PASS estimation of the BAS of EVOO oleuropeins
Predictions were made using the PASS12 refined version of the program (www.genexplain.com/pass), which predicts 1,105 types of biological activities with a mean prediction accuracy in leave-one-out cross-validation (LOOCV) of 96%. PASS prediction tools are constructed using principal compounds from the MDDR database (produced by Accelrys and Prous Science), which is continuously updated with biologically relevant compounds. The PASS training set consisted of 287,633 known biologically active substances (e.g., drugs, drug-candidates, lead compounds, toxic compounds) compiled from various sources, including publications, patents, chemical databases, and “gray” literature [33-43].
Figs. 2 and 3 show only the activities predicted at a Pa (probability “to be active”) > 0.200 for the hemiacetalic and dialdehidic forms of OA (Fig. 2) and DOA (Fig. 3), grouped using PharmaExpert (http://genexplain.com/pharmaexpert/), a tool that was developed to analyze the biological activity spectra of substances predicted by PASS. PharmaExpert software analyzes the relationships between biological activities (“mechanism-effect(s)” and “effect-mechanism(s)”), identifies probable drug-drug interactions, and searches for compounds acting on multiple targets [33-43].
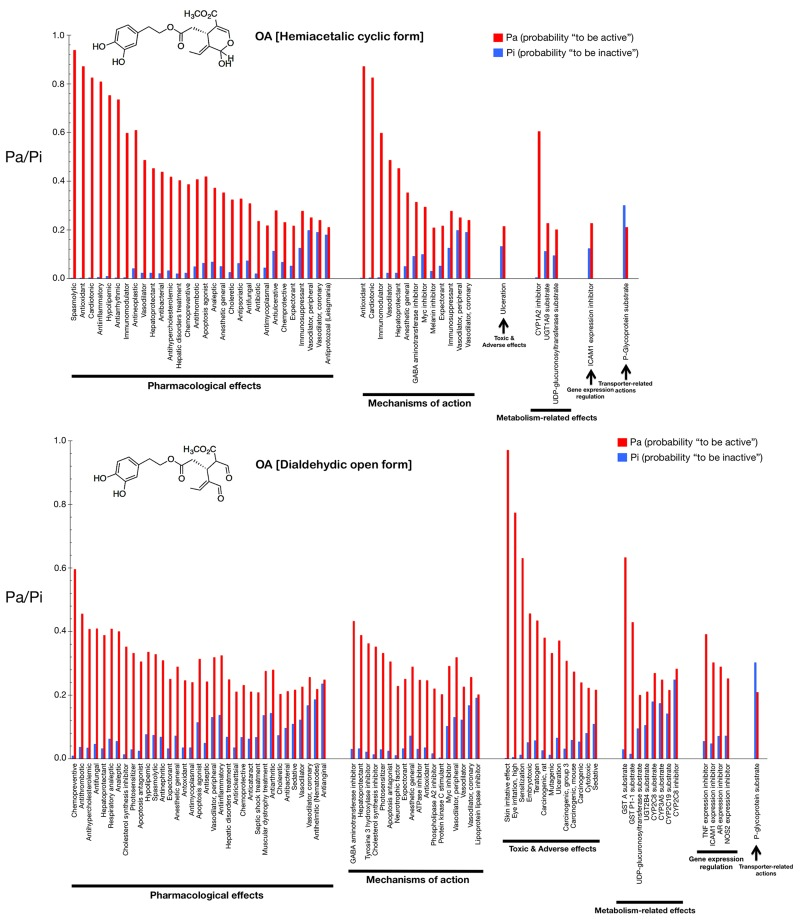
Figure 2. Biological activity spectra of the gerosuppressant olive oil oleuropein OA. The results of predicted activity spectra generated by PASS are presented as a bar graph of biological activities with the probabilities “to be active” (Pa) and “to be inactive” (Pi) calculated for each activity. The values vary from 0.000 to 1.000; the higher a Pa value is the lower is the predicted probability of obtaining false positives in biological testing. The lists are arranged in descending order of Pa-Pi; therefore, more probable biological activities are at the top of the list. The list can be shortened at any desirable cutoff value, but PASS uses the criteria Pa=Pi as the as the default threshold, i.e., only biological activities with Pa > Pi are considered as probable for a particular compound. If we choose to use rather high value of Pa as cutoff for selection of probable activities, the chance to confirm the predicted activities is high too, but many existing activities will be lost. For instance, if one selects for consideration particular biological activities predicted with Pa > 0.9, then about 90% of actual activities will be lost (i.e., the expected probability to find inactive compounds in the selected set is very low but about 90% of active compounds will be missed). If one lowers the Pa threshold to 0.8, the probability to find inactive compounds is still low, but about 80% of active compounds will be missed, etc. Another important aspect of PASS predictions is the compounds' novelty. If one limits to high Pa values, one may find close analogues of known biologically active substances among the tested compounds. For instance, for Pa > 0.7, the chance to experimentally find the biological activity is high, but some of the activities may be close analogue of known pharmaceutical agents. If one chooses 0.5
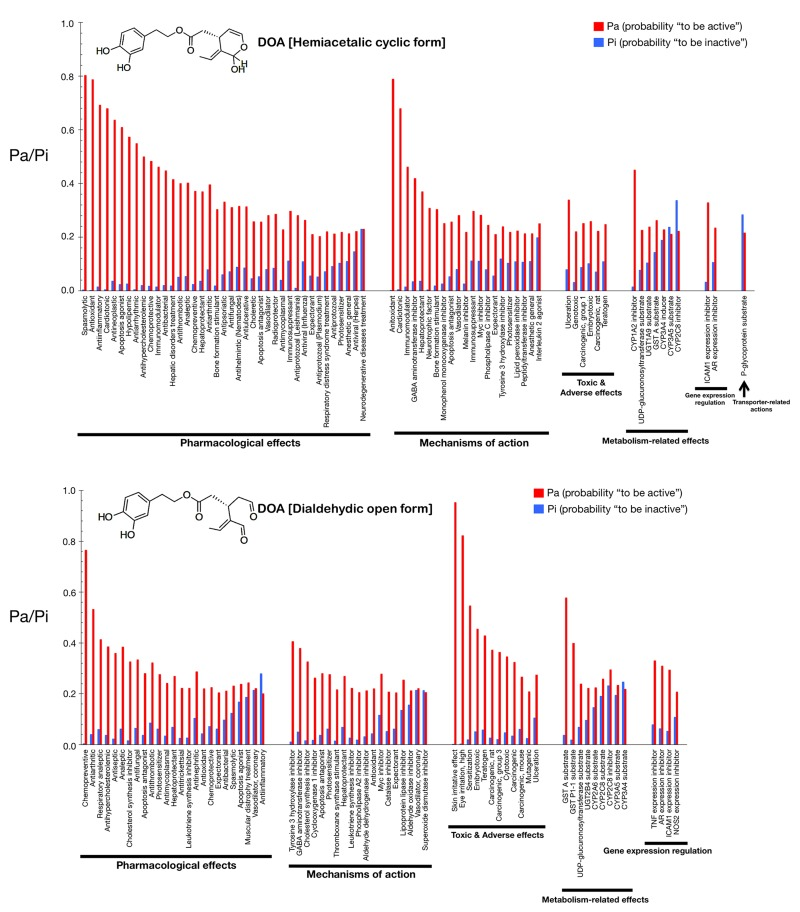
Figure 3. Biological activity spectra of the gerosuppressant olive oil oleuropein DOA. (see Fig. 2 text for details).
Different types of biological activities are divided into six classes: mechanisms of action, pharmacological effects, metabolism-related actions, transporter terms, gene expression terms, and toxic/adverse effects.
The mechanisms of action reflect the interactions of biologically active compounds with biological entities at the macromolecular level. In addition to the expected antioxidant mechanism of action of OA (Pa = 0.872), PASS predicts some new mechanisms for the cyclic hemiacetalic form of OA, including cardiotonic (Pa = 0.826), immunomodulator (Pa = 0.598), vasodilator (Pa = 0.487), and hepatoprotectant (Pa = 0.453) activities. The cyclic hemiacetalic form of DOA is similarly predicted to exhibit an expected antioxidant mechanism of action (Pa = 0.790) while lacking all of the cardiotonic, immunomodulator, and hepatoprotectant mechanisms predicted for the cyclic hemiacetalic form of OA.
The open dialdehydic form of OA is predicted to lack all of the cardiotonic, immunomodulator, and hepatoprotectant mechanisms of action suggested for the cyclic hemiacetalic form of OA. Moreover, the expected antioxidant mechanism of OA is notably reduced when PASS predictions are used to estimate the probable mechanisms of action of the open dialdehydic form of OA (Pa = 0.247). The top predicted mechanism of action for the open dialdehydic form of OA is GABA aminotransferase inhibitor activity (Pa = 0.433). The open dialdehydic form of DOA is predicted to lack (Pa = 0.221) the highly predicted antioxidant mechanism of action of the cyclic hemiacetalic form of DOA, while gaining new mechanisms such as acting as a tyrosine 3 hydroxylase inhibitor (Pa = 0.407) and cholesterol synthesis inhibitor (Pa = 0.380).
The pharmacological effects reflect the pharmacological action or pharmacotherapeutic application of the compound. Some of the most likely anti-aging/anti-cancer pharmacological effects, showing higher Pa values in the predictions for the cyclic hemiacetalic form of OA, include anti-antioxidant (Pa = 0.872), anti-inflammatory (Pa = 0.809), anti-neoplastic (Pa = 0.610), apoptosis agonist (Pa = 0.419), and chemopreventive (Pa = 0.387) effects. The cyclic hemiacetalic form of DOA conserves all the anti-aging/anti-cancer pharmacological effects observed in the prediction for the cyclic hemiacetalic form OA, but displaying somewhat lower Pa values (i.e., anti-oxidant [Pa = 0.790], anti-inflammatory [Pa = 0.693], and anti-neoplastic [Pa = 0.637] effects). The sole exception is the apoptosis agonist effect, which is predicted to occur with a higher probability (Pa = 0.610) for the cyclic hemiacetalic form of DOA.
Remarkably, the open dialdehydic form of OA exclusively conserves the chemopreventive effect of the cyclic hemiacetalic form of OA, and with an even higher Pa value (0.596). Similarly, the open dialdehydic form of DOA notably gains a strong anti-aging/anti-cancer-related chemopreventive effect (Pa = 0.766) compared with the weaker effect observed in the activity prediction for the cyclic hemiacetalic form of DOA (Pa = 0.372).
The metabolism-related actions reflect interactions of chemical compounds with metabolic enzymes. The cyclic hemiacetalic forms of OA and DOA are predicted to function as CYP1A2 inhibitors (Pa = 0.605 and Pa = 0.451, respectively).
The open dialdehydic form of OA is predicted to function as a substrate of GST A and GST P1-1 (Pa = 0.633 and 0.430, respectively). Similarly, the open dialdehydic form of DOA is predicted to function as a GST A and GST P1-1 substrate (Pa = 0.579 and 0.400, respectively).
The transporter terms reflect the interaction of chemical compounds with transporters (e.g., P-glycoprotein substrate, P-glycoprotein inhibitor, P-glycoprotein inducer). The cyclic and open forms of OA as well as the cyclic form of DOA are all predicted to function as P-glycoprotein substrates, but with a low Pa (~0.220); this activity is not predicted for the open dialdehydic form of DOA.
The gene expression terms reflect the influence of chemical compounds on the expression of certain genes. The cyclic hemiacetalic form of OA is predicted to negatively regulate ICAM1 gene expression with a low Pa (0.228). The cyclic hemiacetalic form of DOA is similarly predicted to inhibit the expression of ICAM1 (Pa = 0.329) and AR (Pa = 0.235) genes.
In addition to inhibiting the expression of the ICAM1 gene (Pa = 0.303) and AR (Pa = 0.290), as indicated for the cyclic hemiacetalic form of OA, the open dialdehydic form is predicted to inhibit the expression of the TNF (Pa = 0.392) and NOS2 (Pa = 0.253) genes. The open dialdehydic form of DOA, in addition to inhibiting the ICAM1 (Pa = 0.295) and AR (Pa = 0.310) genes, as indicated for its cyclic hemiacetalic form, is also predicted to inhibit the expression of the TNF (Pa = 0.331) and NOS2 (Pa = 0.208) genes.
The toxic/adverse effects reflect the specific toxicities or adverse reactions of the chemical compounds. No highly predicted toxic/adverse effects are indicated for the cyclic hemiacetalic form of OA beyond ulceration (Pa = 0.215). Interestingly, other indirectly related anti-aging/anti-cancer pharmacological effects with low Pa values are predicted for the cyclic hemiacetalic form of DOA, specifically embryotoxic (Pa = 0.260) and teratogen (Pa = 0.248) effects.
The open dialdehydic form of OA, in addition to showing skin irritation (Pa = 0.971) and eye irritation (Pa = 0.774) effects, is predicted to exhibit embryotoxic (Pa = 0.457) and teratogenic (Pa = 0.435) toxicities with Pa values that are somewhat higher than those predicted for its cyclic hemiacetalic form. A very similar pattern of toxic and adverse effects is predicted for the open dialdehydic form of DOA, including skin irritation (Pa = 0.954) and eye irritation (Pa = 0.823) effects as well as embryotoxic (Pa = 0.456), and teratogenic (Pa = 0.429) activities.