With aging, the risk of the onset of pancreatic cancer, particularly PDAC, is increasing. Among the patients, less than 10% appear having an inherited predisposition, which is in a familial setting with a low penetrance [8, 9]. Besides the possible link to genetic abnormality, factors that contribute to the pathology of pancreatic tumors have generally been shown to be pancreatitis, bad diet habits, tobacco smoking (possible e-cigarette smoking), and excessive alcohol consumption [10–13].
Genetic alterations during pancreatic tumorigenesis
Biological and biochemical analyses have demonstrated high genomic complexity in pancreatic cancer, in which mutations are frequently detected in somatic cells, and the tumors present highly heterogeneous with various genetic changes [14, 15]. These studies reveal high frequencies of genetic alterations in the tumors, which are noticed as changes of expressions of tumor suppressor genes and oncogenes, such as K-ras, T53, p16INK4A/ARF, MLH1, LKB1, PRSS1, and BRCA2 [16–18]. Among these genetic changes, we here focus on describing the oncogenic K-ras and, tumor suppressors’ p53 and p16INK (CDKN2, MTS1).
Mutant K-ras is detected in more than 90% of pancreatic cancer patients and considered as one of the possibly early elements in pancreatic cancer pathogenesis [16, 19–21]. K-ras is located on chromosome 12 and its encoded protein belongs to the Ras family of GTP-associating proteins. Ras family consists of three major forms, K-, H. and N-Ras and the active form of Ras transmits signals to promote various cellular activities, including cell growth, differentiation, survival and apoptosis under certain circumstances. In most of pancreatic cancer, the single mutation is detected at the position G12 of the amino acid sequence of K-Ras, which is substituted glycine to aspartic acid or valine [22, 23]. Less frequent missense mutations of K-ras are at the codons of 13, 59, 61 or 63 [24–26]. Balletic mutations of this oncogene are also revealed by deep sequencing of exomes [27]. Studies reveal the peri-ductal lymphocyte infiltration and gastric mucous neck cell hyperplasia in the pancreases of the genetically modified mutant K-ras mice [28, 29]. Furthermore, the specific mice are generated by crossing the knock-in mice in which a Cre-activated KrasG12D is knocked into the endogenous K-ras locus with mice expressing Cre recombinase that is expressed by a Pdx1 (pancreatic islet specific) or Ptf1-p48 (pancreatic acinar specific) [30–32]. These transgenic mice express Cre recombinase under the control of the mouse Pdx1 (pancreatic and duodenal homeobox 1) promoter. Mosaic Cre recombinase activity is detected in the pancreatic epithelium, antral stomach and duodenum in neonates and in pancreatic beta islet cells in adults. Specifically, early lesions in the mice are detected, in which the Notch pathways is noticeably activated with over-expressions of cyclooxygenase-2 (COX-2) or MMP-7, sometimes accompanied with metastasis. Thereafter, approximately one year after the development of PanIN, some of these genetic engineered pancreatic cancer mice develop pancreatic cancer (mainly PDAC), in which the origin of the tumors appears from acinar cells or acinar precursors. The spontaneous knockout or mutations of p53 or p16 permits a full penetrance of the cancer in the animals. Thus, these studies suggest the close connection among mutant K-ras, PanIN and pancreatic tumors.
Mutations in K-ras prohibit its encoded onco-protein to associate with and further be inhibited by GTPase-activating proteins (GAPs), leading to mutant Ras staying in a persistently or constitutively active status. As the result, multiple downstream effector pathways of Ras, such as Raf/ERK1/2/MEK or PI3K/Akt, are activated for promoting uncontrollable cell growth, desensitizing cell death, remodeling cellular metabolism, escaping from immune surveillance and increasing cell invasion. Studies showed that pancreatic tumor cells can be shed and further circulate in the blood stream. Therefore, mutant K-ras can be detected in circulating tumor cells, which has been used to facilitate clinical diagnostic imaging analyses [33].
p53 is located on chromosome 17 and its encoded protein serves as a tumor suppressor for protecting the integrity of the genome of cells. This tumor suppressor is often inactivated or mutated in about 70% of pancreatic cancer and most of mutations are the missense mutations, especially at the locus of R248, R273 or R175 [34]. Using animal models, studies demonstrate that the loss of heterozygosity (LOH) of p53 is an important factor for driving pancreatic cancer progression [35, 36]. Specifically, loss of p53 appears to cooperate with oncogenic K-ras-induced pancreatic cancer initiation and progression, by perturbing cell cycle progression, impairing DNA damage repair, augmenting survival activities and hindering apoptosis in cells [35, 36]. p53 mutations are more commonly detected in mutant K-ras pancreatic tumors than that expressing wild-type p53, suggesting that K-ras mutations developed in early stages of pancreatic tumorigenesis, creates a genetic background favoring p53 mutations. The cooperation of these two oncogenes or proteins promotes uncontrollable cell growth, cell cycle progression, improper damage repair and establishment of genetic instability, which promotes pancreatic tumorigenesis [35, 36]. p16INK (CDKN2, MTS1) is another tumor suppressor and its inactive form is found in a large number of pancreatic cancer patients [16, 37]. This suppressor gene is anchored on chromosome 9 and the encoded protein regulates the cell cycle by preventing cells from improperly entering the S phase through inhibiting cyclin-dependent kinase (CDK) 4/6. Due to the inactivation of p16INK by the promoter methylation, missense mutation and deletion, its related cell cycle checkpoint is perturbed, which allows pancreatic cancer cells to improperly progress from G1 to S phase without repairing potential damages. As the result, risks of genetic instability in cells are increased. This is further demonstrated by the animal study that the knockout of p16INK causes the deregulation of the cell cycle transition and rapid advance of pancreatic tumorigenesis a K-ras transgenic mouse strain [38]. Overall, mutations of p16INK and p53 are frequently observed in pancreatic tumors and the linear relationship of these two tumor suppressors in pancreatic tumorigenesis remains unclear.
Epigenetic abnormalities that alter DNA methylation, histone modification or microRNA expression are other factors to change gene functions in driving and promoting pancreatic tumorigenesis [32]. In some pancreatic cancers, tumor suppressor or DNA repair genes (such as CDKN2A, CDH1 and MLH1) are found to be silenced by methylation [16]. The over-expressions of microRNAs in pancreatic cancer have also been revealed, which seemed to participate in pancreatic neoplastic development [39–44].
Pathological precursors of pancreatic cancer and its subtypes
During the development of cancer in the pancreases, the conventional model of the progression of pancreatic cancer suggests that early genetic changes initiate tumor-prone activities in a cell or few cells that then undergo clonal expansion to achieve a full transformation. Another scenario is that pancreatic cancer cells disseminate early and then undergo transformation independently [38]. It is also being suggested that pancreatic cancer is originated from acinar cells that undergo the process of the acinar-to-ductal metaplasia (ADM), during which K-ras mutations are acquired [39–41]. Overall, genetic-initiated alterations are considered as the bases for the classification of different subtypes of pancreatic tumors. In recent years, using advanced biological techniques, such as genomic, transcriptomic and proteomic assays that enable to identify different characteristic clusters of tumor cells with different gene expressions and mutations, studies demonstrated that pancreatic tumors can be divided into different subtypes [39–41]. In particular, global gene expression analysis reveals three subtypes of PC as: classical, quasi-mesenchymal, and exocrine-like subtypes [39]. It also pointed out that the prognosis and therapeutic responses of these subtypes are different. The classical subtype of pancreatic cancer expresses high levels of adhesion-associated and epithelial genes. The quasi-mesenchymal subtype shows an augmented expression of mesenchyme-related genes. The exocrine-like subtype contains the tumor cells that express high levels of digestive enzyme genes. Three metabolic subtypes were also identified in pancreatic cancer patient samples by metabolomics analysis, as slow proliferating, glycolytic and lipogenic subtypes [42]. The classifications of the subtypes of the cancer by these methods correlate well with each other. In addition, the analyses of the genomic sequencing plus copy number variation measurement demonstrated the mutation landscapes of the cancer and four subtypes are accordingly classified as stable, locally rearranged, scattered and unstable [43]. The tumor-specific and stroma-specific subtypes of pancreatic cancer were also classified by RNA sequencing and computational analysis [44]. It is noticeable that various immunological features of the tumors are recently included for determining the tumor subtypes [41]. Taken together, the uses of current available modern techniques permit better understandings of pancreatic cancer subtypes at molecular levels and more accurate predictions for outcomes of treatments.
Although advanced technology helps us obtain the molecular insight into pancreatic cancer, it still remains enigma about the association of PanINs to the onset of pancreatic cancer, as inflammatory lesions are frequently detected in pancreatic cancer patients in early stages of the malignancy, but not in all PanIN patients. It is known after pancreatic inflammation or injury, acinar cells in pancreatic ducts start to lose their properties gradually and form lesions with changes around the ductal, which can be categorized pathologically in four grades (PanIN 1A, 1B, 2 and 3) [16, 38, 45]. The lesions of PanIN 1 are recognized by consisting of columnar epithelial cells with basally aligned nuclei [46]. The lesions are flat as PanIN 1A or papillary as PanIN 1B. PanIN 2 lesions have more changes in the nuclei manifested as loss of nuclear polarity, nuclear pleomorphism, hyperchromasia, or pseudo-stratification. In PanIN 3 lesions, a large degree of dysplasia exists, which alter architectures, such as formation of papillae or clusters of cells from the epithelium invading into the lumen of the duct, accompanied with various nuclear changes. Therefore, current research approaches for obtaining better defining subtypes, mutations of the cancer, together with external elements, certainly provide better distinguish of benign PanINs from those inflammation-associated neoplasia or tumorigenesis.
Overall, it is clear that through precursor lesions, pancreatic tumors in some PanIN patients are evolved [16, 38, 45, 46]. Along with the progression of a clone carrying neoplastic precursors to malignancy, mutations of K-ras and tumor suppressors drive tumorigenesis, which worsens with arising genomic instability or genetic heterogeneity for malignant advancing [47–49]. A better understanding of molecular alterations in pancreatic cancer development should provide early diagnostic opportunities for recognizing primary cancer lesions as well as early onset periods for effective clinic interventions. Various growth-related signaling pathways driven by oncogenes and mutated genes are involved in promoting the formation of invasive pancreatic tumors. The major aberrant signaling pathways driven by these mutant factors in pancreatic cancer initiation and progression are summarized in Figure 1.
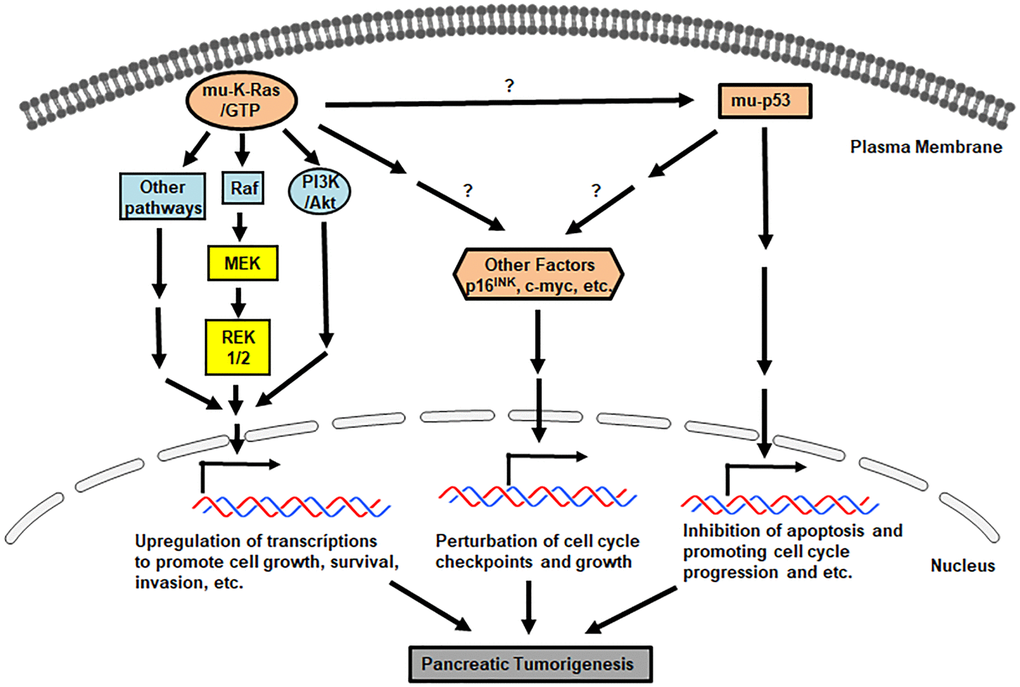
Figure 1. Pro-pancreatic tumorigenic, aberrant genes/proteins and pathways. Signals mediated by mu-K-ras, mu-p53 and loss of p16INK promote various uncontrollable cell growth, cell cycle progression and etc.