Protein kinase A is a target for aging and the aging heart
Abstract
PKA is an important mediator of signal transduction downstream of G-protein-coupled receptors and plays a key role in the regulation of metabolism and triglyceride storage. It is a ubiquitous cellular kinase that phosphorylates serine and threonine residues in response to cAMP. PKA consists of two regulatory subunits, RI and RII, that are activated by cAMP to release two catalytic subunits, Cα and Cβ. We have shown that C57/BL6J male mice lacking the regulatory RIIβ subunit have extended lifespan and are resistant to age-related conditions including cardiac decline. In addition to being protected from diet-induced pathologies, PKA Cβ null mutant mice are protected from age-related problems such as weight gain and enlarged livers, as well as cardiac dysfunction and hypertrophy. Several possible mechanisms for the age sparing effects of PKA inhibition are discussed including A kinase anchoring protein signaling, alterations in the β-adrenergic pathway, and activation of AMPK. Since PKA is a major metabolic regulator of gene signaling, the human gene homologs are potential pharmacological targets for age-related conditions including heart disease associated with declining cardiac performance.
Loss
of function of Protein kinase A (PKA) mediates anti-aging effects
PKA is an important mediator of signal
transduction downstream of G-protein-coupled receptors and plays a key role in
the regulation of metabolism and triglyceride storage. It is a ubiquitous
cellular kinase that phosphorylates serine and threonine residues in response to
cAMP [1]. PKA is dependent upon cAMP for functional activation. Adenyl cyclase
(AC) is an upstream regulator of cAMP and PKA. PKA consists of two regulatory
subunits and two catalytic subunits (Figure 1). cAMP binds to the regulatory subunits,
releasing the catalytic subunits which are then free to interact with and
phosphorylate downstream targets. There are four isoforms of the regulatory
subunit (RIα, RIβ, RIIα, RIIβ) and three types of catalytic
subunits (Cα, Cβ, Cγ), each of which demonstrates different
patterns of tissue expression and subcellular localization [2,3]. Our published studies have shown that
C57/BL6J male
mice lacking the regulatory RIIβ subunit have extended lifespan and are
also resistant to age-related conditions including cardiac decline [4],
summarized in Table 1. There was no lifespan advantage seen in PKA RIIβ
females. Young RIIβ null and WT littermates weigh about the same and have
about the same amount of body fat and lean body mass, but with age, there is a
striking difference in these parameters between the two genotypes for either
gender. Both genotypes eat about the same amount, so the body composition
differences cannot be attributed to differences in food intake. RIIβ null males are
more insulin sensitive that WT littermates, regardless of age, but old (17 mos)
RIIβ null females are also extremely insulin sensitive compared to WT
littermates, suggesting that this is not the physiological factor responsible
for the longevity phenotype observed exclusively in males.
In contrast, our data has
shown that body composition, including body weight, percent body fat mass, and
percent lean body mass, are correlated with lifespan in WT C57/BL6J male, but
not female mice [4] suggesting that body composition may be a physiological
factor contributing to the difference in lifespan phenotypes between mutant
males and females.
Cardiac
aging is delayed in PKA RIIβ null mutant mice
Echocardiographic
parameters in mice, particularly left ventricular hypertrophy, diastolic
dysfunction and impaired myocardial performance index, show progressive and
highly reproducible changes with advancing age which parallel those of human
aging [5]. Aging is accompanied by slowly progressive and irreversible
structural changes and functional declines in the heart. Echocardiography in
healthy populations from the Framingham Study and the Baltimore Longitudinal
Study on Aging showed an age-dependent increase in the prevalence of left
ventricular hypertrophy, a decline in diastolic function, and relatively
preserved systolic function at rest but a decline in exercise capacity, as well
as an increase in the prevalence of atrial fibrillation (reviewed in [6]).
Diastolic heart failure, defined as symptoms of heart failure in the setting of
diminished diastolic function, is pervasive in older individuals and markedly
increases the risk of mortality [7]. Greater
than half of individuals over the age
of 75 with validated congestive heart failure had diastolic dysfunction and in
many individuals
this was clinically unrecognized and untreated. Diastolic dysfunction is also a
major contributor to exercise intolerance in the elderly population. An
age-dependent impairment of myocardial performance index (MPI) has also been
shown [8].
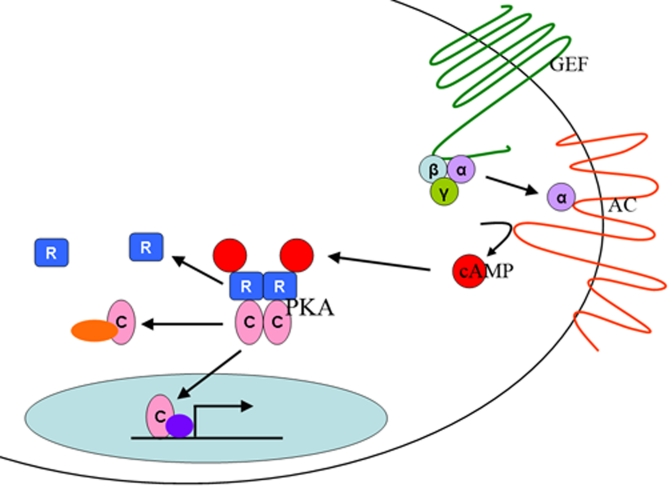
Figure 1. The PKA
pathway.
The
PKA pathway is a nutrient sensing pathway. In mammals, nutrients are
sensed by a G-protein (GEF) that activates an adenylyl cylase (AC). AC
produces cAMP, which binds to the regulatory subunits (R) of the PKA
holoenzyme, releasing the catalytic subunits (C), which are then free to
enter the nucleus of the cell and activate gene transcription or to
interact with other signaling proteins in the cell.
Using
echocardiography, we have seen cardiac dysfunction as early as 10-12 months of
age in wild type C57BL/6 mice, which continually progresses with increasing
age. Heart weights of young PKA RIIβ mutant mice are similar to WT
littermates, but at 24 months of age, mutants showed significantly lower left
ventricular masses compared to WT mice [4]. Doppler imaging on these older
mice, employed to measure the velocity of the mitral valve annulus, isovolumic
contraction and relaxation times and ejection times, also showed superior Ea/Aa
ratios in the mutants, indicating a resistance to age-related diastolic
dysfunction, and a lower average myocardial performance index (MPI) indicative
of superior global ventricular function [8]. These observations indicate a
cardiac protective affect of the RIIβ deletion and suggest a possible
connection with the Yan et al. findings [9] that the absence of AC5 is
protective of cardiac function. The fact that RIIβ is not expressed, or
expressed at very low levels in cardiac tissue suggests that signaling from
adipose tissue or the brain may be involved. It is also possible that the
delayed cardiac function is a secondary effect due to lack of adiposity.
Table 1. Summary of aging phenotypes in end of life RIIbeta nulle males.
| Phenotype | Males | Females |
|
Lifespan
|
Extended
|
No extension
|
|
Body fat gain
|
Suppressed
|
Suppressed
|
|
Insulin resistance
|
Suppressed
|
Suppressed
|
|
Cardiac dysfunction
|
Suppressed
|
To be determined
|
|
Cardiac hypertrophy
|
Suppressed
|
Suppressed
|
PKA
plays multiple roles in heart function. Its phosphorylation in the cardiac
myocyte regulates many processes including contraction, metabolism, ion fluxes,
and the transcription of many different genes [10]. Altered PKA signaling has
been implicated in a number of physiological problems leading to
cardiomyopathy. For example, the onset of cardiac hypertrophy is influenced by
alterations in muscle-specific A-kinase Anchoring Protein (mAKAP) signaling in
myocytes. AKAPs bind to PKA regulatory subunits such as RIIβ, in order to subcellularly localize and modulate interactions between
PKA and its downstream targets [11]. PKA is also involved in the downstream
regulation of the β-adrenergic pathway. Stimulation of β-adrenergic
receptors (β-ARs) in the heart leads to the PKA-dependent phosphorylation
of multiple intracellular targets in cardiac myocytes including the L-type Ca2+
channel in the sarcolemma, the ryanodine receptor (RyR2), and phospholamban in
the sarcoplasmic reticulum [12, 13]. Deficiencies in this pathway have been
linked to increased baseline myofibrillar Ca2+ sensitivity and subsequent
cardio-myopathy in humans, due to reduced phosphorylation of downstream targets
such as cardiac troponin I [14]. The β-adrenergic pathway
is known to be enhanced in RIIβ null mice [15], which could
help provide a possible mechanism for the cardiac sparing effects in seen in
these mice. Paradoxically, activated βAR signaling has also been
implicated in the failing heart. Chronic heart failure is associated with an
increase in circulating catecholamines [16], PKA phosphorylation of RyR2 is
markedly increased in failing human hearts [17], and mice with constitutive
activation of PKA show hyperphosphorylation of RyR2 and dilated cardiomyopathy
[13]. Investigation of the downstream targets
of PKA and how they affect cardiac function in aged RIIβ null mice will be
a productive area of aging research.
Deletion
of the Cβ catalytic subunit of PKA results in delayed aging
We have studied PKA catalytic Cβ subunit
null mutant mice to establish correlations with age-delaying benefits [18].
Female PKA Cβ null mice fed a high caloric diet (HCD) showed robust obesity
resistance. The significant increase in body weight in wild type littermates
was shown by quantitative magnetic resonance (QMR) imaging to be due to an
increase in fat mass. Generally, there was no difference in the amount of food
consumed by either genotype. When individual fat depots were weighed there was
a sparing effect in visceral fat in both female and male PKA Cβ null mice
consistent with observations indicating that accumulation of visceral fat is a
high risk factor for age-related disease. Mutants of both genders also showed
dramatic fat sparing effects in the liver, showing that PKA Cβ null mice are
resistant to the hepatic steatosis-like condition associated with ingesting a
high caloric diet. Blood glucose was elevated in wild type littermates, but not
PKA Cβ null mice, as early as 4 weeks on the HCD. A glucose tolerance test
showed that PKA Cβ null mice on a HCD maintain their tolerance to glucose in
contrast to wild type littermates. Hyperinsulinemia was seen as early as seven
weeks into the HCD diet in wild type littermates, but not in PKA Cβ null mice.
An insulin sensitivity test showed that PKA Cβ null mice do not develop insulin
resistance associated with the high caloric diet as wild type littermates do.
In
addition to being protected from diet-induced pathologies, PKA Cβ null
mutant mice are protected from age-related problems such as weight gain and
enlarged livers, as well as cardiac dysfunction and hypertrophy. As with
RIIβ, we have used echocardiography and doppler imaging to look at
diastolic function and myocardial performance index, and have found superior
Ea/Aa ratios and MPIs in the mutants compared to WT as early as 9 months of
age, continuing up to 24 months of age. By 24 months, we have observed other
evidence of worsening diastolic function in WT mice, including significantly
higher injection response times, reduced fractional shortening percentages, and
enlarged left atria compared to mutants. By end of life, we have found that WT
mice have significantly larger hearts than
littermates lacking PKA Cβ (manuscript in preparation). The mechanisms
for these observations are not known since PKA Cβ is detectable only at
very low levels in cardiac tissue of the mouse. We know that PKA Cβ is
expressed in the liver and could help provide a
correlation with cardiac protective effects since PKA has been shown to
phosphorylate and inactivate AMPKα in order to regulate the activity of lipolytic enzymes
such as hormone-sensitive lipase [19]. Increases in AMPKα have been linked to fatty liver
resistance, as well as a reduction in cardiac protein synthesis and delayed
hypertrophy [20, 21]. Interestingly, we have data to show that Cβ null
mice have increased levels of phosphorylated AMPK. Transcription of the gene
for carboyhydrate-response-element-binding protein ChREBP, a master regulator
of lipid metabolism, is known to be AMPK-mediated, and we have found that
levels of this protein are lower in livers of PKA Cβ disrupted mice.
Increased fatty acid oxidation and lipolysis, and decreased fatty acid and
protein synthesis through the AMPK pathway may be possible mechanisms by which
PKA Cβ disruption leads to obesity resistance and healthy aging (Figure 2).
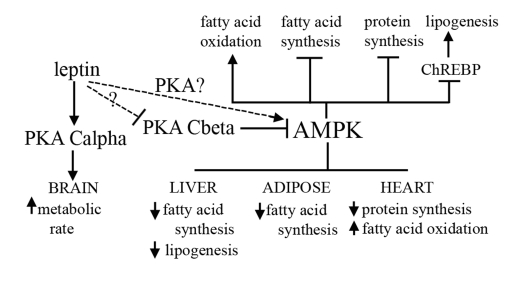
Figure 2. Proposed mechanism for how the PKA Cβ deletion
results in resistance to obesity, fatty liver, and heart disease.
Activation of AMPK is known to affect different aspects of lipid metabolism,
and to play a role in protein synthesis. PKA inhibits activity of AMPK, and
we have shown that loss of Cβ results in decreased levels of ChREBP.
Our model proposes that disruption of Cβ and concomitant increased AMPK
activity leads to a decrease in fatty acid and protein synthesis and an
increase in lipolysis and fatty acid oxidation in select tissues. Leptin
sensitivity caused by disruption of Cβ may also play a role in the
observed increase in AMPK activity in our mutants. A compensatory increase
by Cα in the brain also results in an increase in overall energy
expenditure.
The high levels of
Cβ expression in the brain, and the discrete neural expression of Cβ
variants hints at a specific functional role in neuronal signaling. Disruption
of Cβ causes a 26% decrease in basal PKA activity in the brain despite a
reported compensatory increase in the amount of Cα protein [22]. While the
catalytic subunits Cα and Cβ are 91% identical in amino acid
sequence, their amino acid differences are highly conserved across species and
they are thus believed to have unique functions [23]. Therefore, a shift from
Cβ to Cα activity may still represent an increase in a particular
type of PKA catalytic function. Since our current studies suggest that PKA
Cβ null mice are leptin sensitive, one possibility is an enhanced response
to the activation of leptin-sensitive melanocortin receptors, resulting in
increased energy expenditure compared to WT. The arcuate nucleus region of the
hypothalamus contains leptin-responsive neurons that control feeding and energy
expenditure through the activation of Gs-coupled melanocortin receptors. These
receptors are thought to decrease food intake and increase energy expenditure
through stimulation of the cAMP pathway and activation of PKA [24]. The
implications for aging are highly relevant since aging is known to be
characterized by a decline in metabolic function, and is associated with
resistance to the effects of leptin on the modulation of fat accumulation and
distribution [25]. Interestingly, the AMPK pathway is also stimulated by
leptin [26], suggesting another potential mechanism by which leptin sensitivity
caused by deletion of PKA Cβ might lead to the obesity, fatty liver, and
heart disease resistance phenotypes observed in our mice (Figure 2).
PKA
subunit genes are potential anti-aging targets
Since PKA is a major metabolic regulator of gene
signaling, the human gene homologs are potential pharmacological targets for
age-related conditions. Therefore, our studies in the mouse are directly
applicable to advancing new knowledge in the treatment and prevention of
diseases associated with progressive aging [27]. The heart would appear to be
an excellent PKA inhibitory target since PKA null mutant mice are robustly protected
from age-related cardiac decline. Through the mouse models we have
characterized, we hope to explore several possible mechanisms that may explain
the positive health benefits of PKA inactivation or down-regulation. Studies on
PKA subunit genes can be carried out and confirmed to more specially define the
intervention targets and realistically predict biological outcomes in human
clinical trials.
Conflicts of Interest
The authors of this manuscript have no conflict of
interests to declare.
References
-
1.
Niswender
CM
, Ishihara
RW
, Judge
LM
, Zhang
C
, Shokat
KM
and McKnight
GS.
Protein engineering of protein kinase A catalytic subunits results in the acquisition of novel inhibitor sensitivity.
J Biol Chem.
2002;
277:
28916
-28922.
[PubMed]
.
-
2.
McKnight
GS
Differential expression of mRNAs for protein kinase inhibitor isoforms in mouse brain.
Curr Opin Cell Biol.
1991;
3:
213
-217.
[PubMed]
.
-
3.
Brandon
EP
, Idzerda
RL
and McKnight
GS.
PKA isoforms, neural pathways, and behaviour: making the connection.
Curr Opin Neurobiol.
1997;
7:
397
-403.
[PubMed]
.
-
4.
Enns
L
, Morton
J
, Treuting
P
, Emond
M
, Wold
N
, McKnight
GS
, Rabinovitch
P
and Ladiges
W.
Disruption of protein kinase A in mice enhances healthy aging.
PLoS.
2009;
4:
e5963
.
-
5.
Dai
DF
, Santana
LF
, Vermulst
M
, Tomazela
DM
, Emond
MJ
, Maccoss
MJ
, Gollahon
K
, Martin
GM
, Loeb
LA
, Ladiges
WC
and Rabinovitch
PS.
Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging.
Circulation.
2009;
119:
2789
-2797.
[PubMed]
.
-
6.
Lakatta
EG
and Levy
D.
Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease.
Circulation.
2003;
107:
346
-354.
[PubMed]
.
-
7.
Redfield
MM
, Jacobsen
SJ
, Burnett
JC
, Mahoney
DW
, Bailey
KR
and Rodeheffer
RJ.
Burden of Systolic and Diastolic Ventricular Dysfunction in the Community: Appreciating the Scope of the Heart Failure Epidemic.
JAMA.
2003;
289:
194
-202.
[PubMed]
.
-
8.
Spencer
KT
, Kirkpatrick
JN
, Mor-Avi
V
, Decara
JM
and Lang
RM.
Age-dependency of the Tei index of myocardial performance.
J Am Soc Echocardiogr.
2004;
17:
350
-352.
[PubMed]
.
-
9.
Yan
L
, Vatner
DE
, O'Connor
JP
, Ivessa
A
, Ge
H
, Chen
W
, Hirotani
S
, Ishikawa
Y
, Sadoshima
J
and Vatner
SF.
Type 5 adenylyl cyclase disruption increases longevity and protects against stress.
Cell.
2007;
130:
247
-58.
[PubMed]
.
-
10.
Walsh
DA
and Van
Patten WM.
Multiple pathway signal transduction by the cAMP-dependent protein kinase.
FASEB J.
1994;
8:
1227
-1236.
[PubMed]
.
-
11.
McConnachie
G
, Langeberg
LK
and Scott
JD.
AKAP signaling complexes: getting to the heart of the matter.
Trends in Molecular Medicine.
2006;
12:
317
-323.
[PubMed]
.
-
12.
Kass
RS
and Moss
AJ.
Long QT syndrome: novel insights into the mechanisms of cardiac arrhythmias.
J Clin Invest.
2003;
112:
810
-815.
[PubMed]
.
-
13.
Antos
CL
, Frey
N
, Marx
SO
, Reiken
S
, Gaburjakova
M
, Richardson
JA
, Marks
AR
and Olson
EN.
Dilated cardiomyopathy and sudden death resulting from constitutive activation of Protein Kinase A.
Circ Res.
2010;
89:
997
-1004.
[PubMed]
.
-
14.
Zakhary
DR
, Moravec
CS
, Stewart
RW
and Bond
M.
1999. Protein Kinase A (PKA)-dependent Troponin-I phosphorylation and PKA regulatory subunits are decreased in human dilated cardiomyopathy.
Circulation.
1999;
99:
505
-605.
[PubMed]
.
-
15.
McKnight
GS
, Cummings
DE
, Amieux
PS
, Sikorski
MA
and Brandon
EP.
Cyclic AMP, PKA, and the physiological regulation of adiposity.
Recent Prog Hormone Res.
1998;
53:
139
-161.
.
-
16.
Packer
M
, Lee
WH
, Kessler
PD
, Gottlieb
SS
, Bernstein
JL
and Kukin
ML.
Role of neurohormonal mechanisms in determining survival in patients with severe chronic heart failure.
Circulation.
1987;
75:
IV80
-IV92.
[PubMed]
.
-
17.
Marx
SO
, Reiken
S
, Hisamatsu
Y
, Jayaraman
T
, Burkhoff
D
, Rosenblit
N
and Marks
AR.
PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts.
Cell.
2000;
101:
365
-376.
[PubMed]
.
-
18.
Enns
LC
, Morton
JF
, Mangalindan
RS
, McKnight
GS
, Schwartz
MW
, Kaeberlein
MR
, Kennedy
BK
, Rabinovitch
PS
and Ladiges
WC.
Attenuation of age-related metabolic dysfunction in mice with a targeted disruption of the Cβ subunit of protein kinase A.
J Gerontol.
2009;
64:
1221
-1231.
.
-
19.
Djouder
N
, Tuerk
RD
, Suter
M
, Salvioni
P
, Thali
RF
, Scholz
R
, Vaahtomeri
K
, Auchli
Y
, Rechsteiner
H
, Brunisholz
RA
, Viollet
B
, Makela
TP
, Wallimann
T
, Neumann
D
and Krek
W.
PKA phosphorylates and inactivates AMPK alpha to promote efficient lipolysis.
EMBO J.
2010;
29:
469
-481.
[PubMed]
.
-
20.
Chan
AYM
and Dyck
JRB.
Activation of AMP-activated protein kinase (AMPK) inhibits protein synthesis: a potential strategy to prevent the development of cardiac hypertrophy.
Can J Physiol Pharmacol.
2005;
83:
24
-28.
[PubMed]
.
-
21.
Viollet
B
, Guigas
B
, Leclerc
J
, Hebrard
S
, Lantier
L
, Mounier
R
, Andreelli
F
and Foretz
M.
AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives.
Acta Physiol.
2009;
196:
81
-98.
.
-
22.
Howe
DG
, Wiley
JC
and McKnight
GS.
Molecular and behavioural effects of a null mutation in all PKA Cβ isoforms.
Mol Cell Neurosci.
2002;
20:
515
-524.
[PubMed]
.
-
23.
Gamm
DM
, Baude
EJ
and Uhler
MD.
The major catalytic subunit isoforms of cAMP-dependent protein kinase have distinct biochemical properties in vitro and in vivo.
J Biol Chem.
1996;
271:
15736
-15742.
[PubMed]
.
-
24.
Czyzyk
TA
, Sikorski
MA
, Yang
L
and McKnight
GS.
Disruption of the RIIβ subunit of PKA reverses the obesity syndrome of agouti lethal yellow mice.
Proc Natl Acad Sci USA.
2008;
105:
276
-281.
[PubMed]
.
-
25.
Ma
XH
, Muzumdar
R
, Yang
XM
, Gabriely
I
, Berger
R
and Barzilai
N.
Aging is associated with resistance to effects of leptin on fat distribution and insulin action.
J Gerontol A Biol Sci Med Sci.
2002;
57:
B225
-B231.
[PubMed]
.
-
26.
Minokoshi
Y
, Kim
Y-B
, Peroni
OD
, Fryer
LGD
, Müller
C
, Carling
D
and Kahn
BB.
Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase.
Nature.
2002;
415:
339
-343.
[PubMed]
.
-
27.
Ladiges
W
, Van
Remmen H
, Strong
R
, Ikeno
Y
, Treuting
P
, Rabinovitch
P
and Richardson
A.
Lifespan extension in genetically modified mice.
Aging Cell.
2009;
8:
346
-52.
[PubMed]
.