Identification and validation of prognostic autophagy-related genes associated with immune microenvironment in human gastric cancer
Abstract
Autophagy-related genes (ATGs) play critical roles in tumorigenesis and progression in gastric cancer (GC). The present study aimed to identify immune-based prognostic ATGs and verify their functions in tumor immune microenvironment (TIME) in GC. Macrophage infiltration was found to negatively correlate with prognosis in GC patients. After stratifying by infiltration levels of macrophages, we screened The Cancer Genome Atlas and Human Autophagy Database to identify the differentially expressed ATGs (DE-ATGs). Of 1,433 differentially expressed genes between the two groups, seven genes qualified as DE-ATGs. Of these, CXCR4, DLC1, and MAP1LC3C, exhibited strong prognostic prediction ability in Kaplan-Meier survival–log-rank test. High expression of these genes correlated with increased occurrence of advanced grade 3 tumors and poor prognoses. Furthermore, GSEA indicated that they were significantly associated with oncogenic and immune-related pathways. The comprehensive evaluation of TIME via GEPIA, ESTIMATE, CIBERSORT, and TIMER suggested that the three DE-ATGs were closely associated with immune condition, both in terms of immune cells and immune scores. Thus, the outcome of this study may aid in better understanding of the ATGs and their interaction with the immune microenvironment, which would allow the development of novel inhibitors, personalized treatment, and immunotherapy in gastric cancer.
Introduction
Gastric cancer (GC) is a common malignancy and is the third leading cause of cancer-related deaths worldwide [1]. The poor survival in patients with GC is primarily due to late diagnosis and suboptimal therapies. The development of immunotherapy has helped improve outcome in patients with GC [2, 3]. However, majority of the patients with GC fail to respond to immunotherapy, while the initial responders may develop resistance to the treatment [4]. Genetic changes play a critical role in immune evasion and suppression in the tumor microenvironment (TME) that may further regulate the occurrence and development of tumors [4, 5]. Therefore, combining targeted gene therapy with immunotherapy may enhance the treatment effect and overcome resistance to immunotherapy in GC.
Autophagy, similar to a “double-edged sword,” plays a dual role in inhibiting and promoting tumor formation and resistance to treatment [6, 7]. Studies suggest that autophagy can modulate immune responses by influencing immune cells and release of cytokines in the TME [8]. Additionally, the fundamental effects of autophagy on tumor progression and immune responses are mediated by autophagy-related genes (ATGs) [9]. ATGs perform widespread physiological functions in autophagy and other biological pathways, and thus, may provide us with novel therapeutic targets in GC [9, 10]. Studies have demonstrated that combining therapy targeting ATGs (induction or inhibition of autophagy) with immunotherapy may enhance the antitumor effects of immunotherapy and overcome immune resistance [8, 11].
The macrophages function as a connection between autophagy and immunity [11, 12]. As a major component of the TME, tumor-associated macrophages closely resemble the M2 macrophages that are related to immunosuppression and tumor progression in GC [13]. Moreover, using Tumor Immune Estimation Resource (TIMER) (https://cistrome.shinyapps.io/timer/), we found that macrophage infiltration was significantly negatively correlated with patient prognosis in GC, and patients with GC with high macrophage infiltration had poor survival outcome. Additionally, it is possible to screen prognostic ATGs related to GC immunity based on the infiltration of macrophages.
Here, using the Cancer Genome Atlas (TCGA) datasets, we performed differential expression analysis and survival analysis with ATGs related to GC immunity in patients stratified by macrophage infiltration levels. We explored the underlying biological pathways of ATGs and their clinical utility as prognostic signatures. Furthermore, we verified correlation between prognostic ATGs and the tumor immune microenvironment (TIME). We identified potential prognostic targets that may provide a foundation for subsequent immune-related work, such as enhancing antitumor effects of immunotherapy or selecting patients who may benefit from the treatment.
Results
Identification of differentially expressed autophagy-related genes (DE-ATGs) related to GC immunity
First, using TIMER, we investigated the correlation between overall survival (OS) and abundance of the six types of immune cells (B cells, CD4+ T cells, CD8+ T cells, neutrophils, macrophages, and dendritic cells). The analysis showed that macrophage infiltration was significantly negatively correlated with prognosis in patients with GC (Figure 1A). Based on the median macrophage infiltration levels, patients were segregated into high-infiltration and low-infiltration groups. Patients in the high-infiltration group had higher ESTIMATE scores, immune scores and stromal scores than those in low-infiltration group, indicating that macrophage infiltration levels could accurately reflect the TME (Figure 1B). Next, we performed gene expression data analysis in GC patient samples with variable macrophage infiltration levels, and identified 1,433 differentially expressed genes (DEGs). Of these, 1,337 genes were significantly upregulated and 96 were downregulated in the high-infiltration group than in low-infiltration group (Figure 1C, 1D). Furthermore, seven DEGs were screened as DE-ATGs related to GC immunity (Figure 1E).
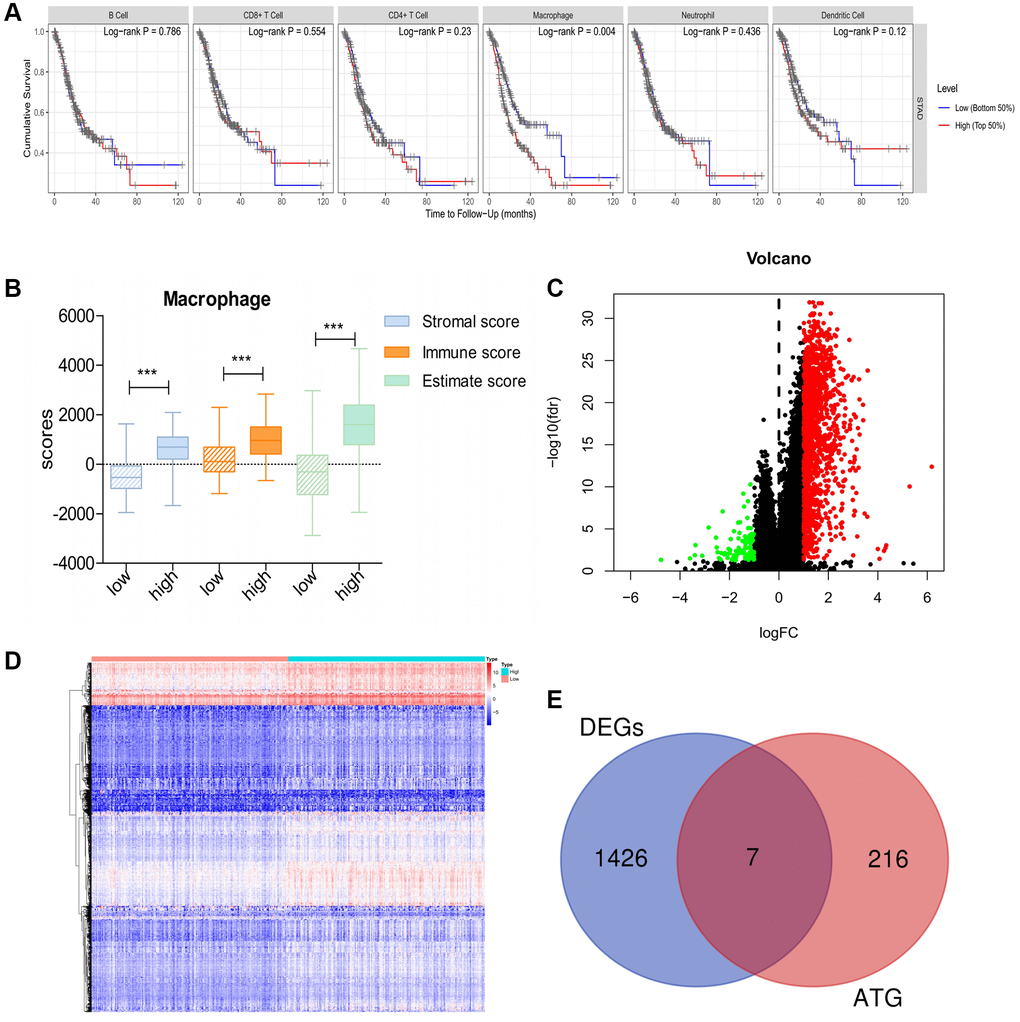
Figure 1. DE-ATGs related to GC immunity. (A) Macrophage infiltration is significantly negatively correlated with prognosis in patients with GC. (B) Distribution of ESTIMATE scores, immune scores, and stromal scores between high- and low-macrophage infiltration groups. (C, D) Volcano plot and Heatmap of the DEGs between high- and low-macrophage infiltration groups (Upregulated genes are indicated in red dots; downregulated genes are indicated in green dots). (E) Venn diagram analysis of DE-ATGs between DEGs and ATGs.
Prognostic significance of DE-ATGs in GC
To assess the predictive significance of the DE-ATGs, we performed Kaplan-Meier survival analysis for the seven DE-ATGs related to GC immunity. The analysis identified three genes as prognostic factors in patients with GC, and that patients with low expression of CXCR4, DLC1, and MAP1LC3C, (p = 0.001, p = 0.046, and p = 0.021) had better prognoses than those with high expression (Figure 2A–2C). The expression levels of CXCR4, DLC1, and MAP1LC3C were significantly different in the high-infiltration and low-infiltration groups (Figure 2D–2F). Moreover, all three genes (CXCR4, DLC1, MAP1LC3C) showed significant prognostic capability in the GEPIA analysis (p < 0.05; hazard ratio (HR) > 1; Figure 2G–2I). Furthermore, the multivariate regression Cox analysis suggests that all three genes (CXCR4, DLC1, MAP1LC3C), age and tumor stage are important factors that correlate with survival outcome in GC patients (p < 0.05; hazard ratio (HR) > 1; Figure 2J–2L). And, both these analyses demonstrate the effective prognostic prediction by CXCR4, DLC1 and MAP1LC3C.
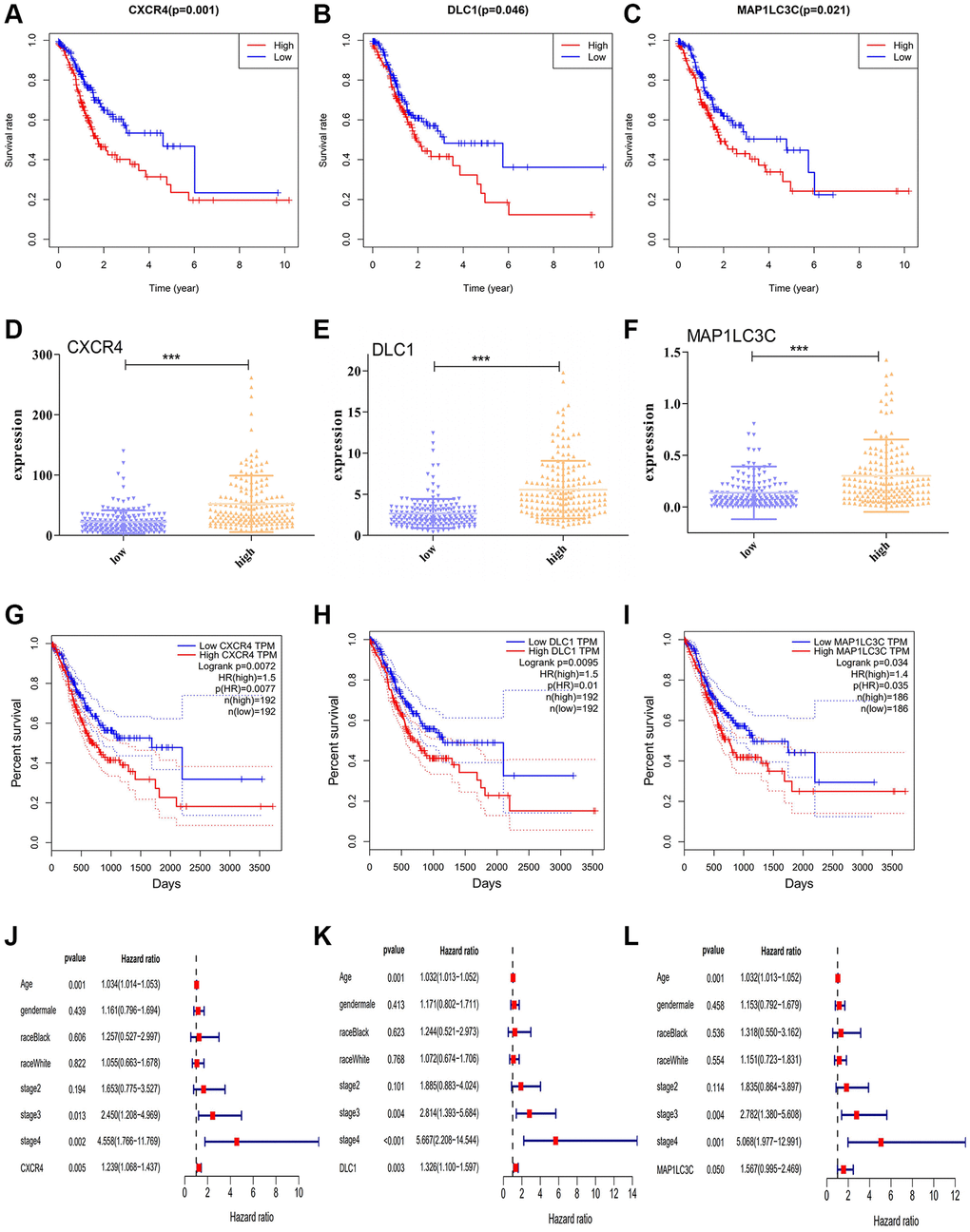
Figure 2. Prognostic significance of DE-ATGs in GC. (A–C) Kaplan-Meier curves for three prognostic DE-ATGs (CXCR4, DLC1, and MAP1LC3C) related to immunity in patients with GC (P < 0.05). (D–F) The expression level of CXCR4, DLC1, and MAP1LC3C in high- and low-macrophage infiltration groups. (G–I) GEPIA-based validation that DE-ATGs (CXCR4, DLC1, and MAP1LC3C) are effective prognostic indicators and risk factors for GC. (J–L) Tree diagram of a multivariate regression analysis for CXCR4, DLC1, and MAP1LC3C with other clinical variables.
Clinical utility of prognostic DE-ATGs (CXCR4, DLC1, and MAP1LC3C) in patients with GC
As high expression of CXCR4, DLC1, and MAP1LC3C was significantly related to worse OS, we further analyzed the relationship of these genes with clinical features in GC, such as grade, clinical stage, and TNM stage. Consistent with outcome of the survival analysis, CXCR4 was significantly upregulated in GC patients with younger ages and worse tumor status, including the grade 3 (p = 0.0062) and M1 stage, than in patients with other tumor statuses (p = 0.0194; Figure 3A). Moreover, DLC1 and MAP1LC3C also showed a higher expression in the grade 3 group than in other groups (p = 0.0006 and p = 0.0012; Figure 3B, 3C). CXCR4 and MAP1LC3C also showed a higher expression in the younger patients (age ≤ 65, p < 0.01; Figure 3A, 3C).
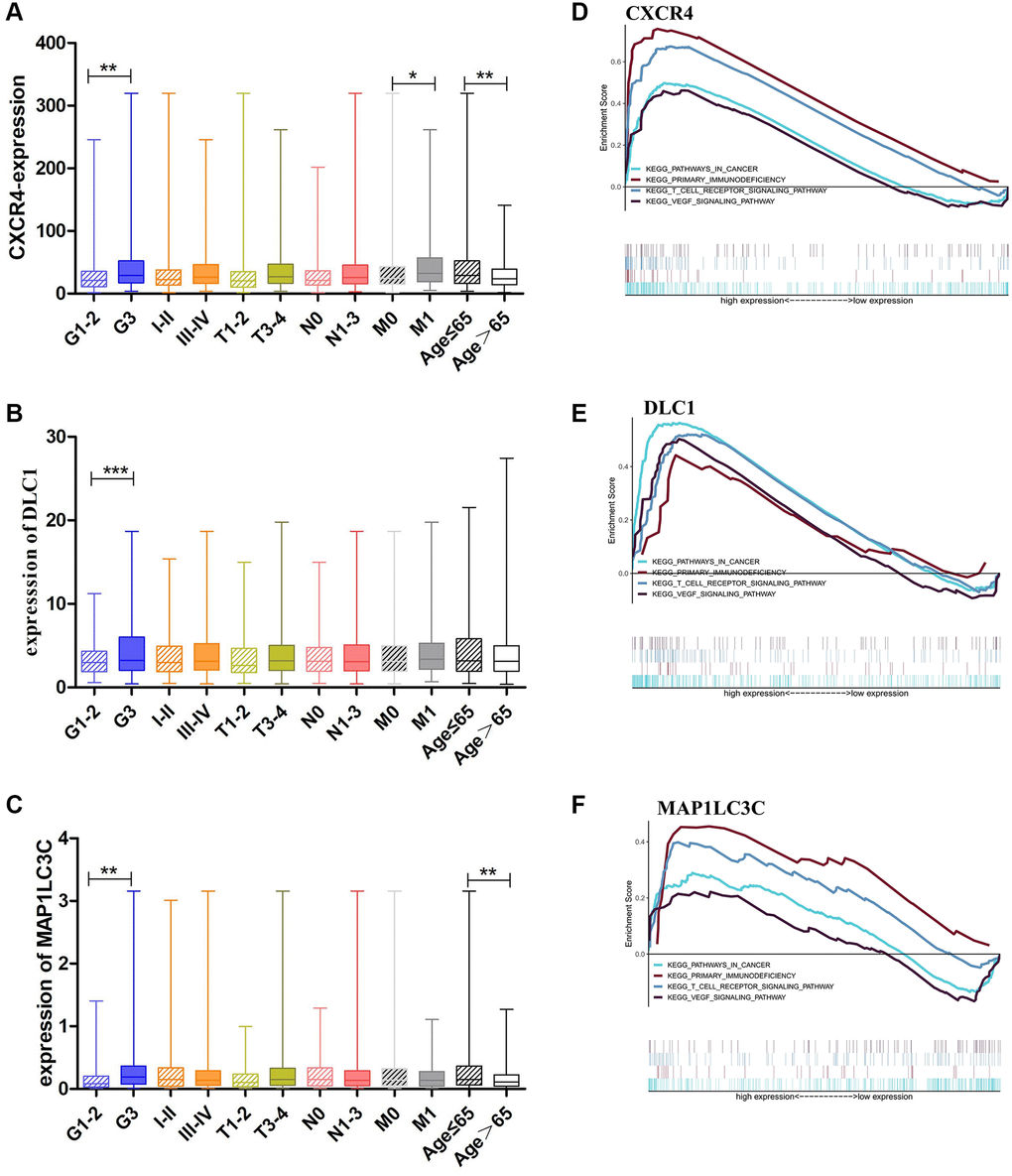
Figure 3. Clinical evaluation and identification of signaling pathways associated with CXCR4/DLC1/MAP1LC3C. (A, B) Expression of CXCR4/DLC1/MAP1LC3C in different grade stage, clinical stage, T stage and age groups in patients with GC. (C–F) CXCR4, DLC1, and MAP1LC3C are significantly enriched in pathways related to immune response and cancer in GSEA (FDR < 0.25, NP < 0.05).
Signaling pathways related to the prognostic DE-ATGs (CXCR4, DLC1, and MAP1LC3C)
We performed Gene Set Enrichment Analysis (GSEA) using the prognostic DE-ATGs in the high and low expression groups in the TCGA GC cohort. The analysis indicated that CXCR4, DLC1, and MAP1LC3C were significantly (FDR < 0.25, NP < 0.05) enriched in pathways related to immune response and cancer. The intersecting immune-related pathways of the three genes included primary immunodeficiency and T cell receptor signaling pathway; and those of the intersecting cancer-related pathways included pathways in cancer and VEGF pathway (Figure 3D–3F).
Correlation of prognostic DE-ATGs (CXCR4, DLC1, and MAP1LC3C) with TIME using CIBERSORT, TIMER, and ESTIMATE
To verify the correlation between the prognostic DE-ATGs and immunity, we first investigated the differences between 22 subpopulations of tumor-infiltrating immune cells (TIICs) in DE-ATGs high and low expression groups using the CIBERSORT Algorithm [14]. Filtering with CIBERSORT p < 0.05, we selected 96 GC samples for the subsequent analysis (Figure 4A). The analysis suggested that compared with proportion in CXCR4 low expression group, high CXCR4 expression group had higher proportions of regulatory T cells (Treg cells, p = 0.007), and relatively lower proportions of memory B cells (p = 0.007; Figure 4B, 4C). Further, compared with proportions in low DLC1 expression group (Figure 5A, 5B), the proportion of resting memory CD4+ T cells (p = 0.013) and Treg cells (p = 0.047) was significantly higher (p = 0.029), and that of plasma cells (p = 0.006), follicular helper T cells (p = 0.002) and M1 macrophages (p = 0.039) was significantly lower in high DLC1 expression group. However, no significant differences in the proportions of immune cells between MAP1LC3C high- and low-expression groups were observed (Figure 6A, 6B).
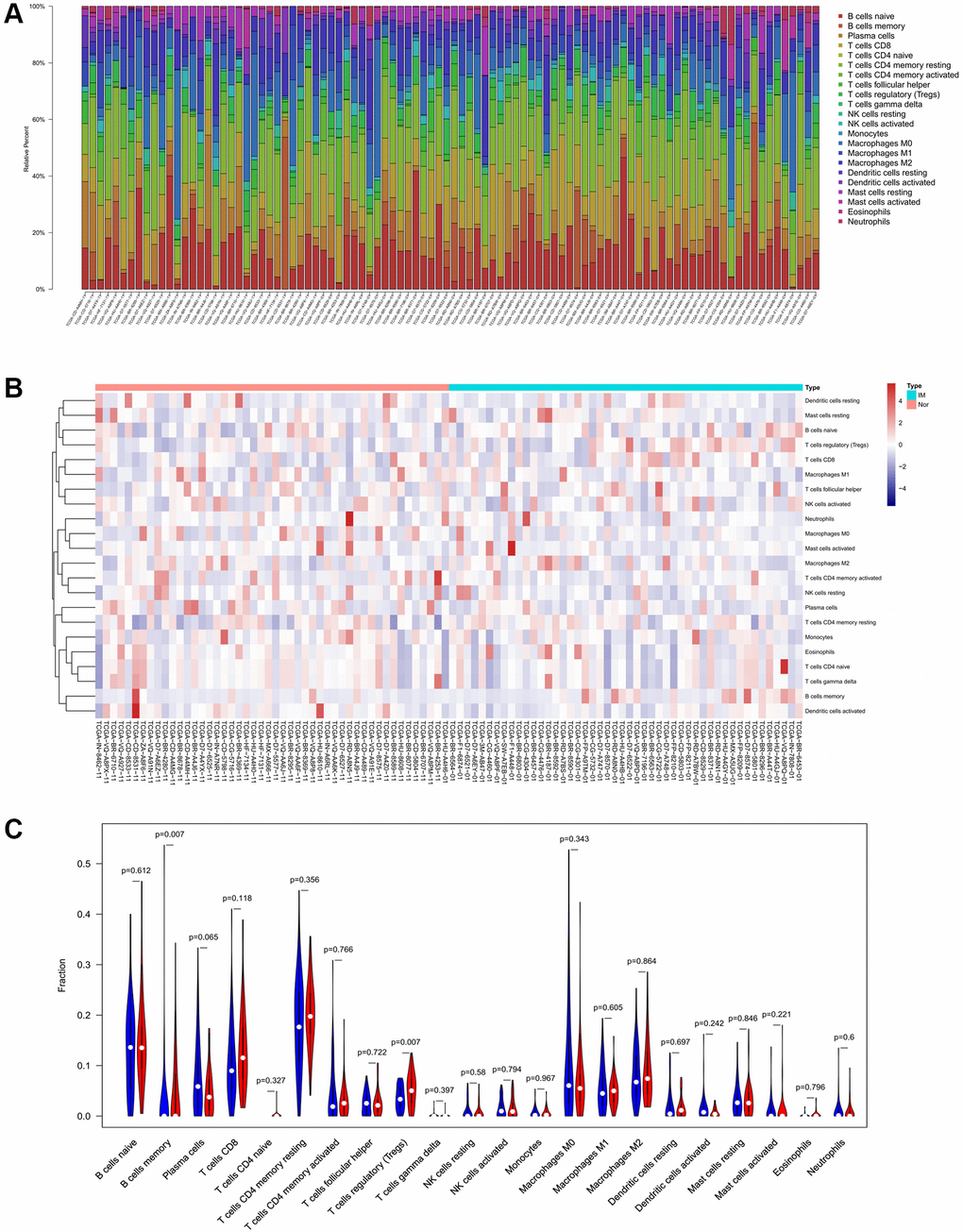
Figure 4. Composition of 22 TIICs in the TCGA cohort with CIBERSORT p < 0.05 for all qualified samples. (A) Fractions of 22 immune cells in qualified tumor samples (n = 96) in the TCGA. (B and C) Heatmap and violin plot comparing the immune cells between high- and low-CXCR4 expression groups.
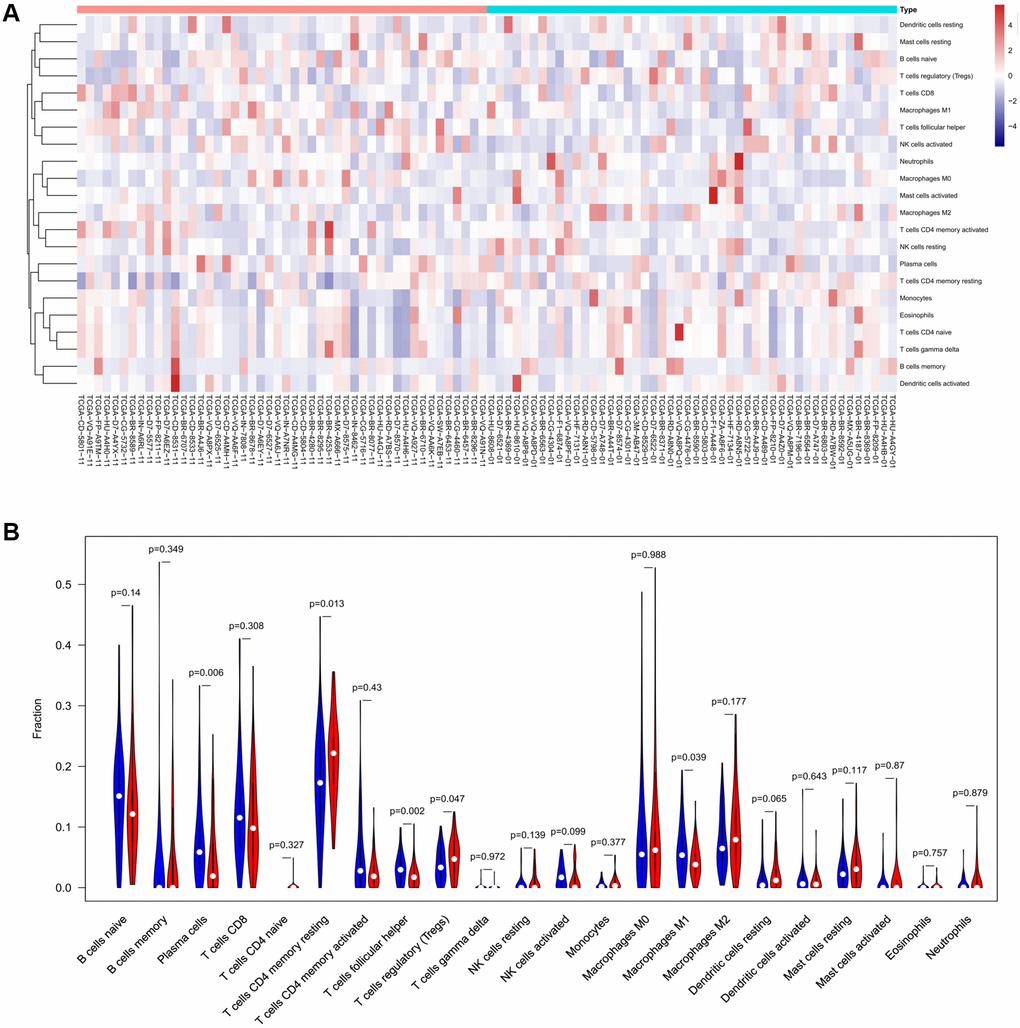
Figure 5. The distinct compositions of 22 TIICs in the high- and low-DLC1 expression groups are shown using (A) heatmap and (B) violin plot and analyzed with CIBERSORT.
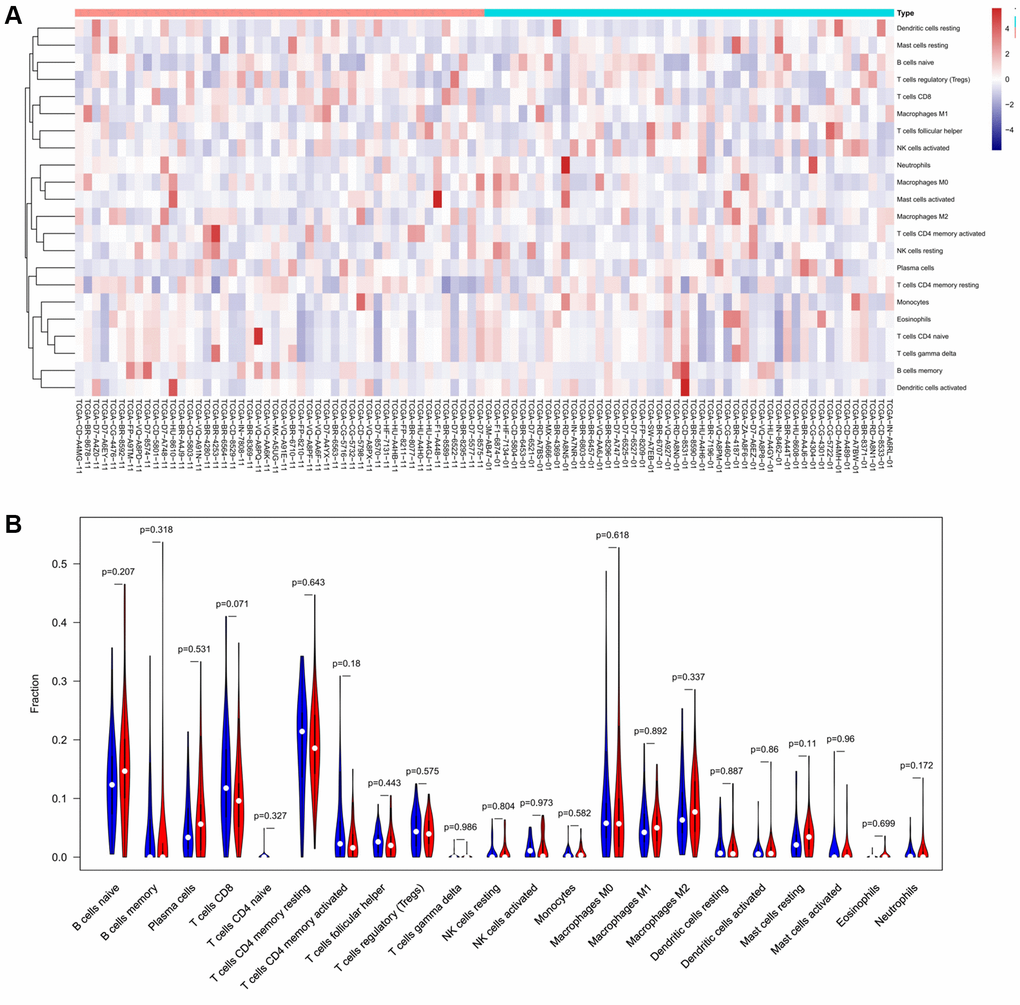
Figure 6. The distinct compositions of 22 TIICs in the high- and low-MAP1LC3C expression groups are shown using (A) heatmap and (B) violin plot and analyzed with CIBERSORT.
Next, we checked the correlation of DE-ATGs with TIICs using TIMER. The analysis showed that expression signature of CXCR4 and MAP1LC3C had significant positive association with CD8+ T cells infiltration, CD4+ T cells infiltration, macrophage infiltration, neutrophil infiltration, and dendritic cells infiltration (Correlation coefficient >0.3; p < 0.05; Figure 7A, 7C). Moreover, the expression signature of DLC1 was significantly positively correlated with CD4+ T cells infiltration, macrophage infiltration, and dendritic cells infiltration (Correlation coefficient > 0.3; p < 0.05; Figure 7B).
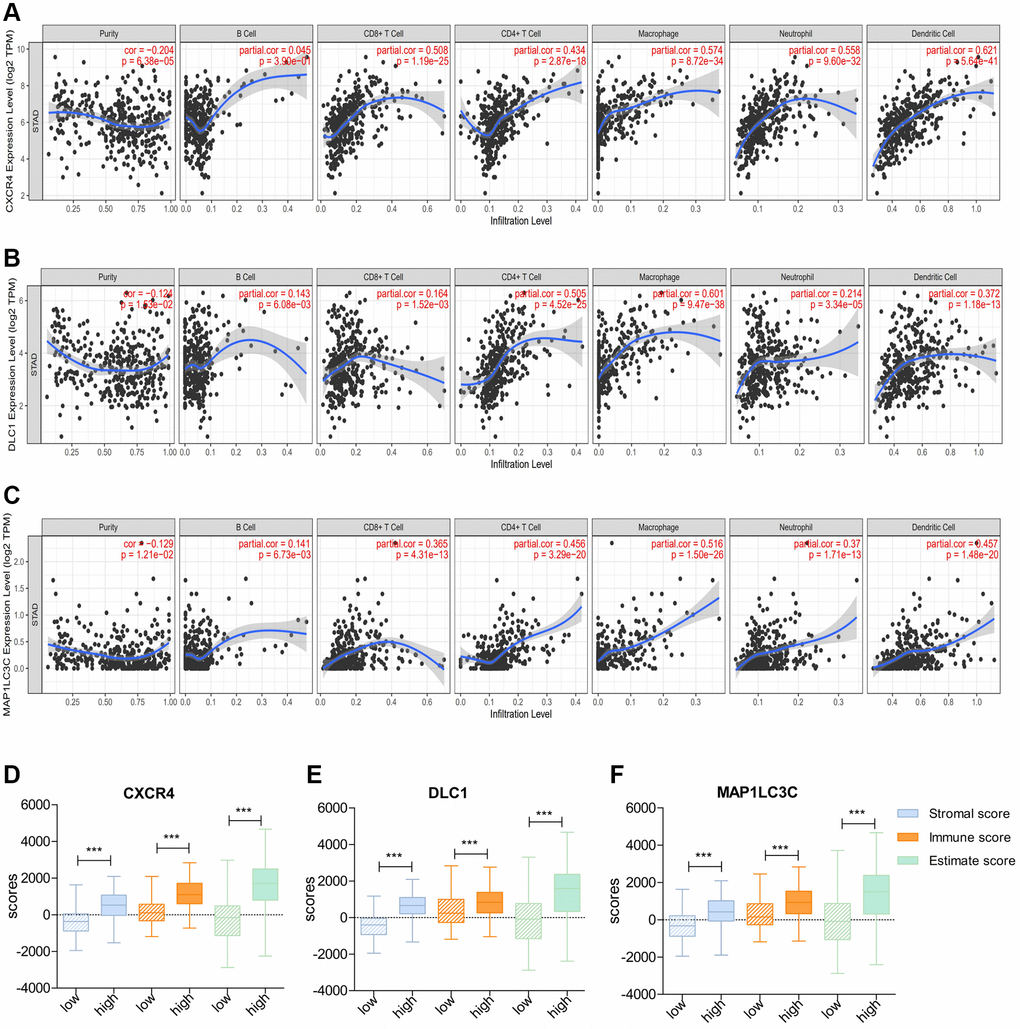
Figure 7. Correlation of prognostic immune-related ATGs with characterization of the tumor immune environment. (A–C) Relationship of CXCR4/DLC1/MAP1LC3C with B cells, CD8+ T cells, CD4+ T cells, macrophages, neutrophils, and dendritic cells using TIMER. (D–F) Distribution of ESTIMATE scores, immune scores, and stromal scores between high- and low-CXCR4/DLC1/MAP1LC3C-expression groups.
Consistent with above results, patients with high expression of all three DE-ATGs (CXCR4, DLC1, and MAP1LC3C) had a higher stromal score, immune score, and estimate score than those with low expression; this suggested that high-expression samples were infiltrated with more immune cells than low-expression samples (p < 0.05; Figure 7D–7F).
Correlation of prognostic DE-ATGs (CXCR4, DLC1, and MAP1LC3C) with immune checkpoint inhibitor genes or surface molecules of immune cells
Further, we checked correlation between prognostic DE-ATGs and immune checkpoint inhibitor genes or surface molecules of immune cells, using GEPIA, to elucidate the function of prognostic DE-ATGs in the immune checkpoint blockade (ICB) therapy. The analysis indicated that expression of CXCR4 was significantly positively correlated to expression of CD274 (R = 0.31; p < 0.05), PDCD1 (R = 0.47; p < 0.05), CTLA4 (R = 0.62, p < 0.001), LAG3(R = 0.36; p < 0.05), CSF1(R = 0.53; p < 0.05) and NT5E (R = 0.14; p < 0.05)in GC (Figure 8A); and, expression of DLC1 was significantly positively correlated to expression of CD274 (R = 0.14; p < 0.05), PDCD1 (R = 0.19; p < 0.05) , CTLA4 (R = 0.25, p < 0.001), CSF1 (R = 0.69; p < 0.05) and NT5E (R = 0.26; p < 0.05) in GC (Figure 8B). The expression of MAP1LC3C was significantly positively correlated to expression of PDCD1 (R = 0.31; p < 0.05), CTLA4 (R = 0.26, p < 0.001), LAG3 (R = 0.16; p < 0.05), CSF1 (R = 0.46; p < 0.05) and NT5E (R = 0.13; p < 0.05) in GC (Figure 8C). Therefore, CXCR4, PDCD1 and MAP1LC3C may function as non-negligible factors in cancer immunity.
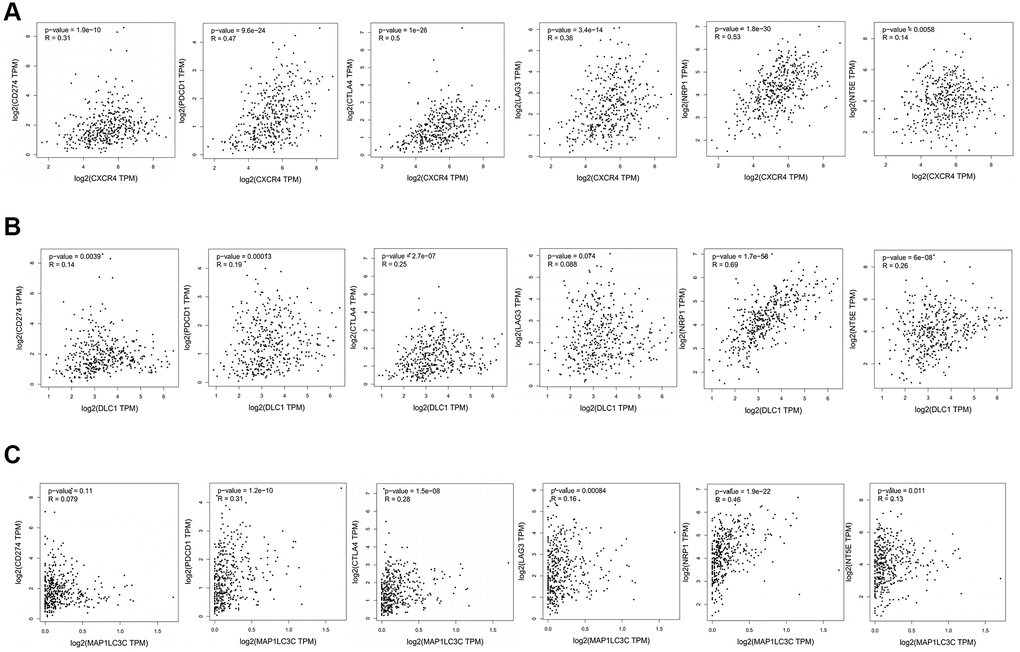
Figure 8. Association between CXCR4/DLC1/MAP1LC3C and crucial immune checkpoint genes or surface molecules. Analyses of association of ICB-related genes or surface molecules (CD274/PDCD1/CTLA4/LAG-3/NRP-1/CD73) with (A) CXCR4, (B) DLC1, and (C) MAP1LC3C.
Discussion
GC is a common malignancy and a leading cause of cancer-related mortality worldwide [1]. Moreover, despite the encouraging advancements made in cancer immunotherapy targeting the TME, only few patients with GC have achieved satisfactory therapeutic effects. The factors contributing to the poor curative effect in GC include tumor recurrence, metastasis, insensitivity to immunotherapy, heterogeneous molecular characterization, and poor selection of target genes [15, 16]. Therefore, it is important to develop accurate and powerful molecular biomarkers to elucidate novel effects of immunotherapy in GC.
Autophagy is shown to play a key role in physiological and pathological processes. Moreover, dysregulated expression of ATGs influences tumorigenesis, progression, and therapeutic resistance in multiple cancers, including GC [9, 17, 18]. Therefore, DE-ATGs can be employed as new prognostic indicators and prospective therapeutic targets. Moreover, the use of new therapeutic avenues targeting autophagy may enhance the antitumor effects of immunotherapy, and improve the treatment outcome [8, 19]. In the present study, we found that macrophages that function as a bridge between autophagy and immunity [6, 7] were significantly negatively correlated with prognosis in GC. Further, three genes—CXCR4, DLC1, and MAP1LC3C—were identified as prognostic DE-ATGs that were associated with immune conditions via microphages (Figure 9).
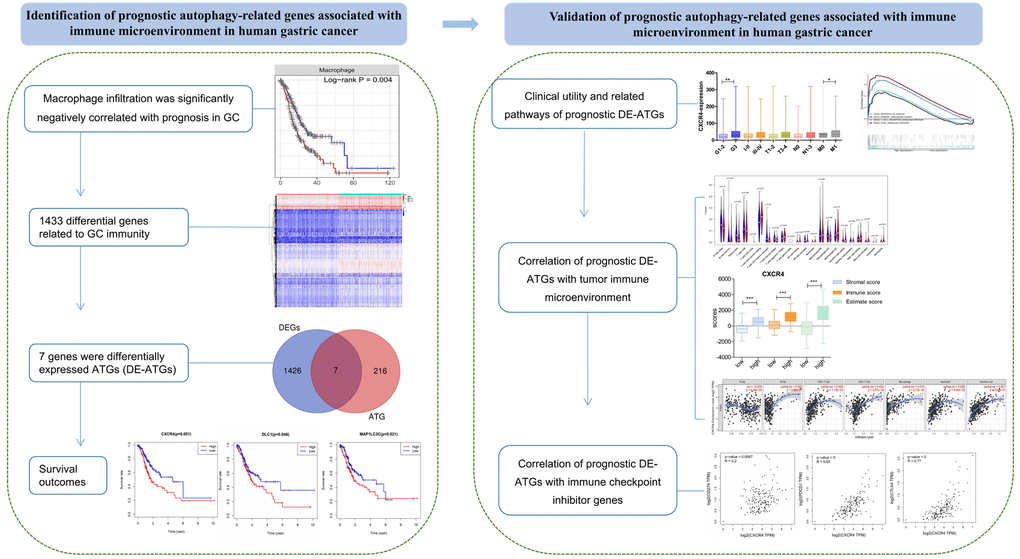
Figure 9. The workflow of the study. Construction and validation of the prognostic autophagy-related genes.
Of the three prognostic DE-ATGs, CXCR4 was found to be significantly upregulated and suggested poor prognosis in many cancer types, including GC [20, 21]. CXCR4 performs important functions in regulating tumor growth, proliferation, metastasis, autophagy, and immune responses in cancers [22]. Studies have focused on the CXCL12-CXCR4 chemokine axis due to its effects on macrophage recruitment and polarization, and immune cell migration [23, 24]. The effects of AMD3100, a CXCR4 antagonist, in combination with PD-L1 and PD-1 inhibition have been tested as an ICB therapy in pre-clinical models [25–27]. Some of our findings are consistent with these previous bioinformatics or experimental observations that can be mutually complementary and verified [28–30]. But these studies are not comprehensive, and on top of that, we further checked the correlation between CXCR4 and surface markers of immune cells to elucidate the interaction between the CXCR4 and the potential related immune cells that may help improve strategies for CXCR4 blockade in combinatorial immune-based therapies.
For DLC1, which is known to function as a GTPase-activating protein for Rho family members, plays an important role in cancer development and progression [31, 32]. In various cancer types, DLC1 was identified as a potential tumor suppressor, however, the effects of DLC1 do not work in only one direction [33]. The expression of DLC1 has been reported to be reduced in gastric cancer, but the sample size in these studies was small [34, 35]. Regardless of the clear role of DLC1 or its underlying mechanism remains elusive. The present study further clarified the prognostic value of DLC1 and analyzed its interaction with the immune microenvironment.
Few existing studies have shown that the human Atg8-family protein-MAP1LC3C may be essential in particular biological responses, including cell autophagy, motility and invasion [36, 37]. Some bioinformatics analyses have revealed the potential value of MAP1LC3C in cancer [38, 39], but its role and mechanism in GC has seldom been discussed systematically up to now. The present study filled some gaps in the existing research, which identified higher expression of MAP1LC3C to be significantly associated with poorer prognoses and more advanced tumor grade 3. Moreover, the expression signature of MAP1LC3C had significant positive association with CD8+ T cells infiltration, CD4+ T cells infiltration, macrophage infiltration, neutrophil infiltration, and dendritic cells infiltration. Therefore, studying MAP1LC3C may provide newer insights for the prognosis and design of immune-based therapies in gastric cancer.
GSEA indicated that these prognostic genes were strongly associated with oncogenic and immune-related pathways. Then we found that all three prognostic DE-ATGs were closely associated with immune condition, both in terms of immune cells and immune scores. Various immune cells play different role in the immunosuppression and tumor progression, which are also related to prognosis of GC. Infiltration of some immune cells in tumors, such as CD8+ T cells, CD4+ T cells and NK cells, usually portends a more favorable prognosis. In contrast, tumors infiltrated by neutrophils and macrophages generally tend to have a poor prognosis [40]. Effector CD8+ T cells could produce a variety of chemokines to regulate tumor growth and development [41, 42]. As a major component of the TME, CD4+ T cells may attack tumor cells directly through cytolytic responses or indirectly through regulating other lymphocytes, such as strengthening B cell and cytotoxic T cells (CTLs) responses [43, 44]. Studies showed that N2 neutrophils inhibited the activity of anti-tumor CD8+ T cells by producing iNOS in breast cancer [45, 46]. DCs are also associated with immunosuppression and tumor progression via the initiation of CD8+ and CD4+ T cells [47]. However, the significant heterogeneity in the location and density of immune cells may affect their prognostic assessment [40]. As surface marker for CD8+ T cells, CD4+ T cells, and NK cells, LAG-3 can suppress their proliferation and effector activity [47–49]. NRP-1 is expressed in T cells and dendritic cells (DCs) and is associated with their activity and interaction [50]. CD73 (NT5E) is a potential surface marker for macrophage [51]. ICB immunotherapy (CD274, CTLA4, and PDCD1) is also being integrated into first-line therapy and gaining greater importance [3, 52, 53]. As the significant correlation between DE-ATGs and immune cells or surface makers of immune cells, we hypothesized DE-ATGs may connect autophagy with immunity via different surface markers (Supplementary Figure 1).
The bioinformatics-based studies on ATGs have focused on prognostic role of ATGs in GC [28, 54]. In contrast, the present study evaluated the link between autophagy and cancer immunity via macrophages to screen immune-related prognostic ATGs, which has not been elucidated previously. We believe that the identified immune-related prognostic ATGs may aid in developing combined immunotherapy and predict the response rate to immunotherapy in GC patients. However, the study has a few limitations. First, the study was heavily dependent on the publicly available datasets that provide less information about the ICB therapy administered in patients. Second, the function and significance of ATGs identified in the study remained to be elucidated; but we will attempt to clarify their role in future experimental in vitro and in vivo validation studies.
In summary, the present study provides immune-related ATGs that predict the OS in patients with GC. We also explored the clinical utility and verified the comprehensive landscape of TIME for these prognostic immune-related ATGs. We believe that the outcome of the present study may aid in better understanding of the ATGs and their interaction with the immune microenvironment, which would allow the development of novel inhibitors, personalized treatment, and immunotherapy in GC.
Materials and Methods
Identification of DEGs
A correlation between the levels of TIICs and survival in patients with GC was established using TIMER (https://cistrome.shinyapps.io/timer/, till October 18, 2020). Further, we distributed patients with GC into two groups in accordance with the macrophage infiltration levels that were negatively correlated with patients’ OS. Adjusted P-value (adj.P) < 0.01 and |fold change (FC)| > 1.0 were applied to identify DEGs between the two groups using edgeR package in R version 4.0.3. Heatmap and volcano plots of DEGs were generated using pheatmap2 package in R software. DE-ATGs were obtained by intersection of the DEGs and ATGs, and visualized using Venn diagrams.
Survival analysis and prognostic DE-ATGs
To identify potential prognostic DE-ATGs, we distributed patients with GC into high- and low-expression groups in accordance with the median expression levels of DE-ATGs. Further, log-rank test was assessed using R software to estimate the correlation between expression levels of DE-ATGs and OS, and the results were visualized using Kaplan-Meier (K-M) survival curve. Prognostic independence was validated using multivariate Cox Proportional Hazards model of the DE-ATGs and other clinic-pathological factors.
Gene set enrichment analysis (GSEA)
GSEA (http://www.broadinstitute.org/gsea/) was performed to assess related pathways and molecular mechanisms in patients with GC. For each analysis, we performed 1,000 times gene set permutations to obtain a normalized enrichment score (NES) and an enrichment score (ES). Enriched gene sets with a normalized P (NP) < 0.01 and false discovery rate (FDR) < 0.25 were considered statistically significant.
Correlation of prognostic DE-ATGs with characterization of TIME
The abundance of 22 types of TIICs—naive B cells, memory B cells, plasma cells, CD8+ T cells, naive CD4+ T cells, resting memory CD4+ T cells, activated memory CD4+ T cells, follicular helper T cells, Treg cells, gamma delta T cells, resting NK cells, activated NK cells, monocytes, M0 macrophages, M1 macrophages, M2 macrophages, resting dendritic cells, activated dendritic cells, resting mast cells, activated mast cells, eosinophils, and neutrophils—was determined using the CIBERSORT algorithm. CIBERSORT uses a previously reported statistical method to quantify the infiltrated immune cell composition fractions on the basis of gene signature matrix [14]. Only 96 GC samples with CIBERSORT p < 0.05 were selected for the subsequent analysis. Further, these 96 GC samples were distributed into high- and low-expression groups in accordance with the median expression levels of prognostic DE-ATGs. The Wilcoxon test was performed to analyze differential infiltrations of the 22 TIICs in the two groups. Correlations between different immune cells were tested by R package corrplot.
Correlation between expression levels of prognostic DE-ATGs and abundance of TIICs
We selected ICB-related genes or surface molecules for immunotherapy: programmed death ligand 1 (PD-L1 or CD274), programmed death 1 (PD-1 or PDCD1), cytotoxic T-lymphocyte antigen 4 (CTLA-4), LAG-3, NRP-1, and CD73(NT5E). Further, we analyzed their expression levels between low- and high-expression prognostic DE-ATGs groups using Gene Expression Profiling Interactive Analysis (GEPIA, http://gepia.cancer-pku.cn/).
Statistical analysis
All analyses were performed using R software version 4.03. For all statistical tests, p < 0.05 was considered as statistically significant. The DEGs were evaluated with Wilcoxon test using R software. Wilcoxon test was performed to analyze the different expression levels of prognostic DE-ATGs in several subgroups of clinical features.
Ethics approval and consent to participate
The databases are publicly available and open to access, so this study did not need the approval from the ethics committee.
Abbreviations
GC: Gastric cancer;
DEG: Differentially-expressed genes;
ATGs: Autophagy-related genes;
DE-ATGs: Differentially expressed ATGs;
TCGA: The Cancer Genome Atlas;
OS: Overall survival;
TIME: Tumor immune microenvironment;
TIICs: Tumor-infiltrating immune cells;
ICB: Immune checkpoint blockade.
Author Contributions
Ruyue Tian conceived the project and wrote the manuscript. Ruyue Tian, Ya Sun, Xuedi Han, Jiajun Wang and Hongli Gu participated in data analysis, discussion and language editing. Wenhai Wang and Lei Liang reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors thank members of Department of Ultrasound, Aero Space Central Hospital and Department of Gastroenterology, Beijing Friendship Hospital for data processing. We also appreciated TCGA database for providing the original study data.
Conflicts of Interest
The authors declare no conflicts of interest related to this study.
Funding
This study was fully supported by Hospital level project of Aerospace Center Hospital (YN202105), Beijing Association of Science and Technology Golden Bridge Project Seed Fund (ZZ21054), Aerospace Medical and Health Science and Technology Group, (2021YK01) and Scientific research and cultivation plan of health development in Haidian District (HP2021-32-50702).
Editorial Note
&
This corresponding author has a verified history of publications using a personal
email address for correspondence
References
-
1.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. https://doi.org/10.3322/caac.21492 [PubMed]
-
2.
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020; 396:635–48. https://doi.org/10.1016/S0140-6736(20)31288-5 [PubMed]
-
3.
Lordick F, Shitara K, Janjigian YY. New agents on the horizon in gastric cancer. Ann Oncol. 2017; 28:1767–75. https://doi.org/10.1093/annonc/mdx051 [PubMed]
-
4.
Olino K, Park T, Ahuja N. Exposing Hidden Targets: Combining epigenetic and immunotherapy to overcome cancer resistance. Semin Cancer Biol. 2020; 65:114–22. https://doi.org/10.1016/j.semcancer.2020.01.001 [PubMed]
-
5.
Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021; 71:264–79. https://doi.org/10.3322/caac.21657 [PubMed]
-
6.
Wu MY, Lu JH. Autophagy and Macrophage Functions: Inflammatory Response and Phagocytosis. Cells. 2019; 9:70. https://doi.org/10.3390/cells9010070 [PubMed]
-
7.
Silva VR, Neves SP, Santos LS, Dias RB, Bezerra DP. Challenges and Therapeutic Opportunities of Autophagy in Cancer Therapy. Cancers (Basel). 2020; 12:3461. https://doi.org/10.3390/cancers12113461 [PubMed]
-
8.
Jiang GM, Tan Y, Wang H, Peng L, Chen HT, Meng XJ, Li LL, Liu Y, Li WF, Shan H. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol Cancer. 2019; 18:17. https://doi.org/10.1186/s12943-019-0944-z [PubMed]
-
9.
Levine B, Kroemer G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell. 2019; 176:11–42. https://doi.org/10.1016/j.cell.2018.09.048 [PubMed]
-
10.
Amaravadi RK, Kimmelman AC, Debnath J. Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 2019; 9:1167–81. https://doi.org/10.1158/2159-8290.CD-19-0292 [PubMed]
-
11.
Zhong Z, Sanchez-Lopez E, Karin M. Autophagy, Inflammation, and Immunity: A Troika Governing Cancer and Its Treatment. Cell. 2016; 166:288–98. https://doi.org/10.1016/j.cell.2016.05.051 [PubMed]
-
12.
Clarke AJ, Simon AK. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat Rev Immunol. 2019; 19:170–83. https://doi.org/10.1038/s41577-018-0095-2 [PubMed]
-
13.
Gambardella V, Castillo J, Tarazona N, Gimeno-Valiente F, Martínez-Ciarpaglini C, Cabeza-Segura M, Roselló S, Roda D, Huerta M, Cervantes A, Fleitas T. The role of tumor-associated macrophages in gastric cancer development and their potential as a therapeutic target. Cancer Treat Rev. 2020; 86:102015. https://doi.org/10.1016/j.ctrv.2020.102015 [PubMed]
-
14.
Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015; 12:453–7. https://doi.org/10.1038/nmeth.3337 [PubMed]
-
15.
Grosser R, Cherkassky L, Chintala N, Adusumilli PS. Combination Immunotherapy with CAR T Cells and Checkpoint Blockade for the Treatment of Solid Tumors. Cancer Cell. 2019; 36:471–82. https://doi.org/10.1016/j.ccell.2019.09.006 [PubMed]
-
16.
Nagaraja AK, Kikuchi O, Bass AJ. Genomics and Targeted Therapies in Gastroesophageal Adenocarcinoma. Cancer Discov. 2019; 9:1656–72. https://doi.org/10.1158/2159-8290.CD-19-0487 [PubMed]
-
17.
Lorente J, Velandia C, Leal JA, Garcia-Mayea Y, Lyakhovich A, Kondoh H, LLeonart ME. The interplay between autophagy and tumorigenesis: exploiting autophagy as a means of anticancer therapy. Biol Rev Camb Philos Soc. 2018; 93:152–65. https://doi.org/10.1111/brv.12337 [PubMed]
-
18.
Bhol CS, Panigrahi DP, Praharaj PP, Mahapatra KK, Patra S, Mishra SR, Behera BP, Bhutia SK. Epigenetic modifications of autophagy in cancer and cancer therapeutics. Semin Cancer Biol. 2020; 66:22–33. https://doi.org/10.1016/j.semcancer.2019.05.020 [PubMed]
-
19.
Xia H, Green DR, Zou W. Autophagy in tumour immunity and therapy. Nat Rev Cancer. 2021; 21:281–97. https://doi.org/10.1038/s41568-021-00344-2 [PubMed]
-
20.
Li Y, Wang HC, Wang JS, Sun B, Li LP. Chemokine receptor 4 expression is correlated with the occurrence and prognosis of gastric cancer. FEBS Open Bio. 2020; 10:1149–61. https://doi.org/10.1002/2211-5463.12864 [PubMed]
-
21.
Xiang Z, Zhou ZJ, Xia GK, Zhang XH, Wei ZW, Zhu JT, Yu J, Chen W, He Y, Schwarz RE, Brekken RA, Awasthi N, Zhang CH. A positive crosstalk between CXCR4 and CXCR2 promotes gastric cancer metastasis. Oncogene. 2017; 36:5122–33. https://doi.org/10.1038/onc.2017.108 [PubMed]
-
22.
Lee HJ, Jo DY. The role of the CXCR4/CXCL12 axis and its clinical implications in gastric cancer. Histol Histopathol. 2012; 27:1155–61. https://doi.org/10.14670/HH-27.1155 [PubMed]
-
23.
Tang C, Lei X, Xiong L, Hu Z, Tang B. HMGA1B/2 transcriptionally activated-POU1F1 facilitates gastric carcinoma metastasis via CXCL12/CXCR4 axis-mediated macrophage polarization. Cell Death Dis. 2021; 12:422. https://doi.org/10.1038/s41419-021-03703-x [PubMed]
-
24.
Daniel SK, Seo YD, Pillarisetty VG. The CXCL12-CXCR4/CXCR7 axis as a mechanism of immune resistance in gastrointestinal malignancies. Semin Cancer Biol. 2020; 65:176–88. https://doi.org/10.1016/j.semcancer.2019.12.007 [PubMed]
-
25.
Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013; 110:20212–7. https://doi.org/10.1073/pnas.1320318110 [PubMed]
-
26.
Seo YD, Jiang X, Sullivan KM, Jalikis FG, Smythe KS, Abbasi A, Vignali M, Park JO, Daniel SK, Pollack SM, Kim TS, Yeung R, Crispe IN, et al. Mobilization of CD8+ T Cells via CXCR4 Blockade Facilitates PD-1 Checkpoint Therapy in Human Pancreatic Cancer. Clin Cancer Res. 2019; 25:3934–45. https://doi.org/10.1158/1078-0432.CCR-19-0081 [PubMed]
-
27.
Liu T, Li X, You S, Bhuyan SS, Dong L. Effectiveness of AMD3100 in treatment of leukemia and solid tumors: from original discovery to use in current clinical practice. Exp Hematol Oncol. 2016; 5:19. https://doi.org/10.1186/s40164-016-0050-5 [PubMed]
-
28.
Chen L, Ma G, Wang P, Dong Y, Liu Y, Zhao Z, Guo J, Liang H, Yang L, Deng J. Establishment and verification of prognostic model for gastric cancer based on autophagy-related genes. Am J Cancer Res. 2021; 11:1335–46. [PubMed]
-
29.
Wen F, Lu X, Huang W, Chen X, Ruan S, Gu S, Gu P, Li Y, Liu J, Liu S, Shu P. Characteristics of immunophenotypes and immunological in tumor microenvironment and analysis of immune implication of CXCR4 in gastric cancer. Sci Rep. 2022; 12:5720. https://doi.org/10.1038/s41598-022-08622-1 [PubMed]
-
30.
Xue S, Ma M, Bei S, Li F, Wu C, Li H, Hu Y, Zhang X, Qian Y, Qin Z, Jiang J, Feng L. Identification and Validation of the Immune Regulator CXCR4 as a Novel Promising Target for Gastric Cancer. Front Immunol. 2021; 12:702615. https://doi.org/10.3389/fimmu.2021.702615 [PubMed]
-
31.
Ren G, Li G. Tumor suppressor gene DLC1: Its modifications, interactive molecules, and potential prospects for clinical cancer application. Int J Biol Macromol. 2021; 182:264–75. https://doi.org/10.1016/j.ijbiomac.2021.04.022 [PubMed]
-
32.
Zhang Y, Li G. A tumor suppressor DLC1: The functions and signal pathways. J Cell Physiol. 2020; 235:4999–5007. https://doi.org/10.1002/jcp.29402 [PubMed]
-
33.
Yang X, Hu F, Liu JA, Yu S, Cheung MPL, Liu X, Ng IO, Guan XY, Wong KKW, Sharma R, Lung HL, Jiao Y, Lee LTO, Cheung M. Nuclear DLC1 exerts oncogenic function through association with FOXK1 for cooperative activation of MMP9 expression in melanoma. Oncogene. 2020; 39:4061–76. https://doi.org/10.1038/s41388-020-1274-8 [PubMed]
-
34.
Kim TY, Jong HS, Song SH, Dimtchev A, Jeong SJ, Lee JW, Kim TY, Kim NK, Jung M, Bang YJ. Transcriptional silencing of the DLC-1 tumor suppressor gene by epigenetic mechanism in gastric cancer cells. Oncogene. 2003; 22:3943–51. https://doi.org/10.1038/sj.onc.1206573 [PubMed]
-
35.
Su Y, Lin L, Zhang J, Jiang Y, Pan C, Sun L, Duan J, Liao W. Low expression of DLC1 is predictive of poor therapeutic efficiency of fluoropyrimidine and oxaliplatin as adjuvant chemotherapy in gastric cancer. Mol Med Rep. 2015; 12:5771–9. https://doi.org/10.3892/mmr.2015.4173 [PubMed]
-
36.
Bell ES, Coelho PP, Park M. LC3C mediates selective autophagy of the MET RTK, inhibiting cancer cell invasion. Autophagy. 2020; 16:959–61. https://doi.org/10.1080/15548627.2020.1728099 [PubMed]
-
37.
Bell ES, Coelho PP, Ratcliffe CDH, Rajadurai CV, Peschard P, Vaillancourt R, Zuo D, Park M. LC3C-Mediated Autophagy Selectively Regulates the Met RTK and HGF-Stimulated Migration and Invasion. Cell Rep. 2019; 29:4053–68.e6. https://doi.org/10.1016/j.celrep.2019.11.063 [PubMed]
-
38.
Wang Y, Lin K, Xu T, Wang L, Fu L, Zhang G, Ai J, Jiao Y, Zhu R, Han X, Cai H. Development and validation of prognostic model based on the analysis of autophagy-related genes in colon cancer. Aging (Albany NY). 2021; 13:19028–47. https://doi.org/10.18632/aging.203352 [PubMed]
-
39.
Liu L, Zhang J, Liu H, Shi M, Zhang J, Chen L, Huang L, Li B, Xu P. Correlation of autophagy-related genes for predicting clinical prognosis in colorectal cancer. Biomark Med. 2021; 15:715–29. https://doi.org/10.2217/bmm-2020-0292 [PubMed]
-
40.
Peske JD, Woods AB, Engelhard VH. Control of CD8 T-Cell Infiltration into Tumors by Vasculature and Microenvironment. Adv Cancer Res. 2015; 128:263–307. https://doi.org/10.1016/bs.acr.2015.05.001 [PubMed]
-
41.
Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010; 70:8368–77. https://doi.org/10.1158/0008-5472.CAN-10-1322 [PubMed]
-
42.
St Paul M, Ohashi PS. The Roles of CD8+ T Cell Subsets in Antitumor Immunity. Trends Cell Biol. 2020; 30:695–704. https://doi.org/10.1016/j.tcb.2020.06.003 [PubMed]
-
43.
Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008; 222:129–44. https://doi.org/10.1111/j.1600-065X.2008.00616.x [PubMed]
-
44.
Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W. CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018; 18:635–47. https://doi.org/10.1038/s41577-018-0044-0 [PubMed]
-
45.
Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJM, Ciampricotti M, Hawinkels LJA, Jonkers J, de Visser KE. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015; 522:345–8. https://doi.org/10.1038/nature14282 [PubMed]
-
46.
Jackaman C, Tomay F, Duong L, Abdol Razak NB, Pixley FJ, Metharom P, Nelson DJ. Aging and cancer: The role of macrophages and neutrophils. Ageing Res Rev. 2017; 36:105–16. https://doi.org/10.1016/j.arr.2017.03.008 [PubMed]
-
47.
Wang Y, Xiang Y, Xin VW, Wang XW, Peng XC, Liu XQ, Wang D, Li N, Cheng JT, Lyv YN, Cui SZ, Ma Z, Zhang Q, Xin HW. Dendritic cell biology and its role in tumor immunotherapy. J Hematol Oncol. 2020; 13:107. https://doi.org/10.1186/s13045-020-00939-6 [PubMed]
-
48.
Xie Y, Xie F, Zhang L, Zhou X, Huang J, Wang F, Jin J, Zhang L, Zeng L, Zhou F. Targeted Anti-Tumor Immunotherapy Using Tumor Infiltrating Cells. Adv Sci (Weinh). 2021; 8:e2101672. https://doi.org/10.1002/advs.202101672 [PubMed]
-
49.
Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016; 44:989–1004. https://doi.org/10.1016/j.immuni.2016.05.001 [PubMed]
-
50.
Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, Workman CJ, Vignali DA. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013; 501:252–6. https://doi.org/10.1038/nature12428 [PubMed]
-
51.
Goswami S, Walle T, Cornish AE, Basu S, Anandhan S, Fernandez I, Vence L, Blando J, Zhao H, Yadav SS, Ott M, Kong LY, Heimberger AB, et al. Immune profiling of human tumors identifies CD73 as a combinatorial target in glioblastoma. Nat Med. 2020; 26:39–46. https://doi.org/10.1038/s41591-019-0694-x [PubMed]
-
52.
Smyth EC, Gambardella V, Cervantes A, Fleitas T. Checkpoint inhibitors for gastroesophageal cancers: dissecting heterogeneity to better understand their role in first-line and adjuvant therapy. Ann Oncol. 2021; 32:590–9. https://doi.org/10.1016/j.annonc.2021.02.004 [PubMed]
-
53.
Lee B, Hutchinson R, Wong HL, Tie J, Putoczki T, Tran B, Gibbs P, Christie M. Emerging biomarkers for immunomodulatory cancer treatment of upper gastrointestinal, pancreatic and hepatic cancers. Semin Cancer Biol. 2018; 52:241–52. https://doi.org/10.1016/j.semcancer.2017.12.009 [PubMed]
-
54.
Li J, Pu K, Li C, Wang Y, Zhou Y. A Novel Six-Gene-Based Prognostic Model Predicts Survival and Clinical Risk Score for Gastric Cancer. Front Genet. 2021; 12:615834. https://doi.org/10.3389/fgene.2021.615834 [PubMed]