Living on the edge: stress and activation of stress responses promote lifespan extension
Abstract
Oxidative stress constitutes the basis of physio-pathological situations such as neurodegenerative diseases and aging. However, sublethal exposure to toxic molecules such as reactive oxygen species can induce cellular responses that result in stress fitness. Studies in Schizosaccharomyces pombe have recently showed that the Sty1 MAP kinase, known to be activated by hydrogen peroxide and other cellular stressors, plays a pivotal role in promoting fitness and longevity when it becomes activated by calorie restriction, a situation which induces oxidative metabolism and reactive oxygen species production. Activation of the MAP kinase by calorie restriction during logarithmic growth induces a transcriptional anti-stress response including genes essential to promote lifespan extension. Importantly enough, the lifespan promotion exerted by deletion of the pka1 or sck2 genes, inactivating the two main nutrient-responsive pathways, is dependent on the presence of a functional Sty1 stress pathway, since double mutants also lacking Sty1 or its main substrate Atf1 do not display extended viability. In this Research Perspective, we review these findings in relation to previous reports and extend important aspects of the original study. We propose that moderate stress levels that are not harmful for cells can make them stronger.
Aging and lifespan extension have been a matter of
debate for decades, with huge social interest in the civilized world, and much
personal and financial effort focusing on this hot topic. The molecular
mechanisms that govern cellular aging have been conserved over the course of
evolution, so that pluricellular and unicellular model systems share similar
environmental and genetic strategies for modulating the aging process. Several
reports have indicated earlier that either calorie restriction or the inactivation
of nutrient-dependent pathways (i.e. protein kinase A) is able to promote life
extension in different eukaryotes.
In unicellular fungi, researchers use two
different cellular situations in order to study the mechanisms of aging:
replicative aging refers to the number of descendents that a cell can generate before its
death, whereas chronological lifespan measures the viability of cultures at the
stationary phase of the growth curve. Therefore, chronological aging
constitutes a model for differentiated somatic cells. Recently, Schizosaccharomyces
pombe has been used as a model system for the study of chronological
aging. As described for other eukaryotes, fission yeast cultures grown under
low glucose conditions survive longer at the stationary phase than cultures
grown in the same medium but with higher concentrations of the carbon source.
It is worth pointing out that in both types of medium the concentration of
glucose in the extracellular environment is undetectable soon after reaching
the stationary phase. Therefore, the type of metabolism occurring -during the metabolically -active
logarithmic cultures seems to condition chronological
aging. What is the link between calorie restriction and lifespan extension? When comparing S. pombe cultures
growing in yeast extract-based media with 1% versus 4% glucose, we have
determined that the respiratory rates differ considerably [1]. Indeed, low glucose cultures display significantly
higher oxygen consumption levels, as an indicator of oxidative metabolism, than
those of high glucose cultures. Intracellular production of reactive oxygen
species (ROS) is also more elevated in cells grown under low glucose
conditions. Under this situation, the MAP kinase Sty1, which is also a sensor
of extracellular hydrogen peroxide stress (H2O2), becomes activated to a much higher extent in cells
grown in this respiratory-prone medium, probably as a consequence of elevated
ROS levels. Since its identification in 1995 by Shiozaki, Russell and
Millar groups [2,3], this MAP kinase has been traditionally linked to the
activation of wide transcriptional responses promoting survival under diverse
environmental stresses (for reviews, see [4,5]). The activation of Sty1 at the onset of stationary
phase only under conditions of calorie restriction suggests that the gene
response triggered by this stressful situation may contribute to the
establishment of a quiescent state which would allow survival under a
hypometabolic stage. In fact, cells lacking Sty1 or its main effector, the
transcription factor Atf1 [6-8], display a compromised viability even under calorie
restriction (Figure 1). We believe that growth under low-glucose media
promotes respiration versus fermentation, ROS production, Sty1 phosphorylation/ activation and as a consequence the
induction of a transcriptional stress program which will contribute to the fitness of cells under starvation conditions
(Figure 1).
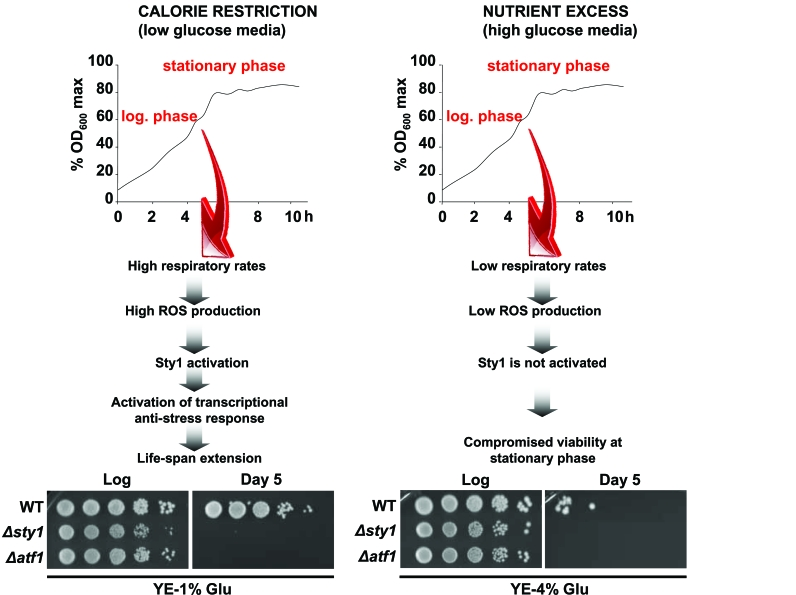
Figure 1. Activation of Sty1 stress response pathway is required for life extension upon calorie restriction. Scheme depicting the role of
the Sty1 pathway on life-span promotion (see text for details).
Strains 972 (WT), AV18 (Δsty1) and AV15 (Δatf1) were grown in YE-1%
glucose media (calorie restriction condition) and YE-4% glucose media
(glucose-rich conditions). At the logarithmic phase (Log) or 120 hours
after reaching the stationary phase (Day 5) serial dilutions of the
cultures were plated onto YE plates.
In the process of chronological aging in fission
yeast, we suggest that oxidative stress is exerting two antagonistic roles. On
one hand, during late logarithmic phase, we report the first side, a
beneficial, signalling role of ROS: growth under calorie restriction allows for
the activation of a ROS-activated, MAP kinase-driven signalling pathway which
promotes a global transcriptional change (up to 400 genes can be regulated by
Sty1) [9,10], meant to induce cellular fitness. This hormetic
effect of mild stresses, able to induce adaptive responses, has been widely reported in several model systems [11-15], and the blockage of
such non-toxic stress, for instance with
antioxidants, may preclude its health-promoting effects [16]. On the other hand, death at the stationary phase
may well be dependent on oxidative stress, as suggested by Rokeach and
colleagues [17] and by ourselves [1]: the levels of ROS of live cells at stationary phase
are higher in cultures from glucose-rich media (Figure 2A), as are the levels
of carbonylated proteins (Figure 2B). We suggest that, as widely reported in
the literature (for a review, see [18]), oxidative stress is the main cause of the molecular damage
associated with death in chronological aging.
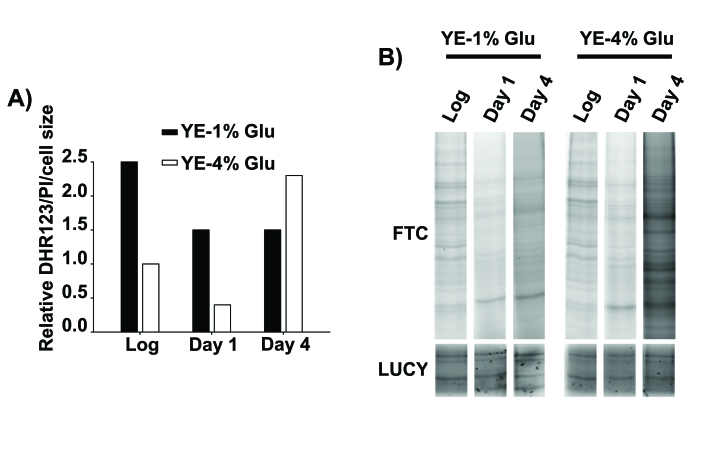
Figure 2. Oxidative stress as a cause of death of stationary phase, glucose-rich cultures.
(A) Relative intracellular H2O2 levels of cells in
logarithmic and stationary phase conditions. Wild type cells were grown in YE-1% and
YE-4% glucose media. At the logarithmic phase (Log) and one or four days after
reaching stationary phase (Day 1 and Day 4) cells were incubated with the redox-sensitive
dye dihydrorhodamine 123 (DHR123) and with the permeability-dependent dye propidium
iodide (PI), and the fluorescence of live cells was analyzed by flow cytometry.
The DHR123 green fluorescence was normalized to the PI red fluorescence and to the
cell size (y axis: Relative DHR123/PI/cell size), and all the values are referred
to that of YE-4% glucose culture in logarithmic phase, with an assigned value of 1.
(B) Protein carbonylation generated during stationary phase in calorie
restriction and rich glucose condition. Cells from the same strains as in A
were collected, protein samples were loaded in a SDS-PAGE gels and protein
carbonylation was detected by fluorescein-5-thiosemicarbazide (Fluka-Sigma)
fluorescence (FTC, top panel). Protein carbonylation detection method was performed
like in [34] with minor modifications. LUCY (Sigma) staining
of total proteins was performed as a loading control (LUCY, bottom panel).
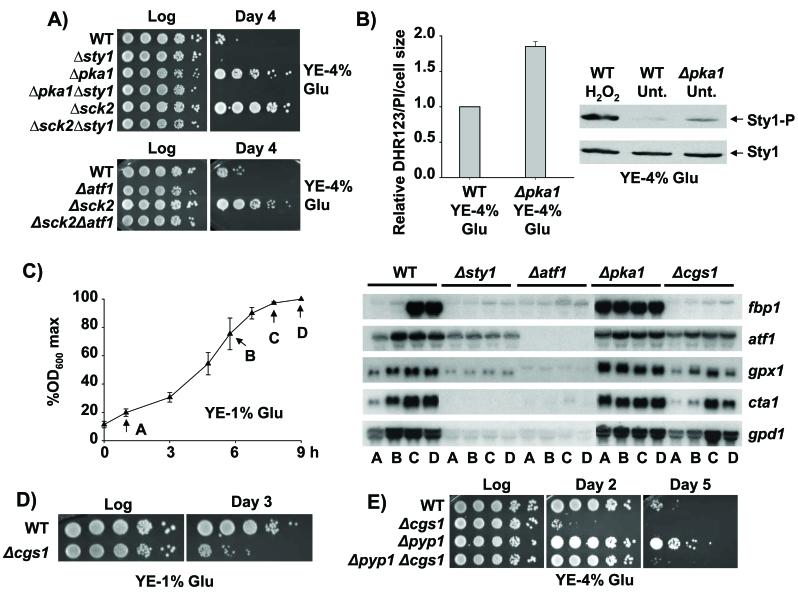
Figure 3. Role of the Sty1, Pka1 and TOR-Sck2 pathways in stationary phase. (A)
Lack of Pka1 and Sck2 kinases promotes stationary phase cell survival under
glucose rich conditions in a Sty1-, Atf1-dependent manner. Strains 972
(WT), AV18 (Δsty1), MC22 (Δpka1), MC24 (Δpka1
Δsty1), MC25 (Δsck2), MC27 (Δsck2Δsty1),
AV15 (Δatf1) and AZ118 (h- sck2::kanMX6
atf1::natMX6; Δsck2Δatf1) were grown in YE-4% glucose
media. Serial dilutions of the logarithmic (Log) and stationary phase (Day
4) cultures were spotted onto YE plates. (B) Loss of function of
the glucose dependent Pka1 kinase triggers enhanced intracellular H2O2
levels and Sty1 activation. Strains 972 (WT) and MC22 (Δpka1) were
grown in YE-4% glucose media. Cells were harvested at an OD600
of 0.5 and relative intracellular H2O2 levels were
analysed as described in Figure 2A. The same cultures were used to
characterize Sty1 phosphorylation from TCA extracts, using anti-p38-P
antibody. Wild type cells treated with 1 mM H2O2 for 10 min (H2O2) were used as a control of
activated Sty1. Anti-Sty1 antibody was used as a loading control. (C)
Activation of the transcriptional stress response at stationary phase is
Sty1-dependent and Cgs1-independent. Strains 972 (WT), AV18 (Δsty1),
AV15 (Δatf1), MC22 (Δpka1) and AZ106 (h-
cgs1::kanMX6 ura4-D18; Δcgs1) were grown in YE-1% glucose media.
The time points of the five growth curve were recorded approximately at the
same percentages of the maximum OD600 of each culture. Standard
deviation for every point is indicated. At the time points indicated (A to
D), cells were collected and RNA samples were obtained and hybridized
against fbp1, atf1, gpx1, cta1 and gpd1.
(D) Pka1 pathway is required for stationary phase survival upon
calorie restriction. Strains 972 (WT) and AZ106 (Δcgs1) were
grown in YE-1% glucose media. At the logarithmic phase (Log) or 72 hours
after reaching the stationary phase (Day 3) serial dilutions of the
cultures were plated onto YE plates. (E) Strains 972 (WT), AZ106 (Δcgs1),
AZ103 (Δpyp1) and AZ115 (h- pyp1::kanMX6
cgs1::natMX6; Δpyp1 Δcgs1) were grown in YE-4% glucose
media. At the logarithmic phase (Log) or several days after reaching
stationary phase (Day 2 and Day 5) serial dilutions of the cultures were
plated onto YE plates.
For any model system studied, it is widely
accepted that the de-repression of pathways
which should only be active upon calorie restriction is a genetic intervention which promotes lifespan extension (for reviews,
see [19-22]). For instance,
both in budding and fission yeasts, deletion of the genes coding for the protein kinase A or the TOR kinase
substrate, SCH9 (S. cerevisiae) / Sck2 (S. pombe) kinases, induces
longevity even under glucose-rich conditions [1,17,23-25] (Figure 3A). Is this genetically-driven lifespan
promotion in any way connected to the Sty1 MAP kinase pathway in fission yeast?
Apparently so, because cells carrying double deletions of the genes pka1 orsck2, coding for two kinases governing the two main nutrient-dependent pathways,
and either the sty1 or the atf1 genes [1] (Figure 3A), display a
highly compromised viability at stationary phase. We have reported that
deletion of the pka1 gene leads to an enhanced oxygen consumption even
with high glucose levels [1], elevated intracellular ROS (Figure 3B) and basal
Sty1 phosphorylation [1] (Figure 3B), and this promotes cell survival without
the need of calorie restriction-driven hormotic activation of stress
responses. In the case of the TOR substrate, Sck2, we suspect that deletion of
its gene may also induce a subtle de-repression of respiration as it has been
reported for the budding yeast homolog SCH9 [26], although we have not been able to experimentally
probe it yet.
It is important to point out that the
glucose-dependent Pka1 pathway has been traditionally linked to the stationary
phase in fission yeast (for a review, see [27]).
In fact, a number of genes such as fbp1 (coding
for the gluconeogenesis regulatory protein
fructose-1,6-bisphosphatase; [28]) are triggered at the onset of stationary phase in a
Pka1-dependent manner. During logarithmic growth, that is, in the presence of
glucose, Pka1 kinase is fully active and phosphorylates and inactivates the
transcription factor Rst2, which cannot trigger fbp1 transcription.
Upon glucose depletion, cAMP levels decrease, and the regulatory subunit of
Pka1, Cgs1, is then free to interact with the kinase, inactivate it and trigger
Rst2-dependent fbp1 transcription. Therefore, whereas deletion of the pka1gene induces lifespan extension by de-repressing its gene expression
program and activating Sty1 (Figure 3ABC), deletion of cgs1 leads to a
severe phenotype under calorie restriction, like the one described for cells
lacking Sty1 or Atf1 (Figure 3D). That indicates, as previously suggested,
that activation of gene responses by both the Sty1-Atf1 pathway and the
Pka1/Cgs1-Rst2 pathways are required for survival at stationary phase.
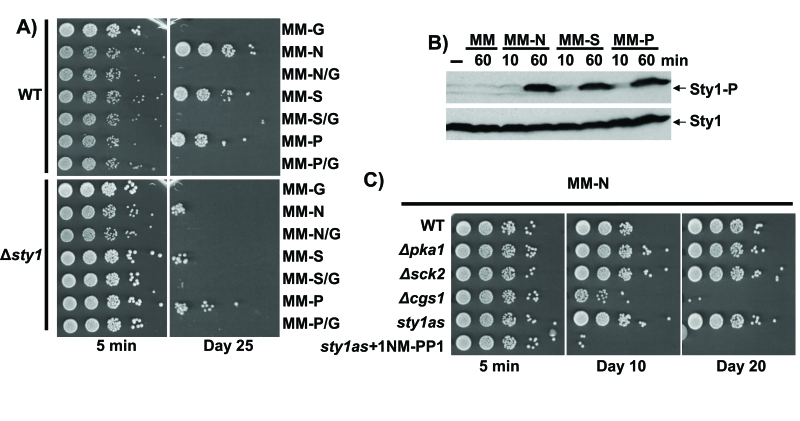
Figure 4. Quiescence establishment upon nitrogen, sulphate or phosphate starvation is glucose- and Sty1-dependent. (A) Sty1 and
carbon source are necessary for lifespan extension upon nutrients starvation.
972 (WT) and AV18 (Δsty1) strains grown in minimal media until
OD600 0.5 were harvested by centrifugation. Then, cells were washed twice with
minimal media without nutrients and resuspended to a final OD600 of 0.1 in
minimal media lacking glucose (MM-G), nitrogen (MM-N), nitrogen and glucose
(MM-N/G), sulphate (MM-S), sulphate and glucose (MM-S/G), phosphate (MM-P),
phosphate and glucose (MM-P/G). Five minutes (5 min) and 25 days (Day 25) after
the shift, serial dilutions of the cultures were plated onto YE plates. (B)
Sty1 is activated upon nutrients depletion. Wild type cells treated like in A
and resuspended in MM, MM-N, MM-S and MM-P with a final OD600 of 0.5 were recollected
10 or 60 minutes after the shift and Sty1 phosphorylation was determined from TCA
extracts using anti-p38-P antibody. Anti-Sty1 antibody was used as a loading control.
(C) Lack of Cgs1 impairs quiescence maintenance upon nitrogen depletion.
Strains 972 (WT), AZ74 (h- pka1::kanMX6), AZ73 (h- sck2::kanMX6), DC3
(h- cgs1::kanMX6) and AZ107, harbouring the as mutation sty1.T97A [1]
and treated or not with 5µM ATP analogue 1NM-PP1 (sty1as + 1NM-PP1 and
sty1as respectively) were grown in minimal media until OD600 0.5.
Addition of the ATP analogue specifically inhibits the Sty1.T97A kinase activity.
Cells were harvested by centrifugation, washed twice with milli Q water and
resuspended to a final OD600 of 0.1 in minimal media lacking nitrogen
(MM-N). Five minutes (5 min), 10 and 20 days (Day 10 and Day 20) after the
shift/resuspention serial dilutions of the cultures were plated onto YE plates.
Activation of fbp1 and other genes
depends on both the Pka1 and the Sty1 pathways [29], whereas activation of the stress genes atf1,
gpx1, cta1 and gpd1 depends mainly on the presence of Sty1 and Atf1
(Figure 3C). We also know now that the activation of the MAP kinase dependent
transcriptional response has a more prominent role than the one of the Pka1
pathway, since constitutive activation of Sty1 (by deletion of the gene coding
for the Sty1 phosphatase Pyp1) can partially overcome the defects of cells
lacking Cgs1, at least at early times (Figure 3E; Day 2); on the contrary, in
the ∆pka1∆sty1 strain the phenotype of the sty1 deletion
predominates (Figure 3A) [1].
In fission yeast, an experimental approach
to study proliferation versus quiescence is to nutritionally starve
logarithmically growing cultures by simply harvesting cells from complete media
and re-suspending them in media depleted of an essential growth component. The
genetic bases for entry into and maintenance of quiescence upon nitrogen
deprivation have been recently characterized [30-32], and we have observed that lack of phosphate or
sulphate can also trigger viability in fission yeast (Figure 4A). It is
important to point out that, in these types of abrupt starvation, extracellular
glucose cannot be depleted, suggesting that during logarithmic growth cells do
not accumulate any energy source reservoir and that quiescent cells remain
metabolically active [30] (Figure 4A). Are the Sty1/Atf1 and the Pka1/Cgs1
pathways essential to promote entry into and maintenance of quiescence using
this experimental approach? Indeed, they are. In a genetic screen to detect
genes required for entry into and maintenance of quiescence upon nitrogen
deprivation, strains lacking Sty1 or its double MAP kinase Wis1 were
consistently isolated [31]. We have determined that the MAP kinase is also
required to promote viability upon sulphate and phosphate starvation (Figure 4A). Whatever the mechanism of activation may be, the MAP kinase becomes
phosphorylated/activated by nitrogen [8], sulphate and phosphate depletion (Figure 4B).
Importantly, gene induction by the Pka1 pathway may also be required to
maintain quiescence, since cells lacking Cgs1 lose viability under nitrogen
starvation (Figure 4C).
In conclusion, using fission
yeast as a model system we confirm that moderate levels of stress due to
oxidative metabolism during the logarithmic growth may prepare cells to
encounter future periods of starvation or inactivity, and that a MAP kinase
pathway has an essential role in linking endogenous stress and the activation
of a genetic fitness program. Similarly, a role for the Sty1 mammalian
ortholog p38 in promoting senescence has been established (for a recent review,
see [33]). In fact, it has also been postulated that the beneficial effect on
replicative aging of human fibroblasts of heat shock-induced hormesis is
concomitant to enhanced levels of some MAP kinases [15]. Whether calorie restriction may exert a beneficial
effect on human cells through activation of basal p38 activity remains to be
demonstrated.
NOTE: Most of the experimental procedures,
media and strains used to perform the figures in this manuscript are fully described
in reference [1]. Only the strains generated for this work are
described in the figure legends (complete genotypes in brackets).
Acknowledgments
We thank Mercè Carmona and other members of
the laboratory for helpful discussions. We
apologize to any authors which find themselves reflected in this work but are
not cited; it is due to space limitations. This work was supported by Dirección General de
Investigación of Spain Grants BFU2006-02610 and BFU2009-06933, Plan E and FEDER, and by the Spanish program Consolider-Ingenio 2010
Grant CSD 2007-0020 to E.H.
Conflicts of Interest
The authors declare that they have no competing
financial interests related to this manuscript.
References
-
1.
Zuin
A
, Carmona
M
, Morales-Ivorra
I
, Gabrielli
N
, Vivancos
AP
, Ayte
J
and Hidalgo
E.
Lifespan extension by calorie restriction relies on the Sty1 MAP kinase stress pathway.
Embo J.
2010;
29:
981
-991.
[PubMed]
.
-
2.
Millar
JB
, Buck
V
and Wilkinson
MG.
Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast.
Genes Dev.
1995;
9:
2117
-2130.
[PubMed]
.
-
3.
Shiozaki
K
and Russell
P.
Cell-cycle control linked to extracellular environment by MAP kinase pathway in fission yeast.
Nature.
1995;
378:
739
-743.
[PubMed]
.
-
4.
Vivancos
AP
, Jara
M
, Zuin
A
, Sanso
M
and Hidalgo
E.
Oxidative stress in Schizosaccharomyces pombe: different H(2)O (2 )levels, different response pathways.
Mol Genet Genomics.
2006;
276:
495
-502.
[PubMed]
.
-
5.
Veal
EA
, Day
AM
and Morgan
BA.
Hydrogen peroxide sensing and signaling.
Mol Cell.
2007;
26:
1
-14.
[PubMed]
.
-
6.
Takeda
T
, Toda
T
, Kominami
K
, Kohnosu
A
, Yanagida
M
and Jones
N.
Schizosaccharomyces pombe atf1+ encodes a transcription factor required for sexual development and entry into stationary phase.
EMBO J.
1995;
14:
6193
-6208.
[PubMed]
.
-
7.
Wilkinson
MG
, Samuels
M
, Takeda
T
, Toone
WM
, Shieh
JC
, Toda
T
, Millar
JB
and Jones
N.
The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast.
Genes Dev.
1996;
10:
2289
-2301.
[PubMed]
.
-
8.
Shiozaki
K
and Russell
P.
Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast.
Genes Dev.
1996;
10:
2276
-2288.
[PubMed]
.
-
9.
Chen
D
, Toone
WM
, Mata
J
, Lyne
R
, Burns
G
, Kivinen
K
, Brazma
A
, Jones
N
and Bahler
J.
Global transcriptional responses of fission yeast to environmental stress.
MolBiolCell.
2003;
14:
214
-229.
.
-
10.
Chen
D
, Wilkinson
CR
, Watt
S
, Penkett
CJ
, Toone
WM
, Jones
N
and Bahler
J.
Multiple pathways differentially regulate global oxidative stress responses in fission yeast.
Mol Biol Cell.
2008;
19:
308
-317.
[PubMed]
.
-
11.
Schulz
TJ
, Zarse
K
, Voigt
A
, Urban
N
, Birringer
M
and Ristow
M.
Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress.
Cell Metab.
2007;
6:
280
-293.
[PubMed]
.
-
12.
Gems
D
and Partridge
L.
Stress-response hormesis and aging: "that which does not kill us makes us stronger".
Cell Metab.
2008;
7:
200
-203.
[PubMed]
.
-
13.
Kharade
SV
, Mittal
N
, Das
SP
, Sinha
P
and Roy
N.
Mrg19 depletion increases S. cerevisiae lifespan by augmenting ROS defence.
FEBS Lett.
2005;
579:
6809
-6813.
[PubMed]
.
-
14.
Rattan
SI
, Sejersen
H
, Fernandes
RA
and Luo
W.
Stress-mediated hormetic modulation of aging, wound healing, and angiogenesis in human cells.
Ann N Y Acad Sci.
2007;
1119:
112
-121.
[PubMed]
.
-
15.
Nielsen
ER
, Eskildsen-Helmond
YE
and Rattan
SI.
MAP kinases and heat shock-induced hormesis in human fibroblasts during serial passaging in vitro.
Ann N Y Acad Sci.
2006;
1067:
343
-348.
[PubMed]
.
-
16.
Ristow
M
, Zarse
K
, Oberbach
A
, Kloting
N
, Birringer
M
, Kiehntopf
M
, Stumvoll
M
, Kahn
CR
and Bluher
M.
Antioxidants prevent health-promoting effects of physical exercise in humans.
Proc Natl Acad Sci U S A.
2009;
106:
8665
-8670.
[PubMed]
.
-
17.
Roux
AE
, Leroux
A
, Alaamery
MA
, Hoffman
CS
, Chartrand
P
, Ferbeyre
G
and Rokeach
LA.
Pro-aging effects of glucose signaling through a G protein-coupled glucose receptor in fission yeast.
PLoS Genet.
2009;
5:
e1000408
[PubMed]
.
-
18.
Muller
FL
, Lustgarten
MS
, Jang
Y
, Richardson
A
and Van
Remmen H.
Trends in oxidative aging theories.
Free Radic Biol Med.
2007;
43:
477
-503.
[PubMed]
.
-
19.
Bishop
NA
and Guarente
L.
Genetic links between diet and lifespan: shared mechanisms from yeast to humans.
Nat Rev Genet.
2007;
8:
835
-844.
[PubMed]
.
-
20.
Kaeberlein
M
, Burtner
CR
and Kennedy
BK.
Recent developments in yeast aging.
PLoS Genet.
2007;
3:
e84
[PubMed]
.
-
21.
Schieke
SM
and Finkel
T.
Mitochondrial signaling, TOR, and life span.
Biol Chem.
2006;
387:
1357
-1361.
[PubMed]
.
-
22.
Blagosklonny
MV
and Hall
MN.
Growth and aging: a common molecular mechanism.
Aging.
2009;
1:
357
-362.
[PubMed]
.
-
23.
Fabrizio
P
, Pozza
F
, Pletcher
SD
, Gendron
CM
and Longo
VD.
Regulation of longevity and stress resistance by Sch9 in yeast.
Science.
2001;
292:
288
-290.
[PubMed]
.
-
24.
Wei
M
, Fabrizio
P
, Hu
J
, Ge
H
, Cheng
C
, Li
L
and Longo
VD.
Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9.
PLoS Genet.
2008;
4:
e13
[PubMed]
.
-
25.
Roux
AE
, Quissac
A
, Chartrand
P
, Ferbeyre
G
and Rokeach
LA.
Regulation of chronological aging in Schizosaccharomyces pombe by the protein kinases Pka1 and Sck2.
Aging Cell.
2006;
5:
345
-357.
[PubMed]
.
-
26.
Lavoie
H
and Whiteway
M.
Increased respiration in the sch9Delta mutant is required for increasing chronological life span but not replicative life span.
Eukaryot Cell.
2008;
7:
1127
-1135.
[PubMed]
.
-
27.
Hoffman
CS
Glucose sensing via the protein kinase A pathway in Schizosaccharomyces pombe.
Biochem Soc Trans.
2005;
33:
257
-260.
[PubMed]
.
-
28.
Hoffman
CS
and Winston
F.
Glucose repression of transcription of the Schizosaccharomyces pombe fbp1 gene occurs by a cAMP signaling pathway.
Genes Dev.
1991;
5:
561
-571.
[PubMed]
.
-
29.
Neely
LA
and Hoffman
CS.
Protein kinase A and mitogen-activated protein kinase pathways antagonistically regulate fission yeast fbp1 transcription by employing different modes of action at two upstream activation sites.
MolCell Biol.
2000;
20:
6426
-6434.
.
-
30.
Su
SS
, Tanaka
Y
, Samejima
I
, Tanaka
K
and Yanagida
M.
A nitrogen starvation-induced dormant G0 state in fission yeast: the establishment from uncommitted G1 state and its delay for return to proliferation.
J Cell Sci.
1996;
109 ( Pt 6):
1347
-1357.
[PubMed]
.
-
31.
Sajiki
K
, Hatanaka
M
, Nakamura
T
, Takeda
K
, Shimanuki
M
, Yoshida
T
, Hanyu
Y
, Hayashi
T
, Nakaseko
Y
and Yanagida
M.
Genetic control of cellular quiescence in S. pombe.
J Cell Sci.
2009;
122:
1418
-1429.
[PubMed]
.
-
32.
Yanagida
M
Cellular quiescence: are controlling genes conserved.
Trends Cell Biol.
2009;
19:
705
-715.
[PubMed]
.
-
33.
Maruyama
J
, Naguro
I
, Takeda
K
and Ichijo
H.
Stress-activated MAP kinase cascades in cellular senescence.
Curr Med Chem.
2009;
16:
1229
-1235.
[PubMed]
.
-
34.
Chaudhuri
AR
, de Waal
EM
, Pierce
A
, Van
Remmen H
, Ward
WF
and Richardson
A.
Detection of protein carbonyls in aging liver tissue: A fluorescence-based proteomic approach.
Mech Ageing Dev.
2006;
127:
849
-861.
[PubMed]
.