MicroRNA profiling in human diploid fibroblasts uncovers miR-519 role in replicative senescence
Abstract
MicroRNAs (miRNAs) are short non-coding RNAs that regulate diverse biological processes by controlling the pattern of expressed proteins. In mammalian cells, miRNAs partially complement their target sequences leading to mRNA degradation and/or decreased mRNA translation. Here, we have analyzed transcriptome-wide changes in miRNAs in senescent relative to early-passage WI-38 human diploid fibroblasts (HDFs). Among the miRNAs downregulated with senescence were members of the let-7 family, while upregulated miRNAs included miR-1204, miR-663 and miR-519. miR-519 was recently found to reduce tumor growth at least in part by lowering the abundance of the RNA-binding protein HuR. Overexpression of miR-519a in either WI-38 or human cervical carcinoma HeLa cells triggered senescence, as measured by monitoring β-galactosidase activity and other senescence markers. These data suggest that miR-519 can suppress tumor growth by triggering senescence and that miR-519 elicits these actions by repressing HuR expression.
Introduction
MicroRNAs
are short (~ 22-nt) RNA molecules that modulate changes in gene expression
[1,2]. They are generated from precursor transcripts (primary microRNAs) which
are exported to the cytoplasm and are cleaved by Dicer; mature miRNAs then
assemble into ribonucleoprotein silencing complexes (RISC) that are recruited
to specific mRNAs [3]. MicroRNAs function primarily as repressors of mRNA
stability and translation [4]. Through their influence on the patterns of
expressed genes, microRNAs have been implicated in numerous physiologic
processes, such as develop-ment of the muscular, immune, neuronal, epithelial
and other systems, and in pathologies including neuro-degeneration and cancer
[5-8]. The latter studies have
revealed a number of miRNAs that can function
as tumor
suppressors (TS-miRNAs) or tumor promoters (oncomiRs) [9].
Cellular
senescence is achieved when cells reach the end of their replicative lifespan
[10,11].
It is believed to represent a tumor-suppressive mechanism and a contributing
factor in aging [12,13]. MicroRNAs have been implicated in replicative
senescence, since loss of miRNA biogenesis through Dicer ablation causes
senescence in primary cells [14]. Several specific miRNAs were reported to be
differentially expressed in senescent cells compared to young, proliferating
cells. For example, miRNA-146a and miR-146b are up-regulated in senescent
cells and modulate inflammatory responses by suppressing secretion of IL-6 and
IL-8 and by downregulating IRAK1 [15].
Recently,
four microRNAs (miR-15b, miR-24, miR-25, and miR-141) that jointly lower
expression of the kinase MKK4 were found to
decline during replicative senescence and to contribute to the senescence
process [16]. miR-24 was also found to regulate translation of the
cyclin-dependent kinase inhibitor p16, thereby allowing increased p16
expression in senescent cells [17].
Several miRNAs differentially expressed with aging have
also been identified. For example, miR-17, miR-19b, miR-20a, and miR-106a were
less abundant in cells from older humans [18]. Reduced expression of miR-103, miR-107, miR-128, miR-130a, miR-155, miR-24, miR-221, miR-496,
and miR-1538 in older individuals was also recently reported [19]. Age-regulated changes in the expression of microRNAs were
also found in mouse liver and brain [20,21]. MicroRNA changes in Ames dwarf mouse liver led to the identification of
microRNAs that might delay aging [22]. Studies in Caenorhabditis
elegans revealed that the microRNA lin-4 represses lin-14 transcripts and lin-14 protein to extend lifespan
by reducing DAF-16; miRNA profiling in C elegans provided evidence
that microRNAs may potently influence the biology of aging
[23-25].
Many studies have focused on the role of microRNAs in
tumorigenesis and age-related diseases. Here, we have studied changes in
expressed microRNAs during replicative senescence of WI-38 human diploid fibroblasts
(HDFs). We identified subsets of microRNAs
that were differentially expressed in young compared with senescent WI-38
cells. miR-519, a microRNA that suppresses tumorigenesis and lowers expression
of RNA-binding protein HuR, was upregulated in senescent cells. Overexpression
of miR-519 induced senescence in WI-38 and HeLa cells. Our data support the
hypothesis that senescence-associated changes in microRNA expression patterns
can affect the susceptibility to age-related diseases such as cancer.
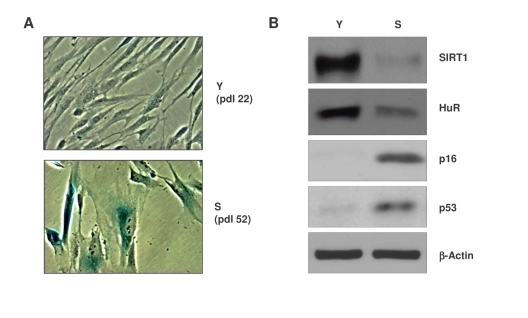
Figure 1. Characterization of early-passage and senescent WI-38 cells. (A)
Micrographs illustrating β-galactosidase activity in young (Y)
early-passage (pdl 22) and senescent (S), late-passage (pdl 52) WI-38
cells. (B) Western blot analysis of the proteins indicated in
whole-cell lysates prepared from Y and S WI-38 populations; β-actin
served as a loading control.
Results
Global
changes in microRNAs between early-passage and senescent WI-38 human diploid
fibroblasts
Compared with early-passage, ‘young' proliferating [Y, at
population doubling (pdl) 22] WI-38 cells, the senescent (S, pdl 52) WI-38
cells displayed a flattened morphology and senescence-associated (SA)
β-galactosidase (SA-β-gal) activity, a widely used senescence marker
[26,27] (Figure 1A). Western blot analysis also revealed that senescent cells
expressed lower levels of SIRT1 and HuR, whereas p16 and p53 were upregulated (Figure 1B), in keeping with reported literature [28-30].
To test how the pattern of expressed microRNAs
is affected by replicative senescence, we studied transcriptome-wide changes in
microRNAs using miRNome arrays (not shown); we then validated individual microRNAs
by reverse transcription (RT) followed by real-time, quantitative (q)PCR
amplification (see Materials and Methods). Depicted in Figures 2 and 3 and in
Supplementary Table 1 are all of the microRNAs validated using sequence-specific qPCR
primers. As shown in Figure 2, several microRNAs were markedly more abundant
in senescent cells (e.g., miR-1204, miR-663, miR-548b-3p and miR-431). Other microRNAs
were expressed at lower levels in senescent cells [e.g., miR-24, miR-141, and
miR-10a (Figure 3, Supplementary Table 1)]. MicroRNAs changing less than
twofold with senescence are listed in the Supplementary Table 1.
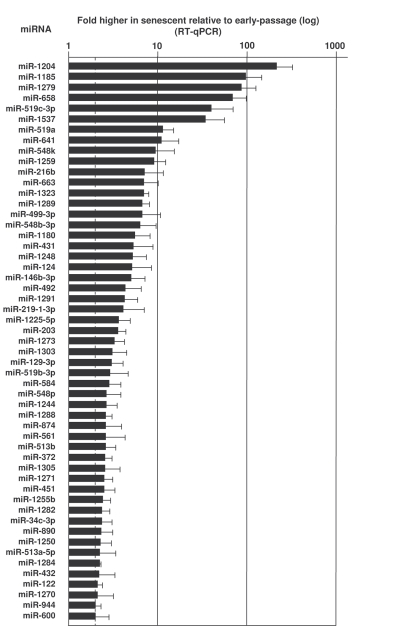
Figure 2. MicroRNAs upregulated in senescent cells.
RNA extracted from Y (pdl 22-25) and S (pdl 50-55) WI-38 cells was used to
measure the levels of the microRNAs listed, using RT-qPCR (Materials and
Methods). MicroRNA abundance was normalized to U1 snRNA levels. Data are
the means and S.D. from three independent experiments.
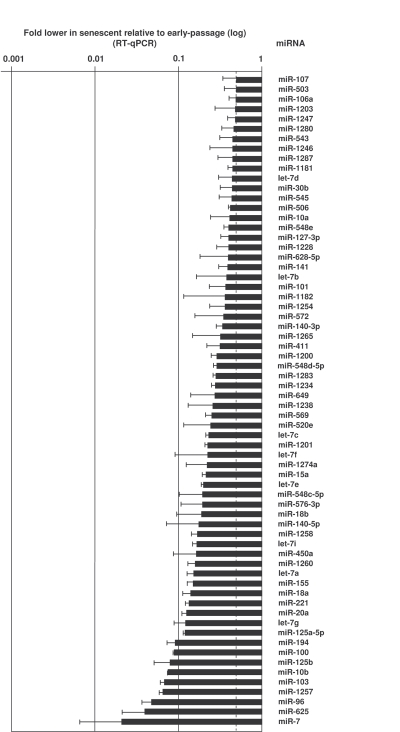
Figure 3. MicroRNAs downregulated in senescent cells.
RNA was extracted and analyzed as explained in the legend of Figure 2.
Data show the means and S.D. from three independent experiments.
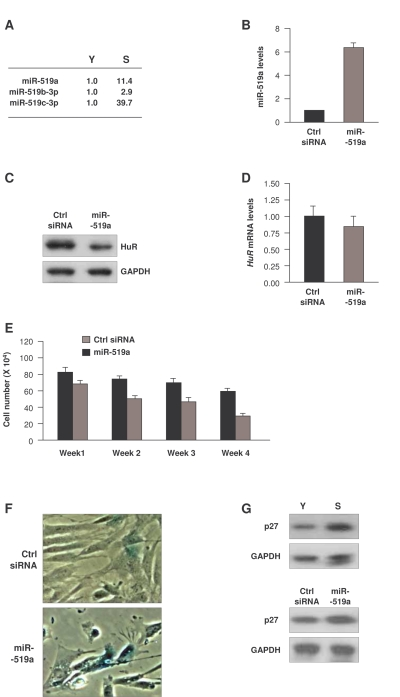
Figure 4. Influence of miR-519 on WI-38 senescence. (A)
Fold differences in miR-519 expression in S relative to Y cells, calculated
as explained in the legend of Figure 2. (B) Forty-eight h after
transfection of either control (Ctrl) siRNA or miR-519a, the levels of
miR-519a were measured by RT-qPCR. (C,D) In cells transfected as
explained in panel (B), the levels of HuR protein and loading control GAPDH
were assessed by Western blot analysis (C), and the levels of HuR
mRNA and normalization control 18S rRNA were measured by RT-qPCR (D). (E)
WI-38 cell numbers in cells transfected as in (B) were counted every 7
days. (F) SA-β-galactosidase activity in WI-38 cells by week 4
after sequential transfection (every 7 days) of either Ctrl siRNA or
miR-519a. (G) Western blot analysis of p27 and loading control
GAPDH in Y and S WI-38 cells (top) or in WI-38 cells by week 4 after
transfection as explained in (E) (bottom). The data in B,D,E
represent the means and S.D. from three independent experiments.
miR-519-induced senescence in HDFs
We were particularly interested in the
miR-519 family. miR-519 was recently found to inhibit translation of the
RNA-binding protein HuR through its interaction with the HuR coding region
[31]. In a separate study, miR-519 suppressed the growth of tumor xenografts
in an HuR-dependent manner [32]. Given that HuR promotes cell proliferation
and decreases senescence [33,34], we hypothesized that the elevated miR-519 in
senescent cells (Figure 4A) might lower HuR expression in WI-38 HDFs, and hence
promote senescence. To test this possibility, we overexpressed miR-519a in
young-HDFs (Figure 4B); western blot analysis confirmed that miR-519a overexpression repressed HuR (Figure 4C). In keeping with earlier results [31], miR-519a
did not influence the levels of HuR mRNA (Figure 4D), in agreement with
the view that miR-519a inhibited HuR mRNA translation without affecting HuR
mRNA stability. Moreover, sustained miR-519a overexpression for 4 weeks caused
a marked reduction in cell number as compared to control transfection groups (Figure 4E). miR-519a-overexpressing cells also showed increased SA-β-gal activity (Figure 4F) and elevated expression of the senescence marker p27 [35,36] (Figure 4G, bottom).
Together, these data indicate that miR-519a induced cellular senescence and
inhibited cell proliferation, resulting in accelerated senescence. They
further suggest that miR-519a-induced senescence may be mediated in part by
repression of HuR.
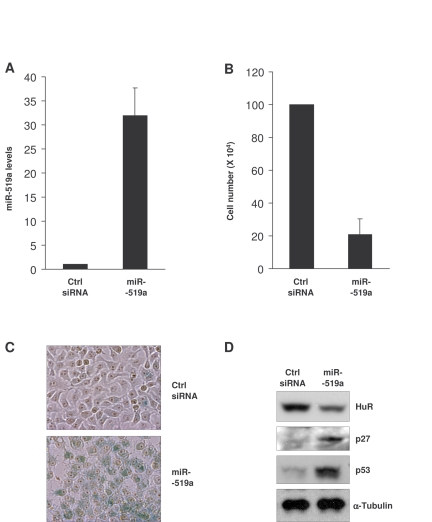
Figure 5. Influence of miR-519 on the senescent phenotype of HeLa cells. (A)
Forty-eight h after transfection of HeLa cells with either control (Ctrl)
siRNA or miR-519a, miR-519a levels were measured by RT-qPCR. (B)
Number of HeLa cells remaining by 72 h after transfection of Ctrl siRNA or
miR-519a as explained in (A). (C) β-galactosidase
activity in HeLa cells 5 days after transfection with either Ctrl siRNA or
miR-519a. (D) Seventy-two hours after transfection as indicated in
(A), the levels of the proteins shown were assessed by Western blot
analysis. The data in A,B represent the means and S.D. from three
independent experiments.
miR-519-induced senescence in HeLa cells
As indicated above, miR-519 was found to suppress tumor growth
[32]. Since cellular senescence is
considered to be an anti-tumorigenic process, we examined the effect of miR-519
on the senescent phenotype of cancer cells. Upon miR-519a overexpression (Figure 5A), HeLa cell numbers declined significantly (Figure 5B). Five days after
transfection of miR-519a, cells showed a strong
increase in SA-β-gal activity compared to the control transfection group (Figure 5C); in addition, miR-519-induced senescence in HeLa cells was
accompanied by increased levels of the senescence markers p53 and p27 (Figure 5D). Together, these data indicate that miR-519
reduced HeLa cell proliferation and promoted HeLa cell senescence.
Accordingly, we postulate that one of the mechanisms by which miR-519 suppress
tumor growth is by inducing senescence, and further propose that miR-519 triggers
senescence -at least in part- by reducing HuR levels.
Discussion
Cells become senescent as a result of factors such as the
accumulation of reactive oxygen species, DNA damage, erosion of telomeres, and
oncogenic activation. Collectively, these triggers cause cells to undergo
morphological changes, to become unable to replicate DNA and to display altered
gene expression patterns [10-13]. Here, we investigated microRNA levels in
WI-38 human diploid fibroblasts by comparing microRNA patterns in senescent
relative to young, proliferating cells. Among the microRNAs showing increasing
abundance with senescence, miR-519 was of particular interest because it was
shown to inhibit translation of HuR and to diminish tumor growth [31,32].
Through its influence on the expression of many genes, HuR plays a key role in
cell proliferation, tumorigenesis, and senescence [37,38]. We found that
overexpression of miR-519a decreased HuR levels, lowered cell proliferation,
and promoted replicative senescence in both WI-38 and HeLa cells.
microRNAs and senescence
We previously used miRNA
microarrays to identify changes in a limited number of microRNAs in senescent
cells [16]. Here, we have expanded this analysis and have verified many
individual microRNAs whose abundance changes with replicative senescence. Many
of them target key proteins implicated in senescence and cancer. For example,
miR-146b is upregulated in senescent cells (Figure 2), in keeping with earlier
findings that miR-146a and miR-146b increased with senescence and repressed the senescence-associated inflammatory
mediators IL-6 and IL-8 [15]. miR-34a regulates SIRT1 expression and induced
senescence of cancer cells [39-41]; here, we observed higher miR-34c (not
miR-34a) in senescent cells, likely a reflection of the variability and
complexity of the senescence process. Several
let-7 members were also upregulated in senescent cells (Figure 2 and
Supplementary Table 1); this observation supports the view that the ability
of let-7
microRNAs can suppress tumor growth [reviewed in 42], which could contribute to
the senescence process. Similarly, upregulation of miR-20 in senescence cells
correlates with the ability of miR-20 to inhibit proliferation of K562 human
erythromyeloblastoid leukemia cells
[43]. In conjunction with the finding that miR-519 reduced tumorigenesis in a
xenograft model [32], we propose that the coordinated action of
senescence-upregulated microRNAs can
suppress tumor growth by reducing the levels of oncogenes or tumor promoters.
Conversely,
many microRNAs were downregulated in senescent cells (Figure 3). Among the
myriad of senescence-associated proteins that they might regulate, these
microRNAs likely repress several tumor suppressors. In this regard, as miR-21
has been shown to lower expression of the tumor suppressor PTEN [44], the
downregulated of miR-21 in senescent cells (Figure 3) could allow increased
PTEN expression, in turn reducing tumor cell proliferation, migration, and
invasion [45].
miR-519a-induced senescence by lowering HuR
We previously reported that miR-519 represses the production of
HuR, an RNA-binding protein which is highly abundant in cancer cells and is low
in untransformed cells [11,38]. HuR overexpression delays the senescent
phenotype while the loss of HuR enhances it [11]. Moreover, while HuR levels
are high in tumors and low in normal tissues, miR-519 levels are high in normal
tissues and low in cancer tissues [32]. Since HuR potently enhances the
expression of cancer-promoting proteins, and reducing HuR levels promotes HDF
senescence [11,38], we propose that miR-519 represses tumor growth at least in
part, by lowering HuR and thereby promoting senescence (Figs. 4 and 5).
Additionally, miR-519 could further repress tumor growth by lowering the
expression of other genes, such as ABCG2
or HIF-1α [46,47].
In
summary, we have identified collections of microRNAs displaying altered
abundance with replicative senescence. As shown here for miR-519, we postulate
that these changes help to meet the needs of senescent cells in eliciting tumor
suppression and growth arrest. Future studies will help to recognize more
fully the proteins and processes modulated by senescence-regulated microRNAs.
Materials and Methods
Cell culture, transfections, and
β
-galactosidase
staining.
Early-passage, proliferating (‘young', ~20
to 30 pdl) and late-passage, senescent (~50 to 55 pdl) WI-38 human diploid fibroblasts (HDFs; Coriell Cell Repositories) were
cultured in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum
and 0.1 mM nonessential amino acids (Invitrogen). HeLa cells were cultured in DMEM supplemented with 10% FBS and
antibiotics. miR-519a
(Ambion) or control siRNA (AATTCTCCGAACGTGTCACGT,
Qiagen) were transfected at a
final concentration of 100 nM using Lipofectamine 2000 (Invitrogen). Where
indicated, transfections were performed every 7 days for 4 weeks. WI-38 HDFs
and HeLa cells were stained with a senescence-associated β-galactosidase (Cell Signaling Technology)
detection kit, according to the manufacturer's protocol.
RNA isolation and miRNA profiling.
Total
cellular RNA was isolated using Trizol (Invitrogen). Isolated RNA was used to
measure miRNA levels in young and senescent cells with a 7900HT real-time PCR
instrument (Applied Biosystems). All microRNAs were measured and validated
using miRNA-specific forward primers (Supplementary Table 2) and a universal
reverse primer (System Biosciences, SBI), according to the manufacturer's
protocol. The levels of U1 snRNA, used for normalization, were determined using
the specific forward primer CGACTGCATAATTTGTGGTAGTGG.
Protein analysis.
Whole-cell lysates were
prepared with RIPA buffer [10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 1 mM
EDTA, 0.1% SDS, and 1 mM dithiothreitol]. Proteins were resolved by SDS-polyacrylamide gel
electrophoresis and transferred to polyvinylidene difluoride membranes
(Invitrogen). After incubation with primary antibodies recognizing SIRT1, HuR,
p16, p53, p27, GAPDH (all from Santa Cruz Biotechnology) or β-actin (Abcam), blots
were incubated with the appropriate secondary antibodies and the signals were
detected by ECL Plus (GE Healthcare).
Supplementary Materials
MicroRNAs showing less than twofold differences in abundance in senescent relative to early-passage cells.
MicroRNAs showing less than twofold differences in abundance in senescent relative to early-passage cells.
Acknowledgments
This research was supported
in full by the National Institute on Aging-Intramural Research Program,
National Institutes of Health.
References
-
1.
Chekulaeva
M
and Filipowicz
W.
Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells.
Curr Opin Cell Biol.
2009;
21:
452
-460.
[PubMed]
.
-
2.
Bartel
DP
MicroRNAs: target recognition and regulatory functions.
Cell.
2009;
8:
215
-233.
[PubMed]
.
-
3.
Winter
J
, Jung
S
, Keller
S
, Gregory
RI
and Diederichs
S.
Many roads to maturity: microRNA biogenesis pathways and their regulation.
Nat Cell Biol.
2009;
11:
228
-234.
[PubMed]
.
-
4.
Valencia-Sanchez
MA
Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs.
Genes Dev.
2006;
20:
515
-524.
[PubMed]
.
-
5.
Ambros
V
The functions of animal microRNAs.
Nature.
2004;
431:
350
-355.
[PubMed]
.
-
6.
Kloosterman
WP
and Plasterk
RH.
The diverse functions of microRNAs in animal development and disease.
Dev Cell.
2006;
11:
441
-450.
[PubMed]
.
-
7.
Baltimore
D
, Boldin
MP
, O'Connell
RM
, Rao
DS
and Taganov
KD.
MicroRNAs: new regulators of immune cell development and function.
Nat Immunol.
2008;
9:
839
-845.
[PubMed]
.
-
8.
Stefani
G
and Slack
FJ.
Small non-coding RNAs in animal development.
Nat Rev Mol Cell Biol.
2008;
9:
219
-230.
[PubMed]
.
-
9.
Croce
CM
Causes and consequences of microRNA dysregulation in cancer.
Nat Rev Genet.
2009;
10:
704
-714.
[PubMed]
.
-
10.
Hayflick
L
The Limited in Vitro Lifetime of Human Diploid Cell Strains.
Exp Cell Res.
1965;
37:
614
-636.
[PubMed]
.
-
11.
Cristofalo
VJ
, Lorenzini
A
, Allen
RG
, Torres
C
and Tresini
M.
Replicative senescence: A critical review.
Mech Ageing Dev.
2004;
125:
827
-848.
[PubMed]
.
-
12.
Smith
JR
and Pereira-Smith
OM.
Replicative senescence: Implications for in vivo aging and tumor suppression.
Science.
1996;
273:
63
-67.
[PubMed]
.
-
13.
Campisi
J
Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors.
Cell.
2005;
120:
513
-522.
[PubMed]
.
-
14.
Mudhasani
R
, Zhu
Z
, Hutvagner
G
, Eischen
CM
, Lyle
S
, Hall
LL
, Lawrence
JB
, Imbalzano
AN
and Jones
SN.
Loss of miRNA biogenesis induces p19Arf-p53 signaling and senescence in primary cells.
J Cell Biol.
2008;
181:
1055
-1063.
[PubMed]
.
-
15.
Bhaumik
D
, Scott
GK
, Schokrpur
S
, Patil
CK
, Orjalo
AV
, Rodier
F
, Lithgow
GJ
and Campisi
J.
MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8.
Aging (Albany NY).
2009;
1:
402
-411.
[PubMed]
.
-
16.
Marasa
BS
, Srikantan
S
, Masuda
K
, Abdelmohsen
K
, Kuwano
Y
, Yang
X
, Martindale
JL
, Rinker-Schaeffer
CW
and Gorospe
M.
Increased MKK4 abundance with replicative senescence is linked to the joint reduction of multiple microRNAs.
Sci Signal.
2009;
2:
ra69
[PubMed]
.
-
17.
Lal
A
, Kim
HH
, Abdelmohsen
K
, Kuwano
Y
, Pullmann
R Jr
, Srikantan
S
, Subrahmanyam
R
, Martindale
JL
, Yang
X
, Ahmed
F
, Navarro
F
, Dykxhoorn
D
, Lieberman
J
and Gorospe
M.
p16(INK4a) translation suppressed by miR-24.
PLoS ONE.
2008;
3:
p
.
-
18.
Hackl
M
, Brunner
S
, Fortschegger
K
, Schreiner
C
and Micutkova
L.
, miR-17, miR-19b, miR-20a, and miR-106a are down-regulated in human aging.
Aging Cell.
2010;
9:
291
-296.
[PubMed]
.
-
19.
Noren
Hooten N
, Abdelmohsen
K
, Gorospe
M
, Ejiogu
N
, Zonderman
AB
and Evans
MK.
microRNA expression patterns reveal differential expression of target genes with age.
PLoS ONE.
2010;
In press
.
-
20.
Maes
OC
, An
J
, Sarojini
H
and Wang
E.
Murine microRNAs implicated in liver functions and aging process.
Mech Ageing Dev.
2008;
129:
534
-541.
[PubMed]
.
-
21.
Li
N
, Bates
DJ
, An
J
, Terry
DA
and Wang
E.
Up-regulation of key microRNAs, and inverse down-regulation of their predicted oxidative phosphorylation target genes, during aging in mouse brain.
Neurobiol Aging.
2009;
[epub]
.
-
22.
Bates
DJ
, Li
N
, Liang
R
, Sarojini
H
, An
J
, Masternak
MM
, Bartke
A
and Wang
E.
MicroRNA regulation in Ames dwarf mouse liver may contribute to delayed aging.
Aging Cell 2009;.
2010;
9:
1
-18.
.
-
23.
Kato
M
and Slack
FJ.
microRNAs: small molecules with big roles - C. elegans to human cancer.
Biol Cell.
2008;
100:
71
-81.
[PubMed]
.
-
24.
Boehm
M
and Slack
F.
A developmental timing microRNA and its target regulate life span in C. elegans.
Science.
2005;
310:
1954
-1957.
[PubMed]
.
-
25.
Ibanez-Ventoso
C
, Yang
M
, Guo
S
, Robins
H
, Padgett
RW
and Driscoll
M.
Modulated microRNA expression during adult lifespan in Caenorhabditis elegans.
Aging Cell.
2006;
5:
235
-246.
[PubMed]
.
-
26.
Dimri
GP
, Lee
X
, Basile
G
, Acosta
M
, Scott
G
, Roskelley
C
, Medrano
EE
, Linskens
M
, Rubelj
I
and Pereira-Smith
O.
A biomarker that identifies senescent human cells in culture and in aging skin in vivo.
Proc Natl Acad Sci U S A.
1995;
92:
9363
-9367.
[PubMed]
.
-
27.
Debacq-Chainiaux
F
, Erusalimsky
JD
, Campisi
J
and Toussaint
O.
Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo.
Nat Protoc.
2009;
4:
1798
-806.
[PubMed]
.
-
28.
Abdelmohsen
K
, Pullmann
R Jr
, Lal
A
, Kim
HH
, Galban
S
, Yang
X
, Blethrow
JD
, Walker
M
, Shubert
J
, Gillespie
DA
, Furneaux
H
and Gorospe
M.
Phosphorylation of HuR by Chk2 regulates SIRT1 expression.
Mol Cell.
2007;
25:
543
-557.
[PubMed]
.
-
29.
Rodier
F
, Coppé
JP
, Patil
CK
, Hoeijmakers
WA
, Muñoz
DP
, Raza
SR
, Freund
A
, Campeau
E
, Davalos
AR
and Campisi
J.
Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion.
Nat Cell Biol.
2009;
11:
973
-979.
[PubMed]
.
-
30.
McConnell
BB
, Starborg
M
, Brookes
S
and Peters
G.
Inhibitors of cyclin-dependent kinases induce features of replicative senescence in early passage human diploid fibroblasts.
Curr Biol.
1998;
8:
351
-354.
[PubMed]
.
-
31.
Abdelmohsen
K
, Srikantan
S
, Kuwano
Y
and Gorospe
M.
miR-519 reduces cell proliferation by lowering RNA-binding protein HuR levels.
Proc Natl Acad Sci U S A.
2008;
105:
20297
-20302.
[PubMed]
.
-
32.
Abdelmohsen
K
, Kim
MM
, Srikantan
S
, Mercken
EM
, Brennan
SE
, Wilson
GM
, de Cabo
R
and Gorospe
M.
miR-519 suppresses tumor growth by reducing HuR levels.
Cell Cycle.
2010;
9 [epub]
.
-
33.
Yi
J
, Chang
N
, Liu
X
, Guo
G
, Xue
L
, Tong
T
, Gorospe
M
and Wang
W.
Reduced nuclear export of HuR mRNA by HuR is linked to the loss of HuR in replicative senescence.
Nucleic Acids Res.
2010;
38:
1547
-1558.
[PubMed]
.
-
34.
Wang
W
, Yang
X
, Cristofalo
VJ
, Holbrook
NJ
and Gorospe
M.
Loss of HuR is linked to reduced expression of proliferative genesduring replicative senescence.
Mol Cell Biol.
2001;
21:
5889
-5898.
[PubMed]
.
-
35.
Alexander
K
and Hinds
PW.
Requirement for p27(KIP1) in retinoblastoma protein-mediated senescence.
Mol Cell Biol.
2001;
21:
3616
-3631.
[PubMed]
.
-
36.
Zeng
J
, Wang
L
, Li
Q
, Li
W
, Björkholm
M
, Jia
J
and Xu
D.
FoxM1 is up-regulated in gastric cancer and its inhibition leads to cellular senescence, partially dependent on p27 kip1.
J Pathol.
2009;
218:
419
-427.
[PubMed]
.
-
37.
Hinman
MN
and Lou
H.
Diverse molecular functions of Hu proteins.
Cell Mol Life Sci.
2008;
65:
3168
-3181.
[PubMed]
.
-
38.
Abdelmohsen
K
and Gorospe
M.
Post-transcriptional regulation of cancer traits by HuR.
WIRES RNA.
2010;
In press
.
-
39.
Christoffersen
NR
, Shalgi
R
, Frankel
LB
, Leucci
E
, Lees
M
, Klausen
M
, Pilpel
Y
, Nielsen
FC
, Oren
M
and Lund
AH.
p53-independent upregulation of miR-34a during oncogene-induced senescence represses MYC.
Cell Death Differ.
2010;
17:
236
-245.
[PubMed]
.
-
40.
Tazawa
H
, Tsuchiya
N
, Izumiya
M
and Nakagama
H.
Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells.
Proc Natl Acad Sci U S A.
2007;
104:
15472
-15477.
[PubMed]
.
-
41.
Yamakuchi
M
, Ferlito
M
and Lowenstein
CJ.
miR-34a repression of SIRT1 regulates apoptosis.
Proc Natl Acad Sci U S A.
2008;
105:
13421
-13426.
[PubMed]
.
-
42.
Zhang
B
, Pan
X
, Cobb
GP
and Anderson
TA.
microRNAs as oncogenes and tumor suppressors.
Dev Biol.
2007;
302:
1
-12.
[PubMed]
.
-
43.
Scherr
M
, Venturini
L
, Battmer
K
, Schaller-Schoenitz
M
, Schaefer
D
, Dallmann
I
, Ganser
A
and Eder
M.
Lentivirus-mediated antagomir expression for specific inhibition of miRNA function.
Nucleic Acids Res.
2007;
35; e149
[PubMed]
.
-
44.
Meng
F
, Henson
R
, Wehbe-Janek
H
, Ghoshal
K
, Jacob
ST
and Patel
T.
MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer.
Gastro-enterology.
2007;
133:
647
-658.
.
-
45.
Salmena
L
, Carracedo
A
and Pandolfi
PP.
Tenets of PTEN tumor suppression, Cell.
2008;
133:
403
-414.
.
-
46.
To
KK
, Robey
RW
, Knutsen
T
, Zhan
Z
, Ried
T
and Bates
SE.
Escape from hsa-miR-519c enables drug-resistant cells to maintain high expression of ABCG2.
Mol Cancer Ther.
2009;
8:
2959
-2968.
[PubMed]
.
-
47.
Cha
ST
, Chen
PS
, Johansson
G
, Chu
CY
, Wang
MY
, Jeng
YM
, Yu
SL
, Chen
JS
, Chang
KJ
, Jee
SH
, Tan
CT
, Lin
MT
and Kuo
ML.
MicroRNA-519c suppresses hypoxia-inducible factor-1alpha expression and tumor angiogenesis.
Cancer Res.
2010;
70:
2675
-2685.
[PubMed]
.