Does taking an angiotensin inhibitor increase the risk for COVID-19? – a systematic review and meta-analysis
Abstract
Because SARS-COV2 entry into cells is dependent on angiotensin converting enzyme 2 (ACE2) and angiotensin converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) increase ACE2 activity, the safety of ACEI/ARB usage during the coronavirus disease 2019 (COVID-19) pandemic is a controversial topic. To address that issue, we performed a meta-analysis following The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Searches of the Embase, MEDLINE, PubMed, and Cochrane Library databases identified 16 case-control studies examining the effect of ACEI/ARB on the incidence of COVID-19 and its severity. ACEI/ARB usage was associated with an increased risk of COVID-19 morbidity (odds ratio (OR) 1.20, 95% confidence interval (CI) 1.07-1.33, P=0.001) among the general population but not in a hypertensive population (OR 1.05, 95% CI 0.90-1.21, P=0.553). ACEI/ARB usage was not associated with an increased risk of COVID-19 morbidity (coefficient 1.00, 95% CI 1.00-1.00, P=0.660) when we adjusted for hypertension in the general population. ACEI/ARB usage was also not associated with an increased risk of severe illness (OR 0.90, 95%CI 0.55-1.47, P=0.664) or mortality (OR 1.43, 95%CI 0.97-2.10, P=0.070) in COVID-19 patients. Our meta-analysis revealed that ACEI/ARB usage was not associated with either the increased risk of SARS-COV2 infection or the adverse outcomes in COVID-19 patients.
Introduction
Coronavirus disease 2019 (COVID-19) has become the most devastating infectious disease caused by a coronavirus since the outbreak of severe acute respiratory syndrome in 2003 [1, 2]. The lack of knowledge about this novel coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-COV2), has hindered the effective protection of especially vulnerable populations, as well as treatment for all patients [3, 4]. Recently, however, it was reported that entry of SARS-COV2 into host cells is dependent on angiotensin converting enzyme 2 (ACE2) [5]. This finding opened a new avenue for treatment of the disease, but was controversial in the cardiovascular field. It has been well established that ACE2 is an important member of renin angiotensin system (RAS) and participates in the protection of the cardiovascular system [6]. ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are classical antihypertensive drugs that exert their effects via the RAS. Moreover, ACEIs and ARBs act in part by increasing cardiac ACE2 gene expression or inducing cardiac ACE2 activity [7], which raises the question, do these agents increase the risk of SARS-COV2 infection and/or worsen the prognosis of patients with COVID-19 [8–10]. Unfortunately, only a few retrospective studies have attempted to address these questions.
In the present meta-analysis, we used the results from published literature to try to answer 1) whether ACEI and/or ARB are associated with the risk of SARS-COV2 infection and 2) whether they are associated with adverse outcomes in patients with COVID-19.
Results
A flow diagram of the selection of included studies is shown in Figure 1. A total of 16 studies of COVID-19 conducted in China, Italy, USA, Spain, and Israel satisfied the inclusion criteria for this meta-analysis. These included 8 studies assessing the correlation between ACEI/ARB and the incidence of COVID-19 and 9 studies analyzing the relationship between ACEI/ARB and adverse outcomes in patients with COVID-19 [11–26] (Supplementary Table 2). Participants in 9 studies were from the general population, while the participants in the other 7 were hypertensive population (Supplementary Table 2). Methodological assessment of the included studies using NOS criteria revealed that 3 studies were of low quality and 13 were of high quality (Supplementary Table 1). Ultimately, a total of 116,111 individuals were included in our meta-analysis. The main clinal features of the general population are shown in Supplementary Table 2. The overall pooled prevalence of hypertension was 46.5% (95% CI 34.1%–58.9%), calculated using a random-effects model (P < 0.001, I2 = 98.0%) (Supplementary Figure 2). The overall rate of ACEI/ARB usage was 27% (95% CI 19.0-35.0%) in the general population and 41.0% (95% CI 20.0-62.0%) in the hypertensive population (Supplementary Figure 3).
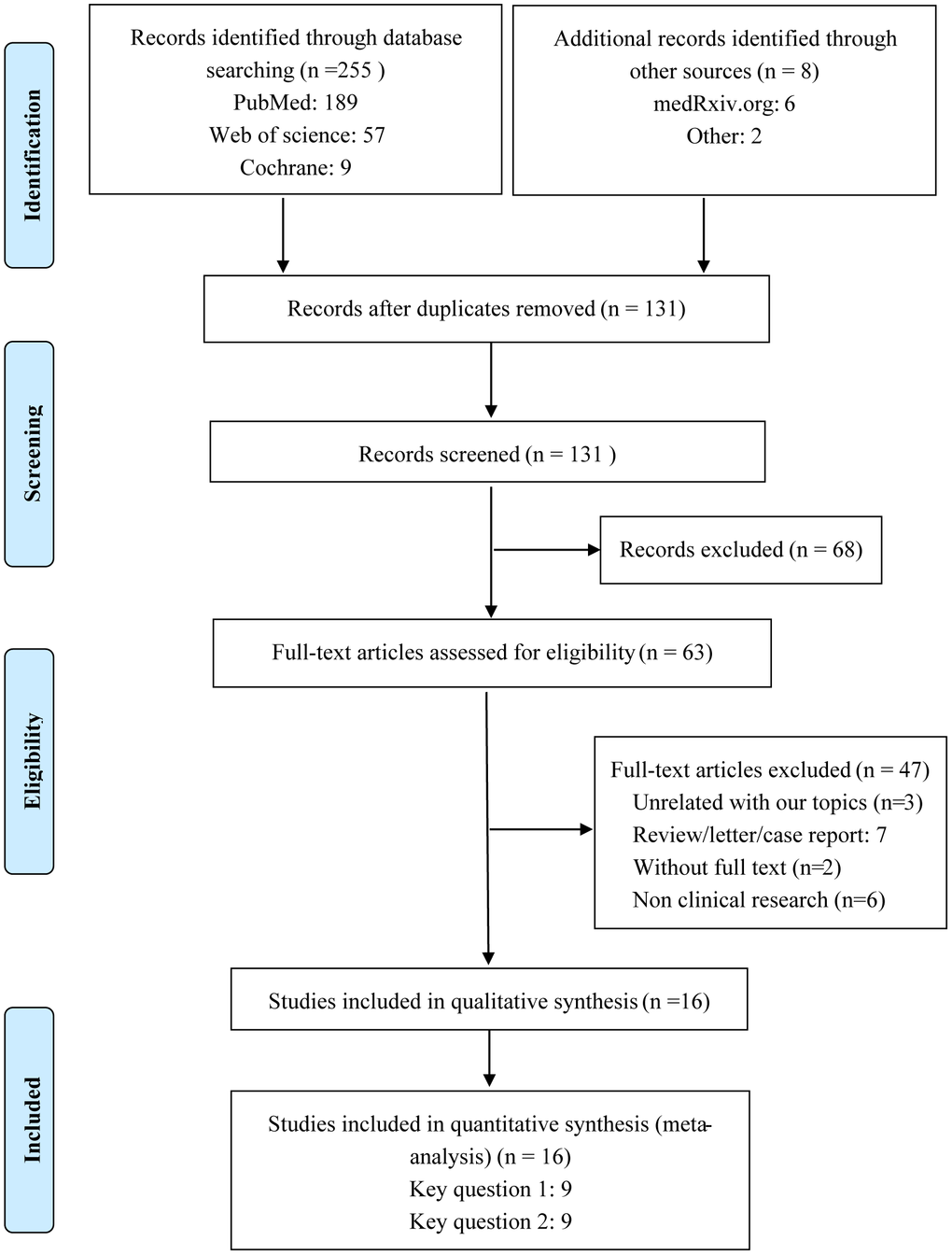
Figure 1. Flow diagram of the process for identification of included studies.
Association between of ACEI/ARB usage and COVID-19 morbidity
A meta-analysis of the odds ratio (OR) for ACEI/ARB-related increases in the risk of COVID-19 morbidity is shown in Figure 2. Among the general population, the usage of ACEI/ARB was associated with higher risk of COVID-19 morbidity (OR = 1.20, 95% CI 1.07-1.33, P=0.001). Intriguingly, in the hypertensive population ACEI/ARB was not associated with increased COVID-19 morbidity (OR = 1.05, 95% CI, 0.90-1.21, P=0.553). Moreover, ACEI/ARB usage was not associated with the increased risk of COVID-19 morbidity in the general population when we adjusted for hypertension using meta-regression (coefficient 1.00, 95% CI 1.00-1.00, P=0.660).
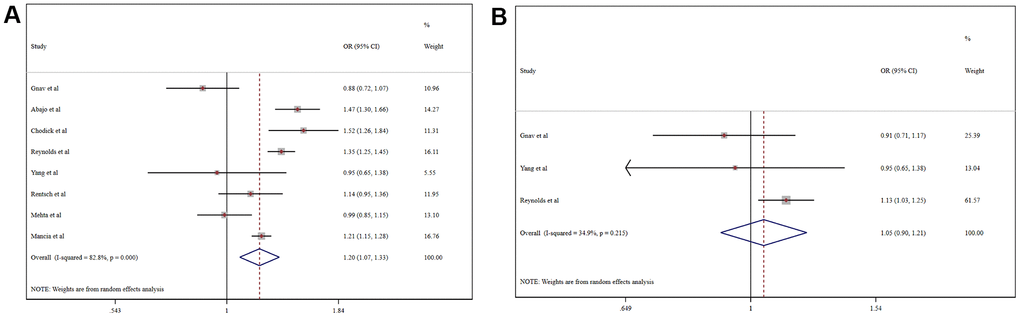
Figure 2. Forest plot of the correlation between ACEIs/ARBs and COVID-19 in the general population (A) and a hypertensive population (B).
Association between ACEI/ARB usage and adverse outcomes in patients with COVID-19
Pooled analysis revealed that the proportion of severe cases among COVID-19 patients was 41.5% (95%CI 26.8%-56.1%), overall mortality was 10.8% (95%CI 6.7%-14.9%), and the prevalence of total adverse outcomes (including severe cases and mortality) was 24.0% (95%CI 18.0%-29.0%) (Supplementary Figure 4). ACEI/ARB usage was not associated with either severity (OR = 0.90, 95%CI 0.55-1.47, P=0.664) or mortality (OR = 1.43, 95%CI 0.97-2.10, P=0.070) in COVID-19 patients (Figure 3). Patients taking an ACER/ARB did not have a higher rate of noninvasive mechanical ventilation, invasive mechanical ventilation or renal replacement therapy, and they had a low overall rate of advanced life support usage (OR = 0.60, 95%CI 0.40-0.90, P<0.001) (Figure 4).
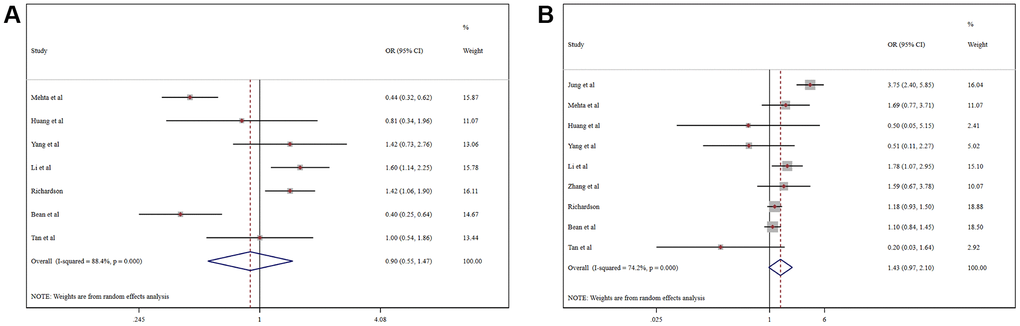
Figure 3. Forest plot of the correlation between ACEIs/ARBs and adverse outcomes in patients with COVID-19: (A) Severe COVID-19; (B) Mortality.
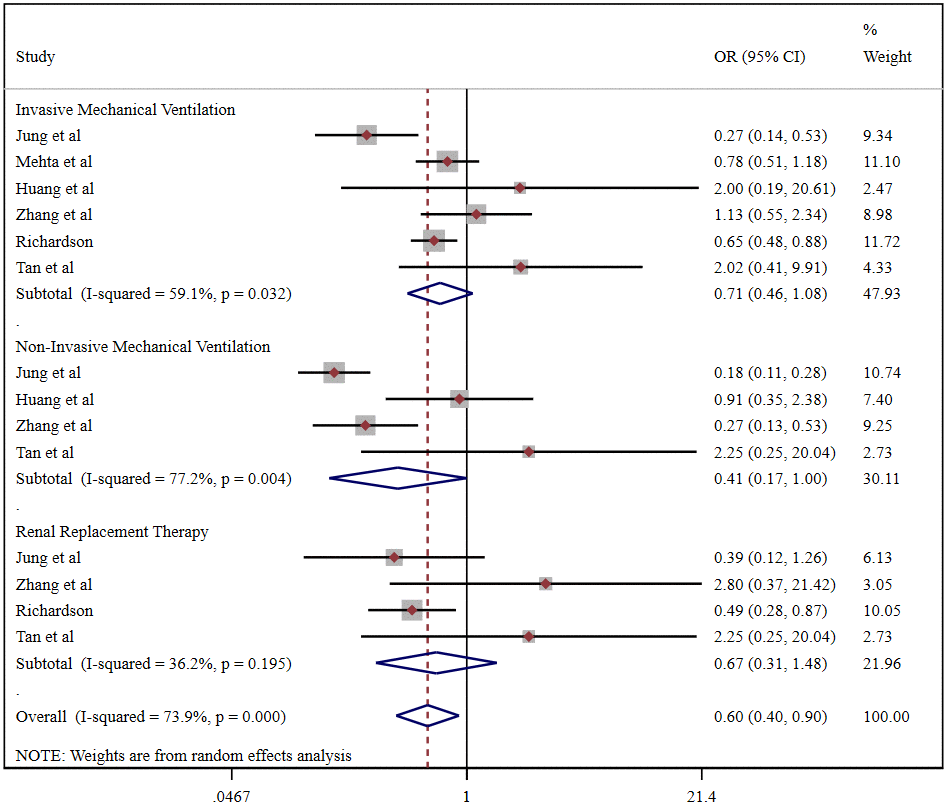
Figure 4. Forest plot of the correlation between ACEIs/ARBs and advanced life support in patients with COVID-19.
Discussion
ACEIs and ARBs are frequently prescribed medications that are used by about 40% of hypertension patients in our meta-analysis. Given the high rate of ACEI or ARB usage and the role of ACE2 in the pathogenesis of COVID-19 [5], a key concern is whether use of an ACEI/ARB increases the risk of SARS-COV2 infection. The results from this meta-analysis indicate that that ACEI/ARB usage correlates positively COVID-19 morbidity in the general population, but that relationship is not detected in a hypertensive population. Moreover, when we adjusted for hypertension in the general population, the correlation between ACEI/ARB and morbidity from COVID-19 disappears. These results suggest the real risk factor for COVID-19 may be hypertension, not the ACEI or ARB used to treat it. Hypertension is a common comorbidity with a higher prevalence among COVID-19 patients than the general population [18]. Hypertension often coexists with other cardiovascular and metabolic diseases, especially in older people [27]. It is well known that chronic low-grade inflammation is a common feature of cardiovascular and metabolic diseases [28, 29], and the association between hypertension and the immune system is now accepted [30]. Changes in both innate and adaptive immunity that cause an imbalance between proinflammatory and anti-inflammatory processes contribute to pathological tissue repair and -remodeling not only in the cardiovascular system, but also in lung tissue [31]. Hypertension-associated low-grade inflammation and immunity imbalance may impair pulmonary circulation and reduce the immune barrier effect, which may, in turn, increase the risk of respiratory infection. Consequently, patients with hypertension may be more susceptible to SARS-COV2 infection than the general population, which is consistent with the results of our meta-analysis and observational studies [13, 18]. But if taking an ACEI or ARB increased the risk of SARS-COV2 infection, the correlation between ACEI/ARB and COVID-19 should be stronger in a hypertensive population than the general population. Our finding that this is not the case suggests ACEI/ARB usage is just a covariate of hypertension rather than a risk factor.
A second concern is whether ACEI/ARB usage is associated with adverse outcomes. We selected advanced life support, severe illness and mortality as end points. Our meta-analysis revealed that ACEI/ARB usage did not increase the risk of severe illness or death. Surprisingly, ACEI/ARB usage decreased the risk of needing advanced life support. ACEIs and ARBs are classes of drugs that effectively inhibit the RAS [32]. Their cardioprotective effects, which include anti-inflammatory, anti-oxidative stress and antifibrotic effects, have been confirmed in a large number of basic and clinical studies [33, 34]. In addition, the relationship between the RAS and pulmonary disease has also attracted attention [35]. Angiotensin II induces pulmonary vasoconstriction, exacerbates pulmonary fibrosis, promotes inflammation and enhances oxidative stress. ACEI/ARB-induced ACE2 expression may inhibit the adverse effects of angiotensin II in lung tissue. In a mouse model, for example, ACE2 protected against ARDS, while an ARB also protected against lung injury by blocking AT1 receptors [36]. Moreover, blocking AT1 receptors can suppress pulmonary fibrosis that increases the risk of severe COVID-19 [37]. Consequently, taking an ACEI or ARB may reduce the risk of severe COVID-19 and ARDS, thereby contributing to a lower usage rate of advanced life support. Whether or not this beneficial effect of ACEIs or ARBs actually exists [9, 38], we did not detect an association between ACEI or ARB usage and severe COVID-19 or mortality in our meta-analysis.
Although a meta-analysis based on randomized controlled trials (RCTs) represents the highest level of evidence, there have been only few RCTs designed to investigate the safety of ACEIs or ARBs for patients with cardiovascular disease during the COVID-19 pandemic. However, one recent study shed light on ramipril’s impact on COVID-19 risk in this vulnerable population [39]. The participants in that study came from The RASTAVI (Renin-Angiotensin System Blockade Benefits in Clinical Evolution and Ventricular Remodeling After Transcatheter Aortic Valve Implantation) trial and were randomly assigned to a ramipril or control group. The results showed that ramipril had no impact on the incidence or severity of COVID-19, which is in line with our results. Additional protocols from RCTs have also been published, and their results deserve attention [40–42]. Thus, our systematic review based on currently available large cohort studies will have clinical value until the results of additional well-designed RCTs with large sample sizes become available.
Our results reveal that ACEI or ARB usage was not associated with either the increased risk of SARS-COV2 infection or the pool prognosis of COVID-19. It is therefore unnecessary to discontinue use of an ACEI or ARB during the COVID-19 pandemic. Nonetheless, given the complexity of the interaction between RAS inhibitors and COVID-19, more in-depth studies to resolve existing controversies are required.
Materials and Methods
Literature search
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [43]. Literature published in English and listed in the Embase, MEDLINE, PubMed, and/or Cochrane Library databases were searched by two independent reviewers from January 1 to August 15, 2020. The search terms ‘angiotensin converting enzyme inhibitor’, ‘angiotensin-converting enzyme inhibitor’, ‘ACEI’, ‘angiotensin receptor blocker’, ‘ARB’, ‘novel coronavirus’, ‘novel coronavirus 2019’, ‘2019nCoV’, ‘COVID-19’, ‘Wuhan coronavirus’, ‘Wuhan pneumonia’, ‘SARS-CoV-2’ and ‘Corona virus disease 2019’ were included in our search strategy. Any inconsistency between the reviewers was resolved by a third independent reviewer.
Eligibility criteria
High-quality cohort and case-control studies were included in this systematic review and meta-analysis. Studies that reported the usage of an ACEI and/or ARB and SARS-cov-2 infection were eligible. Studies that reported an association between ACEI and/or ARB usage and the outcomes of COVID-19 patients were also included. Studies without adverse outcomes, including usage of advanced life support, severe COVID-19 and death, were excluded after reviewing Supplementary Materials.
Data extraction
Two independent reviewers used a predetermined data collection table to extract relevant data. Any divergence between the reviewers was resolved by a third independent reviewer.
Quality assessment
Two of the authors used the Newcastle-Ottawa Quality Assessment Scale (NOS) to independently assess the quality of observational studies [44].
Outcomes of interest
The primary endpoints were case confirmation and adverse outcomes, including severe disease and death, in patents with COVID-19. The secondary outcome was usage of advanced life support by COVID-19 patients during their hospitalization.
Statistical analysis
Meta-analysis was performed using Stata version 15.0 (StataCorp, College Station, TX, USA). Results were presented ORs and CIs on the basis of the Mantel-Haenszel random-effects model. Heterogeneity was evaluated with the I2 statistic. Values of I2 >50% were regarded as indicating considerable heterogeneity. For qualitative evaluation of publication bias toward the endpoint, funnel plots were used; for quantitative assessment, Egger’s linear regression test and Begg’s rank correlation test was used (Supplementary Figure 1). We conducted a sensitivity analysis of the endpoint through sequential removal of each study. Meta-regression analyses based on study-level covariates (age, sex, hypertension history) were conducted to explain any heterogeneity. We calculated the optimal information (sample) size to maintain a 2-sided type I error at 0.05 and a type II error at 0.20 (80% power), with a relative risk reduction of 25% and an incidence of 8.5% endpoint in the central arm.
Author Contributions
XC.Y., Z.M. and MP.W. designed the study, acquired, analyzed, and interpreted data. Z.M. and MP.W. did the literature search. Y.S. and HF.L. independently assessed the quality of the observational studies. H.D. L.L. and Y.Z. extracted relevant data. Z.M. and MP.W. drafted the manuscript. TR.W. and L.Z. revised it critically. XC.Y. gave final approval of the version to be published. All authors read and approved the final manuscript.
Acknowledgments
We thank Dr. Xue-Gong Yu for providing statistical advice. We also thank Xiao-Hong Li and Ming Yang for manuscript review.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Funding
This work was supported by the National Natural Science Foundation of China [81670214; 81970271].
Editorial Note
&
This corresponding author has a verified history of publications using a personal email address for correspondence
References
-
1.
Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020; 55:105924. https://doi.org/10.1016/j.ijantimicag.2020.105924 [PubMed]
-
2.
Singhal T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J Pediatr. 2020; 87:281–86. https://doi.org/10.1007/s12098-020-03263-6 [PubMed]
-
3.
Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020; 109:102433. https://doi.org/10.1016/j.jaut.2020.102433 [PubMed]
-
4.
Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020; 323:1824–36. https://doi.org/10.1001/jama.2020.6019 [PubMed]
-
5.
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020; 181:271–280.e8. https://doi.org/10.1016/j.cell.2020.02.052 [PubMed]
-
6.
Santos RA, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: focus on Angiotensin-(1-7). Physiol Rev. 2018; 98:505–53. https://doi.org/10.1152/physrev.00023.2016 [PubMed]
-
7.
Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005; 111:2605–10. https://doi.org/10.1161/CIRCULATIONAHA.104.510461 [PubMed]
-
8.
Kreutz R, Algharably EA, Azizi M, Dobrowolski P, Guzik T, Januszewicz A, Persu A, Prejbisz A, Riemer TG, Wang JG, Burnier M. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. Cardiovasc Res. 2020; 116:1688–99. https://doi.org/10.1093/cvr/cvaa097 [PubMed]
-
9.
Diaz JH. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med. 2020; 27:taaa041. https://doi.org/10.1093/jtm/taaa041 [PubMed]
-
10.
Sommerstein R, Kochen MM, Messerli FH, Gräni C. Coronavirus Disease 2019 (COVID-19): Do Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers Have a Biphasic Effect? J Am Heart Assoc. 2020; 9:e016509. https://doi.org/10.1161/JAHA.120.016509 [PubMed]
-
11.
Gnavi R, Demaria M, Picariello R, Dalmasso M, Ricceri F, Costa G. Therapy With Agents Acting on the Renin-Angiotensin System and Risk of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Clin Infect Dis. 2020; 71:2291–93. https://doi.org/10.1093/cid/ciaa634 [PubMed]
-
12.
de Abajo FJ, Rodríguez-Martín S, Lerma V, Mejía-Abril G, Aguilar M, García-Luque A, Laredo L, Laosa O, Centeno-Soto GA, Ángeles Gálvez M, Puerro M, González-Rojano E, Pedraza L, et al, and MED-ACE2-COVID19 study group. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020; 395:1705–14. https://doi.org/10.1016/S0140-6736(20)31030-8 [PubMed]
-
13.
Chodick G, Nutman A, Yiekutiel N, Shalev V. Angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers are not associated with increased risk of SARS-CoV-2 infection. J Travel Med. 2020; 27:taaa069. https://doi.org/10.1093/jtm/taaa069 [PubMed]
-
14.
Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, Katz SD, Fishman GI, Kunichoff D, et al. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19. N Engl J Med. 2020; 382:2441–48. https://doi.org/10.1056/NEJMoa2008975 [PubMed]
-
15.
Yang G, Tan Z, Zhou L, Yang M, Peng L, Liu J, Cai J, Yang R, Han J, Huang Y, He S. Effects of Angiotensin II Receptor Blockers and ACE (Angiotensin-Converting Enzyme) Inhibitors on Virus Infection, Inflammatory Status, and Clinical Outcomes in Patients With COVID-19 and Hypertension: A Single-Center Retrospective Study. Hypertension. 2020; 76:51–58. https://doi.org/10.1161/HYPERTENSIONAHA.120.15143 [PubMed]
-
16.
Rentsch CT, Kidwai-Khan F, Tate JP, Park LS, King JT, Skanderson M, Hauser RG, Schultze A, Jarvis CI, Holodniy M, Lo Re V, Akgun KM, Crothers K, et al. Covid-19 Testing, Hospital Admission, and Intensive Care Among 2,026,227 United States Veterans Aged 54-75 Years. medRxiv. 2020; 2020:04.09. https://doi.org/10.1101/2020.04.09.20059964 [PubMed]
-
17.
Mehta N, Kalra A, Nowacki AS, Anjewierden S, Han Z, Bhat P, Carmona-Rubio AE, Jacob M, Procop GW, Harrington S, Milinovich A, Svensson LG, Jehi L, et al. Association of Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020; 5:1020–26. https://doi.org/10.1001/jamacardio.2020.1855 [PubMed]
-
18.
Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-Angiotensin-Aldosterone System Blockers and the Risk of Covid-19. N Engl J Med. 2020; 382:2431–40. https://doi.org/10.1056/NEJMoa2006923 [PubMed]
-
19.
de Lusignan S, Dorward J, Correa A, Jones N, Akinyemi O, Amirthalingam G, Andrews N, Byford R, Dabrera G, Elliot A, Ellis J, Ferreira F, Lopez Bernal J, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020; 20:1034–42. https://doi.org/10.1016/S1473-3099(20)30371-6 [PubMed]
-
20.
Huang Z, Cao J, Yao Y, Jin X, Luo Z, Xue Y, Zhu C, Song Y, Wang Y, Zou Y, Qian J, Yu K, Gong H, Ge J. The effect of RAS blockers on the clinical characteristics of COVID-19 patients with hypertension. Ann Transl Med. 2020; 8:430. https://doi.org/10.21037/atm.2020.03.229 [PubMed]
-
21.
Jung SY, Choi JC, You SH, Kim WY. Association of Renin-angiotensin-aldosterone System Inhibitors With Coronavirus Disease 2019 (COVID-19)- Related Outcomes in Korea: A Nationwide Population-based Cohort Study. Clin Infect Dis. 2020; 71:2121–28. https://doi.org/10.1093/cid/ciaa624 [PubMed]
-
22.
Li J, Wang X, Chen J, Zhang H, Deng A. Association of Renin-Angiotensin System Inhibitors With Severity or Risk of Death in Patients With Hypertension Hospitalized for Coronavirus Disease 2019 (COVID-19) Infection in Wuhan, China. JAMA Cardiol. 2020; 5:825–30. https://doi.org/10.1001/jamacardio.2020.1624 [PubMed]
-
23.
Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, Liu YM, Zhao YC, Huang X, Lin L, Xia M, Chen MM, Cheng X, et al. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circ Res. 2020; 126:1671–81. https://doi.org/10.1161/CIRCRESAHA.120.317134 [PubMed]
-
24.
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, et al, and the Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020; 323:2052–59. https://doi.org/10.1001/jama.2020.6775 [PubMed]
-
25.
Bean DM, Kraljevic Z, Searle T, Bendayan R, Kevin O, Pickles A, Folarin A, Roguski L, Noor K, Shek A, Zakeri R, Shah AM, Teo JT, Dobson RJ. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID-19 infection in a multi-site UK acute hospital trust. Eur J Heart Fail. 2020; 22:967–74. https://doi.org/10.1002/ejhf.1924 [PubMed]
-
26.
Tan ND, Qiu Y, Xing XB, Ghosh S, Chen MH, Mao R. Associations Between Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blocker Use, Gastrointestinal Symptoms, and Mortality Among Patients With COVID-19. Gastroenterology. 2020; 159:1170–72.e1. https://doi.org/10.1053/j.gastro.2020.05.034 [PubMed]
-
27.
Armanios M, de Cabo R, Mannick J, Partridge L, van Deursen J, Villeda S. Translational strategies in aging and age-related disease. Nat Med. 2015; 21:1395–99. https://doi.org/10.1038/nm.4004 [PubMed]
-
28.
Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond). 2014; 126:267–74. https://doi.org/10.1042/CS20130407 [PubMed]
-
29.
Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011; 13:11–22. https://doi.org/10.1016/j.cmet.2010.12.008 [PubMed]
-
30.
Drummond GR, Vinh A, Guzik TJ, Sobey CG. Immune mechanisms of hypertension. Nat Rev Immunol. 2019; 19:517–32. https://doi.org/10.1038/s41577-019-0160-5 [PubMed]
-
31.
Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009; 4:435–59. https://doi.org/10.1146/annurev.pathol.4.110807.092145 [PubMed]
-
32.
Dézsi CA. Differences in the clinical effects of angiotensin-converting enzyme inhibitors and Angiotensin receptor blockers: a critical review of the evidence. Am J Cardiovasc Drugs. 2014; 14:167–73. https://doi.org/10.1007/s40256-013-0058-8 [PubMed]
-
33.
Simões E Silva AC, Teixeira MM. ACE inhibition, ACE2 and angiotensin-(1-7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol Res. 2016; 107:154–62. https://doi.org/10.1016/j.phrs.2016.03.018 [PubMed]
-
34.
Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM. Renin-angiotensin system and cardiovascular risk. Lancet. 2007; 369:1208–19. https://doi.org/10.1016/S0140-6736(07)60242-6 [PubMed]
-
35.
Kuba K, Imai Y, Penninger JM. Angiotensin-converting enzyme 2 in lung diseases. Curr Opin Pharmacol. 2006; 6:271–76. https://doi.org/10.1016/j.coph.2006.03.001 [PubMed]
-
36.
Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005; 436:112–16. https://doi.org/10.1038/nature03712 [PubMed]
-
37.
George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020; 8:807–15. https://doi.org/10.1016/S2213-2600(20)30225-3 [PubMed]
-
38.
Dalan R, Bornstein SR, El-Armouche A, Rodionov RN, Markov A, Wielockx B, Beuschlein F, Boehm BO. The ACE-2 in COVID-19: foe or Friend? Horm Metab Res. 2020; 52:257–63. https://doi.org/10.1055/a-1155-0501 [PubMed]
-
39.
Amat-Santos IJ, Santos-Martinez S, López-Otero D, Nombela-Franco L, Gutiérrez-Ibanes E, Del Valle R, Muñoz-García E, Jiménez-Diaz VA, Regueiro A, González-Ferreiro R, Benito T, Sanmartin-Pena XC, Catalá P, et al. Ramipril in High-Risk Patients With COVID-19. J Am Coll Cardiol. 2020; 76:268–76. https://doi.org/10.1016/j.jacc.2020.05.040 [PubMed]
-
40.
Lopes RD, Macedo AV, de Barros E Silva PG, Moll-Bernardes RJ, Feldman A, D’Andréa Saba Arruda G, de Souza AS, de Albuquerque DC, Mazza L, Santos MF, Salvador NZ, Gibson CM, Granger CB, et al, and BRACE CORONA investigators. Continuing versus suspending angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: Impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)--The BRACE CORONA Trial. Am Heart J. 2020; 226:49–59. https://doi.org/10.1016/j.ahj.2020.05.002 [PubMed]
-
41.
Gommans DH, Nas J, Pinto-Sietsma SJ, Koop Y, Konst RE, Mensink F, Aarts GW, Konijnenberg LS, Cortenbach K, Verhaert DV, Thannhauser J, Mol JQ, Rooijakkers MJ, et al, and Event committee, and Data Safety Monitoring Board, and Steering committee. Rationale and design of the PRAETORIAN-COVID trial: A double-blind, placebo-controlled randomized clinical trial with valsartan for PRevention of Acute rEspiraTORy dIstress syndrome in hospitAlized patieNts with SARS-COV-2 Infection Disease. Am Heart J. 2020; 226:60–68. https://doi.org/10.1016/j.ahj.2020.05.010 [PubMed]
-
42.
Cohen JB, Hanff TC, Corrales-Medina V, William P, Renna N, Rosado-Santander NR, Rodriguez-Mori JE, Spaak J, Andrade-Villanueva J, Chang TI, Barbagelata A, Alfonso CE, Bernales-Salas E, et al. Randomized elimination and prolongation of ACE inhibitors and ARBs in coronavirus 2019 (REPLACE COVID) Trial Protocol. J Clin Hypertens (Greenwich). 2020; 22:1780–88. https://doi.org/10.1111/jch.14011 [PubMed]
-
43.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009; 62:e1–34. https://doi.org/10.1016/j.jclinepi.2009.06.006 [PubMed]
-
44.
Wells G, Shea B, O’connell D. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa, ON: Ottawa Hospital Research Institute. 2009. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.