SIRT1 reduces endothelial activation without affecting vascular
function in ApoE-/- mice
Abstract
Excessive
production of reactive oxygen species (ROS) contributes to progression of
atherosclerosis, at least in part by causing endothelial dysfunction and
inflammatory activation. The class III histone deacetylase SIRT1 has been
implicated in extension of lifespan. In the vasculature,SIRT1
gain-of-function using SIRT1 overexpression or activation has been
shown to improve endothelial function in mice and rats via stimulation of
endothelial nitric oxide (NO) synthase (eNOS). However, the effects of SIRT1
loss-of-function on the endothelium in atherosclerosis remain to be
characterized. Thus, we have investigated the endothelial effects of
decreased endogenous SIRT1 in hypercholesterolemic ApoE-/-
mice. We observed no difference in endothelial relaxation and eNOS (Ser1177)
phosphorylation between 20-week old male atherosclerotic ApoE-/-
SIRT1+/- and ApoE-/- SIRT1+/+ mice.
However, SIRT1 prevented endothelial superoxide production, inhibited
NF-κB signaling, and diminished expression of adhesion molecules.
Treatment of young hypercholesterolemic ApoE-/- SIRT1+/-
mice with lipopolysaccharide to boost NF-κB signaling led to a more
pronounced endothelial expression of ICAM-1 and VCAM-1 as compared to ApoE-/-
SIRT1+/+ mice. In conclusion, endogenous SIRT1 diminishes
endothelial activation in ApoE-/- mice, but does not
affect endothelium-dependent vasodilatation.
Introduction
Inflammation plays a key role in the
development and progression of atherosclerosis. In early stages of the disease,
endothelial cells get activated by circulating proinflammatory molecules such
as cytokines (e.g. TNFα) or modified lipoproteins (e.g. oxidized LDL).
Once activated, these cells express chemokines, cytokines, and adhesion
molecules, which attract and recruit inflammatory cells such as macrophages and
T cells [1,2].
Hypertension, hypercholesterolemia, diabetes, and aging, which may all be
associated with an excessive production of reactive oxygen species (ROS) and
oxidant stress, may contribute to atherosclerosis by affecting endothelial function and inducing
sustained endothelial activation [2-5].
The NAD-dependent class III
histone deacetylase Sir2 was found to increase lifespan in yeast [6]. Its mammalian orthologue SIRT1 senses caloric
restriction, improves insulin secretion in pancreatic beta cells, and reduces
accumulation of fatty acids in white adipose tissue [7-9]. Various other SIRT1 targets
have been identified and characterized in recent years, including PGC-1α,
NF-κB and LXR [10-13]. NF-κB is of special
interest in endothelial cells, since it drives the expression of important
adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1) and
intercellular adhesion molecule-1 (ICAM-1), which recruit blood monocytes to atherosclerotic
lesions [14-16].
Endogenous SIRT1 has been shown to decrease macrophage
foam cell formation and atherogenesis in hypercholesterolemic ApoE-/-
SIRT1+/- mice [17]. In
non-atherosclerotic aortae of rats, dominant-negative SIRT1 transfection
impairs endothelial function via eNOS inhibition ex vivo[18], and
endothelial overexpression of human SIRT1 diminishes atherogenesis in ApoE-/-
mice and improves vascular function [19]. In
addition, activation of SIRT1 prevents hyperglycemia-induced vascular cell
senescence in mice with diabetes, thereby protecting from vascular dysfunction [20].
Nevertheless, the impact of a SIRT1 haploinsufficiency on
endothelium-dependent vaso-motion and endothelial cell activation in
atherosclerotic mice remains to be determined.
In the present study, we therefore investigated the
effects of a single SIRT1 allele on aortic relaxation and endothelial
activation in 20-week-old atherosclerotic ApoE-/- SIRT1+/+
and ApoE-/- SIRT1+/- mice.
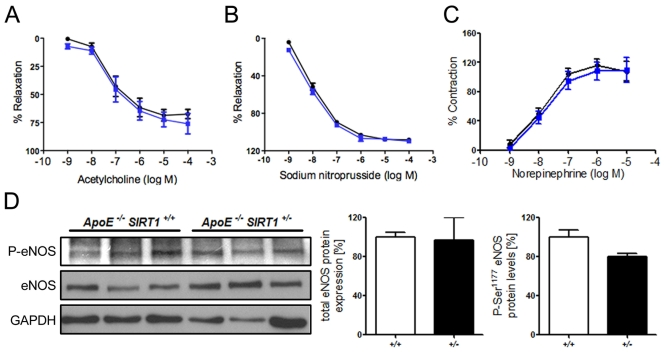
Figure 1. (A) No
difference in relaxation of aortic rings preconstricted with norepinephrine
to the vasodilator acetylcholine. % Relaxation = % of precontraction to
norepinephrine. (B) Relaxation of aortic rings at increasing sodium
nitroprusside concentrations after norepinephrine precontraction. %
Relaxation = % of precontraction to norepinephrine. (C) Contraction
of aortic rings at increasing norepinephrine concentrations. % Contraction
= % of contraction to 80 mM KCl. ApoE-/- SIRT1+/-
(blue line) and ApoE-/- SIRT1+/+ (black line).
(D) Aortic protein levels of total eNOS and phospho-eNOS (Ser1177). ApoE-/-
SIRT1+/- (+/- and black columns) and ApoE-/-
SIRT1+/+ (+/+ and white columns). n=6 per genotype
Results
Endogenous SIRT1 does not alter endothelial function
in ApoE-/- mice Overexpression of human SIRT1 in
mouse endothelial cells has been shown to diminish atherogenesis in ApoE-/-
mice. [19] However, the underlying mechanisms remain to be further characterized. To investigate
the effect of endogenous SIRT1 on endothelium-dependent vasodilatation
and endothelial inflammatory activation, we assessed endothelium-dependent
function and inflammatory pathways in aortic rings from 20-week-old
atherosclerotic ApoE-/- SIRT1+/+ or ApoE-/-
SIRT1+/- mice. Interestingly, the acetylcholine-mediated
relaxation of aortic rings after precontraction with norepinephrine did not
differ between ApoE-/- SIRT1+/+ and the
haploinsufficient ApoE-/- SIRT1+/- mice (Figure 1A). Vasoconstriction with norepinephrine and endothelium-independent
vasodilatation with sodium nitroprusside were normal (Figure 1B, C).
eNOS-derived NO plays an important role in vascular relaxation, and eNOS
activity is mainly regulated by Akt-dependent
Ser1177 phosphorylation [21]. We observed no difference in the Ser1177
phosphorylation status (Figure 1D). Our data indicate that endogenous SIRT1 in
atherosclerotic ApoE-/- mice does not affect endothelial
function.
Silencing of SIRT1 enhances production of endothelial
superoxide Common risk factors predisposing to atherosclerosis,
such as hypercholesterolemia or aging, are associated with oxidant stress at
least in part due to an increased production of ROS [22]. We
measured ROS
production in human
aortic endothelial cells (HAECs) treated with either scrambled- or SIRT1-siRNA.
SIRT1 silencing elevated endothelial ROS levels upon TNFα stimulation,
whereas under basal conditions there was no effect of SIRT1 silencing was
observed (Figure 2).
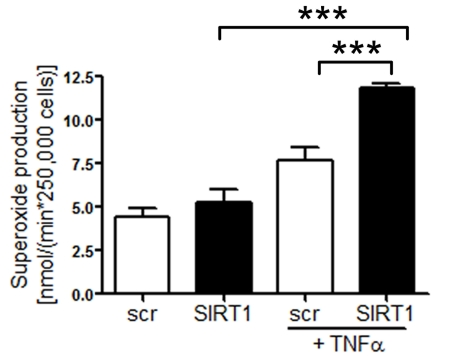
Figure 2. Superoxide production is increased in HAECs after
SIRT1-siRNA compared with scrambled-siRNA-treatment 1 h after TNFα
stimulation. n=2. ***p<0.001.
Enhanced expression of adhesion molecules in ApoE-/-
SIRT1+/- plaques Accumulating evidence suggests that
chronic production of ROS may favor atherogenesis by inducing sustained
endothelial inflammatory activation [2,5].
Expression of endothelial adhesion molecules play an important role in atherogenesis
by promoting monocyte-derived macrophage recruitment and accumulation in the
arterial intima [16].
Interestingly, expression of ICAM-1 and VCAM-1 was increased in atherosclerotic
plaques of ApoE-/- SIRT1+/- compared with ApoE-/-
SIRT1+/+mice (Figure 3). These findings show that SIRT1
prevents adhesion molecule expression, an important step in endothelial cell
activation.
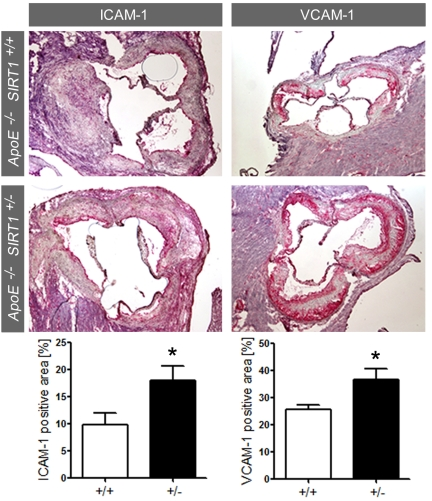
Figure 3.
ICAM-1 and VCAM-1 staining and quantification in plaques from aortic sinus.
Magnifications: X40. ApoE-/- SIRT1+/+ (+/+,
n=6, white columns) and ApoE-/- SIRT1+/- (+/-,
n=6, black columns). *p<0.05.
SIRT1 regulates the expression of endothelial adhesion
molecules via suppression of NF-κB signaling in vitro NF-κB plays a central role in inflammatory
processes and its signaling pathway is inhibited by SIRT1 via deacetylation [12,23].
NF-κB induces expression of adhesion molecules and inflammatory cytokines,
and endothelial-specific inhibition of the NF-κB pathway protects mice
from atherosclerosis [24]. SIRT1 has
been shown to deacetylate the lysine residue K310 of RelA/p65 in human
epithelial lung cells [12]. To test
whether RelA/p65 signaling is suppressed by SIRT1 in HAECs, we quantified
DNA-bound RelA/p65 in TNFα-stimulated and unstimulated cells pretreated
with the SIRT1 inhibitor splitomicin [25]. Binding of
RelA/p65 to naked DNA was enhanced upon treatment with the SIRT1 inhibitor
splitomicin after TNFα stimulation (Figure 4A). To evaluate, if SIRT1 is
also deacetylating K310 of RelA/p65 in HAECs, as previously reported for HEK
293T cells [12], we stimulated SIRT1- or scrambled-siRNA-treated HAECs with
TNFα and performed p65 immunoprecipitations. K310-p65 was increased in
SIRT1-siRNA-treated HAECs (Figure 4B). To further test if suppression of
NF-κB signaling also affects the expression of adhesion molecules, we
analyzed the expression of VCAM-1, a known NF-κB signaling target, in more
detail. SIRT1-siRNA treatment enhanced expression of VCAM-1 in HAECs upon
TNFα stimulation (Figure 4C).
SIRT1 regulates the expression of inflammatory
endothelial molecules in vivo Since SIRT1 suppresses NF-κB signaling in HAECs,
we investigated whether the same concept holds true in mouse aortae. Binding of RelA/p65 to naked DNA was higher in nuclear
extracts of ApoE-/- SIRT1+/- than ApoE-/-
SIRT1+/+ mice (Figure 5A). Importantly, aortic expression of other inflammatory molecules, namely IL-1β,TNFα, and P-Selectin (P-Sel), was also enhanced in ApoE-/-
SIRT1+/- compared to ApoE-/- SIRT1+/+
mice (Figure 5B). The expression of these inflammatory genes is regulated by
NF-κB. Lipopolysaccharides (LPS) induce strong activation of NF-κB
signaling and the expression of target genes [26]. To address
the in vivo relevance of the NF-κB suppression by SIRT1, we
examined the expression of two known NF-κB-dependent genes, ICAM-1 and
VCAM-1, in aortae from young 8-week-old ApoE-/-
SIRT1+/- and ApoE-/- SIRT1+/+ mice
without atherosclerosis in descending thoracic aortae 3 hours after
intraperitoneal injection of LPS. LPS induced an upregulation of both ICAM-1
and VCAM-1 in intimal endothelial cells of aortae from ApoE-/-
SIRT1+/- compared with ApoE-/- SIRT1+/+
mice (Figure 5C, D). These findings indicate that endogenous SIRT1 is
sufficient to prevent adhesion molecule expression in both human and mouse
activated endothelial cells.
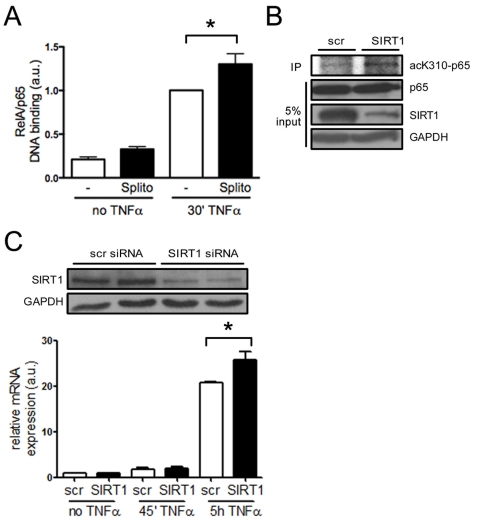
Figure 4. SIRT1 suppresses NF-κB signaling in HAECs. (A)
RelA/p65 DNA binding is higher in HAEC pretreated with splitomicin (Splito)
compared with the untreated group (-), 30 min after TNFα-stimulation.
n=5. (B)
RelA/p65 immunoprecipitation in HAECs reveals more acetyl-K310-RelA/p65
upon SIRT1-siRNA treatment 20 min after TNFα-stimulation compared to
scrambled siRNA-treated cells. n=2. (C) Western blot
showing SIRT1 silencing using siRNA (top), and VCAM-1 mRNA expression 5 h
after TNFα stimulation in SIRT1-siRNA treated HAECs (graph). n=4.
*p<0.05.
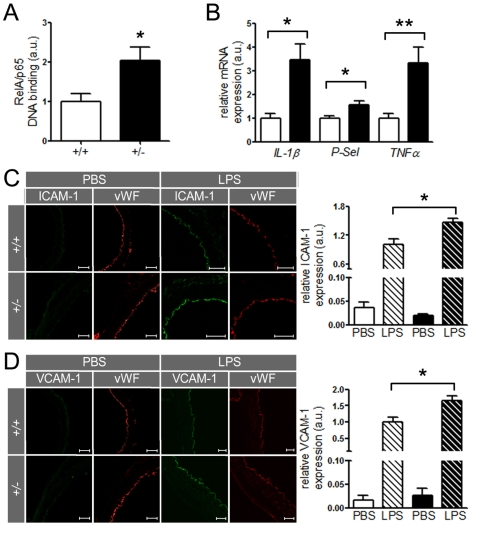
Figure 5. SIRT1 prevents expression of endothelial adhesion molecules.
(A)
RelA/p65 DNA-binding in aortic nuclear extracts from ApoE-/-
SIRT1+/- (+/-, n=8, black column) is elevated in ApoE-/-
SIRT1+/+ (+/+, n=6, white column) mice. (B)
Expression levels of IL-1β, P-Selectin, and TNFα
in aortic lysates of ApoE-/- SIRT1+/+
(white columns) and ApoE-/- SIRT1+/- (black
columns) mice. n=8 per genotype. Enhanced expression of ICAM-1 (C)
and VCAM-1 (D)
is observed in non-atherosclerotic ApoE-/-
SIRT1+/- (+/- and black columns) compared with ApoE-/-
SIRT1+/+ (+/+ and white columns) aortae 3 h post
intra-peritoneal LPS (striped columns) injection. n=6 per genotype. Scale:
50 μm. *p<0.05; **p< 0.01.
Discussion
Enhanced atherogenesis in ApoE-/-
SIRT1+/- mice is causally linked to increased expression of
adhesion molecules in aortae. Indeed, we provide in vitro and in vivo
evidence that underlines this concept by demonstrating that ApoE-/-
SIRT1+/- mice exhibit increased endothelial expression of ICAM-1
and VCAM-1 upon LPS injection. Importantly, upregulation of these adhesion
molecules promotes recruitment of monocytes and T cells to luminal endothelial
cells [27]. In concert
with increased levels of IL-1β, TNFα, and P-Sel in the activated
arterial wall, these molecular events are sufficient to recruit circulating
leukocytes to atherosclerotic lesions, especially monocyte-derived
macrophages and T cells.
At the molecular level, the inhibitory effects of
SIRT1 on adhesion molecule expression may be mediated via RelA/p65 signaling.Our data show that SIRT1 suppresses binding of RelA/p65 to naked DNA,
therefore interfering with a crucial step in the transcriptional activation of
NF-κB. These findings are in line with previous reports showing that SIRT1
deacetylases RelA/p65 at the lysine residue K310 in human epithelial lung cells
[12]. In
agreement with these reports, we demonstrate that this mechanism of RelA/p65
signaling suppression is present in HAECs.
Surprisingly, we observe no endothelial
dysfunction in ApoE-/- SIRT1+/- mice. In contrast,
Pearson et al. showed improved endothelial function in mice kept on a diet with
a very high resveratrol content (2400 mg/kg/food) that could be mediated by
SIRT1 activation [28]. However,
such effects may also be related to activation of AMPK by resveratrol or via
other targets of this compound [29,30].
Furthermore, adenovirus-mediated inhibition of endothelial SIRT1 diminishes
endothelium-dependent vasodilatation in rat aortic rings and decreases
bioavailable NO levels [18]. Others
reported improved relaxation in ApoE-/- mice with endothelialSIRT1 overexpression that were kept on a high-fat diet [19]. However,
in this study WT aortic rings showed also marked endothelial dysfunction by relaxing only up to 50%, thereby casting doubts on
the endothelial integrity of the preparations [19]. In contrast, we observed no change in endothelial
function or aortic eNOS activity between hyper-cholesterolemic ApoE-/-
SIRT1+/- and ApoE-/- SIRT1+/+ mice,
suggesting that the endothelial-protective effects of SIRT1 include factors
other than eNOS-dependent NO production. Indeed, we detected a profound
increase in ROS-production after silencing of SIRT1 in TNFalpha-stimulated
endothelial cells, indicating that endogenous SIRT1 inhibits agonist-induced
ROS production in endothelial cells. Of note, an excessive production of ROS
has been implicated in endothelial inflammatory activation and the pathogenesis
of atherosclerosis [31]. Therefore, inhibition of excessive endothelial
ROS production likely represents an important endothelial-protective action of
endogenous SIRT1.
Taken together, our results show that
SIRT1 does not influence endothelium-dependent vascular function in ApoE-/-
mice, but it prevents superoxide production in endothelial cells and reduces
the expression of inflammatory adhesion molecules by suppressing NF-κB
signaling. Although the specificity of available SIRT1 activators has been
questioned recently [32], it is
likely that SIRT1 activators may prevent atherosclerosis and other inflammatory
diseases by hindering pro-oxidative and inflammatory processes.
Materials
and methods
Animals.
ApoE-/- SIRT1+/- and ApoE-/-
SIRT1+/+ mice were described previously [17]. Male mice
were fed a high-cholesterol diet (1.25% total cholesterol, Research Diets) for
12 weeks starting at the age of 8 weeks. All animal procedures were approved by
the local animal committee and performed in accordance with our institutional
guidelines.
Cell culture.
Human aortic endothelial cells (HAEC, Cambrex Bio Science) were treated
with 100 μM splitomicin (Sigma-Aldrich) to perform analysis of NF-κB
binding to DNA. HAEC were stimulated for 30 minutes with 10 ng/ml human
TNFα (R&D Systems).
siRNA transfection.
Transient transfection siRNA into
HAEC were done with lipofectamin lipofectamin RNAi MAX (Invitrogen). The oligos
used for SIRT1-siRNA have been described previously [9].
Immunohistochemistry and
immunofluorescence.
Serial cryosections from the aortic
sinus were stained with rabbit anti-von Willebrand Factor (Dako), rat
anti-CD31, rat anti-VCAM-1 (BD Biosciences), rat anti-ICAM-1 (Serotec).
Fluorescence was analyzed on a Leica TCS SP2 confocal microscope and means were
taken from n=6 different mice evaluating 6 serial cryosections/tissue from each
mouse.
RNA and protein analysis.
Total RNA isolated from proximal aortae and HAEC was extracted with
TRIZOL (Invitrogen), reverse transcribed, and the cDNA quantified by SYBR green
qPCR using specific primers. For protein analysis, aortic tissue lysates were
blotted and incubated with rabbit anti-SIRT1, rabbit anti-eNOS (Santa Cruz
Biotechnology), and rabbit anti-Phospho-eNOS (Ser1177) (Cell
Signaling Technology).
Endothelial function.
Aortic rings (2-3 mm long) were connected to an
isometric force transducer (MultiMyograph), suspended in a 95% O2/5%
CO2 aerated organ chamber filled with KREBS buffer (118 mM NaCl, 4.7
mM KCl, 1.2 mM MgCl2, 1.2 mM NaH2PO4, 1.2 mM
Na2SO4, 2.5 mM CaCl2, 25 mM NaHCO3,
10 mM glucose, pH to 7.4). Concentration-dependent contractions were
established by using norepinephrine (10−9 to 10−4
mol/liter; Sigma-Aldrich). Concentration-response curves were obtained in a
cumulative fashion. 8 rings cut from the same artery were studied in parallel.
Responses to acetylcholine (10−9 to 10−6
mol/liter; Sigma-Aldrich) were obtained during submaximal contraction to
norepinephrine. The NO donor sodium nitroprusside (10−10 to 10−5
mol/liter; Sigma-Aldrich) was added to test endothelium-independent
relaxations.
LPS assay.
6 8-week-old ApoE-/-
SIRT1+/- and ApoE-/- SIRT1+/+ mice
kept on standard diet were used for this assay. At this age and under normal
diet, ApoE-/- mice do not exhibit plaques in their
thoraco-abdominal aortae. Mice were injected i.p. with 100 μg of LPS
(Sigma) in PBS, sacrificed 3 hours post injection and thoraco-abdominal aortae
embedded in OCT. Cryosections (5 μm) were cut and stained for ICAM-1 or
VCAM-1. Relative expression is given as the ratio of ICAM-1 or VCAM-1 staining
area to von Willebrand Factor (vWF) staining area, respectively. Quantification
of fluorescence was done with analySIS (Olympus) on microscopic images using
identical exposure settings.
Quantification of DNA-bound
RelA/p65.
HAEC were pretreated with
splitomicin for one hour and stimulated with 10 ng/ml TNFα for 30 minutes.
Nuclear extracts of aortic tissue samples were obtained with the Nuclear
Extract kit (ActiveMotif) using a Dounce pestle, and a RelA/p65 transcription
factor assay was performed using the TransAM kit (ActiveMotif) according to the
manufacturer's instructions.
Immunoprecipitation.
HAEC were treated with 50 μM SIRT1 or scrambled siRNA over night,
followed by 10 ng/ml TNFα stimulation for 20 minutes. Cells were then
harvested and protein extracted in lysis buffer (20 mM HEPES, pH
7.5, 80 mM NaCl, 2.5 mM MgCl2, 1 mM EDTA, 100 μM
Splitomicin, 0.5% NP-40, 1 mM PMFS (phenylmethylsulfonyl fluoride),
10 μg/ml aprotinin, and 10 μg/ml leupeptin). 1 mg whole-cell lysates
were immunoprecipitated with rabbit anti-p65 (Santa Cruz) using Protein G
agarose (Millipore). Immunoprecipitated samples were immunoblotted with rabbit
anti-acK310-p65 (Abcam), and the total lysates (5% input) with rabbit
anti-SIRT1 and rabbit anti-p65.
Detection of endothelial cell superoxide production by
electron spin resonance spectroscopy.
The effect of SIRT1 on endothelial cell superoxide
production was assessed in unstimulated and TNFα-stimulated (10 ng/ml) HAEC by ESR spectroscopy
using the spin probe 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetra-methylpyrrolidine
(CMH; Noxygen). HAEC were incubated with 50 nM scrambled or SIRT1-siRNA with or
without TNFα for one hour and resuspended in Krebs-Hepes buffer (pH 7.4;
Noxygen) containing diethyldithiocarbamic acid sodium salt (5 μM, Noxygen)
and deferoxamine methanesulfonate salt (25 μM, Noxygen). ESR spectra were
recorded after addition of CMH (final concentration 200 μM) under stable
temperature conditions using a Bruker e-scan spectrometer (Bruker Biospin). The
ESR instrumental settings were as follows: center field, 3495 G; field sweep
width, 10.000 G; microwave frequency, 9.75 GHz; microwave power, 19.91 mW;
magnetic field modulation frequency, 86.00 kHz; modulation amplitude, 2.60 G;
conversion time, 10.24 msec; detector time constant, 328 msec; nuber of
x-scans, 10.
Statistical analyses.
Data are presented as mean ± SEM. Statistical significance
of differences was calculated using an ANOVA with post hoc Tukey's test or
Student's unpaired t test. Significance was accepted at p<0.05.
Sources and
Funding
This work was funded by grants from the Swiss National
Science Foundation (#31-114094/1, #310030_130626/1, and #3100-068118) and the
University Research Priority Program "Integrative Human Physiology" at the
University of Zurich.
Acknowledgments
We thank the Center for Microscopy and Image Analysis
at the University of Zurich for making their facilities available.
Conflicts of Interest
The authors of this manuscript have no conflict of
interests to declare.
References
-
1.
Hansson
GK
Inflammation, atherosclerosis, and coronary artery disease.
N Engl J Med.
2005;
352:
1685
-1695.
[PubMed]
.
-
2.
Deanfield
JE
, Halcox
JP
and Rabelink
TJ.
Endothelial function and dysfunction: Testing and clinical relevance.
Circulation.
2007;
115:
1285
-1295.
[PubMed]
.
-
3.
Mueller
CF
, Laude
K
, McNally
JS
and Harrison
DG.
Atvb in focus: Redox mechanisms in blood vessels.
Arterioscler Thromb Vasc Biol.
2005;
25:
274
-278.
[PubMed]
.
-
4.
Kawashima
S
and Yokoyama
M.
Dysfunction of endothelial nitric oxide synthase and atherosclerosis.
Arterioscler Thromb Vasc Biol.
2004;
24:
998
-1005.
[PubMed]
.
-
5.
Stokes
KY
, Clanton
EC
, Russell
JM
, Ross
CR
and Granger
DN.
Nad(p)h oxidase-derived superoxide mediates hypercholestero-lemia-induced leukocyte-endothelial cell adhesion.
Circ Res.
2001;
88:
499
-505.
[PubMed]
.
-
6.
Kaeberlein
M
, McVey
M
and Guarente
L.
The sir2/3/4 complex and sir2 alone promote longevity in saccharomyces cerevisiae by two different mechanisms.
Genes Dev.
1999;
13:
2570
-2580.
[PubMed]
.
-
7.
Chen
D
, Steele
AD
, Lindquist
S
and Guarente
L.
Increase in activity during calorie restriction requires sirt1.
Science.
2005;
310:
1641
[PubMed]
.
-
8.
Bordone
L
, Motta
MC
, Picard
F
, Robinson
A
, Jhala
US
, Apfeld
J
, McDonagh
T
, Lemieux
M
, McBurney
M
, Szilvasi
A
, Easlon
EJ
, Lin
SJ
and Guarente
L.
Sirt1 regulates insulin secretion by repressing ucp2 in pancreatic beta cells.
PLoS Biol.
2006;
4:
e31
[PubMed]
.
-
9.
Picard
F
, Kurtev
M
, Chung
N
, Topark-Ngarm
A
, Senawong
T
, Machado
De Oliveira R
, Leid
M
, McBurney
MW
and Guarente
L.
Sirt1 promotes fat mobilization in white adipocytes by repressing ppar-gamma.
Nature.
2004;
429:
771
-776.
[PubMed]
.
-
10.
Lagouge
M
, Argmann
C
, Gerhart-Hines
Z
, Meziane
H
, Lerin
C
, Daussin
F
, Messadeq
N
, Milne
J
, Lambert
P
, Elliott
P
, Geny
B
, Laakso
M
, Puigserver
P
and Auwerx
J.
Resveratrol improves mitochondrial function and protects against metabolic disease by activating sirt1 and pgc-1alpha.
Cell.
2006;
127:
1109
-1122.
[PubMed]
.
-
11.
Rodgers
JT
, Lerin
C
, Haas
W
, Gygi
SP
, Spiegelman
BM
and Puigserver
P.
Nutrient control of glucose homeostasis through a complex of pgc-1alpha and sirt1.
Nature.
2005;
434:
113
-118.
[PubMed]
.
-
12.
Yeung
F
, Hoberg
JE
, Ramsey
CS
, Keller
MD
, Jones
DR
, Frye
RA
and Mayo
MW.
Modulation of nf-kappab-dependent transcription and cell survival by the sirt1 deacetylase.
Embo J.
2004;
23:
2369
-2380.
[PubMed]
.
-
13.
Li
X
, Zhang
S
, Blander
G
, Tse
JG
, Krieger
M
and Guarente
L.
Sirt1 deacetylates and positively regulates the nuclear receptor lxr.
Mol Cell.
2007;
28:
91
-106.
[PubMed]
.
-
14.
Kim
I
, Moon
SO
, Kim
SH
, Kim
HJ
, Koh
YS
and Koh
GY.
Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (icam-1), vascular cell adhesion molecule 1 (vcam-1), and e-selectin through nuclear factor-kappa b activation in endothelial cells.
J Biol Chem.
2001;
276:
7614
-7620.
[PubMed]
.
-
15.
O'Brien
KD
, McDonald
TO
, Chait
A
, Allen
MD
and Alpers
CE.
Neovascular expression of e-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content.
Circulation.
1996;
93:
672
-682.
[PubMed]
.
-
16.
Libby
P
Inflammation in atherosclerosis.
Nature.
2002;
420:
868
-874.
[PubMed]
.
-
17.
Stein
S
, Lohmann
C
, Schäfer
N
, Hofmann
J
, Rohrer
L
, Besler
C
, Rothgiesser
KM
, Becher
B
, Hottiger
MO
, Borén
J
, McBurney
MW
, Landmesser
U
, Lüscher
TF
and Matter
CM.
Sirt1 decreases lox-1-mediated foam cell formation in atherogenesis.
Eur Heart J.
2010;
Apr:
23
.
-
18.
Mattagajasingh
I
, Kim
CS
, Naqvi
A
, Yamamori
T
, Hoffman
TA
, Jung
SB
, DeRicco
J
, Kasuno
K
and Irani
K.
Sirt1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase.
Proc Natl Acad Sci U S A.
2007;
104:
14855
-14860.
[PubMed]
.
-
19.
Zhang
QJ
, Wang
Z
, Chen
HZ
, Zhou
S
, Zheng
W
, Liu
G
, Wei
YS
, Cai
H
, Liu
DP
and Liang
CC.
Endothelium-specific overexpression of class iii deacetylase sirt1 decreases atherosclerosis in apolipoprotein e-deficient mice.
Cardiovasc Res.
2008;
80:
191
-199.
[PubMed]
.
-
20.
Orimo
M
, Minamino
T
, Miyauchi
H
, Tateno
K
, Okada
S
, Moriya
J
and Komuro
I.
Protective role of sirt1 in diabetic vascular dysfunction.
Arterioscler Thromb Vasc Biol.
2009;
29:
889
-894.
[PubMed]
.
-
21.
Dimmeler
S
, Fleming
I
, Fisslthaler
B
, Hermann
C
, Busse
R
and Zeiher
AM.
Activation of nitric oxide synthase in endothelial cells by akt-dependent phosphorylation.
Nature.
1999;
399:
601
-605.
[PubMed]
.
-
22.
Cai
H
and Harrison
DG.
Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress.
Circ Res.
2000;
87:
840
-844.
[PubMed]
.
-
23.
Nichols
TC
, Fischer
TH
, Deliargyris
EN
and Baldwin
AS Jr.
Role of nuclear factor-kappa b (nf-kappa b) in inflammation, periodontitis, and atherogenesis.
Ann Periodontol.
2001;
6:
20
-29.
[PubMed]
.
-
24.
Gareus
R
, Kotsaki
E
, Xanthoulea
S
, van der Made
I
, Gijbels
MJ
, Kardakaris
R
, Polykratis
A
, Kollias
G
, de Winther
MP
and Pasparakis
M.
Endothelial cell-specific nf-kappab inhibition protects mice from atherosclerosis.
Cell Metab.
2008;
8:
372
-383.
[PubMed]
.
-
25.
Bedalov
A
, Gatbonton
T
, Irvine
WP
, Gottschling
DE
and Simon
JA.
Identification of a small molecule inhibitor of sir2p.
Proc Natl Acad Sci U S A.
2001;
98:
15113
-15118.
[PubMed]
.
-
26.
Muller
JM
, Ziegler-Heitbrock
HW
and Baeuerle
PA.
Nuclear factor kappa b, a mediator of lipopolysaccharide effects.
Immunobiology.
1993;
187:
233
-256.
[PubMed]
.
-
27.
Hansson
GK
and Libby
P.
The immune response in atherosclerosis: A double-edged sword.
Nat Rev Immunol.
2006;
6:
508
-519.
[PubMed]
.
-
28.
Pearson
KJ
, Baur
JA
, Lewis
KN
, Peshkin
L
, Price
NL
, Labinskyy
N
, Swindell
WR
, Kamara
D
, Minor
RK
, Perez
E
, Jamieson
HA
and Zhang
Y.
. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span.
Cell Metab.
2008;
8:
157
-168.
[PubMed]
.
-
29.
Pirola
L
and Frojdo
S.
Resveratrol: One molecule, many targets.
IUBMB Life.
2008;
60:
323
-332.
[PubMed]
.
-
30.
Baur
JA
, Pearson
KJ
, Price
NL
, Jamieson
HA
, Lerin
C
, Kalra
A
, Prabhu
VV
, Allard
JS
, Lopez-Lluch
G
, Lewis
K
, Pistell
PJ
and Poosala
S.
Resveratrol improves health and survival of mice on a high-calorie diet.
Nature.
2006;
444:
337
-342.
[PubMed]
.
-
31.
Alom-Ruiz
SP
, Anilkumar
N
and Shah
AM.
Reactive oxygen species and endothelial activation.
Antioxid Redox Signal.
2008;
10:
1089
-1100.
[PubMed]
.
-
32.
Pacholec
M
, Bleasdale
JE
, Chrunyk
B
, Cunningham
D
, Flynn
D
, Garofalo
RS
, Griffith
D
, Griffor
M
, Loulakis
P
, Pabst
B
, Qiu
X
and Stockman
B.
Srt1720, srt2183, srt1460, and resveratrol are not direct activators of sirt1.
J Biol Chem.
2010;
285:
8340
-8351.
[PubMed]
.