Introduction
Benign prostatic hyperplasia (BPH) is one of the most commonly diagnosed urological diseases in men over 50 years of age. BPH is characterized by prostatic stromal cell proliferation, leading to prostatic bladder obstruction (BOO) and lower urinary tract symptoms (LUTS), which together reduce quality of life (QoL). The development of BPH is very often associated with the existence of comorbidities, such as diabetes, cardiovascular diseases, and even neurological diseases [1]. Many studies also indicate a relationship between the metabolic syndrome (MetS) and the risk of LUTS and BPH [2–4]. MetS is defined as the combination of obesity, dyslipidemia, hyperglycemia, high blood pressure and insulin resistance. The factor that contributes to the initiation of pathological changes in the prostate, and consequently its benign hyperplasia, is chronic inflammation resulting, among others, from metabolic disorders [5]. Moreover, MetS is accompanied by sex hormone disorders, which is also one of the etiological factors of BPH [6].
The intestinal microflora is one of the elements of the bacterial ecosystem in mammals. The microorganisms that inhabit the gut are one of the key elements involved in modulating the immune response from the moment of birth. More and more research is currently being carried out on the effect of bacterial metabolites, including short-chain fatty acids (SCFA), on homeostasis, not only in the intestinal microenvironment, but also in cells and tissues of other organs. SCFAs are a group of compounds made up of six carbon atoms (C1 - C6), with the majority of acids being: butyric acid (C4), propionic acid (C3), and acetic acid (C2). These acids are present in humans in certain amounts, but their proportions may change depending on the diet, medications, age, and diseases. The concentrations of SCFAs depend on the ratio of bacteria inhabiting the intestines, and disorders of the intestinal microflora (dysbiosis), which affects the amount of SCFAs produced. These fatty acids play a significant role in maintaining homeostasis—they ensure the appropriate pH of the intestinal microenvironment [7], participate in the maintenance of the intestinal barrier, and are a source of energy for the intestinal epithelium and hepatocytes [8]. These acids are also inhibitors of histone deacetylases (HDACs), thanks to which they regulate epigenetic processes [9] and have an immunomodulating effect not only locally but also in distant tissues of the body [10, 11].
The method of assessing SCFAs in patient stool samples is a quick, cheap and common technique that allows the determination of intestinal microflora disorders [12–14]. Although this method does not assess the composition of the microbiota, it determines the content of its metabolite products, which also allows for an indirect assessment of the intestinal microbiota [15]. In addition, information is also obtained about the type of fermentation taking place in the gut that results in the production of SCFAs [16]. SCFA levels vary depending on the composition of the intestinal microflora and food intake [12]. Differences in the analytical methods and methodology of preparing material for research cause difficulties interpreting the results obtained.
So far, disturbances in the intestinal microflora and its impact on inflammation and prostate diseases have not yet been thoroughly analyzed. The influence of SCFAs on the development of BPH has also not been studied. Only a few publications on the impact of the intestinal microflora on the prostate can be found in the literature. They mainly concern the influence of intestinal bacteria on the synthesis of metabolites and androgens, which may be associated with the development of prostate cancer in humans [17, 18]. There are also reports of the impact of inflammatory bowel disease (IBD) on the risk of prostate cancer [19]. So far, differences in the composition of the intestinal microflora have only been confirmed in a pilot study, in which the composition of the intestinal microflora was analyzed in patients with prostate cancer, and with BPH [20]. The results of the research by Liss et al. [17] indicate that bacteria predominating in men diagnosed with prostate cancer (PCa) were Bacteroides and Streptococcus spp. As reviewed by Shah [21] BPH is not a precursor stage to prostate cancer. Moreover, these two diseases affect different areas of the gland. Nevertheless, it turns out that it is possible to identify risk factors that are common to the development of BPH and PCa [22]. They include genetic factors, androgen signalling, oxidative stress, [21] and inflammation [23, 24]. It is indicated that chronic prostate inflammation predisposes to BPH and PCa, and is not always caused by a bacterial infection, but can be associated with low-grade systemic inflammation [25]. The exact mechanism by which the intestinal microflora affects the prostate gland has not so far been fully elucidated. It seems very likely, however, that disturbed intestinal microflora does not directly affect the prostate gland, but contributes to the development of chronic systemic inflammation. Inflammatory cells and factors (including cytokines) from the intestinal environment, along with the circulation, can get into the gland and there cause ‘local’ inflammation and stimulate the growth factors of the prostate stroma, which in turn may lead to prostatic hyperplasia. The influence of the intestinal microflora and its metabolites entering the systemic circulation has been confirmed in studies on the urinary [26], nervous [27], and respiratory [28] systems, as well as autoimmune diseases [29].
The main aim of this study was to compare the profile of SCFAs between healthy individuals and patients diagnosed with BPH, with regard to MetS as a factor predisposing to the development of prostate hyperplasia. Our study is the first to show changes in the tested SCFAs levels between these two groups. Nevertheless, further research is needed (including testing in animal models) to determine whether there is a ‘microbiota-gut-prostate axis’ and whether the intestinal microflora and its metabolites contribute to the development of BPH.
Results
Factors proving the presence of BPH
The study involved 183 men. The control group included 80 healthy men without BPH (mean PV = 22 ml ± 6.9, mean Qmax = 20.08 ml/s ± 9). The study group consisted of 103 patients with a diagnosis of BPH, qualified for transurethral resection of the prostate (TURP) (mean PV = 61.95 ml ± 29, mean Qmax = 10.39 ml/s ± 6.66).
There were statistically significant differences between the groups (Table 1) regarding the prostate volume (p < 0.001), Qmax (p < 0.001), the results of the IPSS questionnaire (p < 0.001), QoL (quality of life score) (p < 0.001), the results of the ADAM (Androgen Deficiency in Aging Men) questionnaire (p < 0.001).
Table 1. Characteristics of the control group (healthy volunteers, without BPH) and the study group (patients diagnosed with BPH).
| Parametr | Healthy volunteers (without BPH) (n=80) | | Patients with BPH (n=103) | p-value |
| Mean | Median | Min | Max | SD | Mean | Median | Min | Max | SD |
| Age [years] | 54.66 | 54.00 | 45.00 | 72.00 | 6.52 | | 66.46 | 67.00 | 49.00 | 79.00 | 6.50 | < 0.001* |
| PV [ml] | 22.15 | 21.90 | 11.60 | 33.00 | 5.34 | | 65.20 | 60.00 | 35.00 | 120.00 | 20.50 | < 0.001* |
| Qmax [ml/s] | 20.08 | 19.20 | 6.30 | 42.10 | 9.01 | | 10.39 | 9.10 | 2.00 | 40.00 | 6.66 | < 0.001* |
| IPSS | 3.31 | 3.00 | 0.00 | 7.00 | 1.84 | | 19.76 | 21.00 | 3.00 | 35.00 | 7.79 | < 0.001* |
| QoL | 1.43 | 1.00 | 0.00 | 4.00 | 1.07 | | 3.30 | 3.50 | 0.00 | 5.00 | 1.25 | < 0.001* |
| ADAM | 3.61 | 3.00 | 0.00 | 8.00 | 2.24 | | 0.84 | 1.00 | 0.00 | 1.00 | 0.37 | < 0.001* |
| TG [mmol/l] | 163.07 | 156.80 | 110.49 | 306.67 | 40.37 | | 176.32 | 157.58 | 114.05 | 815.43 | 84.51 | 0.250 |
| Cholesterol [mg/dl] | 215.13 | 208.39 | 136.29 | 320.28 | 39.31 | | 204.53 | 200.75 | 143.49 | 404.10 | 38.30 | 0.035* |
| HDL [mg/dl] | 44.76 | 43.98 | 30.32 | 76.62 | 10.64 | | 55.76 | 51.35 | 41.70 | 383.49 | 34.00 | < 0.001* |
| LDL [mg/dl] | 137.76 | 133.17 | 56.32 | 232.63 | 40.48 | | 117.24 | 110.22 | 1.79 | 321.07 | 39.69 | 0.001* |
| FPG [mg/dl] | 100.65 | 99.42 | 85.43 | 161.13 | 10.51 | | 79.14 | 80.19 | 49.43 | 109.17 | 13.67 | < 0.001* |
| BPH – benign prostatic hyperplasia; n – number; Min – minimum; Max – maximum; SD – standard deviation; PV – prostate volume, Qmax - maximum flow rate in uroflowmetry; IPSS - International Prostate Symptom Score; QoL - Quality of Life score; ADAM -Androgen Deficiency in Aging Men questionnaire; TG – triglycerides; HDL – high density lipoprotein; LDL – low density lipoprotein; FPG – fasting plasma glucose; p – statistical significance; * - statistical significant parameter. |
Anthropometric measurements were also performed in the patients. Healthy patients had higher body weight and height than patients with BPH (88.854 vs. 84.825, p = 0.038; 1.767 vs. 1.742, p = 0.005). There were no statistically significant differences in patients' BMI (28.480 vs. 28.051, p = 0.498).
Biochemical parameters
Serum biochemical parameters in the control and the study groups were also measured (Table 1). The levels of total cholesterol (p = 0.035), LDL cholesterol (p = 0.001) and glucose (p < 0.001) were statistically significantly higher in healthy patients. At the same time, statistically significantly lower HDL cholesterol levels (p < 0.001) were found in patients without BPH. There was no relationship in TG levels between the groups, although their mean values were higher in patients with BPH.
SCFA levels in the study and the control groups with regard to MetS
The study shows that the control group had higher levels of acetic and propionic acids, however these results were not statistically significant. Whereas, the levels of isocaproic acid were statistically significantly higher (p = 0.038). Patients with BPH, on the other hand, had statistically significant higher levels of branched-chain SCFAs (BCFAs): isobutyric acid (p = 0.001) and isovaleric acid (p < 0.001) (Table 2).
Table 2. Short-chain fatty acids in patients in the control group (healthy volunteers, without BPH) and the study group (patients diagnosed with BPH), and depending on the presence of MetS.
| SCFAs (%) | Healthy volunteers (without BPH) (n=80) | | Patients with BPH (n=103) | p-value |
| Mean | Median | Min | Max | SD | Mean | Median | Min | Max | SD |
| C 2:0 | 35.148 | 35.210 | 17.408 | 57.556 | 6.792 | | 33.317 | 32.898 | 15.205 | 61.909 | 7.680 | 0.059 |
| C 3:0 | 21.567 | 20.809 | 8.710 | 47.941 | 6.805 | | 20.531 | 19.662 | 0.903 | 36.581 | 6.298 | 0.330 |
| C 4:0 i | 3.814 | 3.726 | 0.266 | 8.293 | 1.947 | | 4.695 | 4.702 | 0.370 | 16.163 | 2.226 | 0.008* |
| C 4:0 n | 24.006 | 22.394 | 3.804 | 47.489 | 8.125 | | 23.216 | 23.734 | 6.094 | 47.467 | 8.395 | 0.631 |
| C 5:0 i | 6.911 | 6.730 | 0.216 | 19.616 | 4.201 | | 9.499 | 9.221 | 0.450 | 33.867 | 4.990 | < 0.001* |
| C 5:0 n | 5.729 | 5.637 | 0.194 | 11.426 | 2.189 | | 6.119 | 6.372 | 0.655 | 16.167 | 2.645 | 0.166 |
| C 6:0 i | 0.359 | 0.174 | 0.015 | 10.799 | 1.198 | | 0.186 | 0.142 | 0.023 | 0.741 | 0.149 | 0.038* |
| C 6:0 n | 2.057 | 1.618 | 0.047 | 7.690 | 1.986 | | 2.120 | 1.396 | 0.088 | 10.709 | 2.132 | 0.732 |
| SCFAs (%) | Healthy volunteers (without BPH) with MetS (n=36) | | Patients with BPH with Mets (n=42) | p-value |
| Mean | Median | Min | Max | SD | Mean | Median | Min | Max | SD |
| C 2:0 | 33.535 | 34.113 | 17.408 | 43.210 | 5.905 | | 33.373 | 32.372 | 19.923 | 61.909 | 8.556 | 0.524 |
| C 3:0 | 24.385 | 23.201 | 10.807 | 47.941 | 7.482 | | 21.175 | 20.295 | 4.921 | 36.581 | 7.000 | 0.059 |
| C 4:0 i | 3.573 | 3.199 | 0.266 | 8.293 | 1.767 | | 4.489 | 4.409 | 0.438 | 9.394 | 2.154 | 0.044* |
| C 4:0 n | 22.897 | 21.641 | 9.093 | 37.315 | 6.253 | | 23.880 | 24.247 | 6.596 | 47.467 | 8.945 | 0.426 |
| C 5:0 i | 6.430 | 5.698 | 0.216 | 19.616 | 3.871 | | 8.902 | 8.637 | 0.450 | 19.674 | 5.050 | 0.029* |
| C 5:0 n | 6.333 | 6.264 | 0.685 | 11.426 | 2.392 | | 5.826 | 6.287 | 0.795 | 12.820 | 2.611 | 0.480 |
| C 6:0 i | 0.492 | 0.154 | 0.018 | 10.799 | 1.773 | | 0.141 | 0.104 | 0.028 | 0.404 | 0.094 | 0.019* |
| C 6:0 n | 1.955 | 1.075 | 0.047 | 7.690 | 2.146 | | 1.943 | 0.881 | 0.123 | 5.911 | 1.955 | 0.869 |
| BPH – benign prostatic hyperplasia; MetS – metabolic syndrome; n – number; Min – minimum; Max – maximum; SD – standard deviation; SCFAs – short-chain fatty acids; C2:0 - acetic acid; C3:0 - propionic acid; C4:0i - isobutyric acid; C4:0n - butyric acid; C5:0i - isovaleric acid; C5:0n - valeric acid; C6:0i - isocaproic acid; C 6:0n - caproic acid; p – statistical significance; * - statistical significant parameter. |
Additionally, patients with BPH and MetS had significantly higher stool BCFAs levels—isobutyric acid (p = 0.044) and isovaleric acid (p = 0.029)—compared to healthy controls. Healthy patients with MetS had significantly higher levels of isocapronic acid (p = 0.019) in comparison to patients with BPH and MetS (Table 2).
Healthy controls without MetS had significantly lower stool levels of propionic acid (C3:0) (p = 0.002) and valeric acid (C5:0n) (p = 0.025) in comparison to healthy controls with MetS (Table 3). Patients diagnosed with BPH without MetS had significantly higher stool levels of isocapronic acid (p = 0.034) (Table 3).
Table 3. Short-chain fatty acids in control patients and in patients diagnosed with BPH, depending on the presence of MetS.
| SCFAs (%) | Healthy volunteers (without BPH) (n=80) | p-value |
| Without MetS (n=44) | | With Mets (n=36) |
| Mean | Median | Min | Max | SD | Mean | Median | Min | Max | SD |
| C 2:0 | 36.468 | 35.730 | 22.598 | 57.556 | 7.240 | | 33.535 | 34.113 | 17.408 | 43.210 | 5.905 | 0.116 |
| C 3:0 | 19.262 | 19.892 | 8.710 | 32.297 | 5.238 | | 24.385 | 23.201 | 10.807 | 47.941 | 7.482 | 0.002* |
| C 4:0 i | 4.010 | 4.085 | 0.438 | 7.801 | 2.082 | | 3.573 | 3.199 | 0.266 | 8.293 | 1.767 | 0.294 |
| C 4:0 n | 24.914 | 24.461 | 3.804 | 47.489 | 9.359 | | 22.897 | 21.641 | 9.093 | 37.315 | 6.253 | 0.431 |
| C 5:0 i | 7.304 | 7.290 | 0.451 | 17.440 | 4.458 | | 6.430 | 5.698 | 0.216 | 19.616 | 3.871 | 0.331 |
| C 5:0 n | 5.235 | 5.482 | 0.193 | 9.567 | 1.895 | | 6.333 | 6.264 | 0.685 | 11.426 | 2.392 | 0.025* |
| C 6:0 i | 0.251 | 0.174 | 0.015 | 1.025 | 0.234 | | 0.492 | 0.154 | 0.018 | 10.799 | 1.773 | 0.525 |
| C 6:0 n | 2.141 | 2.274 | 0.112 | 7.475 | 1.865 | | 1.955 | 1.075 | 0.047 | 7.690 | 2.146 | 0.557 |
| SCFAs (%) | Patients with BPH (n=103) | p-value |
| Without MetS (n=61) | | With MetS (n=42) |
| Mean | Median | Min | Max | SD | Mean | Median | Min | Max | SD |
| C 2:0 | 33.279 | 33.227 | 15.205 | 49.396 | 7.088 | | 33.373 | 32.402 | 19.923 | 61.91 | 8.556 | 0.660 |
| C 3:0 | 20.088 | 19.412 | 0.903 | 35.717 | 5.784 | | 21.175 | 20.031 | 4.921 | 36.58 | 7.000 | 0.562 |
| C 4:0 i | 4.838 | 4.723 | 0.370 | 16.163 | 2.281 | | 4.489 | 4.413 | 0.438 | 9.39 | 2.154 | 0.492 |
| C 4:0 n | 22.759 | 23.054 | 6.094 | 39.532 | 8.039 | | 23.880 | 24.232 | 6.596 | 47.47 | 8.945 | 0.457 |
| C 5:0 i | 9.911 | 9.554 | 1.944 | 33.867 | 4.948 | | 8.902 | 8.842 | 0.450 | 19.67 | 5.050 | 0.336 |
| C 5:0 n | 6.321 | 6.443 | 0.655 | 16.167 | 2.670 | | 5.826 | 6.312 | 0.795 | 12.82 | 2.611 | 0.403 |
| C 6:0 i | 0.217 | 0.167 | 0.023 | 0.7441 | 0.172 | | 0.141 | 0.103 | 0.028 | 0.40 | 0.094 | 0.034* |
| C 6:0 n | 2.242 | 1.578 | 0.088 | 10.709 | 7.253 | | 1.943 | 0.822 | 0.123 | 5.911 | 1.955 | 0.388 |
| BPH – benign prostatic hyperplasia; MetS – metabolic syndrome; n – number; Min – minimum; Max – maximum; SD – standard deviation; SCFAs – short-chain fatty acids; C2:0 - acetic acid; C3:0 - propionic acid; C4:0i - isobutyric acid; C4:0n - butyric acid; C5:0i - isovaleric acid; C5:0n - valeric acid; C6:0i - isocaproic acid; C 6:0n - caproic acid; p – statistical significance; * - statistical significant parameter. |
SCFA levels and biochemical parameters
In all patients from the control group a positive correlation was found only between propionic acid and the levels of triglycerides (R = 0.385, p < 0.05), total cholesterol (R = 0.290, p = 0.010), and LDL cholesterol (R = 0.244, p = 0.030) (Table 4). In the group of patients with BPH, a positive correlation was also demonstrated between the levels of propionic acid and triglycerides (R = 0.232, p = 0.021), and a negative correlation was observed between the levels of isocaproic acid and glucose (R = -0.272, p = 0.007) (Table 4).
Table 4. Correlations between the analyzed SCFAs and biochemical parameters in healthy patients from the control and study group.
| Biochemical parameters | Healthy volunteers (without BPH) (n= 80)
%SCFAs |
| C2:0 | C 3:0 | C 4:0 i | C 4:0 n | C 5:0 i | C 5:0 n | C 6:0 i | C 6:0 n |
| R | p | R | p | R | p | R | p | R | p | R | p | R | p | R | p |
TG
[mmol/l] | -0.068 | 0.551 | 0.385 | < 0.05* | -0.132 | 0.245 | -0.125 | 0.272 | -0.115 | 0.312 | 0.152 | 0.181 | -0.009 | 0.941 | -0.080 | 0.482 |
| Cholesterol [mg/dl] | -0.146 | 0.201 | 0.290 | 0.010* | -0.129 | 0.258 | -0.037 | 0.746 | -0.151 | 0.185 | 0.091 | 0.428 | -0.166 | 0.144 | -0.069 | 0.548 |
HDL
[mg/dl] | 0.200 | 0.077 | -0.128 | 0.260 | -0.056 | 0.624 | -0.076 | 0.507 | -0.072 | 0.527 | -0.040 | 0.724 | -0.008 | 0.946 | 0.021 | 0.857 |
LDL
[mg/dl] | -0.198 | 0.080 | 0.244 | 0.030* | -0.073 | 0.520 | 0.003 | 0.980 | -0.093 | 0.416 | 0.103 | 0.365 | -0.129 | 0.259 | -0.047 | 0.680 |
FPG
[mg/dl] | -0.149 | 0.187 | 0.212 | 0.059 | -0.160 | 0.156 | 0.002 | 0.983 | -0.161 | 0.153 | 0.161 | 0.154 | -0.037 | 0.745 | 0.103 | 0.361 |
| Biochemical parameters | Patients with BPH (n= 103)
%SCFAs |
| C2:0 | C2:0 | C2:0 | C2:0 | C2:0 | C2:0 | C2:0 | C2:0 |
| R | p | R | p | R | p | R | p | R | p | R | p | R | p | R | p |
TG
[mmol/l] | 0.021 | 0.834 | 0.232 | 0.021* | -0.078 | 0.441 | -0.028 | 0.782 | -0.122 | 0.230 | -0.117 | 0.248 | 0.094 | 0.353 | -0.121 | 0.231 |
| Cholesterol [mg/dl] | -0.035 | 0.729 | 0.040 | 0.692 | -0.109 | 0.283 | 0.109 | 0.281 | -0.125 | 0.216 | -0.138 | 0.173 | -0.106 | 0.296 | -0.095 | 0.348 |
HDL
[mg/dl] | -0.020 | 0.843 | -0.058 | 0.571 | -0.032 | 0.757 | 0.073 | 0.470 | -0.013 | 0.900 | 0.023 | 0.819 | -0.107 | 0.291 | -0.019 | 0.855 |
LDL
[mg/dl] | -0.034 | 0.742 | -0.043 | 0.675 | -0.095 | 0.351 | 0.107 | 0.294 | -0.104 | 0.308 | -0.092 | 0.368 | -0.170 | 0.094 | -0.049 | 0.635 |
FPG
[mg/dl] | -0.053 | 0.602 | 0.062 | 0.543 | -0.067 | 0.509 | 0.043 | 0.674 | -0.079 | 0.436 | 0.021 | 0.836 | -0.272 | 0.007* | 0.026 | 0.802 |
| BPH – benign prostatic hyperplasia; n – number; SCFAs – short-chain fatty acids; C2:0 - acetic acid; C3:0 - propionic acid; C4:0i - isobutyric acid; C4:0n - butyric acid; C5:0i - isovaleric acid; C5:0n - valeric acid; C6:0i - isocaproic acid; C 6:0n - caproic acid; TG – triglycerides; HDL – high density lipoprotein; LDL – low density lipoprotein; FPG – fasting plasma glucose; R - correlation coefficient; p – statistical significance; * - statistical significant parameter. |
In the group of healthy controls analyzed with regard to MetS, a positive correlation between the levels of propionic acid, triglycerides (R = 0.331, p = 0.049) and total cholesterol (R = 0.399, p = 0.016) was only observed in patients diagnosed with MetS (Table 5).
Table 5. Correlations between the analyzed SCFAs and the biochemical parameters in healthy control patients without and with metabolic syndrome.
| Biochemical parameters | Healthy volunteers (without BPH) without MetS (n=44)
%SCFAs |
| C2:0 | C 3:0 | C 4:0 i | C 4:0 n | C 5:0 i | C 5:0 n | C 6:0 i | C 6:0 n |
| R | p | R | p | R | p | R | p | R | p | R | p | R | p | R | p |
TG
[mmol/l] | 0.041 | 0.794 | 0.224 | 0.149 | -0.010 | 0.949 | -0.071 | 0.652 | 0.028 | 0.860 | -0.063 | 0.686 | 0.137 | 0.383 | -0.067 | 0.671 |
| Cholesterol [mg/dl] | 0.067 | 0.669 | -0.078 | 0.620 | 0.018 | 0.907 | -0.008 | 0.957 | -0.023 | 0.883 | 0.053 | 0.735 | -0.033 | 0.831 | 0.088 | 0.573 |
HDL
[mg/dl] | 0.296 | 0.054 | -0.078 | 0.617 | -0.151 | 0.333 | -0.145 | 0.353 | -0.154 | 0.323 | -0.026 | 0.870 | -0.183 | 0.239 | 0.197 | 0.205 |
LDL
[mg/dl] | -0.090 | 0.564 | -0.039 | 0.806 | 0.113 | 0.469 | 0.031 | 0.845 | 0.076 | 0.627 | 0.090 | 0.566 | 0.080 | 0.609 | 0.005 | 0.974 |
FPG
[mg/dl] | -0.204 | 0.185 | 0.151 | 0.329 | -0.150 | 0.332 | 0.123 | 0.425 | -0.175 | 0.256 | 0.013 | 0.931 | 0.004 | 0.979 | 0.115 | 0.458 |
| Biochemical parameters | Healthy volunteers (without BPH) with MetS (n=36)
%SCFA |
| C2:0 | C 3:0 | C 4:0 i | C 4:0 n | C 5:0 i | C 5:0 n | C 6:0 i | C 6:0 n |
| R | p | R | p | R | p | R | p | R | p | R | p | R | p | R | p |
TG
[mmol/l] | -0.013 | 0.939 | 0.331 | 0.049* | -0.171 | 0.318 | -0.128 | 0.455 | -0.172 | 0.316 | 0.104 | 0.546 | 0.069 | 0.688 | -0.134 | 0.437 |
| Cholesterol [mg/dl] | -0.240 | 0.158 | 0.399 | 0.016* | -0.233 | 0.171 | 0.033 | 0.847 | -0.245 | 0.150 | 0.009 | 0.957 | -0.187 | 0.274 | -0.076 | 0.659 |
HDL
[mg/dl] | 0.011 | 0.947 | 0.019 | 0.912 | -0.033 | 0.846 | 0.021 | 0.904 | -0.077 | 0.654 | 0.053 | 0.758 | 0.132 | 0.444 | -0.183 | 0.284 |
LDL
[mg/dl] | -0.243 | 0.154 | 0.323 | 0.055 | -0.178 | 0.299 | 0.046 | 0.791 | -0.188 | 0.271 | 0.044 | 0.801 | -0.220 | 0.197 | 0.020 | 0.909 |
FPG
[mg/dl] | 0.118 | 0.492 | 0.023 | 0.896 | -0.107 | 0.535 | -0.107 | 0.533 | -0.081 | 0.637 | 0.146 | 0.397 | -0.009 | 0.960 | 0.212 | 0.214 |
| BPH – benign prostatic hyperplasia; MetS – metabolic syndrome; n – number; SCFAs – short-chain fatty acids; C2:0 - acetic acid; C3:0 - propionic acid; C4:0i - isobutyric acid; C4:0n - butyric acid; C5:0i - isovaleric acid; C5:0n - valeric acid; C6:0i - isocaproic acid; C 6:0n - caproic acid; TG – triglycerides; HDL – high density lipoprotein; LDL – low density lipoprotein; FPG – fasting plasma glucose; R - correlation coefficient; p – statistical significance; * - statistical significant parameter. |
In the group diagnosed with and treated for BPH, on the other hand, a positive correlation was only found in the group of patients without MetS, and it concerned the levels of propionic acid and triglycerides (R = 0.302, p = 0.024) (Table 6).
Table 6. Correlations between the analyzed SCFAs and biochemical parameters in patients with BPH without MetS and with MetS.
| Biochemical parameters | Patients with BPH without MetS (n=61)
%SCFA |
| C2:0 | C 3:0 | C 4:0 i | C 4:0 n | C 5:0 i | C 5:0 n | C 6:0 i | C 6:0 n |
| R | p | R | p | R | p | R | p | R | p | R | p | R | p | R | p |
TG
[mmol/l] | 0.166 | 0.222 | 0.302 | 0.024* | -0.084 | 0.539 | -0.138 | 0.312 | -0.128 | 0.348 | -0.143 | 0.294 | 0.139 | 0.306 | -0.195 | 0.150 |
| Cholesterol [mg/dl] | -0.084 | 0.534 | 0.225 | 0.093 | -0.202 | 0.131 | 0.155 | 0.251 | -0.201 | 0.134 | -0.094 | 0.486 | -0.215 | 0.108 | -0.181 | 0.178 |
HDL
[mg/dl] | -0.046 | 0.731 | -0.180 | 0.180 | 0.111 | 0.409 | 0.064 | 0.637 | 0.120 | 0.372 | 0.130 | 0.335 | -0.167 | 0.214 | -0.033 | 0.810 |
LDL
[mg/dl] | -0.113 | 0.410 | 0.163 | 0.234 | -0.247 | 0.069 | 0.229 | 0.093 | -0.241 | 0.076 | -0.084 | 0.542 | -0.245 | 0.072 | -0.157 | 0.251 |
FPG
[mg/dl] | -0.141 | 0.295 | 0.047 | 0.731 | -0.146 | 0.279 | 0.111 | 0.411 | -0.137 | 0.309 | 0.146 | 0.278 | -0.205 | 0.126 | 0.154 | 0.253 |
| Biochemical parameters | Patients with BPH with MetS (n=42)
%SCFA |
| C2:0 | C 3:0 | C 4:0 i | C 4:0 n | C 5:0 i | C 5:0 n | C 6:0 i | C 6:0 n |
| R | p | R | p | R | p | R | p | R | p | R | p | R | p | R | p |
TG
[mmol/l] | -0.180 | 0.254 | 0.141 | 0.374 | -0.009 | 0.955 | 0.055 | 0.728 | -0.027 | 0.867 | 0.096 | 0.545 | 0.132 | 0.405 | 0.075 | 0.637 |
| Cholesterol [mg/dl] | 0.001 | 0.996 | -0.098 | 0.538 | -0.070 | 0.661 | 0.150 | 0.342 | -0.129 | 0.417 | -0.217 | 0.167 | -0.030 | 0.852 | -0.020 | 0.899 |
HDL
[mg/dl] | 0.034 | 0.833 | 0.130 | 0.412 | -0.239 | 0.128 | 0.087 | 0.582 | -0.185 | 0.241 | -0.213 | 0.175 | -0.166 | 0.294 | -0.083 | 0.600 |
LDL
[mg/dl] | 0.028 | 0.860 | -0.212 | 0.178 | 0.032 | 0.842 | 0.088 | 0.578 | -0.039 | 0.808 | -0.137 | 0.389 | -0.143 | 0.367 | 0.009 | 0.957 |
FPG
[mg/dl] | 0.055 | 0.731 | 0.050 | 0.755 | 0.100 | 0.528 | -0.079 | 0.618 | 0.084 | 0.596 | -0.056 | 0.725 | -0.293 | 0.060 | -0.061 | 0.700 |
| BPH – benign prostatic hyperplasia; MetS – metabolic syndrome; n – number; SCFAs – short-chain fatty acids; C2:0 - acetic acid; C3:0 - propionic acid; C4:0i - isobutyric acid; C4:0n - butyric acid; C5:0i - isovaleric acid; C5:0n - valeric acid; C6:0i - isocaproic acid; C 6:0n - caproic acid; TG – triglycerides; HDL – high density lipoprotein; LDL – low density lipoprotein; FPG – fasting plasma glucose; R - correlation coefficient; p – statistical significance, * - statistical significant parameter. |
In healthy volunteers, depending on BMI, the percentage value of SCFAs negatively correlated with acetic acid (R = -0.221, p = 0.048) and positively with valeric acid (R = -0.287, p = 0.010), and in patients with BPH, it negatively correlated with isocaproic acid (R = -0.246, p = 0.014) (Supplementary Table 1). There were no statistically significant differences in BMI values between the patients. However, in the analysis of the correlation between SCFAs and MetS, this parameter was taken into account. In the entire study sample divided according to MetS, the presence of MetS negatively correlated with the level of propionic acid and BMI (R = -0.265, p = 0.020) (Supplementary Table 2).
In healthy patients without MetS, BMI did not correlate with SCFA levels, while in patients with MetS, it negatively correlated with propionic acid (C3: 0) (R = -0.517, p = 0.001) and positively with caproic acid (C6: 0n) (R = 0.329, p = 0.05) (Supplementary Table 3). In patients with BPH, no correlation between the levels of SCFAs and the presence of MetS was found (Supplementary Table 3).
Discussion
In our study, we indirectly examined the relationship between BPH, MetS and the intestinal microbiota, or more precisely its products - SCFAs. Analyzing the intestinal microbiota through testing its products is a relatively new method. The identification of SCFAs in the stool provides information not only about the composition of the intestinal microbiota, but also improves the understanding of how they interact not only in the gut but also in distant tissues and organs.
To the best of our knowledge, this is the first study investigating the association between the synthesis of SCFAs by the gut microbiota and BPH in aging men. The relationship between SCFAs and serum biochemical parameters in men with MetS have also been demonstrated. Our study showed that among the analyzed SCFAs, mainly isobutyric acid (C4:0i), isovaleric acid (C5:0i) and isocaproic acid (C6:0i) are those that very likely influence factors predisposing men to BPH.
We found that isobutyric acid was significantly elevated in men with BPH compared to healthy controls (% SCFAs - mean: 4.695, median: 4.702 vs. 3.814, 3.726; p = 0.008). The intestinal microbiota of BPH patients produced more isovaleric acid (% SCFAs - mean: 9.499, median: 9.221 vs. 6.911, 6.730; p < 0.001). The acid that predominated among the acids isolated from the feces of healthy controls was isocaproic acid (% SCFAs - mean: 0.359, median: 0.174 vs. 0.186, 0.142; p = 0.038).
SCFAs produced by gut microbiota - primarily acetate, propionate, butyrate play a key role in maintaining homeostasis in humans [7]. These three most common acids account for 95% in total SCFAs. In large intestine and stool samples SCFAs are present in an approximate molar ratio of acetic: propionic: butyric acid amounting to 60:20:20 [30]. The levels of SCFAs in the intestines range from 20 to 140 mM, and depend on the intestinal microflora composition, absorption of SCFAs from the intestines, and the fiber content in the diet [31]. Acetic, propionic and butyric acids are produced as a result of saccharolytic fermentation and has health promoting benefits. SCFAs are regarded as mediators in the communication between the intestinal microbiome and the immune system. The signal they produce is transferred, among others, in immune cells via free fatty acid receptors (FFARs), which belong to the family of G protein-coupled receptors (GPCRs) [32, 33]. It has been also confirmed that SCFAs inhibit the activity of histone deacetylase (HDAC) – an enzyme involved in post-translational modifications [9], namely the process of deacetylation and, what is new, the process of histone crotonylation [34]. These properties of SCFAs have an effect on their immunomodulatory potential i.e. maintaining the anti-proinflammatory balance. SCFAs act not only locally in the intestines colonized by commensal bacteria, but also influence the intestinal immune cells, and modulate immune response by multi-protein inflammasome complexes [35, 36]. Moreover, main SCFAs may affect fatty acids, glucose, and cholesterol metabolism.
The disturbances or changes in SCFAs levels may contribute to the development of many diseases. Currently conducted studies concern not only intestinal diseases, such as irritable bowel syndrome (IBS) [37], inflammatory bowel disease (IBD) [38], and diarrhea [39], but also mental health problems like depression [40], autism [41], neurodegenerative diseases (Parkinson’s disease) [14], multiple sclerosis [42], and even autoimmune diseases [43, 44]. The main SCFAs (acetate, propionate, butyrate) have immunomodulatory potential, and therefore may be helpful in the prevention of chronic but persistent low-grade inflammation. It is also worth noting that the concentrations of SCFAs and BCFAs fluctuate in a healthy population throughout life - from newborns to aging people [45]. It is influenced by: the composition of the intestinal microflora, age and health of patients. Many studies have confirmed that the intestinal microflora changes with the aging of the body [46, 47]. It turns out that the positive impact of microflora (characterized by a decrease in the taxonomic diversity of the intestinal microbiota) on the human body, decreases with age [48]. These changes are also reflected in the physiology of the host organism, and manifested as, among others, an increase in inflammation [49]. These differences are noted between adult men (mean age of 42 years) and elderly men (mean age of 77 years) [50]. In young people, bacteria that predominate in the composition of the intestinal microflora are those with immunomodulatory potential, such as Clostridiales and Bifidobacterium. In aging people, on the other hand, bacterial communities are enriched with pathobionts, e.g. Enterobacteriaceae [51].
Propionic acid (C3:0) is one of the main SCFAs. Its natural production takes place in the large intestine with the participation of Bacteroidetes spp, Roseburia spp., Firmicutes, Roseburia inulinivorans, Ruminococus spp., Cllostridium spp., Clostridiales bactrium, Eubacterium spp, Coprococcus spp., Dialister succinatiphilus, Phascolarctobaterium succinatutens, Akkermansia muciniphila (succinate pathway), Clostridium sp., Clostridiales bacterium, Coproccus catus, Clostridium sp., (acrylate pathway), Roseburia insulinivorans, Ruminococus spp., Eubacterium halli, and Clostridium sp. (propanediol pathway) [52]. Propionic acid plays a role in the metabolism of lipids in the liver and affects the biosynthesis of cholesterol in this organ [53]. This acid is also used as a natural mold growth inhibitor and is therefore used as a preservative in food (cheese and bread) and animal feed. Moreover, it has been proven that propionic acid is a precursor in gluconeogenesis, while acetate and butyrate are involved in the regulation of cholesterol synthesis [54]. In vivo studies [55] have shown that propionic acid can inhibit de novo fatty acids and cholesterol. In addition, propionic acid has been shown to significantly reduce the levels of pro-inflammatory cytokines (TNF-α and CCL5), and increase the expression of lipoprotein lipase and GLUT4, thus influencing the lipogenesis process and glucose uptake [56]. It has been also confirmed that propionate significantly stimulates the production of peptides in the large intestine: peptide YY (PYY) and glucagon like peptide-1 (GLP-1), which are responsible for the regulation of appetite in adults, thus reducing the amount of food intake and weight gain in obese people [57].
Butyric acid (C4:0) is an important factor influencing the metabolic processes, which has been confirmed both in animal models and in humans, yet the exact mechanism of its action still requires a more detailed explanation. It has been proven that butyric acid supplementation prevents obesity, hyperinsulinemia and hypertriglyceridemia, and may also reduce appetite and activate brown adipose tissue (BAT) through vagal nerve signaling [58]. Butyric and valeric acids have also been shown to be class I histone deacetylase inhibitors [59].
There is also little information about the effects of caproic acid (C6:0) or isocaproic acid (C6:0i) on BPH. In the research conducted so far, the impact of caproic acid on prostate cancer cells has been confirmed in the studies conducted so far on cell lines. The C6 acid has been shown to have cytotoxic properties against neoplastic cells [60] Studies on a rat model, on the other hand, showed that in the feces of animals with nonbacterial chronic prostate inflammation (CPI), the levels of butyric, valeric, and caproic SCFAs were decreased. This study also indicates that prostate inflammation is associated with specific changes in the gut microflora, and hence with changes in SCFA levels. [61]. Additionally, it can be mentioned that caproic acid is one of the compounds found in saw palmetto, which is used in natural BPH therapy [62].
Branched SCFAs — branched short-chain fatty acids (BCFAs), such as, isobutyric acid (C4:0i) and isovaleric acid (C5:0i), which are the end-products of aliphatic amino acid catabolism, valine and leucine respectively [63]. Isobutyric acid (C4:0i) is a geometrical isomer of butyric acid, which results in its different physical but not chemical properties. In the human intestine, the fermentation of branched chain amino acids is carried out mainly by genera Bacteroides and Clostridium [64]. It has also been found that gut-derived BCFAs - mainly isovaleric acid, may be one of the contributors to depressive disorders [65], or even be associated with the occurrence of acute ischemic stroke [66]. Among the bacterial populations that participate in the fermentation of peptides and amino acids, there are also those bacteria that are responsible for the production of BCFAs—0.6% of the population is involved in the synthesis of isovaleric acid, and up to 40% of bacteria in the synthesis of isobutyric acid [67]. The highest levels of BCFAs are found in the distal parts of the large intestine (colon). Among short-chain fatty acids, branched forms account for 5-10%. The concentrations of BCFAs in the feces, as well as the concentrations of SCFAs, may be modified depending on the food consumed [68]. Little is known so far about the effects of BCFAs on the host organism. However, there is evidence that these acids can oxidize if the amount of butyric acid is insufficient and then they can be a source of energy for colonocytes [69].
An upsurge in the amount of BCFAs may indicate an increased proteolytic fermentation, which suggests that there is a higher amount of proteins in the large intestine. This in turn may be caused by a high supply of protein in the diet or its disturbed absorption. Moreover, during proteolytic fermentation, along with the increase in BCFAs, harmful metabolites are produced such as: ammonia, phenols and hydrogen sulphides [67]. The resulting components may contribute to the initiation of inflammation and the excessive proliferation of colonocytes, and thus influence the local disease states. Apart from inducing epithelial cell proliferation, inflammation may also affect tight junctions (TJ). This happens in the case of infections (including Helicobakter pylorii) and gastric epithelial disruption [70]. Damage to tight junctions results in the leakage and dysfunction of cellular barriers, and is the cause of ‘leaky states’ and chronic inflammation, which are observed in cancers of the digestive system [71, 72]. Impairment of the function of tight junction in the digestive tract (mainly in the intestines) may also be caused by the state of intestinal dysbiosis and reduced amounts of SCFAs, resulting, among others, from using antibiotics [73]. SCFAs, mainly butyric acid, strengthen the intestinal barrier by regulating the transcription of claudin -1 that is a protein building tight junctions [74]. The leakage of the intestinal barrier results in the penetration of bacterial particles and factors, pro-inflammatory cytokines, immune cells, toxins and antigens into the bloodstream and their movement from the digestive tract to distant places, including the prostate [75] (Figure 1).
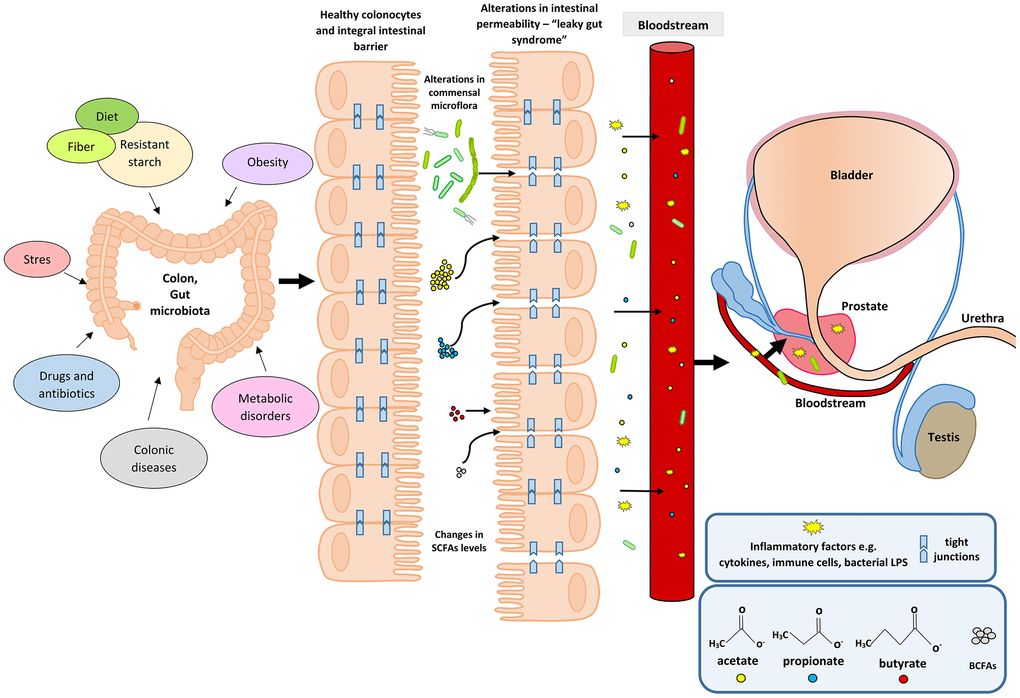
Figure 1. Participation of the intestinal microflora in inflammation and the development of BPH. Disturbances in the intestinal microflora (dysbiosis) can be caused by many factors, among them bad diet that is poor in plant fiber and starch sources. Additionally, the state of intestinal eubiosis may be disturbed by taking antibiotics and other medications, e.g. in the treatment of diabetes or depressive disorders. The specific intestinal microflora—‘obese microbiota’—is found in overweight people and it additionally affects metabolic processes in these people. A different gut microbiome is also observed in people with inflammatory bowel diseases (IBS, IBD). Proper intestinal microflora and its metabolites produced in the fermentation process, including short-chain fatty acids (SCFAs) contribute to the maintenance of intestinal epithelial cell homeostasis, and are a source of energy for colonocytes (mainly butyric acid). Intestinal dysbiosis and changes in SCFAs levels are factors that reduce the protective mucus layer, weaken tight junctions between intestinal epithelial cells, and cause leakage of the intestinal barrier. When the intestinal barrier is disturbed, pathogenic factors, inflammatory factors (immune cells and cytokines) and bacterial metabolites produced in varying amounts, e.g. SCFAs and toxic metabolites enter the bloodstream and migrate to distant tissues and organs. Inflammatory and microbiological factors, along with the peripheral circulation, may also reach the prostate gland, where they cause local inflammation. The inflammatory process in the prostate can activate signaling pathways involving growth factors, thus resulting in the prostate proliferation.
Many studies have confirmed that SCFAs are involved in the pathophysiology of IBD and may be a prognostic marker of the disease state [76]. Jaworska et al. [76] demonstrated that compared with healthy individuals, the ratio of acids in serum to acids in feces (acetate, valerate, isocaproic, caproic and propionic acids) is statistically significantly higher in patients with IBD. These data indicate that SCFAs may be involved in the disturbance of the intestinal barrier function [76]. The research by Huda-Jaujan et al. [38] also confirmed the role of SCFAs in the pathogenesis of IBD. Their analysis showed that in people with inflammatory bowel disease, the levels of the main fatty acids—acetic, butyric and propionic— drop dramatically compared to healthy people (162.0, 86.9, 65.6 μmol/g vs. 209.7, 176.0, 93.3 μmol/g) [38]. Tian et al. [37] reported that serum levels of propionic and butyric acids are increased in diarrhea-predominant IBS (IBS-D) patients compared to healthy controls. Such a dependence was not observed in relation to SCFA levels in patients’ feces. These data indicate that elevated acid levels may influence the development of IBS-D [37]. A meta-analysis of studies on IBS patients showed [77] that the levels of propionic acid in their stools were significantly higher than in the feces of healthy people. Additionally, butyric acid predominated in patients with IBS-D type [77]. The study also confirmed statistically significant differences in the percentages of propionic acid (20.20 vs. 17.85, p = 0.007) and butyric acid (15.58 vs. 19.00, p = 0.003) in the stool samples between patients with IBS and healthy individuals, which indicates that SCFAs are good, non-invasive markers of gut disease [78].
Changes in fecal short-chain fatty acids have also been observed in patients with morbid obesity after surgical interventions. As they lost weight and changed their diet, the total amount of SCFAs decreased. A similar effect was obtained for the relative amounts of straight chain SCFAs, namely acetic, propionic and butyric acids. At the same time, the levels of BCFAs (isobutyric, isovaleric, and isocaproic acids) increased [79]. These changes suggest predominant proteolytic fermentation that may have adverse health effects. A therapy may be to change a high-protein diet to a diet rich in carbohydrates, fiber, and polysaccharides in order to increase saccharolytic fermentation and the level of main straight SCFAs (acetate, propionate, and butyrate), having health-promoting properties [79].
The effect of the intestinal microflora and its metabolism products (SCFAs, including BCFAs) on BPH has not been studied so far. However, there are studies showing that IBD may affect prostate disease and increase the risk of prostate cancer [19]. In the pilot study by Golombos et al. [20], differences in the composition of the intestinal microflora were observed between patients with BPH and PCa, which may indicate the role of the intestinal microflora in the pathogenesis of prostate diseases. In the study by Liss et al. [17], fecal microbiome of men with and without prostate cancer was analyzed. There were no differences in the species composition of bacteria between the studied groups—so far no ‘intestinal microbiological profile’ predisposing to cancer development has been found. However, it has been noticed that in patients diagnosed with PCa, the natural production of vitamin B and folic acid by intestinal bacteria is disturbed [17]. Moreover, long-term intestinal inflammation may increase circulating pro-inflammatory cytokines, which may also contribute to inflammation in the prostate. It has already been confirmed that chronic inflammation in the body, not always associated with microbial infection of the genitourinary system in men, but resulting from an excess of adipose tissue and MetS, can contribute to the development of PCa [80]. Chronic inflammation is a common etiological factor for both BPH [81–84] and PCa [23, 85–88]. Previous studies [18] indicate differences in the composition of the intestinal microflora between healthy individuals and patients with prostate cancer treated with androgen axis-targeted therapies. It has been found that oral hormone therapy for prostate cancer may disrupt the normal intestinal microflora and additionally affect the clinical response of patients to other therapies, including immunological ones [18].
In our study, we also observed a statistically significant relationship between the percentage of some SCFAs—propionic acid (C3: 0, C5: 0n, and C6: 0n), and the presence of MetS, both in the control group and patients with BPH. Patients with BPH and MetS had significantly higher stool BCFA levels— isobutyric acid (p = 0.044) and isovaleric acid (p = 0.029) and lower isocaproic acid (p = 0.019) compared to healthy controls. We showed no differences in the amount of C4: 0. This is due to the fact that butyric acid is used very quickly by intestinal epithelial colonocytes as a source of energy. We found a significant correlation between biochemical parameters and SCFA levels in men without BPH and with MetS— triglycerides and cholesterol, and propionic acid (R = 0.331, p = 0.049; R = 0.399, p = 0.016), and in patients with BPH and without MetS— triglycerides and propionic acid (R = 0.302, p = 0.024). In addition, in patients without BPH, irrespective of MetS, propionic acid correlated with triglycerides, cholesterol, and low-density lipoproteins (LDL) (R = 0.385, p < 0.005; R = 0.290, p = 0.010; R = 0.244, p = 0.030).
There are many studies showing that the intestinal microflora and the bacterial metabolites it produces are involved in the metabolic processes in the body. There is also an association between intestinal inflammation and MetS [89]. Studies carried out in animal models indicate that the presence of microorganisms inhabiting the intestines in the population, e.g. the Lactobacillus (L.) rhamnosus BFE5264 strain, increases the levels of propionate in the blood serum of animals. Moreover, these changes are accompanied by lowering cholesterol levels. These data suggest that both the respective bacterial strains and the metabolites produced with their participation (e.g. SCFAs) may influence the biosynthesis of cholesterol. It is worth noting that the tested L. rhamnosus BFE5264 strain comes from Maasai fermented milk consumed by this social group, which, despite a diet rich in animal fats, has low blood serum cholesterol levels [90]. Tirosh et al. [91] proved that propionate, both in mice and in humans, contributes to metabolic disorders and may even cause gradual weight gain. Propionate, in too high a concentration, via catecholamines (insulin antagonists), can activate signaling pathways that lead to an increase in hepatic glucose production. It can also reduce its uptake and use by peripheral tissues, which in turn may lead to insulin resistance and hyperinsulinemia [91]. In turn, other studies have shown that BCFAs - mainly isobutyric acid, significantly increased glucose uptake and may contribute to increased insulin sensitivity in people with disorders of its metabolism, which was also shown for propionic acid [92]. Research by Granado-Serrano et al. [93] showed that in patients with hypercholesterolaemia, the SCFAs profile isolated from patients' faeces were dominated by: isobutyric and isovaleric acid. Moreover, isobutyric acid positively correlated with Odoribacter and blood lipid parameters. Further research is needed in order to elucidate the mechanisms of action of BCFA in health and disease.
In a prospective cohort study [94] on the Danish population (893 participants), 34 bacterial taxa related to BMI and blood lipids were identified. It was shown that, irrespective of age, sex, and genetic factors, the intestinal microbiota can affect BMI, triglycerides and high-density lipoproteins (HDL), and have a slight impact on low-density lipoproteins (LDL) and total cholesterol [94]. These data indicate that the intestinal microflora, including its metabolites, may be a potential therapeutic target in obesity and lipid disorders, which are components of MetS contributing to the development of BPH. The appropriate proportions and concentrations of SCFAs, produced by the microbiota, affect the homeostasis of metabolism, and therefore may help prevent MetS and type II diabetes [94]. It has also been confirmed that propionic acid supplementation in adults significantly reduces weight gain. It also affects the distribution of abdominal adipose tissue and reduces the content of lipids inside the liver cells in people without non-alcoholic fatty liver disease, and inhibits the development of insulin sensitivity [57]. Research by Bouter et al. [54] also indicate a positive contribution of SCFAs (the study analyzed butyrate) on glucose metabolism in lean people. Propionate produced by the gut microflora has also been confirmed to correlate with a reduced likelihood of developing MetS and its more effective treatment, as well as obesity-related diseases. This is a direct, anti-inflammatory effect of C3: 0 acid on the visceral adipose tissue and an increase in lipogenesis and glucose uptake [95]. Other studies [96], also conducted on humans, reveal differences in SCFAs levels between obese and lean people. In overweight people, the levels of acetate, propionate, butyrate, valerate, and total SCFAs are higher than in lean participants, but no differences have been found between the identified bacterial strains. The available data confirm the hypothesis that between obese and lean people, despite the lack of nutritional differences, colonic fermentation is different, which reflects the presence of the ‘obese microbiome’, and contributes to the observed changes in SCFAs levels [96]. The diet and the composition of the intestinal microflora have a significant effect on the quantitative and qualitative composition of the produced metabolites, including SCFAs, and thus on the development of MetS. Studies in which adult patients diagnosed with MetS were subjected to dietary interventions have confirmed this thesis [57, 97, 98]. Dietary interventions involved the supply of food with a high level of polysaccharides modifying the composition of the intestinal microflora, and increasing the production of SCFAs, mainly acetate and butyrate [97]. The authors of this study also noted that a proper diet significantly reduces the levels of BCFAs (isobutyrate and isovalerate), which additionally indicates a reduction in protein fermentation and the production and accumulation of metabolites damaging the intestinal epithelium and cellular connections [97]. The bacteria predominating in the intestinal microflora of lean people are specific, probiotic bacteria, namely Bifidobacterium and Akkermansia muciniphila, producing health-promoting SCFAs—they, among others, increase insulin sensitivity and maintain the intestinal barrier, which supports the homeostasis of intestinal cells and reduces local and systemic inflammation [99].
W.R., M.L conceived and designed the experiments; W.R, A.M., performed the experiments; W.R., A.R. analyzed the data; M.P., M.L. contributed reagents; W.R., A.M., M.S. supervised the recruitment of patients and data collection; A.M., M.S., O.S. contributed materials and analysis tools; W.R., M.L. wrote the paper.
The authors declare that they have no conflicts of interest.
Financial resources for the project and funds for covering the costs to publication come exclusively from the Pomeranian Medical University in Szczecin (grant number WNoZ-322-03/S/16/2020).