
-398.
[PubMed]
.
-
66.
Eide
EJ
, Woolf
MF
, Kang
H
, Woolf
P
, Hurst
W
, Camacho
F
, Vielhaber
EL
, Giovanni
A
and Virshup
DM.
Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation.
Mol Cell Biol.
2005;
25:
2795
-2807.
[PubMed]
.
-
67.
Eide
EJ
, Kang
H
, Crapo
S
, Gallego
M
and Virshup
DM.
Casein kinase I in the mammalian circadian clock.
Methods Enzymol.
2005;
393:
408
-418.
[PubMed]
.
-
68.
Preitner
N
, Damiola
F
, Lopez-Molina
L
, Zakany
J
, Duboule
D
, Albrecht
U
and Schibler
U.
The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator.
Cell.
2002;
110:
251
-260.
[PubMed]
.
-
69.
Yin
L
and Lazar
MA.
The orphan nuclear receptor Rev-erbalpha recruits the N-CoR/histone deacetylase 3 corepressor to regulate the circadian Bmal1 gene.
Mol Endocrinol.
2005;
19:
1452
-1459.
[PubMed]
.
-
70.
Sato
TK
, Panda
S
, Miraglia
LJ
, Reyes
TM
, Rudic
RD
, McNamara
P
, Naik
KA
, FitzGerald
GA
, Kay
SA
and Hogenesch
JB.
A functional genomics strategy reveals Rora as a component of the mammalian circadian clock.
Neuron.
2004;
43:
527
-537.
[PubMed]
.
-
71.
Ueda
HR
, Hayashi
S
, Chen
W
, Sano
M
, Machida
M
, Shigeyoshi
Y
, Iino
M
and Hashimoto
S.
System-level identification of transcriptional circuits underlying mammalian circadian clocks.
Nat Genet.
2005;
37:
187
-192.
[PubMed]
.
-
72.
Froy
O
The relationship between nutrition and circadian rhythms in mammals.
Front Neuroendocrinol.
2007;
28:
61
-71.
[PubMed]
.
-
73.
Green
CB
, Takahashi
JS
and Bass
J.
The meter of metabolism.
Cell.
2008;
134:
728
-742.
[PubMed]
.
-
74.
Hirota
T
and Fukada
Y.
Resetting mechanism of central and peripheral circadian clocks in mammals.
Zoolog Sci.
2004;
21:
359
-368.
[PubMed]
.
-
75.
Kohsaka
A
and Bass
J.
A sense of time: how molecular clocks organize metabolism.
Trends Endocrinol Metab.
2007;
18:
4
-11.
[PubMed]
.
-
76.
La Fleur
SE
, Kalsbeek
A
, Wortel
J
and Buijs
RM.
A suprachiasmatic nucleus generated rhythm in basal glucose concentrations.
J Neuroendocrinol.
1999;
11:
643
-652.
[PubMed]
.
-
77.
La Fleur
SE
Daily rhythms in glucose metabolism: suprachiasmatic nucleus output to peripheral tissue.
J Neuroendocrinol.
2003;
15:
315
-322.
[PubMed]
.
-
78.
Davidson
AJ
, Castanon-Cervantes
O
and Stephan
FK.
Daily oscillations in liver function: diurnal vs circadian rhythmicity.
Liver Int.
2004;
24:
179
-186.
[PubMed]
.
-
79.
Ramsey
KM
, Marcheva
B
, Kohsaka
A
and Bass
J.
The clockwork of metabolism.
Annu Rev Nutr.
2007;
27:
219
-240.
[PubMed]
.
-
80.
Froy
O
Metabolism and Circadian Rhythms--Implications for Obesity.
Endocr Rev.
2009;
In press
.
-
81.
Yang
X
, Downes
M
, Yu
RT
, Bookout
AL
, He
W
, Straume
M
, Mangelsdorf
DJ
and Evans
RM.
Nuclear receptor expression links the circadian clock to metabolism.
Cell.
2006;
126:
801
-810.
[PubMed]
.
-
82.
Ruiter
M
, La Fleur
SE
, van
Heijningen C
, van der Vliet
J
, Kalsbeek
A
and Buijs
RM.
The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior.
Diabetes.
2003;
52:
1709
-1715.
[PubMed]
.
-
83.
Ando
H
, Yanagihara
H
, Hayashi
Y
, Obi
Y
, Tsuruoka
S
, Takamura
T
, Kaneko
S
and Fujimura
A.
Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue.
Endocrinology.
2005;
146:
5631
-5636.
[PubMed]
.
-
84.
De
Boer SF
and Van
der Gugten J.
Daily variations in plasma noradrenaline, adrenaline and corticosterone concentrations in rats.
Physiol Behav.
1987;
40:
323
-328.
[PubMed]
.
-
85.
Ahima
RS
, Prabakaran
D
and Flier
JS.
Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function.
J Clin Invest.
1998;
101:
1020
-1027.
[PubMed]
.
-
86.
Bodosi
B
, Gardi
J
, Hajdu
I
, Szentirmai
E
, Obal
F Jr
and Krueger
JM.
Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation.
Am J Physiol Regul Integr Comp Physiol.
2004;
287:
R1071
-R1079.
[PubMed]
.
-
87.
Kalra
SP
, Bagnasco
M
, Otukonyong
EE
, Dube
MG
and Kalra
PS.
Rhythmic, reciprocal ghrelin and leptin signaling: new insight in the development of obesity.
Regul Pept.
2003;
111:
1
-11.
[PubMed]
.
-
88.
Kalsbeek
A
, Fliers
E
, Romijn
JA
, La Fleur
SE
, Wortel
J
, Bakker
O
, Endert
E
and Buijs
RM.
The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels.
Endocrinology.
2001;
142:
2677
-2685.
[PubMed]
.
-
89.
Guan
XM
, Hess
JF
, Yu
H
, Hey
PJ
and van der Ploeg
LH.
Differential expression of mRNA for leptin receptor isoforms in the rat brain.
Mol Cell Endocrinol.
1997;
133:
1
-7.
[PubMed]
.
-
90.
Yi
CX
, van der Vliet
J
, Dai
J
, Yin
G
, Ru
L
and Buijs
RM.
Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus.
Endocrinology.
2006;
147:
283
-294.
[PubMed]
.
-
91.
Zigman
JM
, Jones
JE
, Lee
CE
, Saper
CB
and Elmquist
JK.
Expression of ghrelin receptor mRNA in the rat and the mouse brain.
J Comp Neurol.
2006;
494:
528
-548.
[PubMed]
.
-
92.
Prosser
RA
and Bergeron
HE.
Leptin phase-advances the rat suprachiasmatic circadian clock in vitro.
Neurosci Lett.
2003;
336:
139
-142.
[PubMed]
.
-
93.
Yi
CX
, Challet
E
, Pevet
P
, Kalsbeek
A
, Escobar
C
and Buijs
RM.
A circulating ghrelin mimetic attenuates light-induced phase delay of mice and light-induced Fos expression in the suprachiasmatic nucleus of rats.
Eur J Neurosci.
2008;
27:
1965
-1972.
[PubMed]
.
-
94.
Yannielli
PC
, Molyneux
PC
, Harrington
ME
and Golombek
DA.
Ghrelin effects on the circadian system of mice.
J Neurosci.
2007;
27:
2890
-2895.
[PubMed]
.
-
95.
Rutter
J
, Reick
M
, Wu
LC
and McKnight
SL.
Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors.
Science.
2001;
293:
510
-514.
[PubMed]
.
-
96.
Rutter
J
, Reick
M
and McKnight
SL.
Metabolism and the control of circadian rhythms.
Annu Rev Biochem.
2002;
71:
307
-331.
[PubMed]
.
-
97.
Zvonic
S
, Floyd
ZE
, Mynatt
RL
and Gimble
JM.
Circadian rhythms and the regulation of metabolic tissue function and energy homeostasis.
Obesity (Silver Spring).
2007;
15:
539
-543.
[PubMed]
.
-
98.
Loboda
A
, Kraft
WK
, Fine
B
, Joseph
J
, Nebozhyn
M
, Zhang
C
, He
Y
, Yang
X
, Wright
C
, Morris
M
, Chalikonda
I
, Ferguson
M
, Emilsson
V
, Leonardson
A
, Lamb
J
, Dai
H
, Schadt
E
, Greenberg
HE
and Lum
PY.
Diurnal variation of the human adipose transcriptome and the link to metabolic disease.
BMC Med Genomics.
2009;
2:
7
[PubMed]
.
-
99.
Shimba
S
, Ishii
N
, Ohta
Y
, Ohno
T
, Watabe
Y
, Hayashi
M
, Wada
T
, Aoyagi
T
and Tezuka
M.
Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis.
Proc Natl Acad Sci U S A.
2005;
102:
12071
-12076.
[PubMed]
.
-
100.
Chawla
A
and Lazar
MA.
Induction of Rev-ErbA alpha, an orphan receptor encoded on the opposite strand of the alpha-thyroid hormone receptor gene, during adipocyte differentiation.
J Biol Chem.
1993;
268:
16265
-16269.
[PubMed]
.
-
101.
Bray
MS
and Young
ME.
Circadian rhythms in the development of obesity: potential role for the circadian clock within the adipocyte.
Obes Rev.
2007;
8:
169
-181.
[PubMed]
.
-
102.
Torra
IP
, Tsibulsky
V
, Delaunay
F
, Saladin
R
, Laudet
V
, Fruchart
JC
, Kosykh
V
and Staels
B.
Circadian and glucocorticoid regulation of Rev-erbalpha expression in liver.
Endocrinology.
2000;
141:
3799
-3806.
[PubMed]
.
-
103.
Fontaine
C
, Dubois
G
, Duguay
Y
, Helledie
T
, Vu-Dac
N
, Gervois
P
, Soncin
F
, Mandrup
S
, Fruchart
JC
, Fruchart-Najib
J
and Staels
B.
The orphan nuclear receptor Rev-Erbalpha is a peroxisome proliferator-activated receptor (PPAR) gamma target gene and promotes PPARgamma-induced adipocyte differentiation.
J Biol Chem.
2003;
278:
37672
-37680.
[PubMed]
.
-
104.
Duez
H
and Staels
B.
Rev-erb alpha gives a time cue to metabolism.
FEBS Lett.
2008;
582:
19
-25.
[PubMed]
.
-
105.
Oishi
K
, Shirai
H
and Ishida
N.
CLOCK is involved in the circadian transactivation of peroxisome-proliferator-activated re-ceptor alpha (PPARalpha) in mice.
Biochem J.
2005;
386:
575
-581.
[PubMed]
.
-
106.
Inoue
I
, Shinoda
Y
, Ikeda
M
, Hayashi
K
, Kanazawa
K
, Nomura
M
, Matsunaga
T
, Xu
H
, Kawai
S
, Awata
T
, Komoda
T
and Katayama
S.
CLOCK/BMAL1 is involved in lipid metabolism via transactivation of the peroxisome proliferator-activated receptor (PPAR) response element.
J Atheroscler Thromb.
2005;
12:
169
-174.
[PubMed]
.
-
107.
Canaple
L
, Rambaud
J
, Dkhissi-Benyahya
O
, Rayet
B
, Tan
NS
, Michalik
L
, Delaunay
F
, Wahli
W
and Laudet
V.
Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock.
Mol Endocrinol.
2006;
20:
1715
-1727.
[PubMed]
.
-
108.
Kersten
S
, Desvergne
B
and Wahli
W.
Roles of PPARs in health and disease.
Nature.
2000;
405:
421
-424.
[PubMed]
.
-
109.
Lefebvre
P
, Chinetti
G
, Fruchart
JC
and Staels
B.
Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis.
J Clin Invest.
2006;
116:
571
-580.
[PubMed]
.
-
110.
Liu
C
, Li
S
, Liu
T
, Borjigin
J
and Lin
JD.
Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism.
Nature.
2007;
447:
477
-481.
[PubMed]
.
-
111.
Grimaldi
B
and Sassone-Corsi
P.
Circadian rhythms: metabolic clockwork.
Nature.
2007;
447:
386
-387.
[PubMed]
.
-
112.
Carling
D
AMP-activated protein kinase: balancing the scales.
Biochimie.
2005;
87:
87
-91.
[PubMed]
.
-
113.
Hardie
DG
, Hawley
SA
and Scott
JW.
AMP-activated protein kinase--development of the energy sensor concept.
J Physiol.
2006;
574:
7
-15.
[PubMed]
.
-
114.
Canto
C
, Gerhart-Hines
Z
, Feige
JN
, Lagouge
M
, Noriega
L
, Milne
JC
, Elliott
PJ
, Puigserver
P
and Auwerx
J.
AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity.
Nature.
2009;
458:
1056
-1060.
[PubMed]
.
-
115.
Canto
C
and Auwerx
J.
Caloric restriction, SIRT1 and longevity.
Trends Endocrinol Metab.
2009;
20:
325
-331.
[PubMed]
.
-
116.
Um
JH
, Yang
S
, Yamazaki
S
, Kang
H
, Viollet
B
, Foretz
M
and Chung
JH.
Activation of 5'-AMP-activated kinase with diabetes drug metformin induces casein kinase Iepsilon (CKIepsilon)-dependent degradation of clock protein mPER2.
J Biol Chem.
2007;
282:
20794
-20798.
[PubMed]
.
-
117.
Lamia
KA
, Sachdeva
UM
, DiTacchio
L
, Williams
EC
, Alvarez
JG
, Egan
DF
, Vasquez
DS
, Juguilon
H
, Panda
S
, Shaw
RJ
, Thompson
CB
and Evans
RM.
AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation.
Science.
2009;
326:
437
-440.
[PubMed]
.
-
118.
Vieira
E
, Nilsson
EC
, Nerstedt
A
, Ormestad
M
, Long
YC
, Garcia-Roves
PM
, Zierath
JR
and Mahlapuu
M.
Relationship between AMPK and the transcriptional balance of clock-related genes in skeletal muscle.
Am J Physiol Endocrinol Metab.
2008;
295:
E1032
-E1037.
[PubMed]
.
-
119.
Martin
TL
, Alquier
T
, Asakura
K
, Furukawa
N
, Preitner
F
and Kahn
BB.
Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle.
J Biol Chem.
2006;
281:
18933
-18941.
[PubMed]
.
-
120.
Kola
B
Role of AMP-activated protein kinase in the control of appetite.
J Neuroendocrinol.
2008;
20:
942
-951.
[PubMed]
.
-
121.
Kola
B
and Korbonits
M.
Shedding light on the intricate puzzle of ghrelin's effects on appetite regulation.
J Endocrinol.
2009;
202:
191
-198.
[PubMed]
.
-
122.
Minokoshi
Y
, Shiuchi
T
, Lee
S
, Suzuki
A
and Okamoto
S.
Role of hypothalamic AMP-kinase in food intake regulation.
Nutrition.
2008;
24:
786
-790.
[PubMed]
.
-
123.
Blander
G
and Guarente
L.
The Sir2 family of protein deacetylases.
Annu Rev Biochem.
2004;
73:
417
-435.
[PubMed]
.
-
124.
Dali-Youcef
N
, Lagouge
M
, Froelich
S
, Koehl
C
, Schoonjans
K
and Auwerx
J.
Sirtuins: the 'magnificent seven', function, metabolism and longevity.
Ann Med.
2007;
39:
335
-345.
[PubMed]
.
-
125.
Mair
W
and Dillin
A.
Aging and survival: the genetics of life span extension by dietary restriction.
Annu Rev Biochem.
2008;
77:
727
-754.
[PubMed]
.
-
126.
Haigis
MC
and Guarente
LP.
Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction.
Genes Dev.
2006;
20:
2913
-2921.
[PubMed]
.
-
127.
Imai
SI
"Clocks" in the NAD World: NAD as a metabolic oscillator for the regulation of metabolism and aging.
Biochim Biophys Acta.
2009;
In press
.
-
128.
Asher
G
, Gatfield
D
, Stratmann
M
, Reinke
H
, Dibner
C
, Kreppel
F
, Mostoslavsky
R
, Alt
FW
and Schibler
U.
SIRT1 regulates circadian clock gene expression through PER2 deacetylation.
Cell.
2008;
134:
317
-328.
[PubMed]
.
-
129.
Nakahata
Y
, Kaluzova
M
, Grimaldi
B
, Sahar
S
, Hirayama
J
, Chen
D
, Guarente
LP
and Sassone-Corsi
P.
The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control.
Cell.
2008;
134:
329
-340.
[PubMed]
.
-
130.
Hirayama
J
, Sahar
S
, Grimaldi
B
, Tamaru
T
, Takamatsu
K
, Nakahata
Y
and Sassone-Corsi
P.
CLOCK-mediated acetylation of BMAL1 controls circadian function.
Nature.
2007;
450:
1086
-1090.
[PubMed]
.
-
131.
Belden
WJ
and Dunlap
JC.
SIRT1 is a circadian deacetylase for core clock components.
Cell.
2008;
134:
212
-214.
[PubMed]
.
-
132.
Nakahata
Y
, Sahar
S
, Astarita
G
, Kaluzova
M
and Sassone-Corsi
P.
Circadian Control of the NAD+ Salvage Pathway by CLOCK-SIRT1.
Science.
2009;
324:
654
-657.
[PubMed]
.
-
133.
Turek
FW
, Joshu
C
, Kohsaka
A
, Lin
E
, Ivanova
G
, McDearmon
E
, Laposky
A
, Losee-Olson
S
, Easton
A
, Jensen
DR
, Eckel
RH
, Takahashi
JS
and Bass
J.
Obesity and metabolic syndrome in circadian Clock mutant mice.
Science.
2005;
308:
1043
-1045.
[PubMed]
.
-
134.
Rudic
RD
, McNamara
P
, Curtis
AM
, Boston
RC
, Panda
S
, Hogenesch
JB
and Fitzgerald
GA.
BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis.
PLoS Biol.
2004;
2:
e377
[PubMed]
.
-
135.
Kennaway
DJ
, Owens
JA
, Voultsios
A
, Boden
MJ
and Varcoe
TJ.
Metabolic homeostasis in mice with disrupted Clock gene expression in peripheral tissues.
Am J Physiol Regul Integr Comp Physiol.
2007;
293:
R1528
-R1537.
[PubMed]
.
-
136.
Oishi
K
, Atsumi
G
, Sugiyama
S
, Kodomari
I
, Kasamatsu
M
, Machida
K
and Ishida
N.
Disrupted fat absorption attenuates obesity induced by a high-fat diet in Clockmutant mice.
FEBS Lett.
2006;
580:
127
-130.
[PubMed]
.
-
137.
Lamia
KA
, Storch
KF
and Weitz
CJ.
Physiological significance of a peripheral tissue circadian clock.
Proc Natl Acad Sci U S A.
2008;
105:
15172
-15177.
[PubMed]
.
-
138.
Yang
S
, Liu
A
, Weidenhammer
A
, Cooksey
RC
, McClain
D
, Kim
MK
, Aguilera
G
, Abel
ED
and Chung
JH.
The role of mPer2 clock gene in glucocorticoid and feeding rhythms.
Endocrinology.
2009;
150:
2153
-2160.
[PubMed]
.
-
139.
Cassone
VM
and Stephan
FK.
Central and peripheral regulation of feeding and nutrition by the mammalian circadian clock: implications for nutrition during manned space flight.
Nutrition.
2002;
18:
814
-819.
[PubMed]
.
-
140.
Honma
KI
, Honma
S
and Hiroshige
T.
Critical role of food amount for prefeeding corticosterone peak in rats.
Am J Physiol.
1983;
245:
R339
-R344.
[PubMed]
.
-
141.
Grasl-Kraupp
B
, Bursch
W
, Ruttkay-Nedecky
B
, Wagner
A
, Lauer
B
and Schulte-Hermann
R.
Food restriction eliminates preneoplastic cells through apoptosis and antagonizes carcinogenesis in rat liver.
Proc Natl Acad Sci U S A.
1994;
91:
9995
-9999.
[PubMed]
.
-
142.
Mistlberger
RE
Circadian food-anticipatory activity: formal models and physiological mechanisms.
Neurosci Biobehav Rev.
1994;
18:
171
-195.
[PubMed]
.
-
143.
Hara
R
, Wan
K
, Wakamatsu
H
, Aida
R
, Moriya
T
, Akiyama
M
and Shibata
S.
Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus.
Genes Cells.
2001;
6:
269
-278.
[PubMed]
.
-
144.
Boulamery-Velly
A
, Simon
N
, Vidal
J
, Mouchet
J
and Bruguerolle
B.
Effects of three-hour restricted food access during the light period on circadian rhythms of temperature, locomotor activity, and heart rate in rats.
Chronobiol Int.
2005;
22:
489
-498.
[PubMed]
.
-
145.
Hirao
J
, Arakawa
S
, Watanabe
K
, Ito
K
and Furukawa
T.
Effects of restricted feeding on daily fluctuations of hepatic functions including p450 monooxygenase activities in rats.
J Biol Chem.
2006;
281:
3165
-3171.
[PubMed]
.
-
146.
Saito
M
, Murakami
E
and Suda
M.
Circadian rhythms in disaccharidases of rat small intestine and its relation to food intake.
Biochim Biophys Acta.
1976;
421:
177
-179.
[PubMed]
.
-
147.
Comperatore
CA
and Stephan
FK.
Entrainment of duodenal activity to periodic feeding.
J Biol Rhythms.
1987;
2:
227
-242.
[PubMed]
.
-
148.
Stephan
FK
The "other" circadian system: food as a Zeitgeber.
J Biol Rhythms.
2002;
17:
284
-292.
[PubMed]
.
-
149.
Stephan
FK
, Swann
JM
and Sisk
CL.
Anticipation of 24-hr feeding schedules in rats with lesions of the suprachiasmatic nucleus.
Behav Neural Biol.
1979;
25:
346
-363.
[PubMed]
.
-
150.
Oishi
K
, Miyazaki
K
and Ishida
N.
Functional CLOCK is not involved in the entrainment of peripheral clocks to the restricted feeding: entrainable expression of mPer2and Bmal1 mRNAs in the heart of Clock mutant mice on Jcl:ICR background.
Biochem Biophys Res Commun.
2002;
298:
198
-202.
[PubMed]
.
-
151.
Horikawa
K
, Minami
Y
, Iijima
M
, Akiyama
M
and Shibata
S.
Rapid damping of food-entrained circadian rhythm of clock gene expression in clock-defective peripheral tissues under fasting conditions.
Neuroscience.
2005;
134:
335
-343.
[PubMed]
.
-
152.
Damiola
F
, Le
Minh N
, Preitner
N
, Kornmann
B
, Fleury-Olela
F
and Schibler
U.
Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus.
Genes Dev.
2000;
14:
2950
-2961.
[PubMed]
.
-
153.
Stokkan
KA
, Yamazaki
S
, Tei
H
, Sakaki
Y
and Menaker
M.
Entrainment of the circadian clock in the liver by feeding.
Science.
2001;
291:
490
-493.
[PubMed]
.
-
154.
Lin
JD
, Liu
C
and Li
S.
Integration of energy metabolism and the mammalian clock.
Cell Cycle.
2008;
7:
453
-457.
[PubMed]
.
-
155.
Mieda
M
, Williams
SC
, Richardson
JA
, Tanaka
K
and Yanagisawa
M.
The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker.
Proc Natl Acad Sci U S A.
2006;
103:
12150
-12155.
[PubMed]
.
-
156.
Gooley
JJ
, Schomer
A
and Saper
CB.
The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms.
Nat Neurosci.
2006;
9:
398
-407.
[PubMed]
.
-
157.
Landry
GJ
, Simon
MM
, Webb
IC
and Mistlberger
RE.
Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats.
Am J Physiol Regul Integr Comp Physiol.
2006;
290:
R1527
-R1534.
[PubMed]
.
-
158.
Landry
GJ
, Yamakawa
GR
, Webb
IC
, Mear
RJ
and Mistlberger
RE.
The dorsomedial hypothalamic nucleus is not necessary for the expression of circadian food-anticipatory activity in rats.
J Biol Rhythms.
2007;
22:
467
-478.
[PubMed]
.
-
159.
Davidson
AJ
, Cappendijk
SL
and Stephan
FK.
Feeding-entrained circadian rhythms are attenuated by lesions of the parabrachial region in rats.
Am J Physiol Regul Integr Comp Physiol.
2000;
278:
R1296
-R1304.
[PubMed]
.
-
160.
Mistlberger
RE
and Mumby
DG.
The limbic system and food-anticipatory circadian rhythms in the rat: ablation and dopamine blocking studies.
Behav Brain Res.
1992;
47:
159
-168.
[PubMed]
.
-
161.
Mendoza
J
, Angeles-Castellanos
M
and Escobar
C.
Differential role of the accumbens Shell and Core subterritories in food-entrained rhythms of rats.
Behav Brain Res.
2005;
158:
133
-142.
[PubMed]
.
-
162.
Davidson
AJ
Search for the feeding-entrainable circadian oscillator: a complex proposition.
Am J Physiol Regul Integr Comp Physiol.
2006;
290:
R1524
-R1526.
[PubMed]
.
-
163.
Comperatore
CA
and Stephan
FK.
Effects of vagotomy on entrainment of activity rhythms to food access.
Physiol Behav.
1990;
47:
671
-678.
[PubMed]
.
-
164.
Mistlberger
RE
and Marchant
EG.
Enhanced food-anticipatory circadian rhythms in the genetically obese Zucker rat.
Physiol Behav.
1999;
66:
329
-335.
[PubMed]
.
-
165.
Pitts
S
, Perone
E
and Silver
R.
Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice.
Am J Physiol Regul Integr Comp Physiol.
2003;
285:
R57
-R67.
[PubMed]
.
-
166.
Pendergast
JS
, Nakamura
W
, Friday
RC
, Hatanaka
F
, Takumi
T
and Yamazaki
S.
Robust food anticipatory activity in BMAL1-deficient mice.
PLoS ONE.
2009;
4:
e4860
[PubMed]
.
-
167.
Storch
KF
and Weitz
CJ.
Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock.
Proc Natl Acad Sci U S A.
2009;
106:
6808
-6813.
[PubMed]
.
-
168.
Feillet
CA
, Ripperger
JA
, Magnone
MC
, Dulloo
A
, Albrecht
U
and Challet
E.
Lack of food anticipation in Per2 mutant mice.
Curr Biol.
2006;
16:
2016
-2022.
[PubMed]
.
-
169.
Mistlberger
RE
Circadian rhythms: perturbing a food-entrained clock.
Curr Biol.
2006;
16:
R968
-R969.
[PubMed]
.
-
170.
LeSauter
J
, Hoque
N
, Weintraub
M
, Pfaff
DW
and Silver
R.
Stomach ghrelin-secreting cells as food-entrainable circadian clocks.
Proc Natl Acad Sci U S A.
2009;
106:
13582
-13587.
[PubMed]
.
-
171.
Wu
MW
, Li
XM
, Xian
LJ
and Levi
F.
Effects of meal timing on tumor progression in mice.
Life Sci.
2004;
75:
1181
-1193.
[PubMed]
.
-
172.
Filipski
E
, Innominato
PF
, Wu
M
, Li
XM
, Iacobelli
S
, Xian
LJ
and Levi
F.
Effects of light and food schedules on liver and tumor molecular clocks in mice.
J Natl Cancer Inst.
2005;
97:
507
-517.
[PubMed]
.
-
173.
Masoro
EJ
Overview of caloric restriction and ageing.
Mech Ageing Dev.
2005;
126:
913
-922.
[PubMed]
.
-
174.
Spindler
SR
Caloric restriction: From soup to nuts.
Ageing Res Rev.
2009;
In press
.
-
175.
Colman
RJ
, Anderson
RM
, Johnson
SC
, Kastman
EK
, Kosmatka
KJ
, Beasley
TM
, Allison
DB
, Cruzen
C
, Simmons
HA
, Kemnitz
JW
and Weindruch
R.
Caloric restriction delays disease onset and mortality in rhesus monkeys.
Science.
2009;
325:
201
-204.
[PubMed]
.
-
176.
Roth
GS
, Mattison
JA
, Ottinger
MA
, Chachich
ME
, Lane
MA
and Ingram
DK.
Aging in rhesus monkeys: relevance to human health interventions.
Science.
2004;
305:
1423
-1426.
[PubMed]
.
-
177.
Fontana
L
Modulating human aging and age-associated diseases.
Biochim Biophys Acta.
2009;
1790:
1133
-1138.
[PubMed]
.
-
178.
Masoro
EJ
Caloric restriction-induced life extension of rats and mice: a critique of proposed mechanisms.
Biochim Biophys Acta.
2009;
1790:
1040
-1048.
[PubMed]
.
-
179.
Harman
D
Aging: A theory based on free radical and radiation chemistry.
J Gerontol.
1956;
11:
298
-300.
[PubMed]
.
-
180.
Sohal
RS
and Weindruch
R.
Oxidtive stress, caloric restriction, and aging.
Science.
1996;
273:
59
-63.
[PubMed]
.
-
181.
Van
Remmen H
, Ikeno
Y
, Hamilton
M
, Pahlavani
M
, Wolf
N
, Thorpe
SR
, Alderson
NL
, Baynes
JW
, Epstein
CJ
, Huang
TT
, Nelson
J
, Strong
R
and Richardson
A.
Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging.
Physiol Genomics.
2003;
16:
29
-37.
[PubMed]
.
-
182.
Perez
VI
, Bokov
A
, Van
Remmen H
, Mele
J
, Ran
Q
, Ikeno
Y
and Richardson
A.
Is the oxidative stress theory of aging dead.
Biochim Biophys Acta.
2009;
1790:
1005
-1014.
[PubMed]
.
-
183.
Salmon
AB
, Perez
VI
, Bokov
A
, Jernigan
A
, Kim
G
, Zhao
H
, Levine
RL
and Richardson
A.
Lack of methionine sulfoxide reductase A in mice increases sensitivity to oxidative stress but does not diminish life span.
FASEB J.
2009;
23:
3601
-3608.
[PubMed]
.
-
184.
Blagosklonny
MV
Aging: ROS or TOR.
Cell Cycle.
2008;
7:
3344
-3354.
[PubMed]
.
-
185.
Jiang
W
, Zhu
Z
and Thompson
HJ.
Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver.
Cancer Res.
2008;
68:
5492
-5499.
[PubMed]
.
-
186.
Selman
C
, Tullet
JM
, Wieser
D
, Irvine
E
, Lingard
SJ
, Choudhury
AI
, Claret
M
, Al-Qassab
H
, Carmignac
D
, Ramadani
F
, Woods
A
, Robinson
IC
, Schuster
E
, Batterham
RL
, Kozma
SC
, Thomas
G
, Carling
D
, Okkenhaug
K
, Thornton
JM
, Partridge
L
, Gems
D
and Withers
DJ.
Ribosomal protein S6 kinase 1 signaling regulates mammalian life span.
Science.
2009;
326:
140
-144.
[PubMed]
.
-
187.
Harrison
DE
, Strong
R
, Sharp
ZD
, Nelson
JF
, Astle
CM
, Flurkey
K
, Nadon
NL
, Wilkinson
JE
, Frenkel
K
, Carter
CS
, Pahor
M
, Javors
MA
, Fernandez
E
and Miller
RA.
Rapamycin fed late in life extends lifespan in genetically heterogeneous mice.
Nature.
2009;
460:
392
-395.
[PubMed]
.
-
188.
Cao
R
, Lee
B
, Cho
HY
, Saklayen
S
and Obrietan
K.
Photic regulation of the mTOR signaling pathway in the suprachiasmatic circadian clock.
Mol Cell Neurosci.
2008;
38:
312
-324.
[PubMed]
.
-
189.
Duffy
PH
, Feuers
RJ
, Leakey
JA
, Nakamura
K
, Turturro
A
and Hart
RW.
Effect of chronic caloric restriction on physiological variables related to energy metabolism in the male Fischer 344 rat.
Mech Ageing Dev.
1989;
48:
117
-133.
[PubMed]
.
-
190.
Challet
E
, Solberg
LC
and Turek
FW.
Entrainment in calorie-restricted mice: conflicting zeitgebers and free-running conditions.
Am J Physiol.
1998;
274:
R1751
-R1761.
[PubMed]
.
-
191.
Challet
E
, Caldelas
I
, Graff
C
and Pevet
P.
Synchronization of the molecular clockwork by light- and food-related cues in mammals.
Biol Chem.
2003;
384:
711
-719.
[PubMed]
.
-
192.
Mendoza
J
, Graff
C
, Dardente
H
, Pevet
P
and Challet
E.
Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle.
J Neurosci.
2005;
25:
1514
-1522.
[PubMed]
.
-
193.
Swindell
WR
Comparative analysis of microarray data identifies common responses to caloric restriction among mouse tissues.
Mech Ageing Dev.
2008;
129:
138
-153.
[PubMed]
.
-
194.
Froy
O
, Chapnik
N
and Miskin
R.
Long-lived alphaMUPA transgenic mice exhibit pronounced circadian rhythms.
Am J Physiol Endocrinol Metab.
2006;
291:
E1017
-E1024.
[PubMed]
.
-
195.
Froy
O
and Miskin
R.
The interrelations among feeding, circadian rhythms and ageing.
Prog Neurobiol.
2007;
82:
142
-150.
[PubMed]
.
-
196.
Anson
RM
, Guo
Z
, de Cabo
R
, Iyun
T
, Rios
M
, Hagepanos
A
, Ingram
DK
, Lane
MA
and Mattson
MP.
Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake.
Proc Natl Acad Sci U S A.
2003;
100:
6216
-6220.
[PubMed]
.
-
197.
Descamps
O
, Riondel
J
, Ducros
V
and Roussel
AM.
Mitochondrial production of reactive oxygen species and incidence of age-associated lymphoma in OF1 mice: effect of alternate-day fasting.
Mech Ageing Dev.
2005;
126:
1185
-1191.
[PubMed]
.
-
198.
Froy
O
, Chapnik
N
and Miskin
R.
Effect of intermittent fasting on circadian rhythms in mice depends on feeding time.
Mech Ageing Dev.
2009;
130:
154
-160.
[PubMed]
.
-
199.
Goodrick
CL
, Ingram
DK
, Reynolds
MA
, Freeman
JR
and Cider
N.
Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age.
Mech Ageing Dev.
1990;
55:
69
-87.
[PubMed]
.
-
200.
Mattson
MP
and Wan
R.
Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems.
J Nutr Biochem.
2005;
16:
129
-137.
[PubMed]
.
-
201.
Contestabile
A
and Ciani
E.
Dietary restriction differentially protects from neurodegeneration in animal models of excitotoxicity.
Brain Res.
2004;
1002:
162
-166.
[PubMed]
.
-
202.
Mattson
MP
Energy intake, meal frequency, and health: a neurobiological perspective.
Annu Rev Nutr.
2005;
25:
237
-260.
[PubMed]
.
-
203.
Sharma
S
and Kaur
G.
Neuroprotective potential of dietary restriction against kainate-induced excitotoxicity in adult male Wistar rats.
Brain Res Bull.
2005;
67:
482
-491.
[PubMed]
.
-
204.
Ahmet
I
, Wan
R
, Mattson
MP
, Lakatta
EG
and Talan
M.
Cardioprotection by intermittent fasting in rats.
Circulation.
2005;
112:
3115
-3121.
[PubMed]
.
-
205.
Mager
DE
, Wan
R
, Brown
M
, Cheng
A
, Wareski
P
, Abernethy
DR
and Mattson
MP.
Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats.
FASEB J.
2006;
20:
631
-637.
[PubMed]
.
-
206.
Varady
KA
and Hellerstein
MK.
Alternate-day fasting and chronic disease prevention: a review of human and animal trials.
Am J Clin Nutr.
2007;
86:
7
-13.
[PubMed]
.
-
207.
Mattson
MP
, Duan
W
, Wan
R
and Guo
Z.
Prophylactic activation of neuroprotective stress response pathways by dietary and behavioral manipulations.
NeuroRx.
2004;
1:
111
-116.
[PubMed]
.
-
208.
Mattson
MP
Dietary factors, hormesis and health.
Ageing Res Rev.
2008;
7:
43
-48.
[PubMed]
.
-
209.
Duan
W
, Lee
J
, Guo
Z
and Mattson
MP.
Dietary restriction stimulates BDNF production in the brain and thereby protects neurons against excitotoxic injury.
J Mol Neurosci.
2001;
16:
1
-12.
[PubMed]
.
-
210.
Newton
IG
, Forbes
ME
, Legault
C
, Johnson
JE
, Brunso-Bechtold
JK
and Riddle
DR.
Caloric restriction does not reverse aging-related changes in hippocampal BDNF.
Neurobiol Aging.
2005;
26:
683
-688.
[PubMed]
.
-
211.
Andrade
JP
, Mesquita
R
, Assuncao
M
and Pereira
PA.
Effects of food restriction on synthesis and expression of brain-derived neurotrophic factor and tyrosine kinase B in dentate gyrus granule cells of adult rats.
Neurosci Lett.
2006;
399:
135
-140.
[PubMed]
.
-
212.
Maswood
N
, Young
J
, Tilmont
E
, Zhang
Z
, Gash
DM
, Gerhardt
GA
, Grondin
R
, Roth
GS
, Mattison
J
, Lane
MA
, Carson
RE
, Cohen
RM
, Mouton
PR
, Quigley
C
, Mattson
MP
and Ingram
DK.
Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson's disease.
Proc Natl Acad Sci U S A.
2004;
101:
18171
-18176.
[PubMed]
.
-
213.
Xu
B
, Goulding
EH
, Zang
K
, Cepoi
D
, Cone
RD
, Jones
KR
, Tecott
LH
and Reichardt
LF.
Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor.
Nat Neurosci.
2003;
6:
736
-742.
[PubMed]
.
-
214.
Duan
W
, Guo
Z
, Jiang
H
, Ware
M
and Mattson
MP.
Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor.
Endocrinology.
2003;
144:
2446
-2453.
[PubMed]
.
-
215.
Liang
FQ
, Sohrabji
F
, Miranda
R
, Earnest
B
and Earnest
D.
Expression of brain-derived neurotrophic factor and its cognate receptor, TrkB, in the rat suprachiasmatic nucleus.
Exp Neurol.
1998;
151:
184
-193.
[PubMed]
.
-
216.
Liang
FQ
, Allen
G
and Earnest
D.
Role of brain-derived neurotrophic factor in the circadian regulation of the suprachiasmatic pacemaker by light.
J Neurosci.
2000;
20:
2978
-2987.
[PubMed]
.
-
217.
Miskin
R
, Axelrod
JH
, Griep
AE
, Lee
E
, Belin
D
, Vassalli
JD
and Westphal
H.
Human and murine urokinase cDNAs linked to the murine alpha A-crystallin promoter exhibit lens and non-lens expression in transgenic mice.
Eur J Biochem.
1990;
190:
31
-38.
[PubMed]
.
-
218.
Mondino
A
and Blasi
F.
uPA and uPAR in fibrinolysis, immunity and pathology.
Trends Immunol.
2004;
25:
450
-455.
[PubMed]
.
-
219.
Soreq
H
and Miskin
R.
Plasminogen activator in the developing rat cerebellum: biosynthesis and localization in granular neurons.
Brain Res.
1983;
313:
149
-158.
[PubMed]
.
-
220.
Sumi
Y
, Dent
MA
, Owen
DE
, Seeley
PJ
and Morris
RJ.
The expression of tissue and urokinase-type plasminogen activators in neural development suggests different modes of proteolytic involvement in neuronal growth.
Development.
1992;
116:
625
-637.
[PubMed]
.
-
221.
Masos
T
and Miskin
R.
mRNAs encoding urokinase-type plaminogen activator and plasminogen activator inhibitor-1 are elevated in the mouse brain following kainate-mediated excitation.
Brain Res Mol Brain Res.
1997;
47:
157
-169.
[PubMed]
.
-
222.
Bahi
A
, Boyer
F
, gumy
C
, Kafri
T
and Dreyer
JL.
In vivo gene delivery of urokinase-type plasminogen activator with regulatable lentivirus induces behavioural changes in chronic cocaine administration.
Eur J Neurosci.
2004;
20:
3473
-3488.
[PubMed]
.
-
223.
Bahi
A
and Dreyer
JL.
Overexpression of plasminogen activators in the nucleus accumbens enhances cocaine-, amphetamine- and morphine-induced reward and behavioral sensitization.
Genes Brain Behav.
2008;
7:
244
-256.
[PubMed]
.
-
224.
Lahtinen
L
, Lukasiuk
K
and Pitkanen
A.
Increased expression and activity of urokinase-type plasminogen activator during epileptogenesis.
Eur J Neurosci.
2006;
24:
1935
-1945.
[PubMed]
.
-
225.
Miskin
R
, Tirosh
O
, Pardo
M
, Zusman
I
, Schwartz
B
, Yahav
S
, Dubnov
G
and Kohen
R.
alphaMUPA mice: a transgenic model for longevity induced by caloric restriction.
Mech Ageing Dev.
2005;
126:
255
-261.
[PubMed]
.
-
226.
Miskin
R
, Masos
T
, Shoham
Z
and Williams-Simons
L.
Urokinase-type plasminogen activator mRNA is expressed in normal developing teeth and leads to abnormal incisor enamel in alpha MUPA transgenic mice.
Transgenic Res.
2006;
15:
241
-254.
[PubMed]
.
-
227.
Miskin
R
and Masos
T.
Transgenic mice overexpressing urokinase-type plasminogen activator in the brain exhibit reduced food consumption, body weight and size, and increased longevity.
J Gerontol A Biol Sci Med Sci.
1997;
52:
B118
-B124.
[PubMed]
.
-
228.
Miskin
R
, Masos
T
, Yahav
S
, Shinder
D
and Globerson
A.
alphaMUPA mice: a transgenic model for increased life span.
Neurobiol Aging.
1999;
20:
555
-564.
[PubMed]
.
-
229.
Tirosh
O
, Aronis
A
, Zusman
I
, Kossoy
G
, Yahav
S
, Shinder
D
, Abramovitz
R
and Miskin
R.
Mitochondrion-mediated apoptosis is enhanced in long-lived alphaMUPA transgenic mice and calorically restricted wild-type mice.
Exp Gerontol.
2003;
38:
955
-963.
[PubMed]
.
-
230.
Tirosh
O
, Pardo
M
, Schwartz
B
and Miskin
R.
Long-lived alphaMUPA transgenic mice show reduced SOD2 expression, enhanced apoptosis and reduced susceptibility to the carcinogen dimethylhydrazine.
Mech Ageing Dev.
2005;
126:
1262
-1273.
[PubMed]
.
-
231.
Masoro
EJ
, Shimokawa
I
, Higami
Y
, McMahan
CA
and Yu
BP.
Temporal pattern food intake not a factor in the retardation of aging processes by dietary restriction.
J Gerontol A Biol Sci Med Sci.
1995;
50A:
B48
-53.
[PubMed]
.
-
232.
Nelson
W
and Halberg
F.
Meal-timing, circadian rhythms and life span of mice.
J Nutr.
1986;
116:
2244
-2253.
[PubMed]
.
-
233.
Cheney
KE
, Liu
RK
, Smith
GS
, Meredith
PJ
, Mickey
MR
and Walford
RL.
The effect of dietary restriction of varying duration on survival, tumor patterns, immune function, and body temperature in B10C3F1 female mice.
J Gerontol.
1983;
38:
420
-430.
[PubMed]
.
-
234.
Nelson
W
and Halberg
F.
Schedule-shifts, circadian rhythms and lifespan of freely feeding and meal-fed mice.
Physiol Behav.
1986;
38:
781
-788.
[PubMed]
.
-
235.
Heilbronn
LK
, Civitarese
AE
, Bogacka
I
, Smith
SR
, Hulver
M
and Ravussin
E.
Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting.
Obes Res.
2005;
13:
574
-581.
[PubMed]
.
-
236.
Heilbronn
LK
, Smith
SR
, Martin
CK
, Anton
SD
and Ravussin
E.
Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism.
Am J Clin Nutr.
2005;
81:
69
-73.
[PubMed]
.
-
237.
Aksungar
FB
, Topkaya
AE
and Akyildiz
M.
Interleukin-6, C-reactive protein and biochemical parameters during prolonged intermittent fasting.
Ann Nutr Metab.
2007;
51:
88
-95.
[PubMed]
.
-
238.
Beck
B
and Richy
S.
Dietary modulation of ghrelin and leptin and gorging behavior after weight loss in the obese Zucker rat.
J Endocrinol.
2009;
202:
29
-34.
[PubMed]
.
-
239.
Yang
H
, Youm
YH
, Nakata
C
and Dixit
VD.
Chronic caloric restriction induces forestomach hypertrophy with enhanced ghrelin levels during aging.
Peptides.
2007;
28:
1931
-1936.
[PubMed]
.
-
240.
Gonzalez
AA
, Kumar
R
, Mulligan
JD
, Davis
AJ
, Weindruch
R
and Saupe
KW.
Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity.
Am J Physiol Endocrinol Metab.
2004;
287:
E1032
-E1037.
[PubMed]
.
-
241.
Das
M
, Gabriely
I
and Barzilai
N.
Caloric restriction, body fat and ageing in experimental models.
Obes Rev.
2004;
5:
13
-19.
[PubMed]
.
-
242.
Harrison
DE
and Archer
JR.
Genetic differences in effects of food restriction on aging in mice.
J Nutr.
1987;
117:
376
-382.
[PubMed]
.
-
243.
Allison
DB
, Miller
RA
, Austad
SN
, Bouchard
C
, Leibel
R
, Klebanov
S
, Johnson
T
and Harrison
DE.
Genetic variability in responses to caloric restriction in animals and in regulation of metabolism and obesity in humans.
J Gerontol A Biol Sci Med Sci.
2001;
56 Spec No 1:
55
-65.
[PubMed]
.
-
244.
Bordone
L
and Guarente
L.
Calorie restriction, SIRT1 and metabolism: understanding longevity.
Nat Rev Mol Cell Biol.
2005;
6:
298
-305.
[PubMed]
.
-
245.
Masternak
MM
, Al-Regaiey
KA
, Del Rosario
Lim MM
, Jimenez-Ortega
V
, Panici
JA
, Bonkowski
MS
, Kopchick
JJ
and Bartke
A.
Effects of caloric restriction and growth hormone resistance on the expression level of peroxisome proliferator-activated receptors superfamily in liver of normal and long-lived growth hormone receptor/binding protein knockout mice.
J Gerontol A Biol Sci Med Sci.
2005;
60:
1394
-1398.
[PubMed]
.
-
246.
Chen
D
, Bruno
J
, Easlon
E
, Lin
SJ
, Cheng
HL
, Alt
FW
and Guarente
L.
Tissue-specific regulation of SIRT1 by calorie restriction.
Genes Dev.
2008;
22:
1753
-1757.
[PubMed]
.
-
247.
Cohen
HY
, Miller
C
, Bitterman
KJ
, Wall
NR
, Hekking
B
, Kessler
B
, Howitz
KT
, Gorospe
M
, de Cabo
R
and Sinclair
DA.
Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase.
Science.
2004;
305:
390
-392.
[PubMed]
.
-
248.
Boily
G
, Seifert
EL
, Bevilacqua
L
, He
XH
, Sabourin
G
, Estey
C
, Moffat
C
, Crawford
S
, Saliba
S
, Jardine
K
, Xuan
J
, Evans
M
, Harper
ME
and McBurney
MW.
SirT1 regulates energy metabolism and response to caloric restriction in mice.
PLoS One.
2008;
3:
e1759
[PubMed]
.
-
249.
Bordone
L
, Cohen
D
, Robinson
A
, Motta
MC
, van
Veen E
, Czopik
A
, Steele
AD
, Crowe
H
, Marmor
S
, Luo
J
, Gu
W
and Guarente
L.
SIRT1 transgenic mice show phenotypes resembling calorie restriction.
Aging Cell.
2007;
6:
759
-767.
[PubMed]
.
-
250.
Ramadori
G
, Lee
CE
, Bookout
AL
, Lee
S
, Williams
KW
, Anderson
J
, Elmquist
JK
and Coppari
R.
Brain SIRT1: anatomical distribution and regulation by energy availability.
J Neurosci.
2008;
28:
9989
-9996.
[PubMed]
.
-
251.
Taleux
N
, De
Potter I
, Deransart
C
, Lacraz
G
, Favier
R
, Leverve
XM
, Hue
L
and Guigas
B.
Lack of starvation-induced activation of AMP-activated protein kinase in the hypothalamus of the Lou/C rats resistant to obesity.
Int J Obes (Lond).
2008;
32:
639
-647.
[PubMed]
.
-
252.
Veyrat-Durebex
C
, Montet
X
, Vinciguerra
M
, Gjinovci
A
, Meda
P
, Foti
M
and Rohner-Jeanrenaud
F.
The Lou/C rat: a model of spontaneous food restriction associated with improved insulin sensitivity and decreased lipid storage in adipose tissue.
Am J Physiol Endocrinol Metab.
2009;
296:
E1120
-1132.
[PubMed]
.
-
253.
Guarente
L
and Picard
F.
Calorie restriction--the SIR2 connection.
Cell.
2005;
120:
473
-482.
[PubMed]
.