The risk of developing second primary malignancies among colorectal cancer patients
Abstract
Background: The increasing number of young colorectal cancer (CRC) survivors has led to ongoing concerns about the risk of secondary primary malignancies (SPMs). Here, we intended to comprehensively explore the pooled standardized incidence rates (SIRs) for total and site-specific SPMs in CRC survivors with different restriction to lag period.
Methods: Pubmed, Embase, Cochrane Library, and Web of science databases were searched to identify any studies reporting the SIRs of SPM following CRC until August 2021. Total and site-specific SIRs with different restriction to lag period were pooled using fixed/random effect models.
Results: A total of 42 full-text publications with more than 1, 524, 236 CRC survivors and 166, 210 SPM patients were included in the meta-analysis. Pooled data showed an increased SIRs for all SPMs in CRC survivors with different restriction to lag period (no restriction to lag period, SIR = 1.15, 95% CI = [1.08–1.23]; 1-year lag, 1.16 [1.10–1.23]; 5-year lag, 1.18 [1.09–1.28]; 10-year lag, 1.24 [1.11–1.39]). The conclusions were consistent for neoplasms of colorectum, corpus uteri, and small intestine with different restriction to lag period. However, limited evidence was presented for associations between CRC survivors and SPM for prostate, breast (female), ovarian, stomach, urinary bladder, kidney, thyroid, bone and soft tissue.
Conclusion: CRC survivors are associated with an increased risk of SPMs, especially neoplasms of colorectum, corpus uteri, and small intestine. Further studies should explore the risks for these neoplasms in CRC survivors, thus providing the reference for future follow-up care.
Introduction
Colorectal cancer (CRC) is the third most common cancer, with an estimated 1.9 million new cases globally in 2020, accounting for 10.2% of all new cases and 935,000 related deaths [1, 2]. The combination of curative resection and adjuvant chemotherapy has become the standard therapeutic method and has achieved a significant improvement in the overall prognosis of colorectal cancer [3–5]. However, the increasing number of young colorectal cancer (CRC) survivors brought some worries about the risk of second primary malignancies (SPM) [6] which is detrimental to the prognosis of CRC survivors.
Increasing studies have served to define the risk of SPM in CRC survivors. For example, Yang et al., [7] reported that CRC survivors were at increased risk of total SPMs and second primary CRC, while Tanaka et al., [8] demonstrated that the SIRs of total SPMs and CRC cancer were not statistically significant in CRC survivors. Moreover, Ringland et al., [9] noted that the risk of second primary CRC was higher than in the general population in analysis with no restriction for latency time, while the risk was the same when restricted to studies with a lag time of 5 or 10 years. These findings suggested that it still needs to clarify the pooled SIRs for total and site-specific SPM in CRC survivors, especially with different restriction to lag period.
Here, we sought to characterize the pooled SIRs for total and site-specific SPM with restriction for different lag time in CRC survivors. Our finding will provide the reference for further follow-up care in CRC survivors.
Methods
This systematic review was conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline, and the systematic review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO, http://www.crd.york.ac.uk/prospero/) on August 2021 (CRD42021276185).
Literature search
A comprehensive systematic literature search was carried out using Pubmed, Embase, and Cochrane library and Web of Science databases to retrieve any studies that investigated the SIRs of SPM in CRC survivors from inception to August 2021. The detailed search strategies are in Supplementary Table 1. References of eligible articles were also assessed for relevant studies.
Inclusion and exclusion criteria
For the purpose of our meta-analysis, all the studies were screened based on the following inclusion criteria: (1) CRC survivors; (2) second primary malignancies (subsequent or metachronous, not synchronous); all cancers reported subsequent to CRC were defined as second primary malignancies (subsequent or metachronous), and cancers diagnosed before the diagnosis of CRC or on the same day were regarded as synchronous malignancies. (3) Accessible SIR and its 95% confidence interval (CI). Exclusion criteria were as follows: (1) non-CRC patients; (2) studies with smaller sample size from the same authors or hospitals; and (3) patients or studies did not fulfill the inclusion criteria.
Data extraction and quality assessment
Three reviewers (SD, FZ, and HS) summarized the eligible studies and resolved the divisions of opinion by consensus assessment. Study characteristics (first author, publication year, study population, region, gender, and mean age) and relevant data of patient characteristics (site of the second primary tumor, mean age, sex, follow-up period, SIR, 95% confidence interval, sample size, number of incidents, observed and expected patients’ number, latency time) were extracted. When SIR and its 95% CI were not reported, they were calculated based on Poisson distribution using the observed and expected incidents. Standard to identify the possible bias risk, or research rationality, in the individual studies was assessed using the Newcastle-Ottawa Scales. Studies with stars more than six were regarded as high-quality.
Statistical analysis
STATA 16.0 (Stata Corporation, College Station, TX, USA) software was used for all the analyses. The potential heterogeneity was explored by using the Cochran’s Q test and I2 test statistics. The p-value < 0.1 or I2 > 50% indicates significant heterogeneity. The random-effect model was preferentially performed for all the analyses because of the inherent clinical heterogeneity among studies. Fixed-effect model was used to evaluate the consistency of the conclusions. Subgroup analyses stratified by sites of SPM and restriction on different lag time were performed to assess the stability of the conclusions. P-value less than 0.05 was considered statistically significant.
Availability of data and materials
The datasets supporting the conclusions of this article are accessible upon reasonable requests from the corresponding authors.
Results
Literature search and studies characteristics
A total of 15,052 unique publications were retrieved by our literature search. After titles and abstracts screening, 189 articles remained for additional full-text examination (Figure 1). It is worth mentioning that 38 independent studies were eligible based on our selection criteria derived from United States Surveillance, Epidemiology, and End Results (SEER) cohort, while the most representative studies covering the broadest range of years with the largest population was kept for our analysis to avoid duplication. The full-text reviews of these articles were carefully completed and the corresponding reasons are listed in Figure 1. Finally, 42 full publications (ranged from 1969 to 2021) met our eligibility criteria, with more than 1, 524, 236 CRC survivors and 166,210 SPM patients [7–48].
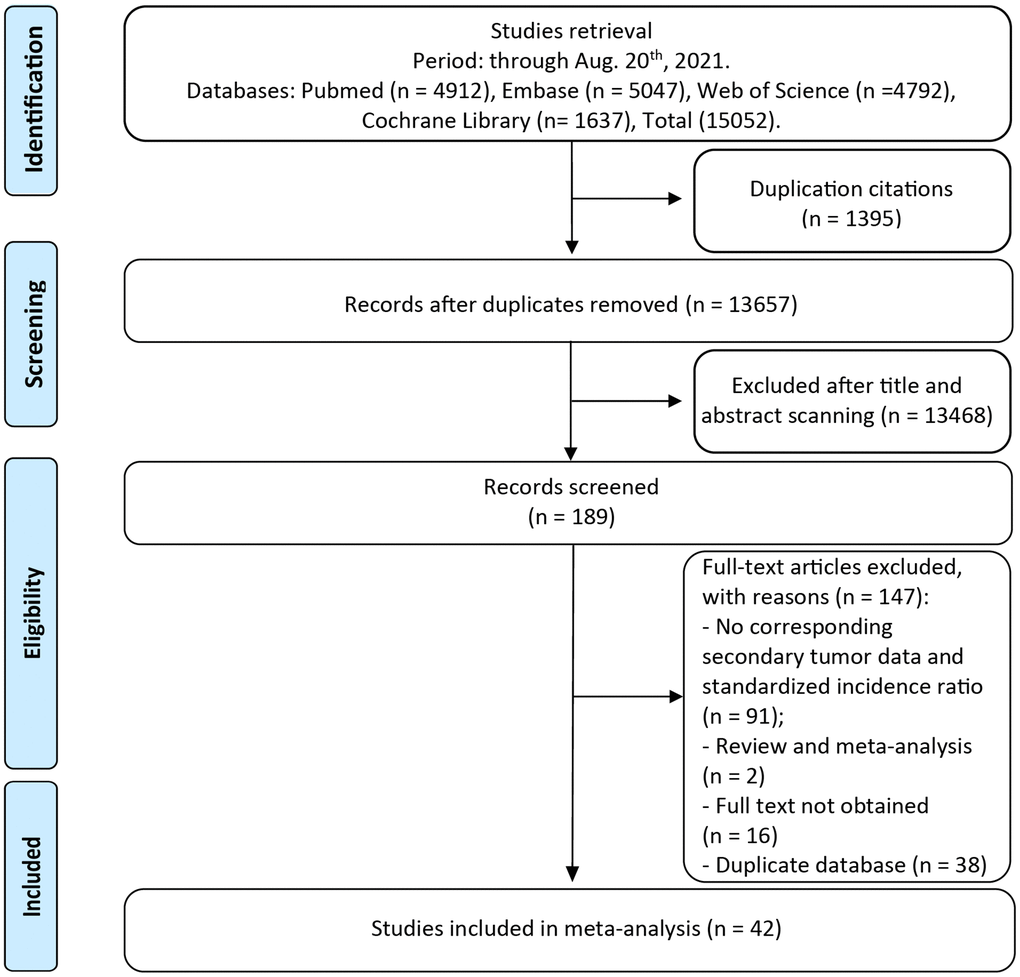
Figure 1. The flow chart of studies with corresponding exclusive reasons.
The main characteristics of the eligible studies are shown in Table 1. Of the 42 publications, the principal meta-analysis on the overall risk for SPMs in CRC survivors was based on 26 studies. Other 16 articles reporting the data of a single tumor contributed to the site-specific SIRs. Thirty-four original investigations were based on regional populations, and other eight studies were conducted from different hospitals or institutions. The mean period of follow-up, reported in 28 studies, ranged from 1.5 to 16.8 years. The mean latency period (the time between diagnosis of CRC and the SPMs) ranged from 1.5 to 6.0 years. Newcastle-Ottawa Scale (NOS) was performed to assess the methodological quality of eligible studies. All studies were identified as high-quality with stars above six (Supplementary Table 2).
Table 1. Characteristics of included studies in meta-analysis of secondary malignancies among colorectal cancer survivals.
| Study (reference) | Publish Date | Registry (study interval) | Country | Mean age at diagnosis, (Range or SD) years | Male: female ratio | Latency,mean or SD (years) | Mean Follow-up (years) | Study Size | No. of Second Malignancies |
| Schottenfeld [10] | 1969 | Memorial Sloan Kettering Cancer Center (1949–1962) | USA | – | – | – | – | 4771 | 190 |
| Teppo [11] | 1985 | Finnish Cancer Registry (1953–1979) | Finland | – | 0.62 | – | – | 21122 | 329 |
| Enblad [12] | 1989 | National Board of Health and Welfare the Swedish Cancer Registry (1960–1981) | Sweden | – | 1.07 | – | – | 61769 | 3845 |
| Tanaka [8] | 1991 | Osaka Cancer Registry (1966–1986) | Japan | 60.1 | 1.15 | – | 3.68 | 14235 | 416 |
| Levi [13] | 1993 | Vaud Cancer Registry (1974–1989) | Switzerland | – | 1.08 | – | – | – | 153 |
| Buiatti [14] | 1997 | Tuscany Tumour Registry (RIT), Ragusa Cancer Registry (RTR), Cancer Registry of Romagna (RTR) (1981–1989) | Italy | 68.6 | – | – | 2.7 | 5238 | 163 |
| McCredie [15] | 1997 | New South Wales Central Cancer Registry (1972–1991) | Australia | 66.5 | 1.08 | – | 3.79 | 42509 | 2098 |
| Malmer [16] | 2000 | The nation-wide Swedish Cancer Registry (1958–1994) | Sweden | 72 | – | – | – | 156872 | 224 |
| Evans [17] | 2001 | The Thames Cancer Registry (1961–1995) | England | – | 0.93 | – | – | 127281 | 4317 |
| Dong [18] | 2001 | The Swedish Family Cancer Database (1958–1996) | Sweden | 67.6 | 1.14 | – | 2.68 | 67899 | 6197 |
| Hemmiki [19] | 2001 | The Swedish Family Cancer Database (1958–1996) | Sweden | 65.55 | 1.17 | – | – | 68084 | 5731 |
| Green [42] | 2002 | U.S. national Cancer Institute (1989–1993) | USA | 63.9 ± 11 (15–87) | 1.22 | 1.5 (0.3–5.8) | 1.5 (0.3–5.8) | 3179 | 42 |
| Moot [20] | 2002 | Victorian Cancer Registry (1982–1993) | Australia | 66.2 | – | – | 7.2 | 13794 | 279 |
| Heard [21] | 2005 | South Australian Cancer Registry (1977–2001) | Australia | – | – | – | – | – | 1472 |
| Bouvier [22] | 2008 | A population-based cancer registry in Burgundy (1976–2002) | France | 71.1 (21.0–99.6) | 1.24 | 3.6 (0.5–22.5) | – | 10801 | 216 |
| Cluze [23] | 2009 | Cancer Registry of Ise `re, France (1989–1997) | France | 69.2 | 1.08 | – | 2.9 | 4944 | 224 |
| Noura [24] | 2009 | Osaka Cancer Registry (1991–1996) | Japan | 61 (33–89) | 1.71 | 2.5 | 6.8 | 301 | 40 |
| Ringland [9] | 2010 | NSW Central Cancer Registry (1987–1996) | Australia | – | 1.22 | Median 3.7 | 5.1 (1.4–10.7) | 29471 | 660 |
| Youlden [25] | 2011 | Queensland Cancer Registry (QCR) (1982–2001) | Australia | – | 1.18 | 5.5 (1.3–10.2) | 5–25 | 27814 | 3046 |
| Raj [26] | 2011 | California Cancer Registry (1990–2005) | USA | 64.7 | 1.19 | 2.67 | 6 | 104257 | 1443 |
| Tabuchi [27] | 2012 | Osaka Cancer Registry (1985–2004) | Japan | – | – | – | 3.9 | – | 2470 |
| Dasgupta [28] | 2012 | Queensland, Australia 1996–2005 | Australia | 64 | 1.36 | 3.1 | 4.2 (2.2–7.3) | 15755 | 1615 |
| Kok [29] | 2012 | Netherlands Cancer Registry (1989–2008) | Netherlands | – | – | – | – | – | – |
| Mulder [30] | 2012 | Rotterdam Cancer registry in the Netherlands (1995–2006) | Netherlands | 70.0 (62–77) | 1.02 | – | 3.9 | 10283 | 135 |
| Levi [31] | 2012 | Vaud Cancer Registry (1974–2008) | Switzerland | – | 1.15 | – | 4.7 | 9389 | 136 |
| Tabuchi [32] | 2013 | Osaka Medical Center for Cancer and Cardiovascular Diseases (1985–2004) | Japan | – | – | – | 5.2 | 2155 | 204 |
| Utada [33] | 2014 | Nagasaki Prefecture Cancer Registry (1985–2007) | Japan | – | – | – | 4.3 | – | 2997 |
| Jégu [34] | 2014 | K2 France nationwide study (1984–2004) | France | 64.2 | – | – | 0.16–18 | – | 2929 |
| Coyte [35] | 2014 | Scottish Cancer Registry (2000–2004) | Scotland | 69.9 ± 11.7 | 1.13 | – | – | 7225 | 324 |
| Lee [36] | 2015 | Taiwan’s national Health Insurance (1996–2011) | China | 67 (56–75) | 1.29 | 4.7 (2.7–7.5) | 4.03 (2.14–7.49) | 98876 | 4259 |
| Liang [37] | 2015 | Taiwan Cancer Registry (TCR) (1995–2005) | China | 66 | 1.32 | – | 4.4 | 65 648 | 3810 |
| Kato [38] | 2016 | Saitama Medical Center (2007–2011) | Japan | 67.4 ± 11.2 | 1.73 | 1.5 (3–61) | 3.69 (1.6) | 1005 | 126 |
| Preyer [39] | 2017 | Tyrol and Vorarlberg Cancer Registries (1988–2005) | Austria | – | – | – | 5.7 (1.4–10.3) | 7138 | 614 |
| Yang [7] | 2017 | SEER (1973–2012) | USA | 68 (14–102) | 1.36 | – | 6.97 | 288390 | 33047 |
| Kim [40] | 2017 | Republic of Korea national Health Insurance System database (2007–2012) | Korea | – | – | – | 5.78 | 85455 | 2005 |
| Chung [41] | 2017 | Severance Hospital (2001–2009) | Korea | 61.0 (45.0–74.0) | 1.62 | 0.3 (0.8–10) | 3.3 (0–30.9) | 4822 | |
| Guan [43] | 2015 | SEER (1992–2012) | USA | – | 1.42 | – | – | 240584 | 27731 |
| He [44] | 2018 | SEER (1973–2013) | USA | – | – | – | – | | 50679 |
| Bright [45] | 2019 | The Teenage and Young Adult Cancer Survivor Study England and Wales (1971–2006) | England | – | 1.03 | – | 16.8 | 5805 | 537 |
| Ahn [46] | 2019 | Support for Serious Illness (SSI) program Korean NHI claims database (2005–2015) | Korean | 65.56 ± 10.51 | 1.58 | 3.08 (1.87–3.44) | – | 251482 | 498 |
| Feller [47] | 2020 | Swiss cantonal cancer registries (1981–2009) | Switzerland | – | – | 6.0 (2.1–10.9) | More than 5 | 35949 | 4441 |
| Tanaka [48] | 2021 | Japan Clinical Oncology Group JCOG0205, JCOG0212 and JCOG0404 | Japan | 62 (23–75) | 1.38 | – | 6.0 (5.0–7.2) | 2824 | 240 |
| Abbreviation: SEER: Surveillance, Epidemiology, and End Results. |
Site-specific prevalence of SPM in CRC survivors
On unadjusted analysis with no restriction to lag period, the pooled site-specific prevalence is 1.506% for prostate cancer, 1.202% for colorectal cancer, 1.060% for breast cancer, with less than 1.00% other malignancies (Supplementary Figure 1).
Overall and site-specific SIRs of SPM in CRC survivors
On pooled analysis with data derived from 26 studies comprising 601,601 CRC survivors and 85,708 SPM patients, we found an increased risk of second malignancies with no restriction to lag period (SIR = 1.15, 95% CI = [1.08–1.23]). The result was similar when we restricted analysis with lag one (SIR = 1.16, 95% CI = [1.10–1.23]), five (SIR = 1.18, 95% CI = [1.09–1.28]) or ten (SIR = 1.24, 95% CI = [1.11–1.39]) years (Figure 2).
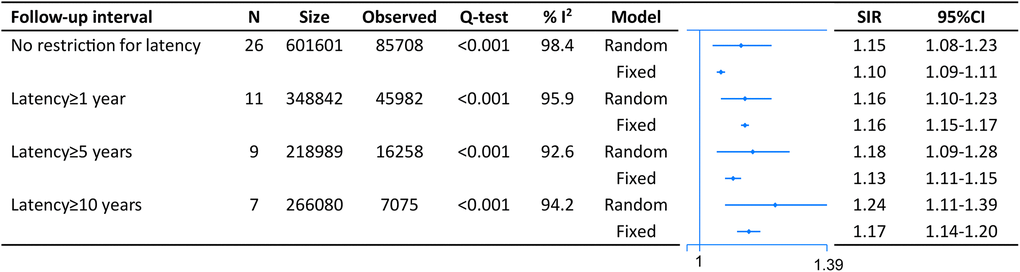
Figure 2. The pooled standardized incidence rates (SIR) for overall second primary malignancies (SPM) in CRC survivors with different restriction to lag time.
Twenty-two studies with no restriction for latency time reported the association between CRC survivors and second primary CRC. The random-effect model showed that CRC survivors had a higher risk of developing second CRC (SIR = 1.59, 95% CI = [1.38–1.83]). Similar results were obtained when the analysis was restricted by one, five-, and ten-years lag (one-year lag, SIR = 1.78, 95% CI = [1.52–2.08]; five-years lag, SIR = 1.45, 95% CI = [1.15–1.82]; and ten-years lag, SIR = 1.69, 95% CI = [1.12–2.54]) (Figure 3).
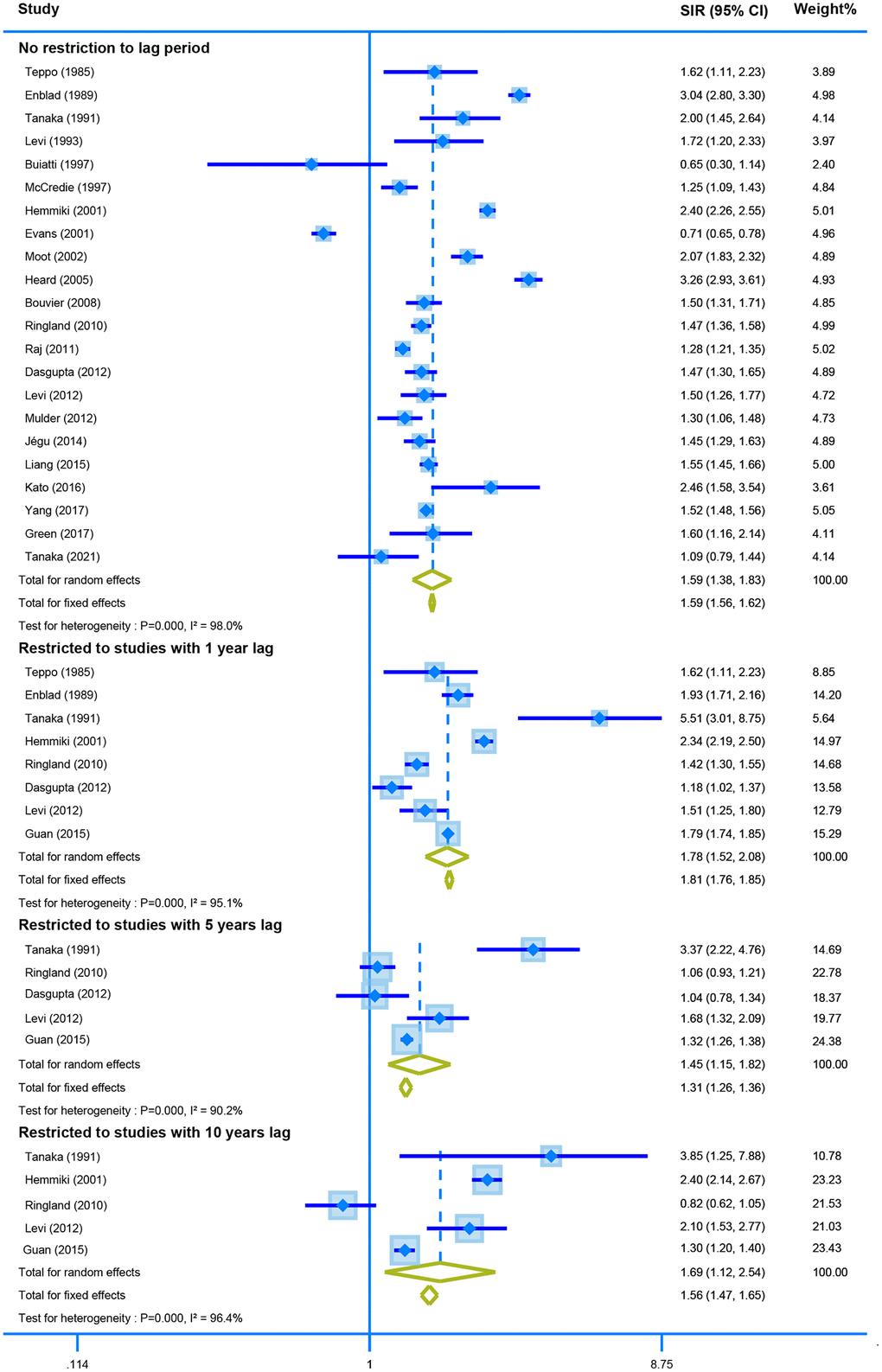
Figure 3. The pooled SIR for second colorectal cancer in CRC survivors with different restriction to lag time.
Fourteen studies reported the SIR of the second neoplasm of uterine corpus in CRC survivors with no restriction for latency time. The pooled data showed a higher risk of developing the malignant neoplasm of corpus uteri using the random model (SIR = 2.11, 95% CI = [1.62–2.76]). Moreover, the SIR was still similar when we calculated the derived data stratified by different lag time (one-year lag, SIR = 2.25, 95% CI = [1.60–3.16]; five-years lag, SIR = 3.61, 95% CI = [1.17–11.16]; and ten--years lag, SIR = 1.53, 95% CI = [1.23–1.91]) (Figure 4).
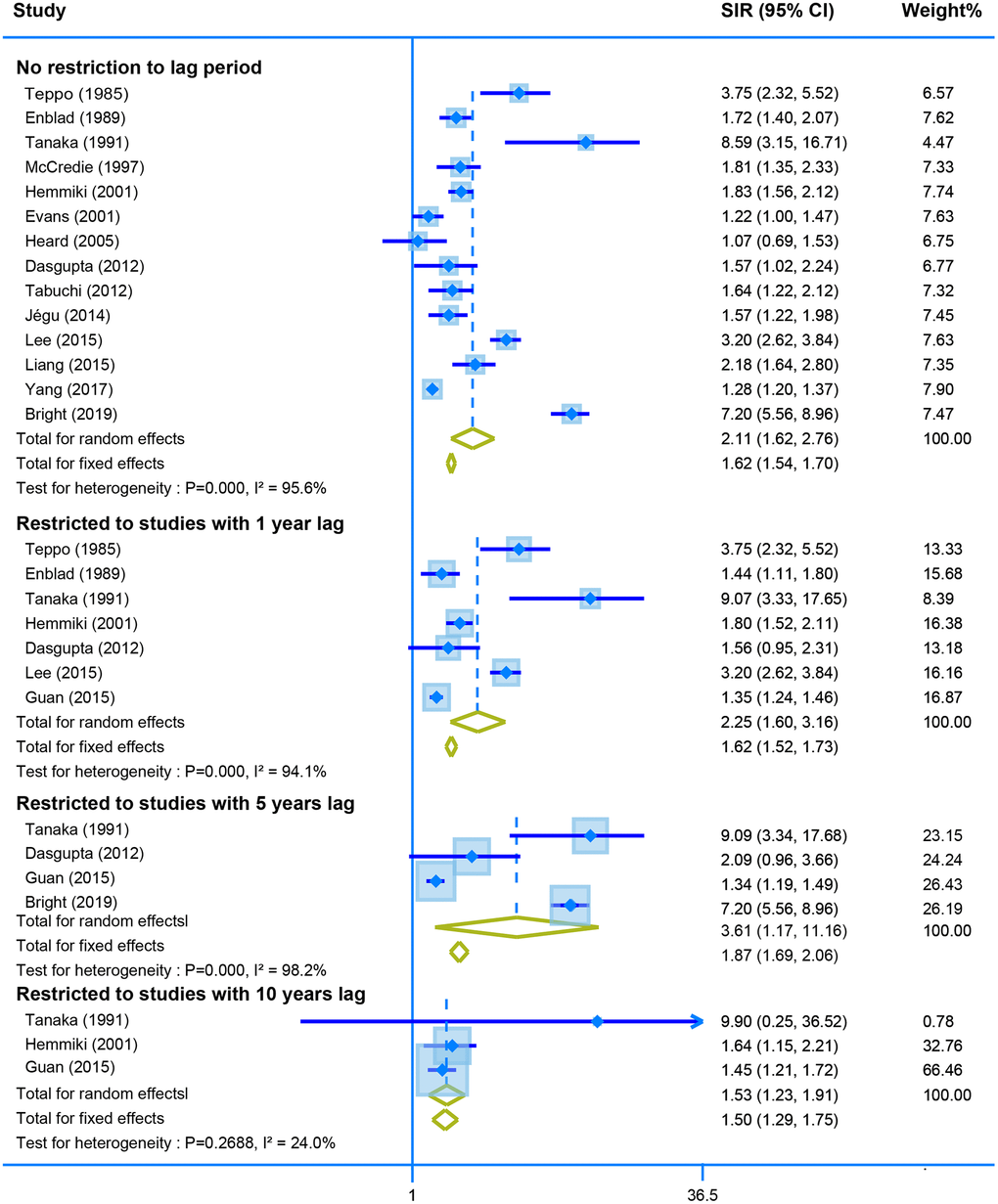
Figure 4. The pooled SIR for second neoplasm of corpus uteri in CRC survivors with different restriction to lag time.
Pooled analysis of seven studies with no restriction to lag time showed a positive association between second malignant of the small intestine and CRC survivors (SIR = 4.00, 95% CI = [2.91–5.49]. Restriction of the analysis with different years lag presents stable results of high risk of small intestine tumor (one-year lag, SIR = 3.38, 95% CI = [3.08–3.71]; SIR = 2.35, 95% CI = [1.99–2.78]; and SIR = 2.75, 95% CI = [2.18–3.47]) (Figure 5).
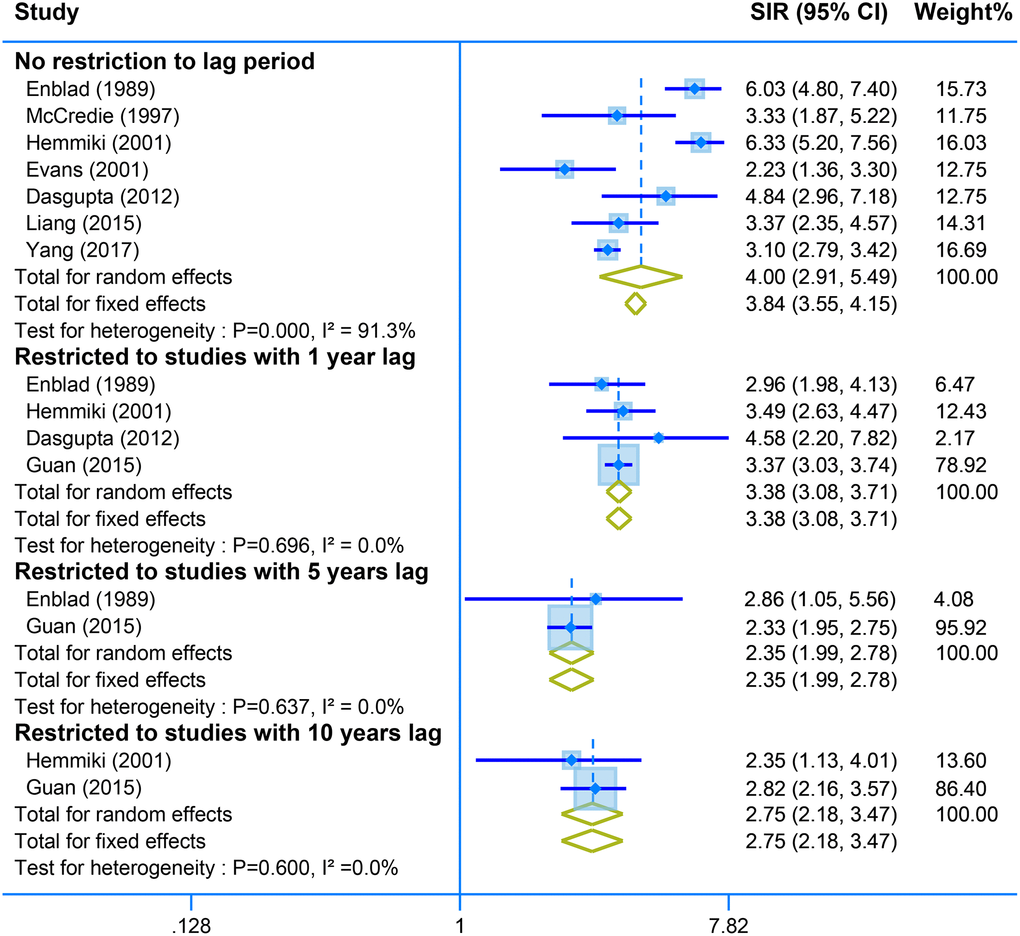
Figure 5. The pooled SIR for second neoplasm of small intestine in CRC survivors with different restriction to lag time.
We also found that CRC survivors are more likely to develop second neoplasms of prostate, breast (female), ovarian, stomach, urinary bladder, kidney, thyroid, bone and soft tissue (Supplementary Figure 1). However, the results of the above tumors failed to be consistent with the analysis stratified by different lag time (Supplementary Figures 2–9). Moreover, there were no increasing association between CRC survivors and neoplasms of stomach, oral, lymphoma, pancreas, leukemia, brain, cervix, esophagus, larynx and gall bladder (Supplementary Figure 1).
Discussion
An increasing number of young CRC survivors are confronted with the threat of developing SPM [6]. Appropriate risk assessment for SPMs has substantial therapeutic implications for long-term patient surveillance and the reduction of morbidity. In this study, based on the 42 publications from comprehensive population databases worldwide, we carried out a meta-analysis to explore the risk of overall SPMs in CRC survivors. Our results demonstrated that CRC survivors were at increased risk of developing second tumors, especially neoplasms of colorectum, corpus uteri, and small intestine.
To our knowledge, this is the first meta-analysis to comprehensively evaluate the risk of SPMs in CRC survivors. Previously, Keegan et al., demonstrated that adolescents and young adults with secondary neoplasms were more likely to experience worse survival compared with adolescents and young adults with the same primary neoplasms [49]. This finding highlights the importance of active surveillance for CRC patients. However, studies conducted by Levi et al., and Youlden et al., mainly focused on overall SPMs risk, but ignored site-specific SIRs [13, 25]. The site-specific SIRs could precisely facilitate the management of disease surveillance in CRC survivors. In this point, our study is of great significance in the field.
Our results confirmed the positive association between SPMs and CRC history [50, 51], and the reasons may be as follows, (1) genetic predisposition and environmental exposures; [9, 30, 52, 53] (2) adjuvant therapy, such as radiotherapy [54] and chemotherapy [55]. Green et al., demonstrated that CRC survivors receiving adjuvant chemotherapy had the high incidence of second primary colorectal cancer. Moreover, direct radiation, radiation scatter or radiation induced genetic alterations with direct exposure might contribute to carcinogenesis due to increased reactive oxygen species and changes of gene expression [56]. Further studies are needed to elucidate underlying mechanisms for the association between CRC survivors and its SPMs.
Inevitably, there are several limitations related to this meta-analysis. First, significant heterogeneity existed among the analysis, while different effect models and subgroup analyses showed a unified result, which further confirmed the conclusion. Second, some SIRs and their 95% CI were estimated based on Poisson distribution using the observed and expected cases, which could cause some bias of results. Finally, we were unable to adjust for any heterogeneity in treatment between studies or evaluate risks by different treatment modalities, as treatment data was not available for the majority of studies.
Conclusion
In summary, for the first time, we reported that CRC survivors are associated with an increased risk of SPMs, especially neoplasms of colorectum, corpus uteri, and small intestine. Further studies should explore the risks for these neoplasms in CRC survivors, thus providing the reference for future follow-up care.
Author Contributions
Furong Zeng, Songtao Du, Bomiao Zhang, and Binbin Cui conceived and designed the study. Songtao Du and Yayun Li analyzed the data. Songtao Du and Yayun Li wrote the manuscript. Huiyan Sun, Yayun Li, Guangtong Deng and Siyuan Tang revised the manuscript. All authors have agreed to publish this manuscript and approved the final version. The content of this manuscript has not been previously published.
Conflicts of Interest
The authors declare no conflicts of interest related to this study.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82102803, 82103183), Youth Natural Science Foundation of Heilongjiang Province (JJ2018QN0724).
References
-
1.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021; 71:209–49. https://doi.org/10.3322/caac.21660 [PubMed]
-
2.
Yoshino T, Argilés G, Oki E, Martinelli E, Taniguchi H, Arnold D, Mishima S, Li Y, Smruti BK, Ahn JB, Faud I, Chee CE, Yeh KH, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis treatment and follow-up of patients with localised colon cancer. Ann Oncol. 2021; 32:1496–510. https://doi.org/10.1016/j.annonc.2021.08.1752 [PubMed]
-
3.
Papamichael D, Audisio RA, Glimelius B, de Gramont A, Glynne-Jones R, Haller D, Köhne CH, Rostoft S, Lemmens V, Mitry E, Rutten H, Sargent D, Sastre J, et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol. 2015; 26:463–76. https://doi.org/10.1093/annonc/mdu253 [PubMed]
-
4.
West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol. 2010; 28:272–8. https://doi.org/10.1200/JCO.2009.24.1448 [PubMed]
-
5.
Seymour MT, Morton D. FOxTROT: an international randomised controlled trial in 1052 patients (pts) evaluating neoadjuvant chemotherapy (NAC) for colon cancer. J Clin Oncol. 2019; 37:3504. https://doi.org/10.1200/JCO.2019.37.15_suppl.3504
-
6.
El-Shami K, Oeffinger KC, Erb NL, Willis A, Bretsch JK, Pratt-Chapman ML, Cannady RS, Wong SL, Rose J, Barbour AL, Stein KD, Sharpe KB, Brooks DD, Cowens-Alvarado RL. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J Clin. 2015; 65:428–55. https://doi.org/10.3322/caac.21286 [PubMed]
-
7.
Yang J, Li S, Lv M, Wu Y, Chen Z, Shen Y, Wang B, Chen L, Yi M, Yang J. Risk of subsequent primary malignancies among patients with prior colorectal cancer: a population-based cohort study. Onco Targets Ther. 2017; 10:1535–48. https://doi.org/10.2147/OTT.S129220 [PubMed]
-
8.
Tanaka H, Hiyama T, Hanai A, Fujimoto I. Second primary cancers following colon and rectal cancer in Osaka, Japan. Jpn J Cancer Res. 1991; 82:1356–65. https://doi.org/10.1111/j.1349-7006.1991.tb01806.x [PubMed]
-
9.
Ringland CL, Arkenau HT, O'Connell DL, Ward RL. Second primary colorectal cancers (SPCRCs): experiences from a large Australian Cancer Registry. Ann Oncol. 2010; 21:92–7. https://doi.org/10.1093/annonc/mdp288 [PubMed]
-
10.
Schottenfeld D, Berg JW, Vitsky B. Incidence of multiple primary cancers. II. Index cancers arising in the stomach and lower digestive system. J Natl Cancer Inst. 1969; 43:77–86. [PubMed]
-
11.
Teppo L, Pukkala E, Saxén E. Multiple cancer--an epidemiologic exercise in Finland. J Natl Cancer Inst. 1985; 75:207–17. [PubMed]
-
12.
Enblad P, Adami HO, Glimelius B, Krusemo U, Påhlman L. The risk of subsequent primary malignant diseases after cancers of the colon and rectum. A nationwide cohort study. Cancer. 1990; 65:2091–100. https://doi.org/10.1002/1097-0142(19900501)65:9%3c2091::aid-cncr2820650934%3e3.0.co;2-m [PubMed]
-
13.
Levi F, Randimbison L, Te VC, Rolland-Portal I, Franceschi S, La Vecchia C. Multiple primary cancers in the Vaud Cancer Registry, Switzerland, 1974-89. Br J Cancer. 1993; 67:391–5. https://doi.org/10.1038/bjc.1993.72 [PubMed]
-
14.
Buiatti E, Crocetti E, Acciai S, Gafà L, Falcini F, Milandri C, La Rosa M. Incidence of second primary cancers in three Italian population-based cancer registries. Eur J Cancer. 1997; 33:1829–34. https://doi.org/10.1016/s0959-8049(97)00173-1 [PubMed]
-
15.
McCredie M, Macfarlane GJ, Bell J, Coates M. Second primary cancers after cancers of the colon and rectum in New South Wales, Australia, 1972-1991. Cancer Epidemiol Biomarkers Prev. 1997; 6:155–60. [PubMed]
-
16.
Malmer B, Tavelin B, Henriksson R, Grönberg H. Primary brain tumours as second primary: a novel association between meningioma and colorectal cancer. Int J Cancer. 2000; 85:78–81. https://doi.org/10.1002/(sici)1097-0215(20000101)85:1<78::aid-ijc14>3.0.co;2-s [PubMed]
-
17.
Evans HS, Møller H, Robinson D, Lewis CM, Bell CM, Hodgson SV. The risk of subsequent primary cancers after colorectal cancer in southeast England. Gut. 2002; 50:647–52. https://doi.org/10.1136/gut.50.5.647 [PubMed]
-
18.
Dong C, Hemminki K. Second primary neoplasms in 633,964 cancer patients in Sweden, 1958-1996. Int J Cancer. 2001; 93:155–61. https://doi.org/10.1002/ijc.1317 [PubMed]
-
19.
Hemminki K, Li X, Dong C. Second primary cancers after sporadic and familial colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001; 10:793–8. [PubMed]
-
20.
Moot AR, Polglase A, Giles GG, Garson OM, Thursfield V, Gunter D. Men with colorectal cancer are predisposed to prostate cancer. ANZ J Surg. 2003; 73:289–93. https://doi.org/10.1046/j.1445-2197.2003.t01-1-02621.x [PubMed]
-
21.
Heard A, Roder D, Luke C. Multiple primary cancers of separate organ sites: implications for research and cancer control (Australia). Cancer Causes Control. 2005; 16:475–81. https://doi.org/10.1007/s10552-004-8023-0 [PubMed]
-
22.
Bouvier AM, Latournerie M, Jooste V, Lepage C, Cottet V, Faivre J. The lifelong risk of metachronous colorectal cancer justifies long-term colonoscopic follow-up. Eur J Cancer. 2008; 44:522–7. https://doi.org/10.1016/j.ejca.2008.01.007 [PubMed]
-
23.
Cluze C, Delafosse P, Seigneurin A, Colonna M. Incidence of second cancer within 5 years of diagnosis of a breast, prostate or colorectal cancer: a population-based study. Eur J Cancer Prev. 2009; 18:343–8. https://doi.org/10.1097/CEJ.0b013e32832abd76 [PubMed]
-
24.
Noura S, Ohue M, Seki Y, Tanaka K, Motoori M, Kishi K, Miyashiro I, Ohigashi H, Yano M, Ishikawa O, Tsukuma H, Murata K, Kameyama M. Second primary cancer in patients with colorectal cancer after a curative resection. Dig Surg. 2009; 26:400–5. https://doi.org/10.1159/000229991 [PubMed]
-
25.
Youlden DR, Baade PD. The relative risk of second primary cancers in Queensland, Australia: a retrospective cohort study. BMC Cancer. 2011; 11:83. https://doi.org/10.1186/1471-2407-11-83 [PubMed]
-
26.
Raj KP, Taylor TH, Wray C, Stamos MJ, Zell JA. Risk of second primary colorectal cancer among colorectal cancer cases: a population-based analysis. J Carcinog. 2011; 10:6. https://doi.org/10.4103/1477-3163.78114 [PubMed]
-
27.
Tabuchi T, Ito Y, Ioka A, Miyashiro I, Tsukuma H. Incidence of metachronous second primary cancers in Osaka, Japan: update of analyses using population-based cancer registry data. Cancer Sci. 2012; 103:1111–20. https://doi.org/10.1111/j.1349-7006.2012.02254.x [PubMed]
-
28.
Dasgupta P, Youlden DR, Baade PD. Multiple primary cancers among colorectal cancer survivors in Queensland, Australia, 1996-2007. Cancer Causes Control. 2012; 23:1387–98. https://doi.org/10.1007/s10552-012-9990-1 [PubMed]
-
29.
Kok DE, van de Schans SA, Liu L, Kampman E, Coebergh JW, Kiemeney LA, Soerjomataram I, Aben KK. Risk of prostate cancer among cancer survivors in the Netherlands. Cancer Epidemiol. 2013; 37:140–5. https://doi.org/10.1016/j.canep.2012.11.004 [PubMed]
-
30.
Mulder SA, Kranse R, Damhuis RA, Ouwendijk RJ, Kuipers EJ, van Leerdam ME. The incidence and risk factors of metachronous colorectal cancer: an indication for follow-up. Dis Colon Rectum. 2012; 55:522–31. https://doi.org/10.1097/DCR.0b013e318249db00 [PubMed]
-
31.
Levi F, Randimbison L, Blanc-Moya R, Maspoli-Conconi M, Rosato V, Bosetti C, La Vecchia C. High constant incidence of second primary colorectal cancer. Int J Cancer. 2013; 132:1679–82. https://doi.org/10.1002/ijc.27780 [PubMed]
-
32.
Tabuchi T, Ito Y, Ioka A, Nakayama T, Miyashiro I, Tsukuma H. Tobacco smoking and the risk of subsequent primary cancer among cancer survivors: a retrospective cohort study. Ann Oncol. 2013; 24:2699–704. https://doi.org/10.1093/annonc/mdt279 [PubMed]
-
33.
Utada M, Ohno Y, Hori M, Soda M. Incidence of multiple primary cancers and interval between first and second primary cancers. Cancer Sci. 2014; 105:890–6. https://doi.org/10.1111/cas.12433 [PubMed]
-
34.
Jégu J, Colonna M, Daubisse-Marliac L, Trétarre B, Ganry O, Guizard AV, Bara S, Troussard X, Bouvier V, Woronoff AS, Velten M. The effect of patient characteristics on second primary cancer risk in France. BMC Cancer. 2014; 14:94. https://doi.org/10.1186/1471-2407-14-94 [PubMed]
-
35.
Coyte A, Morrison DS, McLoone P. Second primary cancer risk - the impact of applying different definitions of multiple primaries: results from a retrospective population-based cancer registry study. BMC Cancer. 2014; 14:272. https://doi.org/10.1186/1471-2407-14-272 [PubMed]
-
36.
Lee YT, Liu CJ, Hu YW, Teng CJ, Tzeng CH, Yeh CM, Chen TJ, Lin JK, Lin CC, Lan YT, Wang HS, Yang SH, Jiang JK, et al. Incidence of Second Primary Malignancies Following Colorectal Cancer: A Distinct Pattern of Occurrence Between Colon and Rectal Cancers and Association of Co-Morbidity with Second Primary Malignancies in a Population-Based Cohort of 98,876 Patients in Taiwan. Medicine (Baltimore). 2015; 94:e1079. https://doi.org/10.1097/MD.0000000000001079 [PubMed]
-
37.
Liang YH, Shao YY, Chen HM, Lai CL, Lin ZZ, Kuo RN, Cheng AL, Yeh KH, Lai MS. Young patients with colorectal cancer have increased risk of second primary cancers. Jpn J Clin Oncol. 2015; 45:1029–35. https://doi.org/10.1093/jjco/hyv137 [PubMed]
-
38.
Kato T, Alonso S, Muto Y, Perucho M, Rikiyama T. Tumor size is an independent risk predictor for metachronous colorectal cancer. Oncotarget. 2016; 7:17896–904. https://doi.org/10.18632/oncotarget.7555 [PubMed]
-
39.
Preyer O, Concin N, Obermair A, Concin H, Ulmer H, Oberaigner W. The relative risk of second primary cancers in Austria's western states: a retrospective cohort study. BMC Cancer. 2017; 17:699. https://doi.org/10.1186/s12885-017-3683-9 [PubMed]
-
40.
Kim HS, Choi YJ, Shin DW, Han KD, Yoon H, Shin CM, Park YS, Kim N, Lee DH. Secondary Primary Prostate Cancer after Colorectal Cancer: A Nationwide Population-based Cohort Study in Korea. J Cancer Prev. 2017; 22:241–7. https://doi.org/10.15430/JCP.2017.22.4.241 [PubMed]
-
41.
Chung JW, Chung MJ, Bang S, Park SW, Song SY, Chung JB, Park JY. Assessment of the Risk of Colorectal Cancer Survivors Developing a Second Primary Pancreatic Cancer. Gut Liver. 2017; 11:728–32. https://doi.org/10.5009/gnl16526 [PubMed]
-
42.
Green RJ, Metlay JP, Propert K, Catalano PJ, Macdonald JS, Mayer RJ, Haller DG. Surveillance for second primary colorectal cancer after adjuvant chemotherapy: an analysis of Intergroup 0089. Ann Intern Med. 2002; 136:261–9. https://doi.org/10.7326/0003-4819-136-4-200202190-00005 [PubMed]
-
43.
Guan X, Jin Y, Chen Y, Jiang Z, Liu Z, Zhao Z, Yan P, Wang G, Wang X. The Incidence Characteristics of Second Primary Malignancy after Diagnosis of Primary Colon and Rectal Cancer: A Population Based Study. PLoS One. 2015; 10:e0143067. https://doi.org/10.1371/journal.pone.0143067 [PubMed]
-
44.
He X, Wu W, Ding Y, Li Y, Si J, Sun L. Excessive risk of second primary cancers in young-onset colorectal cancer survivors. Cancer Med. 2018; 7:1201–10. https://doi.org/10.1002/cam4.1437 [PubMed]
-
45.
Bright CJ, Reulen RC, Winter DL, Stark DP, McCabe MG, Edgar AB, Frobisher C, Hawkins MM. Risk of subsequent primary neoplasms in survivors of adolescent and young adult cancer (Teenage and Young Adult Cancer Survivor Study): a population-based, cohort study. Lancet Oncol. 2019; 20:531–45. https://doi.org/10.1016/S1470-2045(18)30903-3 [PubMed]
-
46.
Ahn HS, Kang TU, Swan H, Kang MJ, Kim N, Kim HJ, Park SM. Incidence and Mortality Rates of Second Pancreatic Cancer Among Survivors of Digestive Cancers: A Nationwide Population-Based Study. Pancreas. 2019; 48:412–9. https://doi.org/10.1097/MPA.0000000000001254 [PubMed]
-
47.
Feller A, Matthes KL, Bordoni A, Bouchardy C, Bulliard JL, Herrmann C, Konzelmann I, Maspoli M, Mousavi M, Rohrmann S, Staehelin K, Arndt V, and NICER Working Group. Correction to: The relative risk of second primary cancers in Switzerland: a population-based retrospective cohort study. BMC Cancer. 2020; 20:87. https://doi.org/10.1186/s12885-020-6584-2 [PubMed]
-
48.
Tanaka K, Ogawa G, Mizusawa J, Kadota T, Nakamura K, Shimada Y, Hamaguchi T, Fujita S, Kitano S, Inomata M, Kanemitsu Y, Fukuda H, and Colorectal Cancer Study Group of Japan Clinical Oncology Group (JCOG). Second primary cancers and recurrence in patients after resection of colorectal cancer: An integrated analysis of trials by Japan Clinical Oncology Group: JCOG1702A. Jpn J Clin Oncol. 2021; 51:185–91. https://doi.org/10.1093/jjco/hyaa184 [PubMed]
-
49.
Keegan THM, Bleyer A, Rosenberg AS, Li Q, Goldfarb M. Second Primary Malignant Neoplasms and Survival in Adolescent and Young Adult Cancer Survivors. JAMA Oncol. 2017; 3:1554–7. https://doi.org/10.1001/jamaoncol.2017.0465 [PubMed]
-
50.
Pirani M, Marcheselli R, Marcheselli L, Bari A, Federico M, Sacchi S. Risk for second malignancies in non-Hodgkin's lymphoma survivors: a meta-analysis. Ann Oncol. 2011; 22:1845–58. https://doi.org/10.1093/annonc/mdq697 [PubMed]
-
51.
Goyal A, O'Leary D, Goyal K, Patel K, Pearson D, Janakiram M. Cutaneous T-cell lymphoma is associated with increased risk of lymphoma, melanoma, lung cancer, and bladder cancer. J Am Acad Dermatol. 2021; 85:1418–28. https://doi.org/10.1016/j.jaad.2020.06.1033 [PubMed]
-
52.
Das A, Chak A, Cooper GS. Temporal trend in relative risk of second primary colorectal cancer. Am J Gastroenterol. 2006; 101:1342–7. https://doi.org/10.1111/j.1572-0241.2006.00580.x [PubMed]
-
53.
Yang L, Xiong Z, Xie QK, He W, Liu S, Kong P, Jiang C, Xia X, Xia L. Second primary colorectal cancer after the initial primary colorectal cancer. BMC Cancer. 2018; 18:931. https://doi.org/10.1186/s12885-018-4823-6 [PubMed]
-
54.
Berrington de Gonzalez A, Curtis RE, Kry SF, Gilbert E, Lamart S, Berg CD, Stovall M, Ron E. Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol. 2011; 12:353–60. https://doi.org/10.1016/S1470-2045(11)70061-4 [PubMed]
-
55.
Allan JM, Travis LB. Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer. 2005; 5:943–55. https://doi.org/10.1038/nrc1749 [PubMed]
-
56.
Oh KS, Sandler HM. CounterPoint: second malignancies after radiotherapy for prostate cancer: keeping perspective. Urology. 2008; 72:971–3. https://doi.org/10.1016/j.urology.2008.07.016 [PubMed]