Circadian disruption induced by light-at-night accelerates aging and promotes tumorigenesis in young but not in old rats
Abstract
We evaluated the effect of exposure to constant light started at the age of 1 month and at the age of 14 months on the survival, life span, tumorigenesis and age-related dynamics of antioxidant enzymes activity in various organs in comparison to the rats maintained at the standard (12:12 light/dark) light/dark regimen. We found that exposure to constant light started at the age of 1 month accelerated spontaneous tumorigenesis and shortened life span both in male and female rats as compared to the standard regimen. At the same time, the exposure to constant light started at the age of 14 months failed to influence survival of male and female rats. While delaying tumors in males, constant light accelerated tumors in females. We conclude that circadian disruption induced by light-at-night started at the age of 1 month accelerates aging and promotes tumorigenesis in rats, however failed affect survival when started at the age of 14 months.
Introduction
Light-at-night
has become an increasing and essential part of modern lifestyle and leads to a
number of health problems, including excess of body mass index, cardiovascular
diseases, diabetes and cancer [1-10]. The International Agency for Research on
Cancer (IARC) Working Group concluded that "shift-work that involves circadian
disruption is probably carcinogenic to humans" (Group 2A) [11]. An increase in
light pollution could be one of causes of the sharp rise of mortality from
breast cancer among Alaskan native peoples (Eskimo, Indian and Aleut) since
1969 [12]. It was shown that there is a
significant positive correlationbetween
geographical latitude and the incidence of breast, colon and endometrial
carcinomas and absence of the correlation in a case of stomach and lung cancers
[13].
According to the circadian disruption
hypothesis, light-at-night might disrupt the endogenous circadian rhythm, and
specifically suppress nocturnal production of pineal hormone melatonin and its
secretion in the blood [9,10,14]. Earlier we have shown that the exposure to
constant illumination started at the age of 1 months accelerated development of
metabolic syndrome and spontaneous tumorigenesis, shortened life span in rats
as compared to the standard (12 hours light/12 hours dark) regimen [15]. In
this paper in the first time it was shown that the exposure to constant
illumination started at the period of natural
switching-off reproductive function has no effect or protective effect on
antioxidant defense system, survival and tumorigenesis in rats.
Results
Effect of light/dark regimen on life
span in rats
In male rats, the exposure to LL regimen
started at the age of 1 month failed significantly influence the mean life span
of all as well as the last of 10% survivors whereas the exposure to LL regimen
started at the age of 14 months increased by 6.7% the mean life span
(p>0.05), by 9.4% (p<0.01) the mean life span of the last 10% survivors
and increases by 3 months the maximum life span of male rats (Table 1).
Table 1. Effect of the exposure to constant light started at the age of 1 month (LL-1) and at the age of 14 months (LL-14) on survival and life span in male rats.
Notes: * Number of rats at the age of 14 months.
Difference with controls (LD) is significant: a, p<
0.05; b, p< 0.01; #, in brackets 95% confidential intervals. MRDT, mortality rate doubling time.
|
Parameters
|
Light/dark regimen
|
|
LD
|
LL-1
|
LL-14
|
|
Number of rats*
|
43
|
34
|
90
|
|
Mean life span, days
|
766 ± 25.4
|
744 ± 28.0
|
818 ± 18.1
(+ 6.7%)
|
|
Maximum life span, days
|
1045
|
1005
|
1141
|
|
Mean life span of last 10%
survivors, days
|
994 ± 9.2
|
1002 ± 1.8
|
1087 ± 8.3
(+ 9.4%)b |
|
α x 103, days-1 |
7.49
(7.20; 7.75)#
|
7.07
(6.90; 7.16)a |
6.58
(6.28; 6.82)a |
|
MRDT, days
|
92.6
(89.4; 96.3)
|
98.1
(96.9; 100.4)a |
105.3
(101.6; 110.4.)a |
Table 2. Effect of the exposure to constant light started at the age of 1 month (LL-1) and at the age of 14 months (LL-14) on survival and life span in female rats.
Notes: * Number of rats at the age of 14 months.
Difference with controls (LD) is significant:
a, p<0.05; b, p<0.01; #,
in brackets 95% confidential intervals. MRDT,
mortality rate doubling time.
|
Parameters
|
Light/dark regimen
|
|
LD
|
LL-1
|
LL-14
|
|
Number of rats*
|
30
|
36
|
71
|
|
Mean life span, days
|
844 ± 33.6
|
658 ± 22.8b
(- 22.0%)
|
811 ± 20.0
|
|
Maximum life span, days
|
1167
|
956
|
1198
|
|
Mean life span of last 10%
survivors, days
|
1129 ± 18.9
|
921 ± 19.7
(-18.4%)
|
1113 ± 24.9
|
|
α x 103, days-1 |
5.74
(5.56; 6.01)
|
4.19
(4.01; 4.38)a |
6.03
(5.79; 6.35)
|
|
MRDT, days
|
120.7
(115.3; 124.6)
|
165.6
(158.4; 173.1)a |
114.9
(109.1; 119.6)
|
At the same time,
the rate of population aging (parameter α in the Gompertz equation) was
slightly decreased in LL-1 and in LL-14 groups as compared with the LD group
males. The survival curve for males of the group LL-1 was significantly
shifted to left in comparison to the survival curve for the group LD (Figure 1A) whereas was not in LL-14 group (Figure 1A).
In female rats, the exposure tothe LL regimen significantly decreased the mean life
span (by 22.0%) and the population aging rate (by 27.0%) when started at the
age of 1 month and failed to change both the mean life span and the aging rate
when it was started at the age of 14 months (Table 2). The survival curve for
females of the group LL-1 was significantly shifted to left in comparison to
the survival curve for the group LD whereas was not in LL-14 group (Figure 1D).
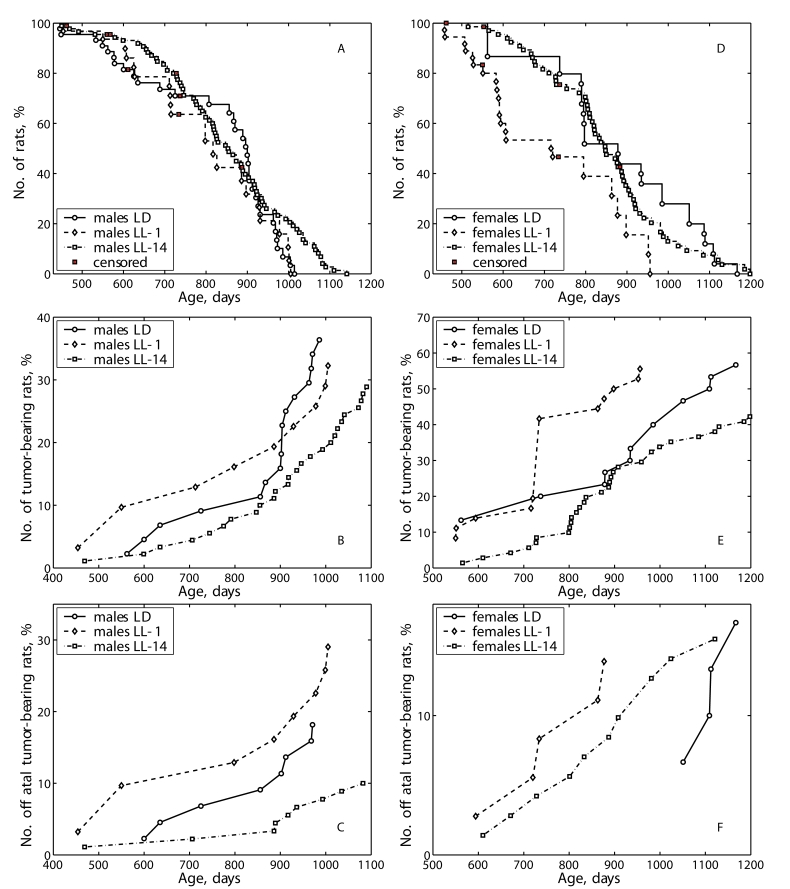
Figure 1. Effect of the exposure to various light regimens on tumorigenesis and survival in rats.
(A) - survival, males; (B) - total tumor incidence, males; (C)
- fatal tumor incidence, males; (D) - survival, females; (E)
- total tumor incidence, females; (F) - fatal tumor incidence,
females.
According to the log-rank test the
conditional life span distributions of rats (given the animals survived the age
of 14 months) kept under alternating day/night and two constant light regimens
starting from one and 14 months of age differ insignificantly for males
(p-value is 1.58E-01, χ2=3.7 on 2 df) and significantly for females
(p-value is 6.31E-04, χ2=14.7 on 2 df). The difference between two groups
of male rats kept under constant light regimens (LL-1 and LL-14) is significant
(p-value is 1.02E-01, χ2=2.7 on 1 df). The life span distribution of
females kept under constant light from the age of one month differs
significantly from the control LD group (p-value is 1.39E-03, χ2=10.2 on 1
df) and from the group subjected to the constant light from the 14th month
(p-value is 1.26E-03, χ2=10.4 on 1 df).
According to the estimated parameters of
the Cox's regression model in males the constant light from older age decreases
the relative risk of death compared to the group kept under the same regiment
from earlier in life. Among the females, the LL-1 regimen increases the risk of
death compared to the control group and the LL-14 decreases the risk of death
compared to the LL-1 group (Table 3).
Effect of light/dark regimen on
spontaneous tumorigenesis in rats
Pathomorphological analysis shows that
benign tumors were most frequent in all groups of males and females. The
significant part of them was represented by testicular Leydig cell tumors in
males and mammary fibroadenomas in females (Tables 4 and 5). Among malignant
tumors lymphomas were most common however some cases of hepatocellular
carcinoma, soft tissues sarcomas and sporadic carcinomas of other organs were
detected.
The exposure to the LL-1 regimen
accelerated sponta- neous tumors development as compared to
the LD group and not influenced their total incidence both in male and female
rats (Tables 4 and 5; Figure 1B and 1E). The first tumor in males of the LL-1
group was detected 5 months earlier than the first tumor in the LD group. The
exposure to the LL-14 regimen did not influence the incidence of spontaneous
tumors in male and female rats.
Table 3. Cox's regression model parameters for experimental groups.
|
All rats
|
β
|
exp(β)
|
se(β)
|
p
|
|
Males LL-1 and LL-14
|
-0.41
|
0.67
|
0.25
|
1.00E-01
|
|
Females LD and LL-1
|
1.02
|
2.78
|
0.34
|
2.30E-03
|
|
Females LL-1 and LL-14
|
-0.82
|
0.44
|
0.26
|
1.70E-03
|
According to the log rank test the
difference in life span distributions among all three groups of male rats with
fatal and non-fatal tumors is significant (p-value is 4.85E-02, χ2=6.1 on
2 df). The pair-vise difference between LD and LL-1 groups is insignificant;
between LD and LD-14 is significant (p-value is 3.32E-02, χ2=4.5 on 1 df);
between LL-1 and LL-14 can be considered as significant (p-value is 1.10E-01,
χ2=2.6 on 1 df). There was no significant difference in life span
distributions among the female tumor-bearing rats.
According to the Cox's regression model
the risk of death among the tumor-bearing male rats subjected to the LL-14
regiment is significantly lower compared to the LD group (β = -0.75;
exp(β) = 0.47; se(β) =0.36; p = 3.60E-02).
According to the log rank test there is
no significant difference in life span distributions among male rats with fatal
tumors subjected to different regiments. In females with fatal tumors the difference
is significant among all three groups of rats (p-value is 8.30E-03, χ2=9.6
on 2 df); between LD and LL-1 groups (p-value is 4.50E-03, χ2=8.1 on 1 df)
and between LD and LL-14 groups (p-value is 1.91E-02, χ2=5.5 on 1 df).
As estimated with
the Cox's regression model the risk of death among female LD-14 rats with fatal
tumors is sig-nificantly greater than for female rats under LD regiment (β
= 1.36; exp(β) = 3.89; se(β) = 0.62; p = 2.80E-02).
Table 4. Effect of the exposure to constant light started at the age of 1 month (LL-1) and at the age of 14 months (LL-14) on tumorigenesis in male rats.
Notes: TBR - tumor-bearing rats.
|
Parameters
|
Light/dark regimen
|
|
LD
|
LL-1
|
LL-14
|
|
Number of rats
|
43
|
34
|
90
|
|
Number
of TBR (%)
|
15 (34.9%)
|
12 (35.3%)
|
26 (28.9%)
|
|
Number of malignant TBR (%)
|
8 (18.6%)
|
10 (29.4%)
|
9 (10%)
|
|
Total number of tumors
|
21
|
13
|
34
|
|
Number of tumors per TBR
|
1.40
|
1.08
|
1.31
|
|
Age at the time of the 1st
tumor detections, days
|
600
|
428
|
469
|
|
Mean life span of TBR, days
|
849 ± 34.7
|
786 ± 65.3
|
897 ± 32.1
|
|
Mean life span of fatal
TBR, days
|
821 ± 52.2
|
794 ± 72.8
|
879 ± 62.5
|
| Localization and type of
tumors |
|
Testes:
Leydigoma
hemangioma
|
7
|
3
|
16
|
|
1
|
-
|
1
|
|
Malignant lymphoma/
leukemia
|
3
|
6
|
3
|
|
Mammary gland:
fibroadenoma
|
-
|
-
|
1
|
|
Liver:
hepatocarcinoma
|
2
|
2
|
1
|
|
Skin
carcinoma
|
-
|
-
|
1
|
|
Soft
trissues: angiofibroma
fibroma
chondroma
sarcoma
malignant fibrous histoiocytoma
|
-
|
-
|
1
|
|
-
|
-
|
1
|
|
-
|
-
|
1
|
|
-
|
-
|
4
|
|
2
|
-
|
-
|
|
Lung:
adenocarcinoma
light-c ell carcinoma
|
-
|
1
|
-
|
|
1
|
-
|
-
|
|
Small bowel:
adenocarcinoma
|
-
|
1
|
-
|
|
Adrenal gland: cortical
adenoma
pheochromocytoma
|
3
|
-
|
3
|
|
1
|
-
|
-
|
|
Urether:
fibroma
|
1
|
-
|
-
|
|
Nervous system:
paraganglioma
|
-
|
-
|
1
|
|
Total:
benign
malignant
|
13
|
3
|
25
|
|
8
|
10
|
9
|
Effect of light/dark regimen on free radical processes in rats
Age-related changes in free radical
processes should be generally described as desynchronization in activity of
antioxidative enzymes and as a decreased antioxidant defense in the majority of
organs. The changes of the functional activity of pineal gland induced by
constant illumination affect both dynamics and level of enzymatic activities.
Most significant effects of the age of start of the exposure to constant light
on differences in the enzymatic activities were
detected in the liver. Thus, the activity of catalase revealed season
cyclicity in rats of the group LD and LL-1. In the group LL-14, the activity
of both catalase and SOD was cyclic and revealed more high level as compared
with the relevant parameters in the group LL-1. Maximum levels of the
enzymatic activity was detected at the age of 24 months whereas in LD and LL-1
groups its where at the age of 12 and 18 months (Figures 2 and 3). There were
age-related decrease in catalase activity in the groups LD and LL-1, but not in
LL-14 group.
There were season changes in dynamics
of activity of antioxidant enzymes. Season variations in the activity of SOD
were observed in heart, lungs and skeletal muscles, whereas the activity of
catalase - in kidney and skeletal muscles. Age-related increase in catalase
activity was observed in the skeletal muscles in rats of all three groups. The activity of SOD in
lungs and spleen of rats in LL-14 group revealed U-shape curve pattern: it
decreased at the age 24 months and increased at the age of 30 months. In the
group LL-1 the decrease in SOD activity in lungs and spleen have been observed
at the age of 12 months (Figures 2 and 3).
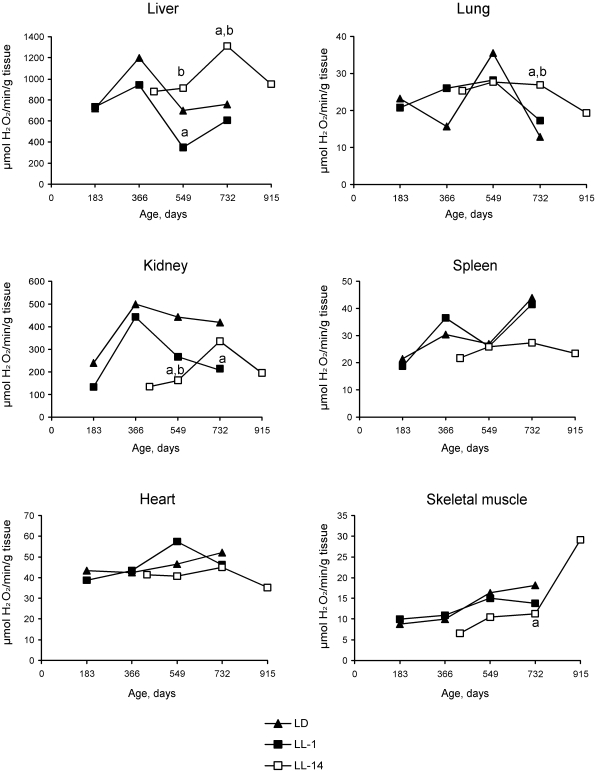
Figure 2. Effect of the exposure to various light regimens on age-related dynamics of the catalase activity in organs of rats. (a) - the
difference with the relevant parameter in the group LD is significant,
p<0.05; (b) - the
difference with the relevant parameter in the group LL-1 is significant,
p<0.05.
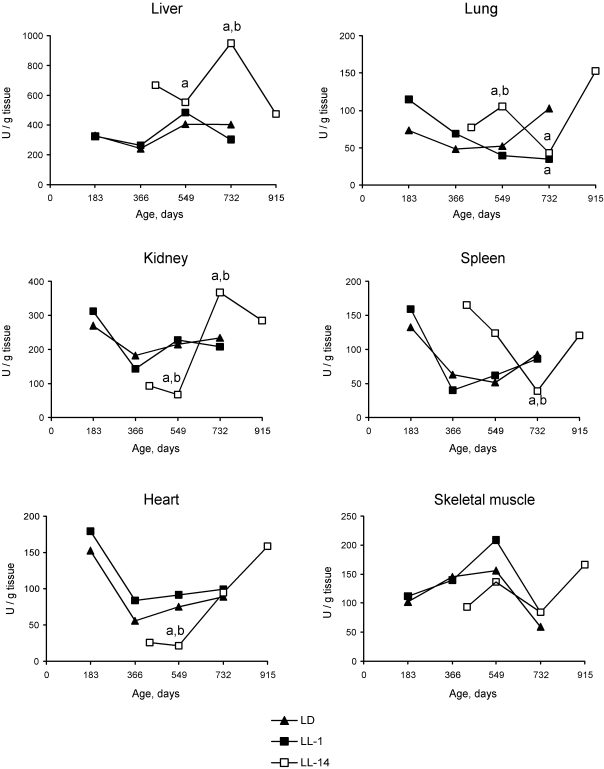
Figure 3. Effect of the exposure to various light regimens on age-related dynamics of the Cu,Zn-superoxide dysmutase (SOD) activity in organs of rats. (a) - the
difference with the relevant parameter in the group LD is significant,
p<0.05;
(b)
- the difference with the relevant parameter in the group LL-1 is
significant, p<0.05.
Discussion
Our present data have shown that
live-long maintenance of male and female rats at the LL regimen started at the
age of 1 month accelerated aging, decreased survival and promoted spontaneous
tumorigenesis, whereas the exposure to constant illumination started at the age
of 14 months failed to reduce life span. Moreover it seems that LL-14 regimen
had rather protective effect on survival and delayed age-related decrease in
activity of antioxidant enzymes, SOD and catalase. Experiments in female
rodents presented significantly evidence that exposure to constant illumination
(24 hours per day) leads to disturbances in estrus function (persistent estrus
syndrome, anovulation) [16-18] and spontaneous tumor development [1,17,19,20]. In all these
studies the exposure to constant illumination has been started at the young
adult age. There are evidences that the exposure to light at night time
inhibits pineal production and secretion of melatonin - key pineal hormone
[5,21,22]. It is worthy of note that old rodents are more susceptible to
modifications of the photoperiod as compared with young ones [23]. In
postmenopausal women, light at night suppressed serum melatonin level in higher
degree then that in young cycling women. The exposure to constant illumination
increases the lipid peroxidation in tissues and decreases both the total
antioxidant activity and SOD activity, whereas treatment with melatonin
inhibits lipid peroxidation, in the brain particularly [19,24-27].
Table 5. Effect of the exposure to constant light started at the age of 1 month (LL-1) and at the age of 14 months (LL-14) on tumorigenesis in female rats.
Notes: TBR - tumor-bearing rats.
|
Parameters
|
Light/dark regimen
|
|
LD
|
LL-1
|
LL-14
|
|
Number of rats
|
30
|
36
|
71
|
|
Number
of TBR (%)
|
17 (56.7%)
|
20 (55.6%)
|
30 (45.3)
|
|
Number of malignant TBR (%)
|
1.47
|
1.75
|
1.37
|
|
Total number of tumors
|
5 (16.7%)
|
5 (13.9%)
|
11 (15.5%)
|
|
Number of tumors per TBR
|
25
|
35
|
41
|
|
Age at the time of the 1st
tumor detections, days
|
562
|
550
|
565
|
|
Mean life span of TBR, days
|
871 ± 51.4
|
732 ± 28.8
|
885 ± 29.3
|
|
Mean life span of fatal
TBR, days
|
1098 ± 21.8
|
758 ± 52.0
|
868 ± 47.3
|
| Localization and type of
tumors |
|
Mammary
gland: fibroma
fibroadenoma
adenocarcinoma
|
2
|
-
|
2
|
|
11
|
19
|
25
|
|
-
|
1
|
-
|
|
No of rats with benign
mammary tumors
|
12 (40.0%)
|
16 (44.4%)
|
19 (26.8%)
|
|
Utery:
polyp
fibroma
fibromyoma
adenocarcinoma
|
2
|
4
|
-
|
|
1
|
1
|
-
|
|
-
|
1
|
-
|
|
-
|
1
|
1
|
|
Skin:
fibroma
|
-
|
-
|
1
|
|
Adrenal gland: cortical
adenoma
pheochromocytoma
|
1
|
3
|
2
|
|
2
|
-
|
-
|
|
Ovary:
fibroma
|
1
|
-
|
-
|
|
Pituitary:
adenoma
|
-
|
1
|
-
|
|
Malignant lymphoma/
leukemia
|
3
|
2
|
7
|
|
Soft
tissues: fibroma
sarcoma
|
-
|
1
|
-
|
|
2
|
1
|
2
|
|
Lung:
adenocarcinoma
|
-
|
-
|
1
|
|
Total:
benign
malignant
|
20
|
30
|
30
|
|
5
|
5
|
11
|
Table 6. Multifactor analysis of variance (MANOVA) evaluation of various factors effect on activity of antioxidant enzymes (Data represented as % of factors influences, F-ratio and p value).
Notes: Only significant data are presented. Empty columns means the absence of effect of a factor on enzyme activity.
| Organ | Factor |
|
Age
|
Season
|
Light regimen
|
Time of the start of
exposure to the constant light
|
| SOD activity |
|
Liver
| | |
9.1%
11.01
0.0016
|
25.5%
26.02
0.0001
|
|
Heart
| |
17.6%
6.67
0.0025
| | |
|
Lungs
| |
11.5%
3.90
0.026
| | |
|
Skeletal muscle
| |
11.7%
3.85
0.027
| | |
| Catalase activity |
|
Liver
|
9.1%
15.2
0.0003
|
30.8%
25.58
0.0001
|
20.1%
33.34
0.0001
|
32.2%
53.47
0.0001
|
|
Kidney
| |
14.0%
7.84
0.001
| | |
|
Skeletal muscle
|
23.0%
20.99
0.0001
|
8.0%
3.65
0.03
| | |
Pierpaoli and Bulian [28] surgically pinealectomized BALB/c mice at the age of 3, 5, 7,
9, 14 and 18 months and evaluated their life span. Results showed that while
pinealectomy at the age of 3 or 5 months promoted acceleration of aging, no relevant effect of pinealectomy was observed when
mice were pinealectomized at the age of 7 or 9 months. The remarkable life
extension was observed when mice were pinealectomized at the age of 14 months.
No effect was observed when the mice were pinealectomized at 18 months of age.
The same aging-promoting or -delaying effects were confirmed in the
hematological and hormonal-metabolic values measured. Evidence from the blood
measurements showed that removal of the pineal gland in mice at the age of 14
months resulted in maintenance of more juvenile hormonal and metabolic patterns
at 4th and 8th months after pinealectomy [28].
On the contrary, a deleterious effect
of pinealectomy was observed in mice subjected to the surgery at the age of 3
or 5 months. The authors suggest that the age of 14 months is the time when
pineal gland accomplished its "aging program" and prevention of and/or recovery
from aging becomes impossible. Our data on effect of "physiological
pinealectomy" induced by the exposure to constant illumination started at the
age of 1 or 14 on survival are in according with the observations of Pierpaoli
and Bulian [28]. The results of our experiments suggest that people at
perimenopausal age could be less susceptible to hazardous effect of constant
illumination. This conclusion is not in contradiction with available data on
age-related differences in susceptibility to carcino-genic agents is some
tissues which were discussed earlier [29-31].
Material and methods
Two hundred sixty seven male and 135
female outbreed LIO rats [32] were born during the first half of May, 2003. At
the age of 25 days they were randomly subdivides into 4 groups (males and
females separately) and kept at 2 different light/dark regimens: 1) standard
alternating regimen (LD) - 12 hours light (750 lux): 12 hours dark; 2) constant
light regimen (LL) - 24 hours light on (750 lux). At the age of 14 months the
part of survived rats kept at the LD regimen were moved in the room with the
constant light regimen (LL). Thus, the were 3 final groups: 1) LD; 2) LL-1
since the age of 1 months; 3) LL-14 since the age of 14 months. Only rats in
each group survived the age of 14 months were included into protocols for
calculations. The full data on the survival and tumorigenesis in control LD
rats and in rats exposed to the LL since the age of 1 months have been
presented elsewhere [15].
Some animals were sacrificed by
decapitation, the appropriate tissues (liver, kidney, heart, lung, spleen and a
skeletal muscle) dissected, weighed, and kept frozen at -25°С before
carrying out of analyses. The samples of tissue of rats groups LD and LL-1 were
collected at age 6, 12, 18 and 24 months, of the group LL-14 - at 14, 18, 24
and 30 months. Prior to enzyme determinations, thawed tissue samples were
homogenized in 20 volumes of ice cold 50mM phosphate buffer (pH 7.4),
centrifuged at 6000 g for 15 min at 5°C. The supernatant fraction was used for
antioxidant enzyme determinations.
All animals were kept in the standard
polypropylene cages at the temperature 21-23 ºC and were given ad libitum
standard laboratory meal [33] and tap water. The study was carried out
according to the recommendations of the Committee on Animal Research of
Petrozavodsk State University about the humane treatment of animals.
The total SOD
activity was measured using the epinephrine-adrenochrome reaction and was
followed kinetically at 480 nm [34]. One unit of SOD was defined as the amount
of enzyme required for 50% inhibition of the spontaneous
epinephrine-adrenochrome transforma-tion. Catalase activity was measured by the
method of Bears and Sizer [35] following the decrease in the absorption spectra
of hydrogen peroxide at 240 nm caused by its decomposition by catalase.
Activity of catalase defined as the amount of hydrogen peroxide in μmol
that decomposed 1 g of tissue per 1 minute.
All
other rats were allowed to survive for natural death and were autopsied. Tumors
as well as the tissues and organs with suspected tumor development were excised
and fixed in 10% neutral formalin. After the routine histological processing
the tissues were embedded into paraffin. 5-7μm thin histological sections
were stained with hematoxylin and eosin and examined microscopically. Tumors
were classified as fatal and non-fatal tumors and morphologically according to
the IARC recommendations [36,37].
Experimental results were statistically
processed by the methods of variation statistics and multifactor analysis of
variance (MANOVA) with the use of STATGRAPH statistic program kit. The
significance of the discrepancies was defined according to the Student
t-criterion, Fischer exact method, χ2, non-parametric
Wilcoxon-Mann-Whitney. Student-Newman-Keuls method was used for all pairwise
multiple comparisons. Coefficient of correlation was estimated by Spearman
method [38]. Differences in tumor incidence were evaluated by the
Mantel-Haenszel log-rank test. Parameters of Gompertz model were estimated
using maximum likelihood method, non-linear optimization procedure [39] and
self-written code in 'Matlab'; confidence intervals for the parameters were
obtained using the bootstrap method [40]. For experimental groups Cox
regression model [41] was used to estimate relative risk of death and tumor
development under the treatment compared to the control group: h(t, z) = h0(t)
exp(zβ), where h(t,z) and h0(t) denote the conditional hazard and baseline
hazard rates, respectively, β is the unknown parameter for treatment
group, and z takes values 0 and 1, being an indicator variable for two samples
− the control and treatment group.
Acknowledgments
The work was supported by grants from
the President of the Russian Federation NSh-306.2008.4, and from the Russian
Foundation for Basic Research 07-04-00546.
Conflicts of Interest
The authors of this manuscript have no conflict of
interest to declare.
References
-
1.
Anisimov
VN
Light pollution, reproductive function and cancer risk.
Neuro Endocrinol Lett.
2006;
27:
35
-52.
[PubMed]
.
-
2.
Ha
M
and Park
J.
Shiftwork and metabolic risk factors of cardiovascular disease.
J Occup Health.
2005;
47:
89
-95.
[PubMed]
.
-
3.
Knutsson
A
Health disorders of shift workers.
Occup Med (Lond).
2003;
53:
103
-108.
[PubMed]
.
-
4.
Knutsson
A
and Boggild
H.
Shiftwork, risk factors and cardiovascular disease: review of disease mechanisms.
Rev Environ Health.
2000;
15:
359
-372.
[PubMed]
.
-
5.
Reiter
RJ
Potential biological consequences of excessive light exposure: melatonin suppression, DNA damage, cancer and neurodegenerative diseases.
Neuro Endocrinol Lett.
2002;
23(Suppl 2):
9
-13.
[PubMed]
.
-
6.
Schernhammer
ES
, Laden
F
and Speizer
FE.
Rotating night shifts and risk of breast cancer in women participating in the nurses' health study.
J Natl Cancer Inst.
2001;
93:
1563
-1568.
[PubMed]
.
-
7.
Schernhammer
ES
, Laden
F
and Spezer
FE.
Night-shift work and risk of colorectal cancer in the Nurses' Health Study'.
J Natl Cancer Inst.
2003;
95:
825
-828.
[PubMed]
.
-
8.
Steenland
K
and Fine
L.
Shift work, shift change, and risk of death from heart disease at work.
Am J Industr Med.
1996;
29:
278
-281.
.
-
9.
Stevens
RG
Circadian disruption and breast cancer. From melatonin to clock genes.
Epidemiology.
2005;
16:
254
-258.
[PubMed]
.
-
10.
Stevens
RG
Artificial lighting in the industrialized world: circadian disruption and breast cancer.
Cancer Causes Control.
2006;
17:
501
-507.
[PubMed]
.
-
11.
Straif
K
, Baan
R
and Grosse
Y.
Carcinogenicity of shift-work, painting, and fire-fighting.
Lancet Oncol.
2007;
8:
1065
-1066.
[PubMed]
.
-
12.
Kelly
JJ
, Lanier
AP
, Alberts
S
and Wiggins
CL.
Differences in cancer incidence among Indians in Alaska and New Mexico and U.S. Whites, 1993-2002. Cancer Epidemiol.
Biomarkers Prev.
2006;
15:
1515
-1519.
.
-
13.
Anisimov
VN
, Baturin
DA
and Ailamazyan
EK.
Pineal gland, light and breast cancer.
Vopr Onkol.
2002;
48:
524
-535.
.
-
14.
Stevens
RG
Light-at-night, circadian disruption and breast cancer: assessment of existing evidence.
Int J Epidemiol.
2009;
38:
963
-970.
[PubMed]
.
-
15.
Vinogradova
IA
, Anisimov
VN
and Bukalev
AV.
Circadian disruption induced by light-at-night accelerates aging and promotes tumorigenesis in rats.
Aging (Albany NY).
2009;
1:
855
-865.
[PubMed]
.
-
16.
Vinogradova
IA
and Chernova
IV.
Effect of light regimen on age-related dynamics of estrous function and blood prolactin level in rats.
Adv Gerontol.
2006;
19:
60
-65.
[PubMed]
.
-
17.
Lazarev
NI
, Ird
EA
and Smirnova
IO.
Moscow
Meditsina
Experimental Models of Endocrine Gynecological Diseases.
1976;
.
-
18.
Prata
Lima MF
, Baracat
EC
and Simones
MJ.
Effects of melatonin on the ovarian response to pinealectomy or continuous light in female rats: similarity with polycystic ovary syndrome.
Brazil J Med Biol Res.
2004;
37:
P987
-995.
.
-
19.
Vinogradova
IA
, Ilyukha
VA
, Fedorova
AS
, Khizhkin
EA
, Unzhakoc
AR
and Yunash
VD.
Age-related changes of physical efficience and some biochemical parameters of rats under the influence of light regimens and pineal preparations.
Adv Gerontol.
2007;
20:
66
-73.
.
-
20.
Baturin
DA
, Alimova
IN
and Anisimov
VN.
Effect of light regime and melatonin on the development of spontaneous mammary tumors in HER-2/neu transgenic mice is related to a downregulation of HER-2/neu gene expression.
Neuro Endocrinol Lett.
2001;
22:
439
-445.
.
-
21.
Blask
DE
, Dauchy
RT
and Sauer
LA.
Light during darkness, melatonin suppression and cancer progression.
Neuro Endocrinol Lett.
2002;
23 (suppl.2):
52
-56,.
[PubMed]
.
-
22.
Lerch
A
Biological rhythma in the context of light at night.
Neuro Endocrinol Lett.
2002;
23 (suppl.2):
23
-27.
.
-
23.
Djeridane
Y
, Charbuy
H
and Touitou
Y.
Old rats are more sensitive to photoperiodic changes. A study on pineal melatonin.
Exp Gerontol.
2005;
40:
403
-308.
[PubMed]
.
-
24.
Moskalev
AA
, Shostal
OA
and Zainullin
VG.
Genetic aspects of different light regime influence on drosophila life span.
Adv Gerontol.
2006;
18:
55
-58.
[PubMed]
.
-
25.
Ilyukha
VA
, Vinogradova
IA
, Fedorova
AS
and Velb
AN.
Effect of light regimens, pineal hormones and age on antioxidant system in rats.
Med Acad J.
2005;
3 (Suppl. 7):
18
-20.
.
-
26.
Voitenkov
VB
, Popovich
IG
and Arutiunian
AV>.
Effect of delta-sleep inducing peptide on free-radical processes in the brain and liver of mice during various light regimens.
Adv Gerontol.
2008;
21:
53
-55.
[PubMed]
.
-
27.
Simonneaux
V
and Ribelayga
C.
Generation of the melatonin endocrine message in mammals: A review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters.
Pharmacol Rev.
2003;
55:
325
-395.
[PubMed]
.
-
28.
Pierpaoli
W
and Bulian
D.
The pineal aging and death program. Life prolongation in pre-aging pinealectomized mice.
Ann N Y Acad Sci.
2005;
1057:
133
-144.
[PubMed]
.
-
29.
Anisimov
VN
Boca Raton, FL
CRC Press, Inc
Carcinogenesis and Aging, Vols. 1 & 2.
1987;
.
-
30.
Anisimov
VN
Biology of aging and cancer.
Cancer Control.
2007;
14:
23
-31.
[PubMed]
.
-
31.
Anisimov
VN
Carcinogenesis and aging 20 years after: Escaping horizon.
Mech Ageing Dev.
2009;
130:
105
-121.
[PubMed]
.
-
32.
Anisimov
VN
, Pliss
GB
and Iogannsen
MG.
Spontaneous tumors in outbreed LIO rats. J Exp Clin.
Cancer Res.
1989;
8:
254
-262.
.
-
33.
Anisimov
VN
, Baturin
DA
and Popovich
IG.
Effect of exposure to light-at-night on life span and spontaneous carcinogenesis in female CBA mice.
Int J Cancer.
2004;
111:
475
-479.
[PubMed]
.
-
34.
Misra
HH
and Fridovich
I.
The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase.
J Biol Chem.
1972;
247:
3170
-3175.
[PubMed]
.
-
35.
Bears
RF
and Sizes
IN.
A spectral method for measuring the breakdown of hydrogen peroxide by catalase.
J Biol Chem.
1952;
195:
133
-140.
[PubMed]
.
-
36.
Gart
JJ
, Krewski
D
, Lee
PN
, Tarone
RE
and Wahrendorf
J.
Lyon
IARC Sci Publ
Statistical methods in cancer research.
1986;
.
-
37.
Turusov
VS
and Mohr
U.
Lyon
IARC Sci Publ
Pathology of tumours in laboratory animals. Vol. 1. Tumours of the rat.
1990;
.
-
38.
Goubler
EV
Leningrad
Meditsina
Computing methods of pathology analysis and recognition.
1978;
.
-
39.
Fletcher
R
New York
Wiley
Practical methods of optimization (2nd ed.).
1987;
.
-
40.
Davison
AC
and Hinkley
DV.
Cambridge
Cambridge Univ Press
Bootstrap methods and their application.
1997;
.
-
41.
Cox
DR
and Oakes
D.
London
Chapman & Hall
Analysis of Survival Data.
1996;
.