Meta-analysis
First, we evaluated the impact of AMA on CPR in infertile women undergoing assisted reproductive technology treatment using the OD model. Seventeen studies were included in this meta-analysis. The results showed a slightly lower CPR in women with AMA than in younger women (RR, 0.92; 95% CI, 0.82, 1.03; P=0.16). I2, which was used to describe the heterogeneity of the included studies, was 52%, indicating statistical heterogeneity in the results (P=0.007). Therefore, the random-effects model was used (Figure 2).
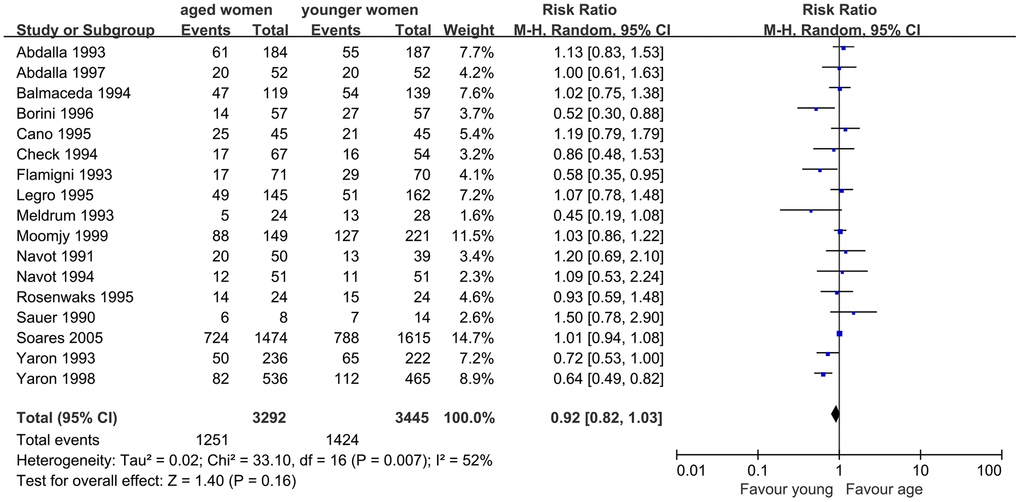
Figure 2. Forest plot showing the results of meta-analysis of studies comparing the effect of AMA on clinical pregnancy rate after OD treatment.
Similarly, nine studies were included to assess the impact of AMA on embryo implantation. The results of the meta-analysis showed similar IR in women with AMA and younger women (RR, 0.85; 95% CI, 0.69, 1.05; P=0.14). I2 was 59%, indicating moderate heterogeneity (P=0.01), and a random-effects model was used (Figure 3).
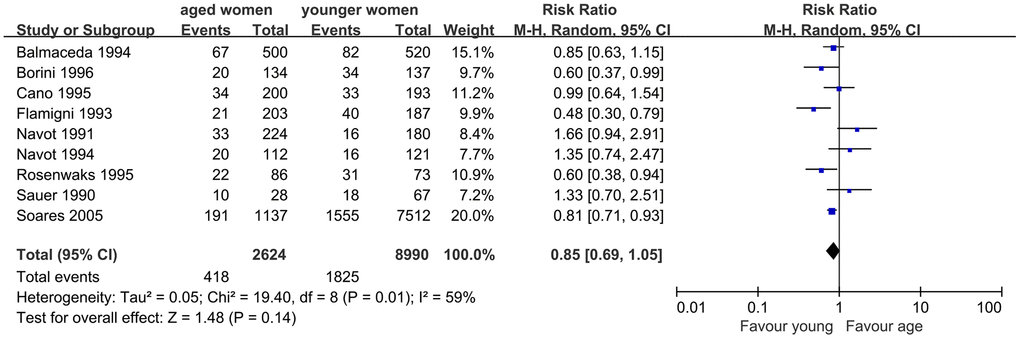
Figure 3. Forest plot showing the results of meta-analysis of studies comparing the effect of AMA on embryo implantation rate after OD treatment.
When we evaluated the impact of AMA on MR, 14 studies were included. The results indicated a significantly higher MR in infertile women with AMA than in younger women. The Q statistic P > 0.1, indicated the homogeneity of the studies (I2=0%, P=0.56). The fixed effects model was used and the pooled RR was 1.37 (95% CI, 1.13, 1.67; P=0.002) (Figure 4).
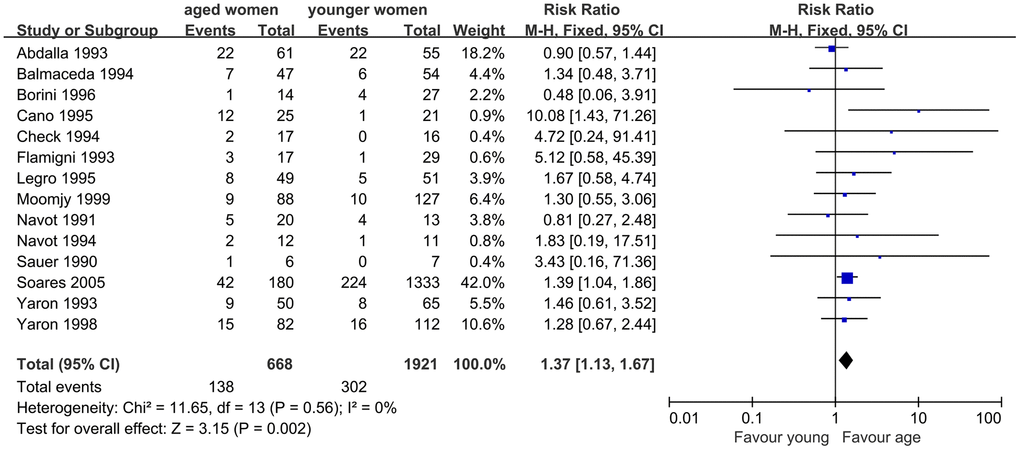
Figure 4. Forest plot showing the results of meta-analysis of studies comparing the effect of AMA on miscarriage rate after OD treatment.
Additionally, LBR was evaluated, and nine studies were included. The results of meta-analysis showed no significant difference in LBR between women with AMA and younger women. Good homogeneity was observed in the results (I2=22%, P=0.25). The fixed effect model combined RR was 0.77 (95% CI, 0.65, 0.91, P=0.002) (Figure 5).
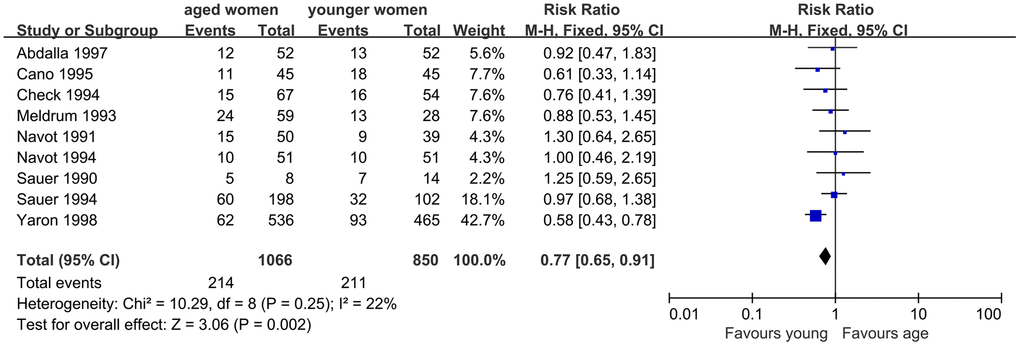
Figure 5. Forest plot showing the results of meta-analysis of studies comparing the effect of AMA on live birth rate after OD treatment.
The results included in this meta-analysis scored medium to high on the Newcastle-Ottawa Scale (not shown). The funnel plots evaluating the impact of AMA on CPR, IR, MR, and LBR suggest a lack of publication bias due to their symmetrical shape (Supplementary Figures 1–4).
To the best of our knowledge, this study is the first systematic review and meta-analysis to assess the impact of AMA on ER using an OD model. The results showed a trend toward a lower IR and CPR without significance; however, significantly increased MR and decreased LBR were observed in women with AMA, indicating that ER was negatively affected by advanced maternal age.
It is well known that fecundity declines in women with AMA are associated with decreased oocyte quality. However, there is no consistent conclusion regarding whether ER is also impaired in women with AMA. OD is considered a perfect model for ascertaining the extent of this relationship. Some studies have evaluated the impact of AMA on endometrial tissues using an OD model.
Early in 1990, one study explored whether ER decreased in older women. In this study, women aged 40-44 years with ovarian failure were enrolled and underwent embryo transfer with OD. These results suggest that the endometrium retains the ability to respond to gonadal steroids and receptivity for embryo implantation, even in older women [6]. Other similar studies also suggested that the age-related decline in female fertility has been attributed to oocyte quality and can be corrected by OD [4], showed similar PR, cumulative PR, and LBR in different age groups, and failed to detect any impact of age on pregnancy outcome in the OD model [15–18].
Other studies have compared pregnancy and implantation rates in oocyte recipients of different ages and showed significant differences in pregnancy and implantation rates according to the age of recipients, suggesting that the ER decreased with age [9, 10, 13]. Some studies reported significantly decreased PR [19] and IR [20, 21], significantly increased MR [11], and worse obstetric outcome [8] in women of advanced age. These discrepancies may be attributed to differences in patients’ age, body mass index (BMI), country, indication of OD, analysis method, and study design.
Although there were discrepancies among the studies, the pooled results suggest that AMA may have a negative effect on the ER. As donated oocytes are obtained from young women, the age-related decline in LBR and increased MR with OD cannot be attributed to oocyte quality. Possible explanations for our findings are as follows.
First, there is an age-related decline in ER. An in vitro experiment found that the expression of HOXA10, a marker of ER, was inversely correlated with uterine age [22]. An animal study compared mRNA levels of endometrial cells in vitro obtained from young and aged cows using next generation sequencing (NGS) and polymerase chain reaction (PCR), and found that endometrial cells of aged cows have higher levels of inflammatory, IFN-signaling, and cell division dysfunction than those of young cows [23]. In human, it has also been reported that placenta in women with AMA is associated with premature senescence during placentation due to SIRT1 deficiency, which promotes epithelial-mesenchymal transition of trophoblast cells and enhances the invasion of trophoblast cells by regulating vimentin acetylation [24]. Older women with decreased serum anti-mullerian hormone (AMH) and antral follicle count (AFC) levels showed significantly lower endometrial vascularization index, flow index, and vascularization flow index, and lower CPR and ongoing PR, indicating impaired ER [25].
Second, another possible explanation for our results may be embryo quality. The oocytes were donated by younger women, which did not contribute to poor embryo quality. However, advanced paternal age may be a reason for poor embryo quality. New dominant mutations, which may be embryologically fatal, are now known to be common in men of advanced age. Thus, it is reasonable to assume that the male partners of older recipients are more likely to be older, and such new dominant mutation may lead to decreased embryo quality [19].
Third, the risk of adverse pregnancy outcomes, such as gestational diabetes, preeclampsia, stillbirth, intrauterine growth restriction, and placenta previa, markedly increased among women with AMA. The increased complications may be related to impaired placentation function and progressive uterine vascular endothelial damage with aging [26–28].
The strength of this study is that it is the first systematic review and meta-analysis to describe the relationship between AMA and ER decline. The sample size was very large (7037 women), which provided an excellent precision for estimates with pooled RRs.
This study had several limitations. First, there was significant between-study heterogeneity, such as different study designs (prospective or retrospective studies), varied definition for AMA (40 years, 42 years, or 45 years), and different endometrium preparation protocol. In addition, most of the studies were retrospective in design, and there were residual confounding factors. Finally, some of the included studies had a small sample size. Despite these drawbacks, this systematic review and meta-analysis provide a valuable analysis and summary of the relevant literatures.
In conclusion, this study found that AMA is related to a decline in ER. Because of the small sample size and the possibility of aneuploidy embryos, further prospective cohort studies using the preimplanation genetic testing-Aneuploid (PGT-A) model are needed to observe the impact of AMA and analyze the possible causes.