Characteristics of the study population and TILs distribution
The WCH set included 316 patients, and the TCGA set included 370 patients. The characteristics of our study population were presented in Supplementary Table 1. Compared to the WCH set, the TCGA set contained a significantly larger proportion of female participants. A statistically significant difference was observed regarding age, race, risk factor, and Ishak score between the two sets (P < 0.001). Specifically, patients from the WCH set tended to be younger and had higher Ishak scores, and more patients from the WCH set than from the TCGA set were Asian and positive for HBV infection. For 1 patient in the WCH set and 27 patients in the TCGA set, TILs could not be assessed due to tissue quality (Supplementary Figure 1). We found that 11% of tumors, in both the WCH set and the TCGA set, had high TILs (TILs ≧ 50%); 33% and 36% of tumors in the WCH set and the TCGA set had intermediate TILs (10% ≦ TILs < 50%), respectively; while 56% and 53% of tumors in the WCH set and the TCGA set had low TILs (TILs < 10%), respectively. We found that compared to the tumors with low TILs, those with high TILs had higher CD8, PD-1, PD-L1 on immune cells, and OX40 expression (Supplementary Table 2).
Prognostic value of TILs
In the WCH set, univariable analysis and survival curves showed that the high- and intermediate-TILs groups had better OS than the low-TILs group (Figure 1A, Supplementary Table 3). The 5-year OS rate of the high-TILs group (79%; 95% CI, 62%-100%) was longer than that of the intermediate-TILs group (74%; 95% CI, 64%-84%) and the low-TILs group (53%; 95% CI, 44%-64%). Similarly, the 5-year DFS of the high-TILs group (61%; 95% CI, 33%-100%) was longer than that of the intermediate-TILs group (49%; 95% CI, 34%-70%) and the low-TILs group (16%; 95% CI, 9%-28%). After adjustment for age, sex, tumor grade and stage, our results confirmed that patients in the high- and intermediate-TILs group had better OS (intermediate: HR, 0.58; 95% CI, 0.36-0.93; high: HR, 0.37; 95% CI, 0.15-0.93; Ptrend, 4.25 × 10-3) and DFS (intermediate: HR, 0.35; 95% CI, 0.22-0.58; high: HR, 0.23; 95% CI, 0.09-0.58; Ptrend, 3.60 × 10-6) than patients in the low-TILs group (Table 1).
Table 1. Mortality risks by intensity of TILs for overall survival and disease-free survival.
| Group | TILs intensity | Overall survival | | Disease-free survival |
No. of
cases/total | Adjusted
HRa | P | | No. of
cases/total | Adjusted
HRa | P |
| Discovery phase (WCH set) |
| Low | TILs < 10% | 67/172 | 1 (reference) | | | 80/103 | 1 (reference) | |
| Intermediate | 10% ≦ TILs <50% | 24/106 | 0.58 (0.36-0.93) | 0.02 | | 21/58 | 0.35 (0.22-0.58) | 4.59 × 10-5 |
| High | TILs ≧ 50% | 5/34 | 0.37 (0.15-0.93) | 0.04 | | 5/22 | 0.23 (0.09-0.58) | 1.83 × 10-3 |
| P for trend | | | 4.25 × 10-3 | | | | 3.60 × 10-6 |
| Validation phase (TCGA set) |
| Low | TILs < 10% | 81/181 | 1 (reference) | | | 100/154 | 1 (reference) | |
| Intermediate | 10% ≦ TILs <50% | 30/123 | 0.42 (0.28-0.66) | 1.13 × 10-4 | | 50/111 | 0.52(0.37-0.74) | 2.78 × 10-4 |
| High | TILs ≧ 50% | 8/39 | 0.41 (0.19-0.86) | 0.02 | | 11/33 | 0.33 (0.17-0.62) | 6.09 × 10-4 |
| P for trend | | | 1.08 × 10-4 | | | | 8.25 × 10-6 |
| aAdjusted for age, sex, tumor stage and tumor grade. |
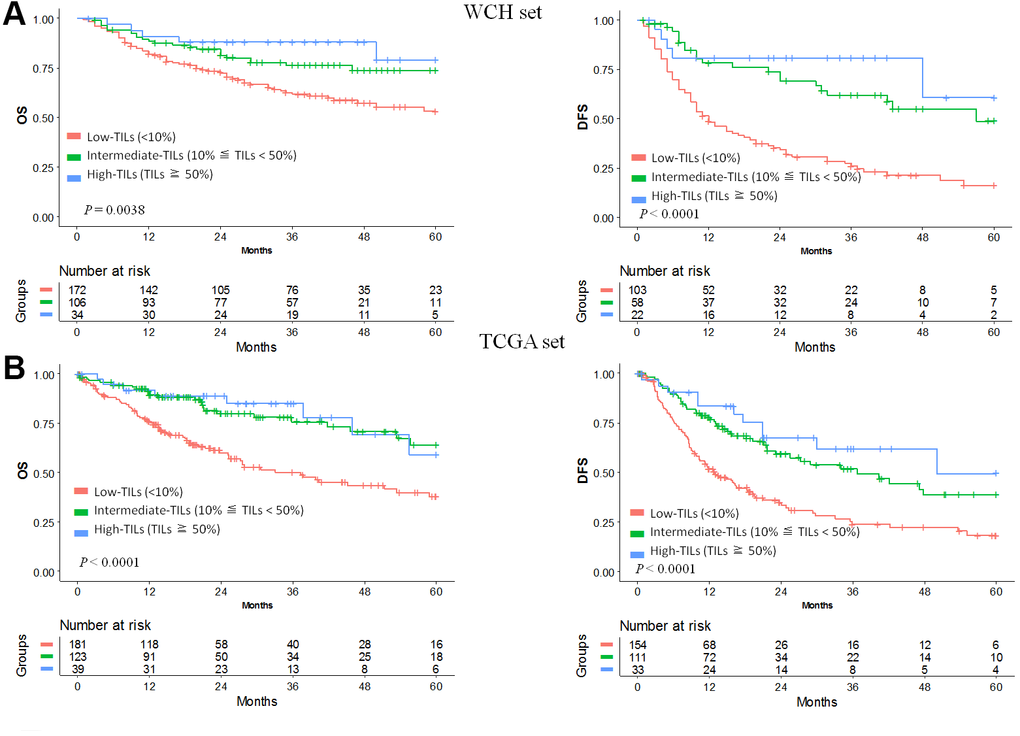
Figure 1. Prognostic value of TILs in hepatocellular carcinoma. Kaplan-Meier curves of estimated overall survival and disease-free survival in the WCH set (A) and the TCGA set (B). Patients without survival information were excluded from the analysis.
We used the TCGA hepatocellular carcinoma cohort to validate the prognostic value of TILs. In line with results in the WCH set, patients in the low-TILs group had the worst 5-year OS and DFS (Figure 1B). The HR of risk for OS (intermediate: HR, 0.42; 95% CI, 0.28-0.66; high: HR, 0.41; 95% CI, 0.19-0.86; Ptrend, 1.08 × 10-4) and DFS (intermediate: HR, 0.52; 95% CI, 0.37-0.74; high: HR, 0.33; 95% CI, 0.17-0.62; Ptrend, 8.25 × 10-6) decreased with the increasing of TILs, confirming the prognostic significance of TILs as a tumor biomarker (Table 1, Supplementary Tables 4, 5).
When TILs was assessed as a continuous variable, the associations between TILs and survival remained in univariable analysis, with estimated HR for each point change in intensity being 0.99 (95%CI, 0.97-1.00; P, 0.05) and 0.98 (95%CI, 0.96-0.99; P, 0.002), respectively, for the WCH cohort. These associations were validated in the TCGA cohort, with HR being 0.98 (95%CI, 0.97-0.99; P, 4.43 × 10-5) and 0.98 (95%CI, 0.97-0.99; P, 4.60× 10-5), respectively (Supplementary Table 6).
To further improve the predictive accuracy of TILs intensity by examining its performance in combination with other known prognostic factors, we constructed a nomogram incorporating TILs intensity, age, sex, tumor stage, and tumor grade for predicting the survival probability in HCC patients. As depicted in Figure 2, in both cohorts, a higher total score based on the sum of the assigned values for each prediction factor in the nomogram was associated with worse 1-year, 3-year, and 5-year OS rates. For instance, in the WCH cohort, a patient with low TIL intensity and BCLC C stage would yield a total of 164 points (64 points for low TIL intensity, and 100 points BCLC C stage), with a predicted 1, year, 3-year and 5-year OS rates of61.0%, 31.0% and 22.0%, respectively.
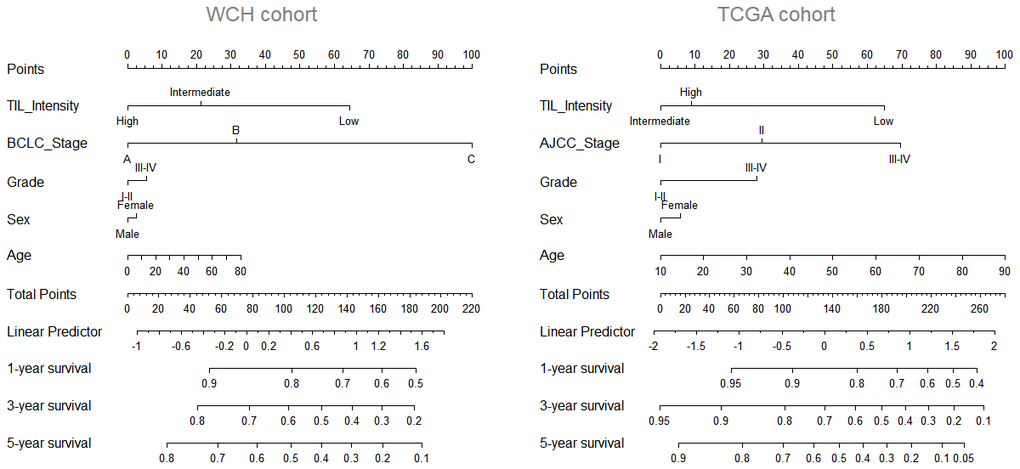
Figure 2. Nomogram predicting survival possibility according to TIL intensity. The values for each prediction factors are marked. From each mark, a vertical line is drawn downward to determine the points, which are subsequently added together and is marked on the Total-points line. Finally, a vertical line is followed downward to the accompanying lines indicating the 1,3 and 5 year survival possibility.
HBV/HCV infection, total mutation burden or neoantigen is not associated with TILs in tumor
A recent study associated several types of oncogenic viruses with increased cytolytic activity in tumors [10]. We investigated whether HBV/HCV infection in HCC patients affected lymphocytic infiltration in tumors and para-tumor liver tissue. In para-tumor tissue, we observed a higher intensity of TILs in samples with HBV or HCV infection compared to those without infection. However, in tumor tissue, no significant difference of TILs was observed between samples with and without HBV or HCV infection (Supplementary Figure 2).
Because neoantigens derived from tumor mutations are likely to trigger an anti-tumor immune response, we tested whether neoantigens in HCC were associated with TILs in the TCGA cohort. The neoantigens data was derived from a previous study [10]. The Spearman correlation test indicated that there was no significant association between neoantigens and TILs intensity (rho = -0.13, P = 0.09). Thus, a lower neoantigens burden alone could not explain the absence of TILs in a major subset of tumors, suggesting that other mechanisms might determine the intensity of TILs in HCC (Supplementary Figure 3).