Abstract
Aging biomarkers may be related to each other through direct co-regulation and/or through being regulated by common processes associated with chronological aging or stress. Klotho is an aging regulator that acts as a circulating hormone with critical involvement in regulating insulin signaling, phosphate homeostasis, oxidative stress, and age-related inflammatory functioning. Both klotho and telomere length are biomarkers of biological aging and decrease with age; however, the relationship between them is not well understood. Here we test the association between klotho levels and the telomere length of specific sorted immune cells among a healthy sample of mothers caregiving for a child with autism spectrum disorder (ASD; i.e., experiencing higher caregiving stress) or a child without ASD, covarying age and body mass index, in order to understand if high stress associated with caregiving for a child with an ASD may be involved in any association between these aging biomarkers. In 178 caregiving women (n = 90 high-stress mothers of children with ASD, n = 88 low-stress mothers of neurotypical children), we found that klotho levels were positively associated with telomere length in PBMCs (an effect driven by CD4+ and CD8+CD28− T cells) among high-stress mothers of children with an ASD but not among low-stress mothers of neurotypical children. There were no significant associations between klotho and telomerase activity in either group, across cell types assessed here. Our results suggest that klotho levels and telomere length may be associated through a coordinated downregulation of longevity factors occurring under higher stress caregiving conditions.
Introduction
Aging biomarkers are often related to each other and this could occur through many different mechanisms. First, age influences aging related biomarkers, by definition, and any study of potential co-regulation must covary chronological age. Secondly, there may be direct co-regulation of each other. Thirdly, they may be regulated by common processes not associated with age, but associated with lifestyle factors such as chronic stress. Here we examine the relationship between two important biomarkers of aging, klotho and telomere length, in a healthy sample stratified into groups based on a combination of (a) stressor exposure and (b) level of perceived stress (i.e., high-stress mothers of children with ASD compared to low-stress mothers of neurotypical children).
Klotho is an aging regulator primarily expressed in the kidneys and choroid plexus of the brain that acts as a circulating hormone once cleaved from its transmembrane form [1–4]. Once in circulation, klotho can regulate functions of cells and tissues that do not express klotho [1, 5]. Klotho may either be overexpressed, which extends lifespan in model organisms [6], or disrupted, which accelerates systemic aging phenotypes (e.g., arterial stiffness, atherosclerosis, chronic obstructive pulmonary disease, infertility, premature mortality) [7–14]. The function of klotho is pleiotropic, with evidence supporting its role in regulating insulin signaling, phosphate homeostasis, oxidative stress, and age-related inflammatory functioning [15–20].
Like klotho, telomere length is increasingly recognized as a key biomarker of biological aging. Telomeres are protective DNA-protein complexes that cap the ends of chromosomes and provide genomic stability [21, 22]. Complete replication of telomeres is not possible due to the end replication problem of the lagging strand, which leads to telomere attrition across cell divisions. Telomeres serve as the protective ends of linear chromosomes so they are not recognized as broken ends; fortunately, telomerase, an inter-cellular enzyme, counteracts telomere attrition by adding DNA sequence repeats [23, 24]. Immune cell telomere length, typically measured in leukocytes or sorted immune cells, is consistently associated and often predictive of age-related chronic disease states, such as cardiovascular-related diseases, as well as early mortality [25–33]. In addition to age-related telomere attrition, telomeres also shorten in response to biochemical stressors (i.e., oxidative stress; [22]) and to various types of psychological stressors in a dose-response manner [34–39].
Despite evidence supporting klotho and telomere length as aging biomarkers, the relationship between them has been examined in only a handful of basic studies and is not well understood. In this regard, in vitro work using human cortical neurons derived from human pluripotent stem cells showed an age-related association between telomere attrition and klotho mRNA expression [40]. In another study using in vitro cultured cells, a 50% inhibition of klotho gene expression led to a shortening of telomere length [41], and in a separate study, the effect of impaired klotho expression on telomere attrition was found to be mediated by klotho’s regulatory effects on telomerase activity [42]. In mouse hippocampal cells, silencing klotho expression before amitriptyline treatment (an antidepressant known to induce neurotoxicity) resulted in DNA damage in telomeres due to oxidative stress and mineral imbalance, suggesting that klotho is involved in key cellular defense mechanisms that protect neurons from amitriptyline-mediated toxicity [43]. Among individuals with obstructive sleep apnea, four single genetic variants (SNPs) of the klotho gene significantly mediated the association between obstructive sleep apnea and short leukocyte telomere length [44]. When investigating relationships between klotho and telomere length among veterans, researchers identified a specific klotho SNP associated with peripheral and neuroimaging biomarkers of aging, but they did not find a significant main effect of the SNP associated with telomere length, nor did they identify a significant interaction between the SNP and PTSD severity associated with telomere length [45]. To date, no study has explored associations between sorted immune cell telomere length, telomerase activity, and circulating klotho in humans.
Chronic stress, such as the stress of caregiving for a child with a neurodevelopmental disability (e.g., autism spectrum disorder (ASD)), is associated with accelerated biological aging and the more rapid development of age-related conditions, including cardiovascular disease [46–49] and premature mortality [50, 51]. Further, chronic psychological stress has been linked to telomere attrition [52, 53], impaired telomerase activity [52, 54], and lower levels of klotho [55] raising the possibility that chronic stress may promote a coordinated downregulation in key processes implicated in longevity. To test this possibility, we explored the associations between telomere length, telomerase, and klotho in a sample of high-stress healthy women caring for a child with ASD and low-stress women caring for a neurotypical child.
Results
Participant characteristics
Full sociodemographic characteristics of the sample (n = 90 high-stress mothers caring for a child with ASD, n = 88 low-stress mothers caring for a neurotypical child) are shown in Table 1. As previously reported [55], there were no significant differences in age, BMI, racial identity or education level between the groups. Perceived stress scores ranged from 7 to 29 in the mothers caring for a neurotypical child and 12 to 33 in the mothers caring for a child with ASD. As expected, mothers caring for a child with ASD reported significantly higher perceived stress (M = 21.90, SD = 4.69) than mothers caring for a neurotypical child (M = 15.73, SD = 4.41; t (174.55) = −9.03, p < .001). For brevity moving forward, we refer to the mothers caring for a child with ASD as the “high-stress” group and the mothers caring for a neurotypical child as the “low-stress” group.
Table 1. Demographic characteristics and descriptive statistics of the study participants (N = 178).
| Variable | N | M | SD |
| 1. Age | 178 | 42.47 | 5.14 |
| 2. Education | 164 | 16.46 | 2.26 |
| 3. BMI | 177 | 25.50 | 5.22 |
| 4. Klothoa | 178 | 891.85 | 324.01 |
| 5. CD4+ TLa | 175 | 1.22 | 0.20 |
| 6. CD8+CD28+ TLa | 175 | 1.24 | 0.24 |
| 7. CD8+CD28− TLa | 173 | 1.02 | 0.25 |
| 8. CD19+ TLa | 175 | 1.71 | 0.29 |
| 9. PBMC TLa | 176 | 1.17 | 0.20 |
| 10. Granulocyte TLa | 178 | 1.00 | 0.16 |
| 11. Whole Blood TL | 173 | 1.08 | 0.16 |
| 12. PBMC TAa | 175 | 5.44 | 3.38 |
| 13. CD4+ TAa | 174 | 4.70 | 2.68 |
| 14. CD8+CD28+ TAa | 169 | 4.63 | 4.84 |
| 15. CD8+CD28− TAa | 50 | 2.22 | 1.66 |
| 16. CD19+ TAa | 157 | 6.34 | 5.59 |
| Abbreviations: TL: telomere length; TA: telomerase activity. aRaw values presented here; values were log-transformed for analyses due to skewness. |
Collapsing across groups, older age was associated with lower klotho levels (r = −.16, p = .031) and shorter telomere length across cell types (rs: −.20– −.37, ps < .01). Older age was also associated with reduced CD8+CD28+ T cell telomerase activity (r = −.16, p = .034), but there were no other significant correlations between age and telomerase activity in any other cell type (ps: .44–.97). Higher BMI was associated with lower klotho levels (r = −.15, p = .048) and shorter telomeres in CD19+ B cells (r = −.18, p = .02), but not shorter telomeres (ps: .19–.52) or telomerase activity (ps: .49–.95) in any other cell types. Education and race were not associated with levels of klotho, telomerase activity, or telomere length in any cell types (ps > .11).
Associations of klotho levels with telomere length (TL) or telomerase activity (TA)
In models unadjusted for covariates, klotho levels were significantly associated with PBMC TL (b = 0.08, 95% CI (0.01, 0.15), p = .025) and whole blood TL (b = 0.21, 95% CI (0.04, 0.37), p = .013). When broken down into specific cell types, there were no statistically significant associations observed for CD4+ T cell TL (b = 0.06, 95% CI (−0.01, 0.13), p = .07), CD8+CD28− T cell TL (b = 0.10, 95% CI (−0.01, 0.21), p = .06), CD8+CD28+ T cell TL (b = 0.05, 95% CI (0.04, 0.37), p = .21), or CD19+ B cells (b = 0.04, 95% CI (−0.03, 0.12), p = .23). Granulocyte TL was also not significantly related to klotho levels in unadjusted models (b = 0.05, 95% CI (−0.02, 0.12), p = .15). After covarying for age and BMI, only klotho levels and whole blood TL had any signal of an association; however, this association was not statistically significant (b = −0.002, 95% CI (−0.02, 0.12), p = .052). There were no other statistically significant associations between klotho and TL across cell types in adjusted models (ps: .12–.67). Regarding telomerase activity (TA), there were no reliable associations between klotho levels and TA across cell types in unadjusted (ps: .14–.57) or adjusted (ps: .14–.55) models.
Higher klotho levels associated with telomere length among high-stress, but not low-stress mothers
We found evidence of a significant klotho by group (high- vs. low-stress) interaction for PBMC TL (unadjusted: b = 0.21, 95% CI (0.06, 0.35), p = .006; adjusted: b = 0.17, 95% CI (0.03, 0.31), p = .019; see Figure 1; see Supplementary Table 1). In an effort to understand this relationship, we explored this interaction in immune cell subsets. In this regard, we identified a significant klotho by group interaction in CD4+ T cell TL (unadjusted: b = 0.18, 95% CI (0.04, 0.33), p = .015, pB-H = .036; adjusted: b = 0.15, 95% CI (0.01, 0.29), p = .039, pB-H = .12; see Figure 2; see Supplementary Table 2), CD8+CD28− T cell TL (unadjusted: b = 0.28, 95% CI (0.06, 0.50), p = .014, pB-H = .036; adjusted: b = 0.23, 95% CI (0.01, 0.45), p = .039, pB-H = .12; see Figure 3; see Supplementary Table 3), and CD8+CD28+ T cell TL (unadjusted: b = 0.20, 95% CI (0.03, 0.36), p = .018, pB-H = .036; adjusted: b = 0.14, 95% CI (−0.02, 0.29), p = .084, pB-H = .17; see Figure 4; see Supplementary Table 4), but not CD19+ B cells (unadjusted: b = 0.08, 95% CI (−0.07, 0.23), p = .30, pB-H = .30; adjusted: b = 0.07, 95% CI (−0.08, 0.22), p = .35, pB-H = .35. There was no evidence of a significant klotho by group interaction in whole blood or for granulocyte TL (ps > .13).
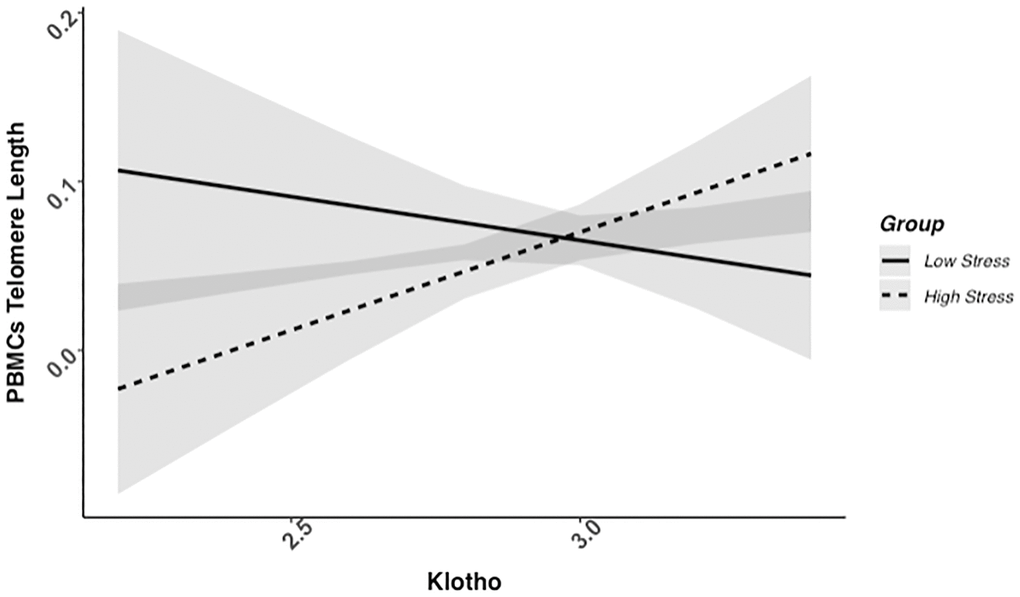
Figure 1. PBMC telomere length as a function of klotho levels and stress group membership. The slope for the high-stress group was significant, but not the slope for the low-stress group (see Table 2).
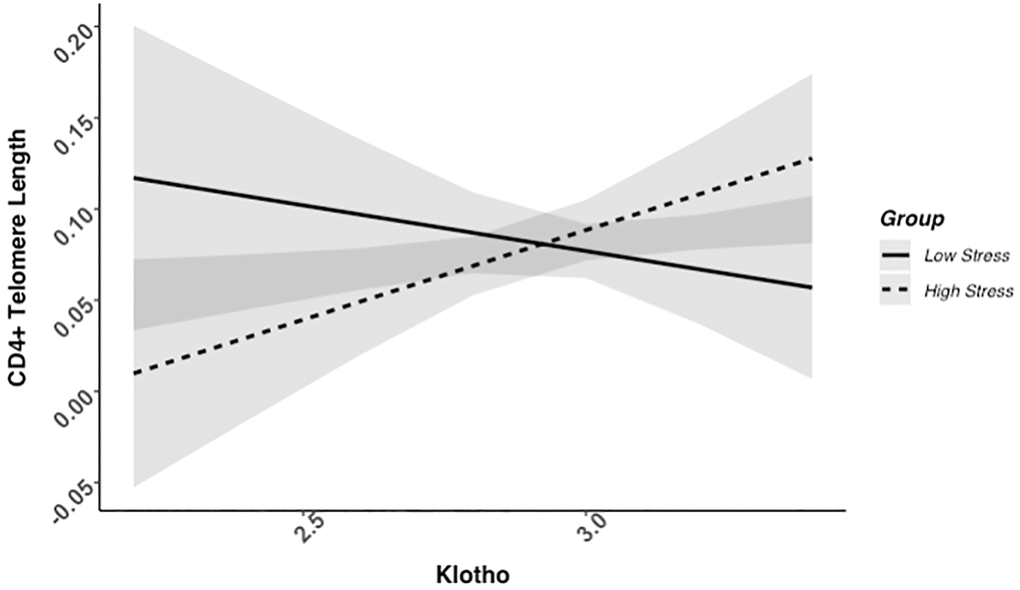
Figure 2. CD4+ telomere length as a function of klotho levels and stress group membership. The slope for the high-stress group was significant, but not the slope for the low-stress group (see Table 2).
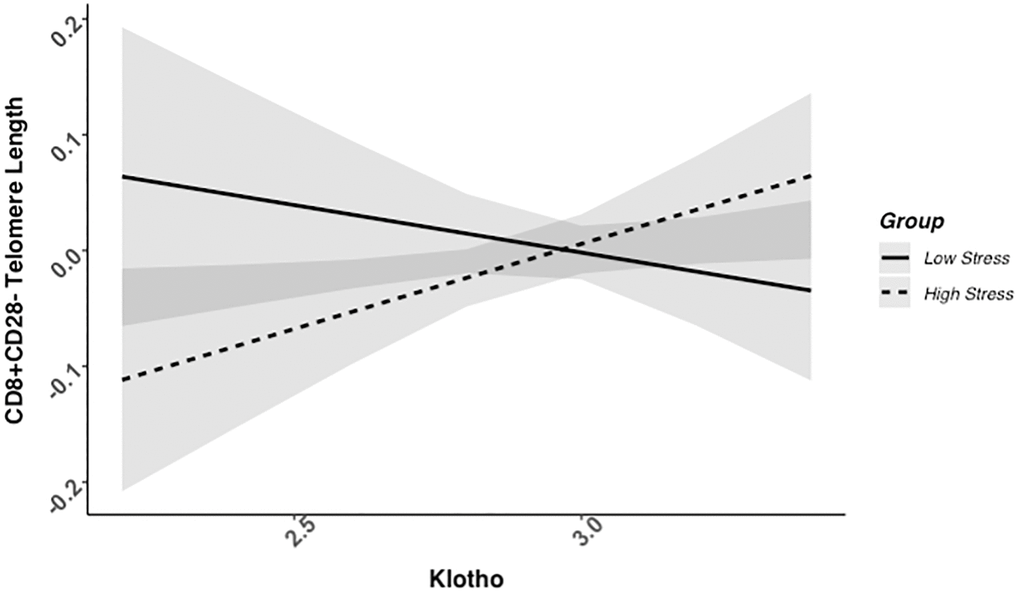
Figure 3. CD8+CD28− telomere length as a function of klotho levels and stress group membership. The slope for the high-stress group was significant, but not the slope for the low-stress group (see Table 2).
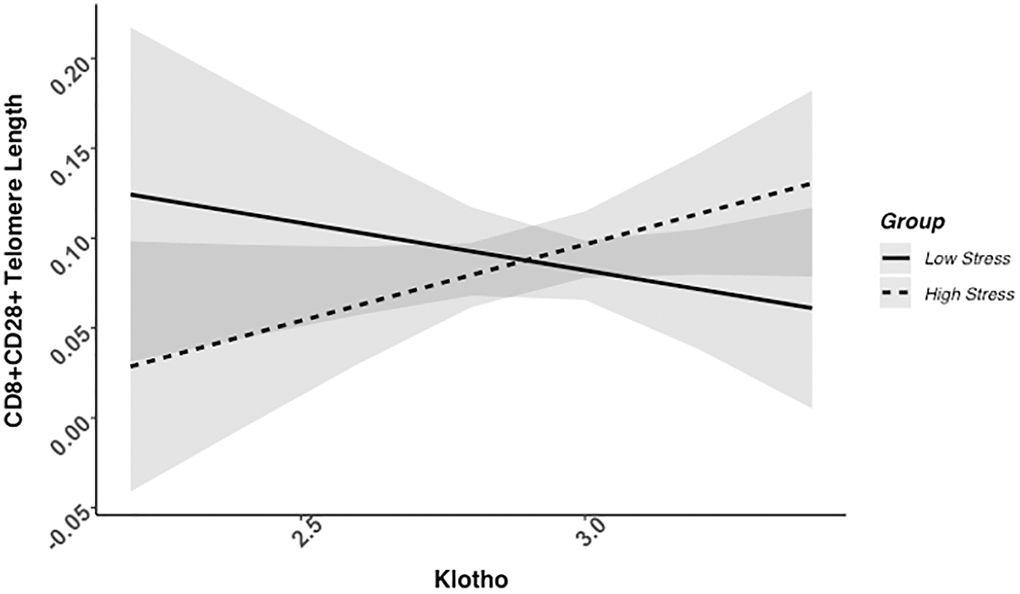
Figure 4. CD8+CD28+ telomere length as a function of klotho levels and stress group membership. Neither slope is significant in this figure (see Table 2).
Examination of simple slopes for the adjusted models indicated that klotho was statistically significantly associated with TL in several cell types for high-stress mothers, but not the low-stress mothers (see Table 2). Specifically, lower klotho was significantly associated with shorter TL in PBMCs, CD4+ T cells, and CD8+CD28− T cells in high- but not low-stress mothers.
Table 2. Simple slopes for significant interactions between klotho and stress group membership by immune cell type.
| Cell type group | Estimate | 95% CI | p |
| LL | UL |
| PBMC Telomere Length |
| Low-stress | −0.04 | −0.15 | 0.06 | .41 |
| High-stress | 0.12 | 0.02 | 0.21 | .019 |
| CD4+ T Cell Telomere Length |
| Low-stress | −0.05 | −0.17 | 0.06 | .36 |
| High-stress | 0.10 | 0.01 | 0.19 | .029 |
| CD8+CD28− T Cell Telomere Length |
| Low-stress | −0.04 | −0.21 | 0.13 | .63 |
| High-stress | 0.15 | 0.01 | 0.29 | .035 |
| CD8+CD28+ T Cell Telomere Length |
| Low-stress | −0.06 | −0.17 | 0.05 | .30 |
| High-stress | 0.08 | −0.03 | 0.19 | .15 |
Associations between klotho and telomerase activity did not differ by stress group status
There was no evidence that associations between levels of klotho and cell-specific telomerase activity differed significantly between the high- and low-stress mothers. Across each cell type assessed for telomerase activity (i.e., PBMCs, CD19+ B cells, CD4+, CD8+CD28−, CD8+CD28+ T cells), there was no evidence of the klotho by group interaction associated with telomerase activity (unadjusted ps: .44−.74; adjusted ps: .44−.87).
Sensitivity analyses
In ancillary analyses to test the robustness of these findings, we examined whether any of the main findings were driven by overly influential observations. As described next, our findings remained consistent after examining influential observations or potential outliers.
There was one significant outlier based on a Bonferroni outlier test of the studentized residuals in the analyses with CD8+CD28+ T cell TL. When this observation was removed the effect of the klotho by group interaction was similar (unadjusted: b = 0.20, 95% CI (0.04, 0.37), p = .017; adjusted: b = 0.14, 95% CI (−0.02, 0.30), p = .081). There were no overly influential observations in this regression analysis based on the DFBETAS values. There were no other overly influential observations or outliers in these analyses.
Discussion
In a sample of 178 healthy caregiving women, klotho levels were positively associated with telomere length in PBMCs for high-stress mothers caring for a child with ASD but not among low-stress mothers caring for a neurotypical child. Specifically, for high-stress mothers of a child with ASD, lower klotho levels were associated with shorter telomeres in PBMCs, with exploratory analyses highlighting that CD4+ and CD8+CD28− T cells contributed to the association across the mixed cells types. There were no significant associations between klotho and telomerase activity, nor between klotho, group (high- vs. low-stress) membership, and telomerase activity across cell types assessed here. This study is the first to explore associations between sorted immune cell telomere length, telomerase activity, and circulating klotho in humans; our results indicate that klotho and telomere length may be linked through a coordinated downregulation of certain longevity factors under higher stress caregiving conditions. Because both klotho and telomere length are associated with insulin signaling, oxidative stress, and age-related inflammatory functioning, the relationship between the klotho and telomere length may be mediated through these common stress- and longevity-related pathways.
Building on past findings in this sample that mothers of a child with ASD had lower klotho levels than mothers of a neurotypical child [55], we identified a positive relationship between klotho and telomere length among high-stress mothers of a child with ASD, but not for low-stress mothers of a neurotypical child. In a study of accelerated aging in chronic obstructive pulmonary disease (COPD) that examined interconnected hallmarks of aging [13, 56], the authors determined that both klotho levels and telomere length were decreased among COPD patients compared to controls, which supports the idea that circulating klotho is lower under disease-induced age-accelerating conditions. Mechanistically, klotho may play a role in both antagonistic and integrative hallmarks of aging by inhibiting the insulin/IGF1 signaling pathway [10, 56]. Across species, there is evidence of an evolutionarily conserved mechanism of life extension through insulin-like signaling pathways [57, 58]. Findings from model organisms that over-express klotho support klotho’s role in these signaling pathways where, in addition to inhibition of insulin/IGF1 pathways, there is also a resistance to oxidative stress that may protect against oxidative damage (Kuro-o, 2009; Kurosu et al., 2005; Yamamoto et al., 2005). Thus, under high-stress conditions, there may be broad disturbances to stress-response systems that disrupt the homeostatic balance between these interacting systems to affect aging biology as we identified here.
For high-stress mothers of a child with ASD, there was preliminary evidence that klotho may be associated with telomere length in specific sorted immune cells that are important for the aging immune system, namely T cells. As people age, telomere shortening happens primarily in CD8+ T cells [59, 60], especially among cells that have lost expression of the co-stimulatory molecule, CD28. Accordingly, CD8CD28− cells have shorter telomeres than CD8+CD28+ telomeres [61]. Critically, shorter telomeres among CD8+CD28− T cells were associated with more severe clinical symptoms among humans experimentally exposed to an upper respiratory virus [62]. A high percentage of CD8+CD28− T cells is a marker of immune senescence [63–65] and associated with poorer responses to immunization [59, 66, 67]. CD4+ T cells serve a similar function as they coordinate immune responses to pathogens [68]. Our results indicate that klotho levels and telomere length in these aged cells are linked under high-stress caregiving conditions.
We found no evidence of reliable associations between klotho and telomerase activity in this sample in either group. The impact of chronic stress itself on telomerase activity is not clear as some studies identify that chronic stress may suppress telomerase activity [52, 54, 69] while others find higher telomerase activity in combination with shorter telomeres [36, 70], which may reflect counter-regulatory attempts to combat telomere shortening occurring under chronic stress conditions. The cell type with the strongest, though still not statistically significant, evidence for an association between klotho levels and telomerase activity under high-stress was CD8+CD28− T cells, which tend to have the lowest telomerase activity of the cell types assessed here [71]. Because our sample size was smallest for this cell type (n = 50), this non-significant effect is worth examining in larger sample sizes.
These findings should be interpreted while recognizing the limitations of the current study design, which also highlight opportunities for important future directions. Although we focus on the stress of caregiving for a child with an ASD in this study, the caregiving experience encompasses far more than just stress [72, 73]. Because the experience of caregiving for a child with an ASD is both heterogenous and presents uniquely rewarding and joyful experiences for caregivers [72, 73], future research should not be limited to solely the stress of the caregiver but rather examine the multifaceted experience of caregiving for a child with ASD with greater focus on the strengths of each child and the structural changes that can be made to support children with ASD and reduce stress for their caregivers [72, 74]. Our specific analyses were based on cross-sectional associations and causality cannot be inferred; moreover, some analyses had smaller samples than others. For example, telomere length variables were well-represented with over 170 people for each cell type whereas there was far more variability in power to detect an effect for telomerase activity with sample sizes ranging from 50–175 across cell types. This sample included exclusively mid-life premenopausal women, so these analyses may not generalize to older or younger ages or men. Lastly, we did not control for length of caregiving or number of other children in the house, which are likely important moderators to consider in the future based on the consistent dose-response effects of chronic stress on telomere length.
These limitations are well-balanced by several key strengths of this study, including carefully matched groups of caregiving women and examining sorted immune cell telomere length. Many studies examining telomere length rely on heterogeneous populations of leukocytes without subdividing these cells; by sorting immune cells for this study we were able to identify where there were clear associations for the high-stress group between klotho and telomere length (PBMCs broadly, with some evidence for CD4+ and CD8+CD28− T cells specifically), where there was some weak evidence of an association between klotho and telomere length following the same pattern (CD8+CD28+ T cells), and where there was no evidence of an association between klotho and telomere length (CD19+ B cells, granulocytes, whole blood).
There appears to be an exciting and fruitful area for future investigations to further understand the impact of caregiving stress on aging biology. There was a wide range of power to detect an effect for telomerase activity related to group membership and klotho levels; thus, for all analyses but especially those with smaller sample sizes (e.g., CD8+ CD28− T cell telomerase activity) future work may replicate or extend these findings with larger sample sizes. Given the importance of both the chronicity and timing of stress for its impact on aging biology [75], future analyses may investigate both factors as they likely impact the associations described here. The experience of severe childhood stress, for example, is particularly impactful for stress-response system processes and may impact the magnitude of any association between stress and aging biology. Beyond characteristics of stress, future studies may also focus on the genetic component of klotho as we focused solely on soluble klotho levels.
In sum, we identified that klotho levels and telomere length of certain immune cells are linked for those experiencing higher stress caregiving conditions but not linked in lower stress caregiving conditions. This may be due to the common and powerful effects of chronic stress on stress related aging pathways. We found no evidence of this pattern of associations for klotho and telomerase activity. Future research is needed to investigate the characteristics of stress exposures that link these two markers of aging and the underlying biological mechanisms driving this connection.
Methods
Participants included 183 mothers recruited via schools, mailings, social media, and ads circulated through child development centers in the San Francisco Bay Area as well as direct recruitment through the University of California, San Francisco Autism Clinic; 178 of the participants had serum samples available for quantifying klotho and of those, all had DNA available for quantifying telomere length. Participants were eligible if they were non-smokers between ages 20 and 50 years, with at least one child between ages of 2 and 16 years. Participants who were caring for a child diagnosed with an autism spectrum disorder (ASD) and reported a score of ≥13 on the Perceived Stress Scale (PSS) were categorized as a high-stress maternal caregiver. Participants who were caring for a neurotypical child (i.e., none of their children had been diagnosed with any conditions, including learning disabilities or ADHD) and reported a PSS score of ≤ 19 were categorized as a low-stress maternal control. This eligibility criteria were based on prior national norms [76, 77]. This overlapping range of PSS scores allowed participants in the low-stress group to be experiencing normal levels of stress associated with parenting that are not due to the chronic stress associated with caregiving for a child with an ASD. All study participants reported being premenopausal and in good health with no major medical conditions, such as history of coronary heart disease, endocrine disorders, epilepsy, brain injury, autoimmune conditions, severe asthma, or lung disease. Potential participants were excluded if they reported a current cancer diagnosis or had undergone chemotherapy or radiation in the past 10 years. Participants with certain current psychiatric conditions that may confound results, were excluded including individuals with bipolar disorder, post-traumatic stress disorder and eating disorders, assessed by Structured Clinical Interviews for Diagnostic and Statistical Manual for Mental Disorders for Axis I Disorders (SCID). Low-stress maternal controls with current major depression were excluded; however, this was not exclusionary for the high-stress maternal caregivers because it is a common sequela of chronic stress. No study participants were taking medications known to affect the immune and endocrine system except for antidepressant medication and oral contraceptives. Participants completed questionnaires, including sociodemographic variables, and submitted to a fasting morning blood draw at the initial study visit. This study was approved by the Institutional Review Board at the University of California, San Francisco; written, informed consent was obtained for each study participant.
Assay of klotho
Procedures for assaying klotho have been described for this sample [55]. Briefly, serum was collected from morning fasting blood samples and stored at −80°C until assay. Soluble α-klotho was measured using a solid-phase sandwich enzyme-linked immunosorbent assay (Immuno-Biological Laboratories, Takasaki, Japan) [78], as previously described [55, 79].
Telomere length assay
Identical methods for determining telomere length in this sample were detailed in earlier papers [80], with the exception of converting T/S ratios to base-pairs, which was not done for the analyses in this paper. Briefly, peripheral blood mononuclear cells were purified by Ficoll gradient and sorted into CD4+, CD8+CD28+, and CD8+CD28− T cells and CD19+ B cells as described previously [71]. The telomere length assay was adapted from the original method by Cawthon [71, 81]. To control for inter-assay variability, eight control DNA samples are included in each run. The T/S ratio of each control DNA was divided by the average T/S for the same DNA from 10 runs to get a normalizing factor in each batch. This was done for all eight samples and the average normalizing factor for all eight samples was used to correct the participant DNA samples to get the final T/S ratio. The T/S ratio for each sample was measured twice. When the duplicate T/S value and the initial value vary by more than 7%, the sample was run the third time and the two closest values were reported. The average coefficient of variation (CV) of this study is 2.3% (±1.8%).
Telomerase activity assay
Telomerase activity was determined identically to how it was previously described [80]. Briefly, gel-TRAP assays were performed by the Telomerase Repeat Amplification Protocol (TRAP) using a commercial kit (TRAPeze Telomerase Detection Kit, Millipore) with modifications [71, 81]. Peripheral blood mononuclear cells were purified by Ficoll gradient and then sorted into CD4+, CD8+CD28+, and CD8+CD28− T cells and CD19+ B cells as described earlier [71]. For each telomerase activity assay reaction, the product/internal value was divided by the product/internal control value from twenty 293T cells and then multiplied by 20 to obtain the final telomerase activity units, defined as 1 unit = the amount of product from one 293T cell/10,000 immune cells. The average intra-assay variability of PBMC samples (N = 6, assayed in triplicate) was 8% and the inter-assay variability of PBMC samples (N = 24, assayed on 2 different days) was 6.7%.
Statistical analyses
All statistical analyses were completed in the R statistical environment using R Studio (v2022.07.2). Linear regressions were performed using the lm() function from the stats package [82]. Leverage statistics for these analyses were computed using the influence.measures() function from the stats package [82]. We examined outliers using the outlierTest() function from the car package, as well as through visual examination using the boxplot() function [82, 83]. Plotting relied on the ggpredict() function from the ggeffects package [84], which builds on ggplot2 capabilities [85]. Descriptive statistics were generated using the describe() function from the psych package [86]. Correlations were generated from the cor.test() function in the stats package [82].
Of the 183 participants in the parent study, 178 had serum available (missingness across relevant variables is described in Table 1). We first identified bivariate associations between sociodemographic variables and our key study variables. To examine whether the influence of klotho varied across groups, we relied on multiple linear regressions. Across primary and exploratory analyses, we first examined the interaction of interest in unadjusted models. Next, we adjusted for key covariates (age, BMI) that were associated with either klotho levels, telomere length, telomerase activity, or all three. For each exploratory model, we examined the level of significance after adjusting for multiple tests using the Benjamini-Hochberg Procedure to reduce the false-discovery rate (noted as pB-H). Lastly, sensitivity analyses tested the robustness of any significant effects by removing any potential outliers or overly influential observations. We used a Bonferonni outlier test to examine studentized residuals of each regression. Regarding leverage statistics, observations were removed if the DFBETAS value was greater than |1.0|, which indicates that a single observation may be influencing the regression parameter estimates’ values. Klotho levels underwent log-10 transformation to approximate a normal distribution. Telomere length was non-normally distributed across all cell types apart from whole blood cells; thus, we used a log-10 transformation to approximate a normal distribution for all measures of telomere length besides whole blood cells. Each measure of telomerase activity was also non-normally distributed and we applied a log-10 transformation to better approximate a normal distribution for analyses.
Abbreviations
ASD: autism spectrum disorder;
PBMC: peripheral blood mononuclear cells;
TL: telomere length;
TA: telomerase activity.
Author Contributions
Ryan Brown: Formal analysis, Writing – original draft, Visualization. Elissa Epel: Conceptualization, Writing – review and editing, Supervision, Funding acquisition. Jue Lin: Investigation, Resources, Writing – review and editing. Dena Dubal: Investigation, Funding acquisition, Resources, Writing – review and editing. Aric Prather: Conceptualization, Writing – review and editing, Supervision, Funding acquisition.
Acknowledgments
We are immensely grateful to the women who agreed to participate and gave their time to this study, in addition to their caregiving responsibilities.
Conflicts of Interest
The Regents of the University of California hold issued and pending patents on methods and compositions for improved cognition involving klotho.
Ethical Statement and Consent
This study was approved by the Institutional Review Board at the University of California, San Francisco, and written, informed consent was obtained for each study participant.
Funding
Support for data collection was provided by NIH grants AG030424 (ESE) and HL117727 (ESE). Support for data and biospecimen analyses was provided by NIH grants HL112961 (AAP), AG034531 (DBD) and by the Coulter-Weeks Foundation (DBD), the Bakar Family Foundation (DBD), the Glenn Foundation for Medical Research (DBD), the American Federation for Aging Research (DBD) and UCSF Research Office of Development (AAP and DBD). The study was also supported by the Marcia and Richard Goldman Foundation. This study was supported by a National Institute of Mental Health grant, T32MH019391 (RLB).
References
-
1.
Wang Y, Sun Z. Current understanding of klotho. Ageing Res Rev. 2009; 8:43–51. https://doi.org/10.1016/j.arr.2008.10.002 [PubMed]
-
2.
Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004; 565:143–7. https://doi.org/10.1016/j.febslet.2004.03.090 [PubMed]
-
3.
Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev. 2015; 36:174–93. https://doi.org/10.1210/er.2013-1079 [PubMed]
-
4.
Wang X, Sun Z. RNAi silencing of brain klotho potentiates cold-induced elevation of blood pressure via the endothelin pathway. Physiol Genomics. 2010; 41:120–6. https://doi.org/10.1152/physiolgenomics.00192.2009 [PubMed]
-
5.
Xie J, Cha SK, An SW, Kuro-O M, Birnbaumer L, Huang CL. Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun. 2012; 3:1238. https://doi.org/10.1038/ncomms2240 [PubMed]
-
6.
Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005; 309:1829–33. https://doi.org/10.1126/science.1112766 [PubMed]
-
7.
Alkalbani M, Prabhu G, Lagbo J, Qayyum R. Serum Klotho and pulse pressure; insight from NHANES. Int J Cardiol. 2022; 355:54–8. https://doi.org/10.1016/j.ijcard.2022.02.021 [PubMed]
-
8.
Gao D, Zuo Z, Tian J, Ali Q, Lin Y, Lei H, Sun Z. Activation of SIRT1 Attenuates Klotho Deficiency-Induced Arterial Stiffness and Hypertension by Enhancing AMP-Activated Protein Kinase Activity. Hypertension. 2016; 68:1191–9. https://doi.org/10.1161/HYPERTENSIONAHA.116.07709 [PubMed]
-
9.
Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, Yamanari T, Kikumoto Y, Uchida HA, Kitamura S, Maeshima Y, Nakamura K, Ito H, Makino H. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One. 2013; 8:e56695. https://doi.org/10.1371/journal.pone.0056695 [PubMed]
-
10.
Kuro-o M. Klotho and aging. Biochim Biophys Acta. 2009; 1790:1049–58. https://doi.org/10.1016/j.bbagen.2009.02.005 [PubMed]
-
11.
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997; 390:45–51. https://doi.org/10.1038/36285 [PubMed]
-
12.
Navarro-González JF, Donate-Correa J, Muros de Fuentes M, Pérez-Hernández H, Martínez-Sanz R, Mora-Fernández C. Reduced Klotho is associated with the presence and severity of coronary artery disease. Heart. 2014; 100:34–40. https://doi.org/10.1136/heartjnl-2013-304746 [PubMed]
-
13.
Rutten EP, Gopal P, Wouters EF, Franssen FM, Hageman GJ, Vanfleteren LE, Spruit MA, Reynaert NL. Various Mechanistic Pathways Representing the Aging Process Are Altered in COPD. Chest. 2016; 149:53–61. https://doi.org/10.1378/chest.15-0645 [PubMed]
-
14.
Yu J, Deng M, Zhao J, Huang L. Decreased expression of klotho gene in uremic atherosclerosis in apolipoprotein E-deficient mice. Biochem Biophys Res Commun. 2010; 391:261–6. https://doi.org/10.1016/j.bbrc.2009.11.046 [PubMed]
-
15.
Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol. 2011; 13:254–62. https://doi.org/10.1038/ncb2167 [PubMed]
-
16.
Utsugi T, Ohno T, Ohyama Y, Uchiyama T, Saito Y, Matsumura Y, Aizawa H, Itoh H, Kurabayashi M, Kawazu S, Tomono S, Oka Y, Suga T, et al. Decreased insulin production and increased insulin sensitivity in the klotho mutant mouse, a novel animal model for human aging. Metabolism. 2000; 49:1118–23. https://doi.org/10.1053/meta.2000.8606 [PubMed]
-
17.
Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005; 280:38029–34. https://doi.org/10.1074/jbc.M509039200 [PubMed]
-
18.
Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, Brobey R, Rosenblatt KP, Tilton RG, Choudhary S. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes. 2011; 60:1907–16. https://doi.org/10.2337/db10-1262 [PubMed]
-
19.
Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003; 17:2393–403. https://doi.org/10.1210/me.2003-0048 [PubMed]
-
20.
Razzaque MS. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009; 5:611–9. https://doi.org/10.1038/nrendo.2009.196 [PubMed]
-
21.
Blackburn EH. Structure and function of telomeres. Nature. 1991; 350:569–73. https://doi.org/10.1038/350569a0 [PubMed]
-
22.
Blackburn EH, Epel ES, Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science. 2015; 350:1193–8. https://doi.org/10.1126/science.aab3389 [PubMed]
-
23.
Chan SW, Blackburn EH. Telomerase and ATM/Tel1p protect telomeres from nonhomologous end joining. Mol Cell. 2003; 11:1379–87. https://doi.org/10.1016/s1097-2765(03)00174-6 [PubMed]
-
24.
Xie Z, Jay KA, Smith DL, Zhang Y, Liu Z, Zheng J, Tian R, Li H, Blackburn EH. Early telomerase inactivation accelerates aging independently of telomere length. Cell. 2015; 160:928–39. https://doi.org/10.1016/j.cell.2015.02.002 [PubMed]
-
25.
De Meyer T, Nawrot T, Bekaert S, De Buyzere ML, Rietzschel ER, Andrés V. Telomere Length as Cardiovascular Aging Biomarker: JACC Review Topic of the Week. J Am Coll Cardiol. 2018; 72:805–13. https://doi.org/10.1016/j.jacc.2018.06.014 [PubMed]
-
26.
Fitzpatrick AL, Kronmal RA, Kimura M, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Hardikar S, Aviv A. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011; 66:421–9. https://doi.org/10.1093/gerona/glq224 [PubMed]
-
27.
Fuster JJ, Andrés V. Telomere biology and cardiovascular disease. Circ Res. 2006; 99:1167–80. https://doi.org/10.1161/01.RES.0000251281.00845.18 [PubMed]
-
28.
Fyhrquist F, Saijonmaa O, Strandberg T. The roles of senescence and telomere shortening in cardiovascular disease. Nat Rev Cardiol. 2013; 10:274–83. https://doi.org/10.1038/nrcardio.2013.30 [PubMed]
-
29.
Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014; 349:g4227. https://doi.org/10.1136/bmj.g4227 [PubMed]
-
30.
Kubben N, Misteli T. Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nat Rev Mol Cell Biol. 2017; 18:595–609. https://doi.org/10.1038/nrm.2017.68 [PubMed]
-
31.
Rehkopf DH, Needham BL, Lin J, Blackburn EH, Zota AR, Wojcicki JM, Epel ES. Leukocyte Telomere Length in Relation to 17 Biomarkers of Cardiovascular Disease Risk: A Cross-Sectional Study of US Adults. PLoS Med. 2016; 13:e1002188. https://doi.org/10.1371/journal.pmed.1002188 [PubMed]
-
32.
Schneider CV, Schneider KM, Teumer A, Rudolph KL, Hartmann D, Rader DJ, Strnad P. Association of Telomere Length With Risk of Disease and Mortality. JAMA Intern Med. 2022; 182:291–300. https://doi.org/10.1001/jamainternmed.2021.7804 [PubMed]
-
33.
Wang Q, Zhan Y, Pedersen NL, Fang F, Hägg S. Telomere Length and All-Cause Mortality: A Meta-analysis. Ageing Res Rev. 2018; 48:11–20. https://doi.org/10.1016/j.arr.2018.09.002 [PubMed]
-
34.
Chae DH, Wang Y, Martz CD, Slopen N, Yip T, Adler NE, Fuller-Rowell TE, Lin J, Matthews KA, Brody GH, Spears EC, Puterman E, Epel ES. Racial discrimination and telomere shortening among African Americans: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Health Psychol. 2020; 39:209–19. https://doi.org/10.1037/hea0000832 [PubMed]
-
35.
Colich NL, Rosen ML, Williams ES, McLaughlin KA. Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis. Psychol Bull. 2020; 146:721–64. https://doi.org/10.1037/bul0000270 [PubMed]
-
36.
Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou Y, Beversdorf DQ, Weng NP. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer's disease patients. J Immunol. 2007; 179:4249–54. https://doi.org/10.4049/jimmunol.179.6.4249 [PubMed]
-
37.
Darrow SM, Verhoeven JE, Révész D, Lindqvist D, Penninx BW, Delucchi KL, Wolkowitz OM, Mathews CA. The Association Between Psychiatric Disorders and Telomere Length: A Meta-Analysis Involving 14,827 Persons. Psychosom Med. 2016; 78:776–87. https://doi.org/10.1097/PSY.0000000000000356 [PubMed]
-
38.
Epel ES, Prather AA. Stress, Telomeres, and Psychopathology: Toward a Deeper Understanding of a Triad of Early Aging. Annu Rev Clin Psychol. 2018; 14:371–97. https://doi.org/10.1146/annurev-clinpsy-032816-045054 [PubMed]
-
39.
Oliveira BS, Zunzunegui MV, Quinlan J, Fahmi H, Tu MT, Guerra RO. Systematic review of the association between chronic social stress and telomere length: A life course perspective. Ageing Res Rev. 2016; 26:37–52. https://doi.org/10.1016/j.arr.2015.12.006 [PubMed]
-
40.
Shaker MR, Aguado J, Chaggar HK, Wolvetang EJ. Klotho inhibits neuronal senescence in human brain organoids. NPJ Aging Mech Dis. 2021; 7:18. https://doi.org/10.1038/s41514-021-00070-x [PubMed]
-
41.
Buendía P, Carracedo J, Soriano S, Madueño JA, Ortiz A, Martín-Malo A, Aljama P, Ramírez R. Klotho Prevents NFκB Translocation and Protects Endothelial Cell From Senescence Induced by Uremia. J Gerontol A Biol Sci Med Sci. 2015; 70:1198–209. https://doi.org/10.1093/gerona/glu170 [PubMed]
-
42.
Ullah M, Sun Z. Klotho Deficiency Accelerates Stem Cells Aging by Impairing Telomerase Activity. J Gerontol A Biol Sci Med Sci. 2019; 74:1396–407. https://doi.org/10.1093/gerona/gly261 [PubMed]
-
43.
Mytych J, Solek P, Tabecka-Lonczynska A, Koziorowski M. Klotho-Mediated Changes in Shelterin Complex Promote Cytotoxic Autophagy and Apoptosis in Amitriptyline-Treated Hippocampal Neuronal Cells. Mol Neurobiol. 2019; 56:6952–63. https://doi.org/10.1007/s12035-019-1575-5 [PubMed]
-
44.
Tempaku PF, D'Almeida V, da Silva SMA, Andersen ML, Belangero SI, Tufik S. Klotho genetic variants mediate the association between obstructive sleep apnea and short telomere length. Sleep Med. 2021; 83:210–3. https://doi.org/10.1016/j.sleep.2021.01.015 [PubMed]
-
45.
Wolf EJ, Morrison FG, Sullivan DR, Logue MW, Guetta RE, Stone A, Schichman SA, McGlinchey RE, Milberg WP, Miller MW. The goddess who spins the thread of life: Klotho, psychiatric stress, and accelerated aging. Brain Behav Immun. 2019; 80:193–203. https://doi.org/10.1016/j.bbi.2019.03.007 [PubMed]
-
46.
Aschbacher K, Milush JM, Gilbert A, Almeida C, Sinclair E, Epling L, Grenon SM, Marco EJ, Puterman E, Epel E. Chronic stress is associated with reduced circulating hematopoietic progenitor cell number: A maternal caregiving model. Brain Behav Immun. 2017; 59:245–52. https://doi.org/10.1016/j.bbi.2016.09.009 [PubMed]
-
47.
Gianaros PJ, Jennings JR. Host in the machine: A neurobiological perspective on psychological stress and cardiovascular disease. Am Psychol. 2018; 73:1031–44. https://doi.org/10.1037/amp0000232 [PubMed]
-
48.
Wirtz PH, von Känel R. Psychological Stress, Inflammation, and Coronary Heart Disease. Curr Cardiol Rep. 2017; 19:111. https://doi.org/10.1007/s11886-017-0919-x [PubMed]
-
49.
Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L, and INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004; 364:937–52. https://doi.org/10.1016/S0140-6736(04)17018-9 [PubMed]
-
50.
Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, Mendes WB. More than a feeling: A unified view of stress measurement for population science. Front Neuroendocrinol. 2018; 49:146–69. https://doi.org/10.1016/j.yfrne.2018.03.001 [PubMed]
-
51.
Montez JK, Hayward MD. Cumulative childhood adversity, educational attainment, and active life expectancy among U.S. adults. Demography. 2014; 51:413–35. https://doi.org/10.1007/s13524-013-0261-x [PubMed]
-
52.
Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004; 101:17312–5. https://doi.org/10.1073/pnas.0407162101 [PubMed]
-
53.
Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011; 73:16–22. https://doi.org/10.1097/PSY.0b013e31820573b6 [PubMed]
-
54.
Epel ES, Lin J, Dhabhar FS, Wolkowitz OM, Puterman E, Karan L, Blackburn EH. Dynamics of telomerase activity in response to acute psychological stress. Brain Behav Immun. 2010; 24:531–9. https://doi.org/10.1016/j.bbi.2009.11.018 [PubMed]
-
55.
Prather AA, Epel ES, Arenander J, Broestl L, Garay BI, Wang D, Dubal DB. Longevity factor klotho and chronic psychological stress. Transl Psychiatry. 2015; 5:e585. https://doi.org/10.1038/tp.2015.81 [PubMed]
-
56.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013; 153:1194–217. https://doi.org/10.1016/j.cell.2013.05.039 [PubMed]
-
57.
Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005; 120:449–60. https://doi.org/10.1016/j.cell.2005.02.002 [PubMed]
-
58.
Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003; 299:1346–51. https://doi.org/10.1126/science.1081447 [PubMed]
-
59.
Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005; 205:147–57. https://doi.org/10.1111/j.0105-2896.2005.00259.x [PubMed]
-
60.
McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009; 21:418–24. https://doi.org/10.1016/j.coi.2009.05.023 [PubMed]
-
61.
Schmid I, Dagarag MD, Hausner MA, Matud JL, Just T, Effros RB, Jamieson BD. Simultaneous flow cytometric analysis of two cell surface markers, telomere length, and DNA content. Cytometry. 2002; 49:96–105. https://doi.org/10.1002/cyto.10163 [PubMed]
-
62.
Cohen S, Janicki-Deverts D, Turner RB, Casselbrant ML, Li-Korotky HS, Epel ES, Doyle WJ. Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults. JAMA. 2013; 309:699–705. https://doi.org/10.1001/jama.2013.613 [PubMed]
-
63.
Effros RB. Kleemeier Award Lecture 2008--the canary in the coal mine: telomeres and human healthspan. J Gerontol A Biol Sci Med Sci. 2009; 64:511–5. https://doi.org/10.1093/gerona/glp001 [PubMed]
-
64.
Effros RB, Allsopp R, Chiu CP, Hausner MA, Hirji K, Wang L, Harley CB, Villeponteau B, West MD, Giorgi JV. Shortened telomeres in the expanded CD28-CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996; 10:F17–22. https://doi.org/10.1097/00002030-199607000-00001 [PubMed]
-
65.
Dagarag M, Evazyan T, Rao N, Effros RB. Genetic manipulation of telomerase in HIV-specific CD8+ T cells: enhanced antiviral functions accompany the increased proliferative potential and telomere length stabilization. J Immunol. 2004; 173:6303–11. https://doi.org/10.4049/jimmunol.173.10.6303 [PubMed]
-
66.
Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001; 75:12182–7. https://doi.org/10.1128/JVI.75.24.12182-12187.2001 [PubMed]
-
67.
Saurwein-Teissl M, Lung TL, Marx F, Gschösser C, Asch E, Blasko I, Parson W, Böck G, Schönitzer D, Trannoy E, Grubeck-Loebenstein B. Lack of antibody production following immunization in old age: association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002; 168:5893–9. https://doi.org/10.4049/jimmunol.168.11.5893 [PubMed]
-
68.
Luckheeram RV, Zhou R, Verma AD, Xia B. CD4+T cells: differentiation and functions. Clin Dev Immunol. 2012; 2012:925135. https://doi.org/10.1155/2012/925135 [PubMed]
-
69.
Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, Dolbier C, Mendes WB, Blackburn EH. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006; 31:277–87. https://doi.org/10.1016/j.psyneuen.2005.08.011 [PubMed]
-
70.
Zalli A, Carvalho LA, Lin J, Hamer M, Erusalimsky JD, Blackburn EH, Steptoe A. Shorter telomeres with high telomerase activity are associated with raised allostatic load and impoverished psychosocial resources. Proc Natl Acad Sci U S A. 2014; 111:4519–24. https://doi.org/10.1073/pnas.1322145111 [PubMed]
-
71.
Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, Wolkowitz O, Mellon S, Blackburn E. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010; 352:71–80. https://doi.org/10.1016/j.jim.2009.09.012 [PubMed]
-
72.
Hillman JL, Anderson CM. It's a Battle and a Blessing: The Experience and Needs of Custodial Grandparents of Children with Autism Spectrum Disorder. J Autism Dev Disord. 2019; 49:260–9. https://doi.org/10.1007/s10803-018-3761-0 [PubMed]
-
73.
Myers BJ, Mackintosh VH, Goin-Kochel RP. “My greatest joy and my greatest heart ache:” Parents’ own words on how having a child in the autism spectrum has affected their lives and their families’ lives. Res Autism Spectr Disord. 2009; 3:670–84. https://doi.org/10.1016/j.rasd.2009.01.004
-
74.
Cidav Z, Marcus SC, Mandell DS. Implications of childhood autism for parental employment and earnings. Pediatrics. 2012; 129:617–23. https://doi.org/10.1542/peds.2011-2700 [PubMed]
-
75.
Mayer SE, Prather AA, Puterman E, Lin J, Arenander J, Coccia M, Shields GS, Slavich GM, Epel ES. Cumulative lifetime stress exposure and leukocyte telomere length attrition: The unique role of stressor duration and exposure timing. Psychoneuroendocrinology. 2019; 104:210–8. https://doi.org/10.1016/j.psyneuen.2019.03.002 [PubMed]
-
76.
Cohen S. Perceived stress in a probability sample of the United States. The social psychology of health. Thousand Oaks, CA, USA: Sage Publications, Inc.; 1988; 31–67.
-
77.
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983; 24:385–96. [PubMed]
-
78.
Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y, Okamoto N, Kurumatani N, Namba N, et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010; 398:513–8. https://doi.org/10.1016/j.bbrc.2010.06.110 [PubMed]
-
79.
Dubal DB, Yokoyama JS, Zhu L, Broestl L, Worden K, Wang D, Sturm VE, Kim D, Klein E, Yu GQ, Ho K, Eilertson KE, Yu L, et al. Life extension factor klotho enhances cognition. Cell Rep. 2014; 7:1065–76. https://doi.org/10.1016/j.celrep.2014.03.076 [PubMed]
-
80.
Lin J, Cheon J, Brown R, Coccia M, Puterman E, Aschbacher K, Sinclair E, Epel E, Blackburn EH. Systematic and Cell Type-Specific Telomere Length Changes in Subsets of Lymphocytes. J Immunol Res. 2016; 2016:5371050. https://doi.org/10.1155/2016/5371050 [PubMed]
-
81.
Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002; 30:e47. https://doi.org/10.1093/nar/30.10.e47 [PubMed]
-
82.
R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. https://www.R-project.org.
-
83.
Fox J, Weisberg S. An {R} Companion to Applied Regression. Third Edition. Thousand Oaks, CA, USA: Sage; 2019. https://socialsciences.mcmaster.ca/jfox/Books/Companion/.
-
84.
Lüdecke D. ggeffects: Tidy Data Frames of Marginal Effects from Regression Models. J Open Source Softw. 2018; 3:772. https://doi.org/10.21105/joss.00772
-
85.
Wickham H. ggplot2: elegant graphics for data analysis. Second edition. Cham: Springer. 2016; 260.
-
86.
Revelle W. psych: Procedures for Psychological, Psychometric, and Personality Research. Northwestern University. 2022. https://CRAN.R-project.org/package=psych.