Age-dependent malignancy of transformed hematopoietic progenitor cells in vivo
As mentioned, BCR-ABLp190+ ALLs have been identified as being originated in cells with the characteristics of a stem cell, and there are many evidences indicating that aging affects both the numbers and the functionality of stem cells and B lymphoid progenitors. Therefore, to investigate if the age of the target cells also has an impact in malignant development we have taken advantage of the doxycline-controlled transgenic CombitTA-BCR-ABLp190 mouse line [20] to induce the expression of the leukaemia-triggering oncogene in mice of different ages. The CombitTA-BCR-ABL-p190 mice expressing the BCR-ABLp190chimeric gene product have previously been described to consistently show the B-ALL pathologic phenotype with which this oncogene is associated in humans [20]. In the present work, BCR-ABLp190 expression was activated in CombitTA-BCR-ABLp190 mice of different ages (4-, 12- and 20-month, respectively) by removing the doxycycline from the drinking water. All mice demonstrated equivalent levels of exogenous BCR-ABL expression, thus precluding any effect from transgene expression changes with age (Figure 2).
In order to test the age-dependent malignancy of transformed hematopoietic progenitor cells in vivo in the least biased manner possible, and to exclude any potential non cell-autonomous, age-related, effect on the disease evolution, bone marrow cells were purified from CombitTA-BCR-ABLp190 sacrificed mice in which BCR-ABL expression had been kept repressed all life. Then, cells from donors sacrificed at either 4-, 12- or 20-months were injected intravenously into syngenic host mice of 4 months of age. This transplantation into a non doxicyclin-treated recipient lead to transgene derepression and expression of the oncogene in a simultaneous manner. 100% of the injected mice developed B-ALL with all the phenotypic characteristics previously described for the CombitTA-BCR-ABLp190 mice [20], namely the presence in the peripheral blood of organ-infiltrating blast cells co-expressing B-cell and myeloid markers (Figure 3). However, B-ALL derived from 12- and 20-month transformed HSCs could be distinguished by two distinct but interrelated relevant features: first, 20-month-ALLs presented increased cellularity of B220, Mac1 co-expressing blasts (Figure 3B-C). Second, and most importantly, animals with B-ALL derived from 4-month-old donors survived nearly twice as long as those with B-ALL from 12- and 20-month-old donors (88 versus 50.5 and 33 days, respectively; log rank P < 0.0001 and 0.0001, respectively; Figure 3A). These findings therefore prove that the malignancy of B-ALL increases with the age of the leukemic-cell-of-origin (i.e., with the age of the normal progenitors from which the disease arises). Leukaemias originating from older progenitors present a more aggressive phenotype, and have a much faster evolution, than leukaemias initiating in young progenitors. Our results also show that, to a great extent, disease evolution is programmed from the beginning, since the starting cell determines the aggressiveness of the phenotype, and also that the decline of the immune response with age is not responsible for the increased development or aggressiveness of the tumours in the elderly. In agreement with these observations it has been recently shown, using a retroviral transduction system, that aged B-cell progenitors present a reduced fitness due to impaired signaling machinery and that this, since the oncogene covers in part the need for kinase signaling, promotes the positive selection for BCR-ABL+ cells [21].
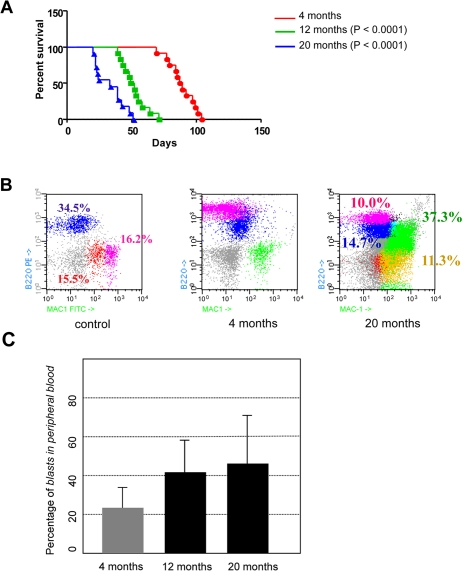
Figure 3. Age-dependent malignancy of transformed hematopoietic progenitor cells in vivo. (A) Kaplan-Meier survival analysis. Animals where BCR-ABL expression was induced at 4-month of age survived significantly longer than animals where BCR-ABL expression was induced at 12- or 20-month of age (median survival of 88 days versus 50.5 and 33 days, respectively; log rank P < 0.0001 and 0.0001, respectively). (B) CombitTA-p190 mice were evaluated for disease progression by flow citometry. Cells from peripheral blood of CombitTA-p190 and control mice were analyzed by flow cytometry with combination of the specific myeloid (Mac1) and B-cell lymphoid (B220) markers. A representative flow cytometry analysis is shown Characteristic blast cells in BCR-ABL B-cell leukaemia co-expressed B-cell and myeloid markers indicated by the presence of Mac+B220+ cells. (C) Variability on the percentage of blast cells in the preripheral blood as a function of donor age.
In summary, our study of B-ALL provides the first in vivo experimental evidence, using a conditional transgenic mouse model, showing that an increase in the age at which transformation occurs has a direct impact in the malignant potential of cancer cells. This is in agreement with clinical observations showing that age-related cell intrinsic factors may play a leading role in the impact of age on B-ALL malignancy [3]. Also, these findings further support the clinical relevance of this model and its uselfulness for future studies to determine how specific oncogenic mechanisms affect age-related malignancy, and to determine how age-related cell-extrinsic host mechanisms such as immune function and others impact B-ALL malignancy.