Longevity biotechnology: bridging AI, biomarkers, geroscience and clinical applications for healthy longevity
Abstract
The recent unprecedented progress in ageing research and drug discovery brings together fundamental research and clinical applications to advance the goal of promoting healthy longevity in the human population. We, from the gathering at the Aging Research and Drug Discovery Meeting in 2023, summarised the latest developments in healthspan biotechnology, with a particular emphasis on artificial intelligence (AI), biomarkers and clocks, geroscience, and clinical trials and interventions for healthy longevity. Moreover, we provide an overview of academic research and the biotech industry focused on targeting ageing as the root of age-related diseases to combat multimorbidity and extend healthspan. We propose that the integration of generative AI, cutting-edge biological technology, and longevity medicine is essential for extending the productive and healthy human lifespan.
Introduction
The landscape of ageing research and interventions targeting age-related diseases has undergone significant advancement in the past decade [1, 2]. Since the first Aging Research and Drug Discovery (ARDD) meeting in 2013, the field has evolved into a multidisciplinary arena, attracting substantial funding, spawning numerous startups, and yielding groundbreaking discoveries. It now encompasses contributions from a broad spectrum of professionals, including biologists, physicians, data scientists, and entrepreneurs, bridging the public and private sectors. This dynamic progression has been greatly accelerated by the advent of cutting-edge technologies, such as artificial intelligence (AI), comprehensive omics analyses, and innovative ageing clocks (Figure 1). These tools have catalysed advancements in our understanding of ageing processes and the development of safe and effective interventions to combat ageing and chronic illness, leading to healthspan extension.
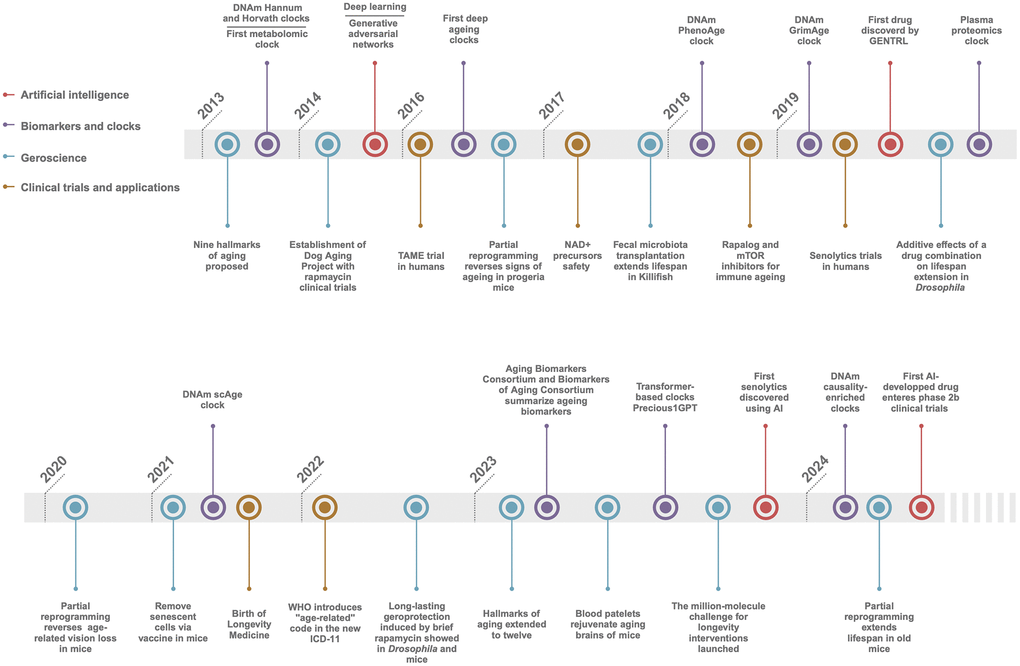
Figure 1. Timeline of longevity biotechnology. Key breakthroughs the AI, Biomarkers and clocks, Geroscience, and Clinical trials and applications in ageing and longevity fields since 2013.
This evolving paradigm is characterized by the synergistic integration of AI and big data analytics, which have emerged as transformative forces in the identification, characterization, and predictive analysis of ageing biomarkers [3]. AI-driven biomarker discovery is increasingly recognized as a cornerstone for advancing personalized medicine and improving healthcare outcomes. Yet, the journey from biomarker discovery to clinical translation encompasses a myriad of challenges, including rigorous validation processes and the harmonization of regulatory standards [4–6]. Overcoming these obstacles necessitates continued investment, collaboration, and innovation, underscoring the pivotal role of biomarkers in the nexus of ageing research, drug discovery, and clinical applications.
Within this context, longevity biotechnology emerges as an interdisciplinary bridge that connects AI, biomarkers discovery and deployment, geroscience, and clinical applications, aiming to redefine healthcare paradigms towards achieving healthy longevity. This review, compiled by the ARDD speakers following the 2023 ARDD event, aims to encapsulate the latest strides in longevity biotechnology. It highlights the convergence of AI-driven approaches with traditional research methodologies, underscoring the potential of this union in addressing the complex challenges posed by ageing and age-related diseases. By delving into AI-empowered biomarker development, mechanisms of ageing, gerotherapeutics, and the landscape of clinical trials and interventions, we endeavour to provide a comprehensive perspective on state of the art in longevity science and its implications for future healthcare strategies.
AI in ageing research
The emergence of longevity biotechnology as a standalone industry has led to the acceleration of the convergence of AI and ageing research [3], which led to notable advancements in longevity science, with AI playing an increasing role in biomarker identification, drug discovery, and clinical practice. The link between longevity and AI is expected to become even stronger, as AI is helping to understand the wider determinants of health, including how the environment influences gene expression, yielding new insights on how to increase the human healthy lifespan [7].
AI-driven biomarker identification
The fusion of AI with biomarker research has markedly revolutionized the way biomarkers are identified and validated in the field of ageing. Machine learning algorithms, deep learning methods, and big data analytics have facilitated the discovery of novel biomarkers of ageing crucial for disease diagnosis, prognosis, and predicting treatment outcomes [8, 9]. For example, deep learning algorithms applied to cellular images across multiple tissues identified nuclear morphology as a new universal senescence marker [10]. Yet, it is often challenging to use singular indicators as ageing biomarkers. Using panels or complex biomarkers that combine data from multiple ‘omic’ technologies is recommended [11]. It is especially important to use explainable AI to create models that can predict chronological age from non-invasive measurements. In the future, the integration of AI with emerging technologies, such as single-cell sequencing and spatial transcriptomics for the biomarker discovery for complex diseases with multifactorial etiology will be at the forefront of ageing research. Notably, it is becoming increasingly important to identify not only biomarkers that predict the onset of age-related disorders and diseases, but also therapeutic biomarkers that change in response to gerotherapeutic interventions that delay, prevent, alleviate, or treat age-related disorders and diseases and that may extend healthspan [5, 12].
AI in drug discovery and longevity science
One of the most prominent applications of AI in the ageing field is drug discovery, where AI techniques are used to identify and design new compounds [13, 14]. Pharmaceuticals have been identified that target some of the hallmarks of ageing, which could be utilized to address age-related multimorbidities. For example, several recent findings showed machine learning or deep neural networks are able to assist in the discovery of senolytic compounds in preclinical models [15, 16], first-in-class preclinical drug candidates against Huntington’s disease [17], and novel mTOR inhibitors and repurposing hypertension drug rilmenidine for extending C.elegans lifespan [18, 19]. This approach has significantly accelerated the process of finding anti-ageing therapeutic solutions.
The usage of AI extends beyond drug discovery. Machine learning techniques have been employed to decode the genetic and epigenetic factors associated with longevity, such as those related to physical fitness and specific genes like ACTN3 [20]. This provides a crucial example of how AI, particularly deep learning and machine learning, accelerate longevity science by uncovering novel biomarkers and elucidating the complex genetic and epigenetic underpinnings of ageing. Furthermore, the application of hybrid quantum-classical machine learning techniques reveals new perspectives on biological age and its determinants and opens new avenues for the development of gerontechnology [21].
Biomarkers and ageing clocks
Biomarkers and clocks play a crucial role in understanding age and age-related disease progression, predicting outcomes, and optimizing treatment strategies. Recent contributions from the Aging Biomarkers Consortium and the Biomarkers of Aging Consortium provided a comprehensive summary of the current state of biomarkers across cellular, organ, and organismal levels of ageing. These groups have also proposed a refined framework for the terminology and characterization of ageing biomarkers, offering valuable insights and data for the field [5, 12]. Furthermore, updates on the hallmarks of ageing delivered extensive information and complex biological datasets, underscoring the necessity of integrating AI with traditional biomarker discovery approaches to derive meaningful insights [1].
Biomarker validation and clinical translation
The biomarkers of ageing need to be widely validated before being incorporated into clinical practice [4]. The ageing process is highly variable across tissues and organs [22, 23]. Moreover, the ageing process varies significantly among individuals, with stochastic variation alone can explain a significant portion of ageing dynamics. The accuracy of predictive biomarkers may plateau as stochasticity increases [24], highlighting the variability in biological ageing among individuals and the value of measuring this difference. Validation is a multistep process that defines the characteristics of biomarkers, including their reliability, accuracy, and ability to predict relevant outcomes [4, 25]. This process requires expertise in various areas, such as the biological mechanisms of ageing, the design and construction of composite biomarkers, and the validation of biomarkers across diverse population samples. Collaboration between basic scientists and clinical investigators is essential for successfully navigating this process.
Initiatives such as Biolearn, methylCIPHER, Estimage, and Clockbase have emerged to facilitate the development, validation, and comparison of ageing biomarkers [26–29]. These platforms provide standardized datasets and evaluation metrics, enabling researchers to benchmark their biomarkers against existing ones and identify areas for improvement. Such collaborative efforts are crucial for accelerating the translation of ageing biomarkers into clinical practice. To enhance rigor in the validation process, guidelines for standardization and harmonization of biomarkers across populations with unique characteristics are needed [4]. Recommendations on metrics for reporting predictive performance should also be established. Systematic validation can accelerate the clinical translation of ageing biomarkers and their use in gerotherapeutic clinical trials.
Ageing clocks and AI
Ageing clocks are a specific type of biomarker designed to predict biological age, typically through computational models. These clocks often use a combination of biomarkers, integrating them through machine learning techniques to estimate the overall ageing rate and biological age of an individual. It has been a decade since the first-generation DNA methylation clocks were created [30]. Since then, many biological clocks have been developed, from clocks based on molecular signatures such as DNA methylation, transcriptome, lipids and glycans to physiological clocks using facial, fundus, and tongue images [31–35].
One of the limitations of most existing clocks is that often reflect changes in blood cell populations rather than intrinsic ageing. Removing CpG sites linked to T cell differentiation could be a way to measure intrinsic ageing more accurately [36]. Another limitation of most existing clocks is their lack of information on causality, which means that while ageing clocks can show correlations with ageing, they are not able to pinpoint which biological changes are causing ageing. DamAge and AdaptAge-clocks are novel epigenetic clocks that are based on causal analysis [37]. These clocks might prove to be more useful for tracking short-term interventions making them suitable and applicable for assessing nuanced effects on geroprotective and rejuvenative interventions.
Beyond the DNA methylation clocks, blood-measurement-based biological age predictors have been developed since 2016, when a deep neural network (DNN) approach was introduced to predict age from blood biochemistry data [3, 38]. Since then, several studies have built upon this concept, refining and expanding the use of blood parameters to predict biological age and health outcomes. For example, the “block clock” employs an array of blood parameters, such as complete blood count (CBC), and other parameters measured with a scale and glucose meter, demonstrating its power to forecast health and survival outcomes in mice [39]. Similarly, a new OMICmAge integrates multi-omics data, including proteomics, metabolomics, clinical information, and DNA methylation, resulting in composite metric, shows promising associations with disease, improved hazard ratios, and higher accuracy in predicting 5-year and 10-year survival rates compared to single-omic clocks [40]. However, despite the great potential of biological clocks supported by AI, the lack of uniform data exchange protocols between laboratories and units conducting development research makes it difficult to create a reliable clinical tool. Many solutions are still based on reading text documents by an AI algorithm, which carries the risk of incorrect data in the analysis.
In sum, there is an increasing demand in the field for the development of more sophisticated ageing biomarkers and clocks. These should not only predict ageing and associated risks but also elucidate the underlying mechanisms and causal relationships. Furthermore, employing AI to integrate multi-omics data for advanced ageing clocks presents a promising strategy to advance ageing research and improve healthcare outcomes.
Mechanisms of ageing, drug discovery and gerotechnology
In recent years, our understanding of the mechanisms of ageing has significantly advanced. Of note, the proposition of twelve hallmarks of ageing includes primary hallmarks which reflect on the molecular-level damages; antagonistic hallmarks which represent the organelle and cellular response to the damage, and integrative hallmarks which reflect alternations at systemic levels [1]. The shift from the pursuit of single unified theories of ageing towards explaining ageing as a complex combination of molecular events and cellular responses has been useful towards the development of novel interventions and treatments targeting age-related diseases.
Primary hallmarks of ageing and potential applications
The primary hallmarks of ageing include genome instability (DNA damage and repair, and genetic mutations), telomere damage and attrition, epigenetic changes to the genetic code and histones that regulate DNA accessibility, and autophagy/proteostasis that ameliorate damage to proteins and organelles [1]. These hallmarks comprise the consequences of normal metabolism and energetics and how well cells respond to that unavoidable stress. The primary hallmarks are dictated by both genetic and environmental factors and thereby are arguably the most heterogeneous and unavoidable consequences of ageing.
Genome integrity
Recent advancements have further delineated the mechanisms associated with the hallmarks of ageing. Among the primary hallmarks, genome instability, predominantly induced by endogenous DNA damage driven by normal metabolism, the accumulation of genetic mutations and the activity of transposable elements, along with telomeric DNA damage and attrition, the latter being the progressive shortening of telomeres at the ends of chromosomes during cell division, plays a key role in the progression of ageing and its associated diseases [1, 41]. Studies using progeroid mouse models, such as Ercc1 mutant, a model of a human progeroid syndrome, have illustrated how particular gene mutations in DNA repair genes can accelerate ageing processes, initiate most of the other hallmarks of ageing, and compromise various organ systems, illustrating the interconnectedness of hallmarks of ageing [42]. The recent discovery of the DREAM complex as a master regulator of DNA repair gene expression provides a potential pharmacological target for boosting genome integrity thus alleviating a causal mechanism that drives the ageing process [43].
Epigenetic alternations
The alteration of epigenetic information with age represents another pivotal mechanism influencing ageing [1]. Innovations in partial reprogramming, using Yamanaka factors and other agents, suggest that the reversal of the epigenetic landscape to a more youthful state may counteract ageing effects [44–48]. This hypothesis is supported by findings that proper maintenance of epigenetic marks is essential for preserving cell identity and mitigating ageing and cancer-related changes in the epigenome [49, 50]. Furthermore, recent research demonstrates that age-associated decline in heterochromatin levels contributes to genomic instability and diminished regenerative capacity, suggesting that interventions aimed at rejuvenating aged cells could be beneficial [51].
Given the therapeutic potential of partial reprogramming, it emerges as a highly promising strategy for combating the effects of ageing and its related diseases. Research targeting specific cell types, such as retinal ganglion cells with reprogramming techniques, reveals the potential to restore cellular function lost with age [52]. Furthermore, the development and application of mRNA-based reprogramming for skin rejuvenation exemplifies the innovative approaches being pursued to reverse age-related cellular changes, offering the non-invasive and transformative potential for anti-ageing interventions [53]. However, safety concerns of partial reprogramming, such as how to protect cell type identity during the reprogramming process or predisposition to cancer may hinder its therapeutic applications [54].
Autophagy and proteostasis
Macroautophagy (we here refer to it as “autophagy”) is a critical cellular process involving the turnover and recycling of organelles and proteins [55]. It plays a pivotal role in maintaining cellular homeostasis and combating the deleterious effects of protein and organelle damage that occurs during ageing. Stimulation of autophagy is sufficient to decelerate age-related pathology and extend lifespan in diverse animal models [1, 56]. Of note, a brief treatment of rapamycin in early adulthood induces sustained autophagy activation and thereby attenuates age-related gut pathology and systemic inflammation markers in mice, shedding light for further testing in clinical trials [57]. Moreover, the integration of AI has yielded promising drug candidates targeting specific autophagic processes, such as mitochondrial autophagy (mitophagy), showcasing positive outcomes in Alzheimer’s disease preclinical models [58].
Parallel to autophagy, proteostasis declines with age, contributing to a range of degenerative diseases including Alzheimer’s [59]. Intervention strategies that modulate protein synthesis, bolster chaperones like heat shock proteins (HSPs), and enhance proteasomal activities decrease toxic protein aggregates and alleviate symptoms of degenerative conditions, illustrating the critical role of proteostasis in maintaining cellular integrity and function [59]. These insights into autophagy and proteostasis deepen our understanding of cellular mechanisms underlying ageing and pave the way for novel therapeutic approaches to combat age-related diseases.
Antagonistic hallmarks and pathways to clinical translation
Antagonistic hallmarks of ageing, including deregulated nutrient sensing, mitochondrial dysfunction, and cellular senescence, play pivotal roles in the ageing process and the development of age-related diseases [1]. These mechanisms comprise the organelle and cellular responses to the primary hallmarks of ageing and stress, and are central to the current research and clinical studies in geroscience, highlighting their significance and potential translatability into therapeutic interventions [60–62].
Nutrient sensing
Research into the deregulation of nutrient-sensing pathways, such as insulin/IGF-1, mTOR, and AMPK, reveals a profound impact on the ageing process and associated diseases. These pathways, when disrupted, contribute significantly to the ageing phenotype, presenting targeted opportunities for intervention. Interventions in this area focus on modulating the activity of these pathways to decelerate ageing and mitigate age-related conditions [63–65]. For example, subtly adjusting mTOR activity maintains muscle strength without promoting excessive growth, indicating a delicate balance between growth and ageing. Similarly, modulating the activity of the AMPK complex may foster metabolic health and support healthy ageing [66].
Nutrient-sensing pathways are well connected with other hallmarks of ageing. Dietary interventions like intermittent fasting and Fasting Mimicking Diet (FMD) have been explored for their potential to slow down the ageing process and prevent the onset of age-related diseases [67]. Notably, circadian alignment can enhance the effect of calorie restriction on lifespan extension in male mice, underscoring the importance of considering timing when optimizing dosing regimens of geroprotectors [68]. Furthermore, pharmacological reagents like metformin, and rapamycin, alongside natural compounds, such as spermidine, alpha-ketobutyrate, alpha-ketoglutarate and taurine, or their synergistic combinations, have been identified for targeting these nutrient-sensing mechanisms to mediate multiple hallmarks of ageing [64, 69–75]. Such interventions have the potential to alter multiple age-related hallmarks, ultimately contributing to the extension of healthy lifespan in preclinical models and potentially in humans as well.
Mitochondrial dysfunction
Mitochondrial dysfunction, which is closely associated with the deregulation of nutrient-sensing pathways, is increasingly recognized as a cornerstone of ageing and its associated illness. A decline in mitochondrial function and a concomitant decrease in NAD+ levels are closely linked with ageing process [76]. Reducing mitochondrial protein folding stress in the dentate gyrus improves neurogenesis and cognitive function in old mice [77]. Therapeutic approaches targeting mitochondrial dysfunction aim to restore or enhance mitochondrial health and energetic efficiency. Research findings underline the significance of CD38 in regulating NAD levels and the role of NAD levels in stem cell fate, suggesting their beneficial impact during ageing processes [78]. Trigonelline is a natural, bioactive pyridine alkaloid that is a precursor of NAD+ and restores mitochondrial function muscle in the muscle of C.elegans and mice [79]. Research suggests that deficiency in cardiolipin, a phospholipid which is exclusively located in mitochondria, increases fatty acid oxidation in glycolytic muscle and accelerates muscle ageing. Furthermore, interventions targeting the mitochondrial matrix, such as the inhibition of manganese-induced coenzyme Q, which regulates ATP and ROS production, hold promise [80]. These findings underscore the importance of maintaining mitochondrial function and integrity for healthy ageing and disease prevention and indicate that natural compound supplementation can be a potential strategy for slowing down muscle ageing.
Cellular senescence
Senescent cells plays a significant role in the physiological decline associated with ageing, making them pivotal targets for anti-ageing interventions [1, 81]. Current research efforts focus on understanding the triggers and consequences of cellular senescence, pinpointing the location of these cells and the communication with neighbouring cells, and uncovering the connection with other hallmarks of ageing [82, 83]. This comprehensive understanding aims to provide foundational knowledge for the development of therapeutics that help alleviate age-related changes that drive vulnerability to chronic diseases.
The therapeutic landscape in this domain is notably enriched by the development of senolytics and senomorphics, which are meticulously crafted to selectively eliminate senescent cells or suppress their deleterious pro-inflammatory secretions, respectively. These innovative approaches have demonstrated promise in mitigating age-associated pathologies and promoting healthspan [83]. Currently, senotherapeutics include repurposing established pharmaceuticals (many from oncology), such as dasatinib and quercetin, delving into natural compounds like fisetin, and pioneering new treatments involving extracellular vesicles (EVs), vaccines, and CAR-T therapies [83–86]. It is possible to rejuvenate senescent cells using partial reprogramming techniques, so the senescence state can be reversed [87]. These diverse approaches enhance the likelihood of tackling cellular senescence safely and effectively. Moreover, the “hit-and-run” approach to targeting senescent cells selectively aligns well with the current timelines and regulatory standards of clinical trials, providing an innovative bridge between the fields of oncology and longevity medicine [83].
In sum, the categorization of antagonistic hallmarks into deregulated nutrient sensing, mitochondrial dysfunction, and cellular senescence provides a structured framework for understanding and targeting the ageing process. Research findings in each category inform the development of targeted interventions, illustrating the promising trajectory from scientific inquiry to clinical application in the field of geroscience. Notably, the majority of clinical trials testing gerotherapeutic interventions currently underway target the antagonistic hallmarks of ageing rather than the upstream primary hallmarks of ageing or the downstream integrative hallmarks [61].
Integrative hallmarks of ageing and therapeutic strategies
Chronic inflammation
Chronic inflammation and inflammaging are closely associated with functional decline and systemic ageing, yet their exact underlying sources and consequences remain to be fully elucidated [1, 88]. Notably, inflammation is often linked with other ageing hallmarks [1]. For example, recent research reveals that ageing leads to a decline in glycolysis and mitochondrial oxidative phosphorylation in myeloid cells, undermining immune cell functions and exacerbating inflammation [89]. Moreover, the response to infection is negatively impacted by the presence of senescent cells, which in turn promote further senescence when infected by viruses. Interestingly, long-lived species such as bats demonstrate remarkable immune responses to infections and viral containment, serving as an extraordinary model for unravelling the complexities of inflammation in the ageing process [90, 91].
Given the intricate relationship between chronic inflammation, age-related immune dysfunction (inflammaging) and immune-related diseases, the development of targeted interventions and treatments to mediate immune function is appealing. Novel strategies, such as inhibitors of key inflammation mediators like NLRP3, peptide-based therapeutics inspired by natural antimicrobial peptides, and immunotherapies for removing senescent or exhausted immune cells and enhancing immune function in ageing are under exploration [92]. AI-powered diagnostics and therapeutic solutions aimed at reversing gut inflammation and promoting gut health in ageing are also being developed [93]. To assess immune function, the immune longevity score (ILS) has been developed as a metric for evaluating the extensive functionality of the immune system, potentially serving as a novel ageing clock.
Microbiome, intercellular communication and stem cell activity
The gut microbiome has emerged as a critical factor influencing host metabolism, behaviour, and ageing [94]. Fecal microbiota transplantation (FMT) from young to old animals is sufficient to extend lifespan in killifish and reverse age-related differences in both peripheral and brain immunity in mice, highlighting the significant role of the microbiome in mediating host health and ageing [95, 96]. Healthy gut microbiota can lead to a less hydrophobic bile acid pool, which benefits liver health, pinpointing the function microbiome of the gut-liver axis in immune and functional ageing [97]. Furthermore, the translocation of Enterobacteriaceae has been observed to reverse premature ageing in SIRT6 knockout mice, suggesting the potential of microbiome replacement or modulation in anti-ageing strategies [98].
Recently, the alternations of extracellular matrix (ECM) have emerged as a driver of ageing [99]. For example, the remodelling of collagen, the most abundant protein in ECM, is required for longevity in C.elegans, suggesting that ECM homeostasis represents a novel mechanism for healthy ageing [100]. Aging is associated with defects in cell turnover and tissue renewal, and reductions in stem cell activity and number, all of which alter with age. These processes are intricately modulated by the “niche” or the local extracellular matrix (ECM) surrounding the cells [101].
Recent research reveals that diapause, a natural phenomenon that happens in many species, including C.elegans, certain insects, and killifish, can be a powerful model to investigate stem cell, rejuvenation and ageing, underscoring the interconnections between different hallmarks of ageing [102].
Other emerging technologies for gerotherapeutics
In the evolving landscape of gerotherapeutics, diverse findings and emerging technologies are reshaping the future of ageing interventions. Of note, the G-alpha protein, Gαq EGL-30, plays a crucial role in enhancing memory and overall healthspan in a conserved manner from C.elegans to mice, implying the potential for unexpected targeted therapies aimed at preserving cognitive function in ageing populations [103]. Similarly, newly developed enzyme-based therapies are targeting age-related macular degeneration, providing hope for individuals at risk of vision loss due to ageing [104]. Cryopreservation is advancing as a method for preserving cells and tissues for regenerative medicine applications, yet it remains under development [105]. Furthermore, novel techniques such as AI, microfluidic systems, and robotic-assisted high-throughput screening methods enable unbiased drug screening and direct measurement of lifespan and healthspan parameters in whole animals, significantly accelerating the study and testing of interventions for ageing [106–108].
Healthy ageing and longevity medicine
Lifestyle interventions, supplements and drugs for healthy ageing
In the evolving landscape of healthy ageing, clinical interventions rooted in exercise, diet, and pharmaceuticals are pivotal [61]. Accumulating evidence suggests that exercise operates beyond mere fitness and acts as a biological modifier by increasing circulating IL-6 levels to regulate inflammation, glucose homeostasis and lipid metabolism [109, 110], showcasing that exercise is not just a physical enhancer but also a potential biochemical modifier beneficial in countering the effects of ageing. Dietary approaches, such as calorie restriction, intermittent fasting, and fasting-mimicking diets (FMDs), have been shown to offer profound health benefits. In clinical settings, these diet strategies have led to notable improvements in patients with chronic conditions such as diabetes and hypertension [63, 111].
The integration of pharmaceuticals further broadens the anti-ageing intervention spectrum. Studies on natural compounds such as NAD+ precursors, alpha-ketobutyrate and urolithin-A showed improvements in mitochondrial function morphology [61, 72, 112]. The repurposing of drugs like rapamycin and other mTOR inhibitors, along with natural compounds like spermidine, can enhance autophagy and improve immune function and vaccine responses [113, 114], suggesting their potential to prevent age-related declines in the immune system. Similarly, combinations such as dasatinib and quercetin show improvements in reduced senescence marks and immune function among aged populations [83], highlighting the potential of pharmacological agents in extending healthspan.
These detailed insights into the multifaceted approaches encompassing exercise, diet, and pharmaceutical interventions reflect the comprehensive nature of interventions targeting ageing. They represent a holistic view that healthspan extension is achievable through targeted, evidence-based strategies grounded in rigorous clinical research and personalised healthcare paradigms.
Transition from sick care to health-oriented longevity medicine
The emergence of longevity medicine, a personalised preventive medicine powered by deep biomarkers of ageing and longevity, is a paradigm shift from traditional, sickness-oriented healthcare towards a more proactive, AI-driven approach [115]. Highlighting the individualisation of patient care, this approach integrates data collection and analysis, from cellular biomarkers to advanced diagnostic techniques, to pave the road for more effective and personalised interventions [116]. Clinics specialising in longevity medicine are now adopting this data-driven approach, establishing longevity medicine protocols and standardized criteria, as well as laying the groundwork for broader accessibility and implementation of longevity interventions in clinical settings worldwide (Figure 2). New commercial platforms leverage the integration of blood biomarkers, DNA, physiological markers, health care records and user-generated data to optimize personal health and healthy longevity strategies [117].
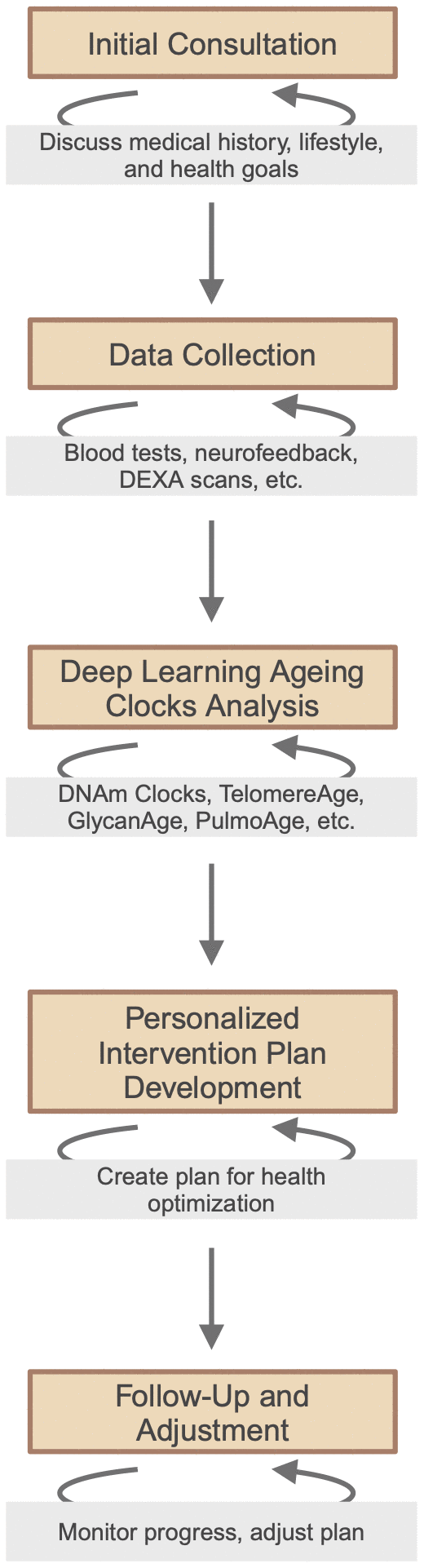
Figure 2. Framework of personalized, data-driven longevity medicine.
The interrelation between longevity and cancer research is considerable, anchored in the shared hallmarks and biological processes between ageing and tumorigenesis [118]. Applying geroscience approaches to cancer and ageing can improve decision-making in oncology, particularly for patients who are not typically included in clinical trials due to age [116]. The cross-section between treatments for chronic diseases and ageing research sets the stage for dual-purpose drug discovery [119, 120], meaning to find drugs that not only treat individual age-related diseases but also target one or multiple hallmarks of ageing, addressing pathological aspects of ageing. For instance, the risk of cancer increases approximately 40 times from the age of 20 to the age of 60, showing that age is the major risk driver, but prevention and early detection can significantly lower its abundance in society before dual-purpose drugs become abundant. To achieve that, the transition from sickness-oriented to health-oriented medicine is crucial and will require the inclusion of healthy longevity medicine in medical education and advanced training for physicians, enriched by the current scientific advancements in ageing research.
Ongoing challenges and future direction
Since 2019, the number of individuals over the age of 65 has surpassed those under 5, marking a significant demographic shift towards an ageing population (https://ourworldindata.org/age-structure). The majority of the elderly will have more than chronic disease associated with ageing, illustrating that curing a single age-related disease, while valuable, will not substantially change the health status of our global population [121, 122]. This transformation underscores the need for a comprehensive approach to extend healthspan and maintain workforce participation in older adults (https://www.un.org/en/global-issues/ageing). The urgency for systemic reforms spanning healthcare, policies, and societal norms is critical to alleviating the burdens posed by an ageing society. Advanced understanding of genetic, epigenetic, and environmental factors becomes increasingly vital to develop targeted health interventions for people across their life course especially in individuals over 50. There is a growing recognition of the importance of factoring in wider environmental, behavioral, and social determinants of health, encapsulated by the ‘exposome’, that have profound effects on the human health trajectory and overall resilience as people age [123].
The economic ramifications highlight ageing as a profound societal challenge, emphasizing that targeting fundamental ageing process is predicted to yield significantly greater economic benefits compared to focusing solely on individual chronic diseases [124]. Consequently, the healthy longevity research and development sector is urged to evolve swiftly by embracing innovative approaches, such as gene and cell therapies, organ replacement, engineered cells, full cell simulation, and reversible cryostasis. Moreover, the development of new tools to measure biology in ever greater detail and precision, such as single-cell proteomics, metabolomics, non-invasive blood chemistry monitoring and more, is essential for advancing our understanding and interventions in ageing. Overcoming challenges related to capital accessibility, quality control, and regulatory barriers is essential for progress. Furthermore, fostering community engagement and advocacy, and leveraging decentralized science and blockchain technology could spur research and innovation, paving the way for a new era of funding and knowledge exchange within the scientific community [125, 126].
The integration of artificial intelligence (AI), biomarkers, ageing biology, and longevity medicine stands as a cornerstone for extending human healthy lifespan. AI innovations offer deeper insights and enable personalised strategies in drug discovery and clinical trials, bridging technology and biology. The integration of traditional biomarkers with advanced deep clocks is steering the shift towards personalised medical interventions. New ‘omics’ technologies coupled with novel AI analyses now enable the study of extended longevity mechanisms evolved in non-canonical model ageing extremists such as bats, whales and naked mole rats, uncovering which pathways are most relevant to humans. Collaboration across disciplines and borders, involving clinicians, biologists, data scientists, funders, policymakers and healthy longevity community, is crucial for the effective integration of scientific findings into practice (Figure 3). This collective approach not only aims to deepen our comprehension of ageing but also to forge new paths for enhancing healthspan, thereby improving the quality of life in later years and reducing the financial strains of age-related conditions.
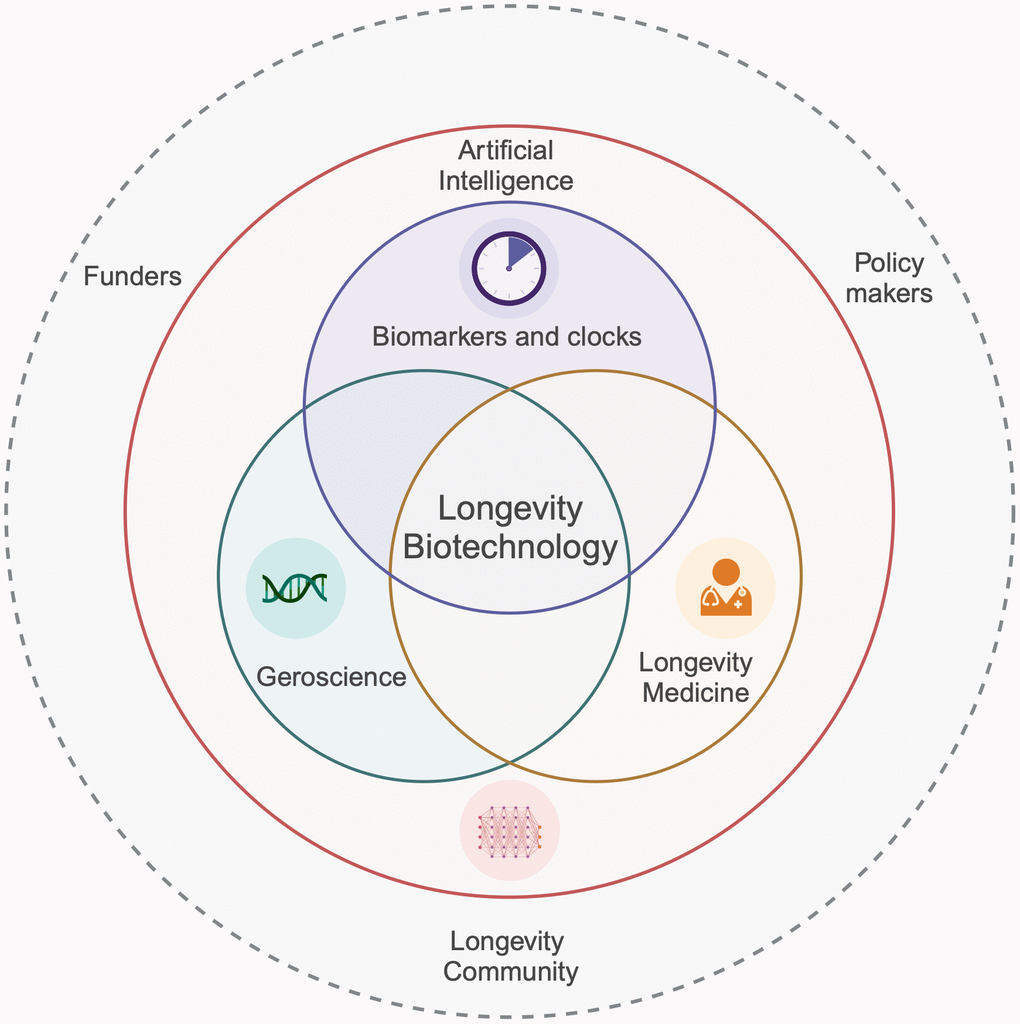
Figure 3. Integration of AI, biomarkers and clocks, geroscience, and longevity medicine in advancing human healthspan.
Acknowledgments
In memory of Judith Campisi and Mikhail Blagosklonny, whose pioneering work laid the foundational stones for this field, we continue to explore the depths of ageing research with a renewed commitment to unraveling the mysteries of longevity. Through this review, we aim to honor their legacy by contributing to the ongoing dialogue and development within the global ageing research community.
Conflicts of Interest
A.G. is affiliated with Haut.AI. A.K.S. is consulting for The Longevity Lab and Primeadine. D.S. is an employee of the Longevity Center Group. J.L.K. has a financial interest related to this research, including patents and pending patents covering senolytic drugs and their uses that are held by Mayo Clinic. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic conflict of interest policies. G.B. is an InsideTracker employee, and InsideTracker is the sole funder of Dr. Blander. G.C is the employee of DSM-Firmenich (Kaiseraugst, Switzerland). J.H. is a founder and scientific advisory board member of LongLifeRx and Aiance. J.S. is affiliated with Private Medical. J.V. is a co-founder of Singulomics Inc. and Mutagentech Inc. K.J.M. holds equity in Lysoclear, Inc. which is commercializing the presented technology. K.P. is funded by the Fondation Santé (19656) and the European Union (European Research Council; ERC), under grant agreement “ERC-GA101077374-SynaptoMitophagy”. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Council. Neither the European Union nor the granting authority can be held responsible for them. M.H. is affiliated with Synthego, Frontier Bio, Ligandal, Longshot Space. M.S-K. is supported by the Novo Nordisk Foundation Challenge Programme (NNF17OC0027812), the Nordea Foundation (02-2017-1749), the Neye Foundation, the Lundbeck Foundation (R324-2019-1492), the Ministry of Higher Education and Science (0238-00003B), VitaDAO and Insilico Medicine. M.Q. is founder and shareholder of Rubedo Life Sciences and Turn Biotechnologies. P.K., F.W.P., A.J. and A.Z. are employed at Insilico Medicine. P.M. was employed at Pompeu Fabra University and currently is employed at Altos Labs. T.F. co-founder and equity holder Generian Pharmaceuticals and Coloma Therapeutics.
Funding
A.K.S. supported by grants from the Wellcome Trust (Investigator award 220784/Z/20/Z). B.K.P The Centre for Physical Activity Research (CFAS) is supported by TrygFonden (grants ID 101390, ID 20045, and ID 125132). C.C. was supported by the Oppenheimer Program, UCLA Jonsson Cancer Center Foundation, Margaret E. Early Medical Research Trust, and National Center for Advancing Translational Sciences UCLA Clinical and Translational Science Institute (CTSI) Grant UL1TR000124. D.C. was supported by NIH R01AG082105, R01DK 117481, R01AG063404, and R01AG 063389. E.C.T. was supported by an Irish Research Council Laureate Award IRCLA/2017/58 and Science Foundation Ireland Future Frontiers 19/FFP/6790. J.V. is funded by grants from the US National Institutes of Health (grants U19AG056278, U01HL145560, U01ES029519, P01AG017242, P01AG047200). J.P. would like to acknowledge NIH grants R01AG068048, R01AG82708, UG3CA268103, and P01 AG062413. J.L.K. is supported by the National Institutes of Health (grants R37AG013925 and R33AG061456), the Connor Fund, the Robert J. and Theresa W. Ryan Fund, and the Noaber Foundation. K.M. was supported by NEI of the National Institutes of Health no. 1R43EY034403-01. L.J.N. and P.D.R. are supported by NIH (R01AG063543, U19AG056278, U54AG076041, U54AG079754). M.H. is funded by Longevity Biotech Fellowship. M.Q. from Rubedo Life Sciences received award from California Institute of Regenerative Medicine (CIRM #DISC-14096) for its pharmacological program on targeting senescent lung stem cells in Idiopathic Pulmonary Fibrosis. P.M. is funded from grant number ERC-2016-AdG-741966. Q.F. received a grant from the Shandong Provincial Natural Science Foundation (ZR2023MH262), Yantai Double Hundred Program, University and Locality Collaborative Program (2021XDHZ082). S.L. was supported by the Healthy Longevity Catalyst Awards (Hong Kong): National Academy of Medicine, USA. T.A.R. are supported by grants from NIH AG68667 and AG82764. V.G. is supported by grants from the US National Institute on Aging, Impetus grants, Michael Antonov Foundation. V.S. research is supported by the NUHS Internal Grant Funding under NUS Start-up grant NUHSRO/2022/047/Startup/11. Y.-X. L. is supported by grants from the Southern University of Science and Technology, China, and from the Max Planck Society, Germany.
References
-
1.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023; 186:243–78. https://doi.org/10.1016/j.cell.2022.11.001 [PubMed]
-
2.
Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019; 571:183–92. https://doi.org/10.1038/s41586-019-1365-2 [PubMed]
-
3.
Zhavoronkov A, Mamoshina P. Deep Aging Clocks: The Emergence of AI-Based Biomarkers of Aging and Longevity. Trends Pharmacol Sci. 2019; 40:546–9. https://doi.org/10.1016/j.tips.2019.05.004 [PubMed]
-
4.
Moqri M, Herzog C, Poganik JR, Ying K, Justice JN, Belsky DW, Higgins-Chen AT, Chen BH, Cohen AA, Fuellen G, Hägg S, Marioni RE, Widschwendter M, et al. Validation of biomarkers of aging. Nat Med. 2024; 30:360–72. https://doi.org/10.1038/s41591-023-02784-9 [PubMed]
-
5.
Bao H, Cao J, Chen M, Chen M, Chen W, Chen X, Chen Y, Chen Y, Chen Y, Chen Z, Chhetri JK, Ding Y, Feng J, et al, and Aging Biomarker Consortium. Biomarkers of aging. Sci China Life Sci. 2023; 66:893–1066. https://doi.org/10.1007/s11427-023-2305-0 [PubMed]
-
6.
Lee M, Ly H, Möller CC, Ringel MS. Innovation in Regulatory Science Is Meeting Evolution of Clinical Evidence Generation. Clin Pharmacol Ther. 2019; 105:886–98. https://doi.org/10.1002/cpt.1354 [PubMed]
-
7.
Marino N, Putignano G, Cappilli S, Chersoni E, Santuccione A, Calabrese G, Bischof E, Vanhaelen Q, Zhavoronkov A, Scarano B, Mazzotta AD, Santus E. Towards AI-driven longevity research: An overview. Front Aging. 2023; 4:1057204. https://doi.org/10.3389/fragi.2023.1057204 [PubMed]
-
8.
Kumar Y, Koul A, Singla R, Ijaz MF. Artificial intelligence in disease diagnosis: a systematic literature review, synthesizing framework and future research agenda. J Ambient Intell Humaniz Comput. 2023; 14:8459–86. https://doi.org/10.1007/s12652-021-03612-z [PubMed]
-
9.
Putin E, Mamoshina P, Aliper A, Korzinkin M, Moskalev A, Kolosov A, Ostrovskiy A, Cantor C, Vijg J, Zhavoronkov A. Deep biomarkers of human aging: Application of deep neural networks to biomarker development. Aging (Albany NY). 2016; 8:1021–33. https://doi.org/10.18632/aging.100968 [PubMed]
-
10.
Heckenbach I, Mkrtchyan GV, Ezra MB, Bakula D, Madsen JS, Nielsen MH, Oró D, Osborne B, Covarrubias AJ, Idda ML, Gorospe M, Mortensen L, Verdin E, et al. Nuclear morphology is a deep learning biomarker of cellular senescence. Nat Aging. 2022; 2:742–55. https://doi.org/10.1038/s43587-022-00263-3 [PubMed]
-
11.
Macdonald-Dunlop E, Taba N, Klarić L, Frkatović A, Walker R, Hayward C, Esko T, Haley C, Fischer K, Wilson JF, Joshi PK. A catalogue of omics biological ageing clocks reveals substantial commonality and associations with disease risk. Aging (Albany NY). 2022; 14:623–59. https://doi.org/10.18632/aging.203847 [PubMed]
-
12.
Moqri M, Herzog C, Poganik JR, Justice J, Belsky DW, Higgins-Chen A, Moskalev A, Fuellen G, Cohen AA, Bautmans I, Widschwendter M, Ding J, Fleming A, et al, and Biomarkers of Aging Consortium. Biomarkers of aging for the identification and evaluation of longevity interventions. Cell. 2023; 186:3758–75. https://doi.org/10.1016/j.cell.2023.08.003 [PubMed]
-
13.
Ren F, Aliper A, Chen J, Zhao H, Rao S, Kuppe C, Ozerov IV, Zhang M, Witte K, Kruse C, Aladinskiy V, Ivanenkov Y, Polykovskiy D, et al. A small-molecule TNIK inhibitor targets fibrosis in preclinical and clinical models. Nat Biotechnol. 2024. https://doi.org/10.1038/s41587-024-02143-0 [PubMed]
-
14.
Arnold C. Inside the nascent industry of AI-designed drugs. Nat Med. 2023; 29:1292–5. https://doi.org/10.1038/s41591-023-02361-0 [PubMed]
-
15.
Wong F, Omori S, Donghia NM, Zheng EJ, Collins JJ. Discovering small-molecule senolytics with deep neural networks. Nat Aging. 2023; 3:734–50. https://doi.org/10.1038/s43587-023-00415-z [PubMed]
-
16.
Smer-Barreto V, Quintanilla A, Elliott RJR, Dawson JC, Sun J, Campa VM, Lorente-Macías Á, Unciti-Broceta A, Carragher NO, Acosta JC, Oyarzún DA. Discovery of senolytics using machine learning. Nat Commun. 2023; 14:3445. https://doi.org/10.1038/s41467-023-39120-1 [PubMed]
-
17.
Galyan SM, Ewald CY, Jalencas X, Masrani S, Meral S, Mestres J. Fragment-based virtual screening identifies a first-in-class preclinical drug candidate for Huntington’s disease. Sci Rep. 2022; 12:19642. https://doi.org/10.1038/s41598-022-21900-2 [PubMed]
-
18.
Bennett DF, Goyala A, Statzer C, Beckett CW, Tyshkovskiy A, Gladyshev VN, Ewald CY, de Magalhães JP. Rilmenidine extends lifespan and healthspan in Caenorhabditis elegans via a nischarin I1-imidazoline receptor. Aging Cell. 2023; 22:e13774. https://doi.org/10.1111/acel.13774 [PubMed]
-
19.
Vidovic T, Dakhovnik A, Hrabovskyi O, MacArthur MR, Ewald CY. AI-Predicted mTOR Inhibitor Reduces Cancer Cell Proliferation and Extends the Lifespan of C. elegans. Int J Mol Sci. 2023; 24:7850. https://doi.org/10.3390/ijms24097850 [PubMed]
-
20.
Wang Z, Emmerich A, Pillon NJ, Moore T, Hemerich D, Cornelis MC, Mazzaferro E, Broos S, Ahluwalia TS, Bartz TM, Bentley AR, Bielak LF, Chong M, et al, and Lifelines Cohort Study. Genome-wide association analyses of physical activity and sedentary behavior provide insights into underlying mechanisms and roles in disease prevention. Nat Genet. 2022; 54:1332–44. https://doi.org/10.1038/s41588-022-01165-1 [PubMed]
-
21.
Gircha AI, Boev AS, Avchaciov K, Fedichev PO, Fedorov AK. Hybrid quantum-classical machine learning for generative chemistry and drug design. Sci Rep. 2023; 13:8250. https://doi.org/10.1038/s41598-023-32703-4 [PubMed]
-
22.
Oh HS, Rutledge J, Nachun D, Pálovics R, Abiose O, Moran-Losada P, Channappa D, Urey DY, Kim K, Sung YJ, Wang L, Timsina J, Western D, et al. Organ aging signatures in the plasma proteome track health and disease. Nature. 2023; 624:164–72. https://doi.org/10.1038/s41586-023-06802-1 [PubMed]
-
23.
Nie C, Li Y, Li R, Yan Y, Zhang D, Li T, Li Z, Sun Y, Zhen H, Ding J, Wan Z, Gong J, Shi Y, et al. Distinct biological ages of organs and systems identified from a multi-omics study. Cell Rep. 2022; 38:110459. https://doi.org/10.1016/j.celrep.2022.110459 [PubMed]
-
24.
Schumacher B, Meyer D. Accurate aging clocks based on accumulating stochastic variation. Research Square. 2023. https://doi.org/10.21203/rs.3.rs-2351315/v1
-
25.
Vicini P, Fields O, Lai E, Litwack ED, Martin AM, Morgan TM, Pacanowski MA, Papaluca M, Perez OD, Ringel MS, Robson M, Sakul H, Vockley J, et al. Precision medicine in the age of big data: The present and future role of large-scale unbiased sequencing in drug discovery and development. Clin Pharmacol Ther. 2016; 99:198–207. https://doi.org/10.1002/cpt.293 [PubMed]
-
26.
Kejun Y, Seth P, Alec E, Siyuan L, Martin PG, Mehrnoosh E, Maximiliano Casas M, Dayoon K, Michael S, Dane G, Jesse RP, Mahdi M, Vadim NG. Biolearn, an open-source library for biomarkers of aging. bioRxiv. 2024: 2023.12.02.569722.
-
27.
Kejun Y, Alexander T, Alexandre T, Hanna L, Mahdi M, Csaba K, Vadim NG. ClockBase: a comprehensive platform for biological age profiling in human and mouse. bioRxiv. 2023: 2023.02.28.530532.
-
28.
Kyra LT, Albert TH-C, Zuyun L, Morgan EL. R methylCIPHER: A Methylation Clock Investigational Package for Hypothesis-Driven Evaluation & Research. bioRxiv. 2022: 2022.07.13.499978.
-
29.
Di Lena P, Sala C, Nardini C. Estimage: a webserver hub for the computation of methylation age. Nucleic Acids Res. 2021; 49:W199–206. https://doi.org/10.1093/nar/gkab426 [PubMed]
-
30.
Field AE, Robertson NA, Wang T, Havas A, Ideker T, Adams PD. DNA Methylation Clocks in Aging: Categories, Causes, and Consequences. Mol Cell. 2018; 71:882–95. https://doi.org/10.1016/j.molcel.2018.08.008 [PubMed]
-
31.
Wang J, Gao Y, Wang F, Zeng S, Li J, Miao H, Wang T, Zeng J, Baptista-Hon D, Monteiro O, Guan T, Cheng L, Lu Y, et al. Accurate estimation of biological age and its application in disease prediction using a multimodal image Transformer system. Proc Natl Acad Sci USA. 2024; 121:e2308812120. https://doi.org/10.1073/pnas.2308812120 [PubMed]
-
32.
Unfried M, Ng LF, Cazenave-Gassiot A, Batchu KC, Kennedy BK, Wenk MR, Tolwinski N, Gruber J. LipidClock: A Lipid-Based Predictor of Biological Age. Front Aging. 2022; 3:828239. https://doi.org/10.3389/fragi.2022.828239 [PubMed]
-
33.
Mijakovac A, Frkatović A, Hanić M, Ivok J, Martinić Kavur M, Pučić-Baković M, Spector T, Zoldoš V, Mangino M, Lauc G. Heritability of the glycan clock of biological age. Front Cell Dev Biol. 2022; 10:982609. https://doi.org/10.3389/fcell.2022.982609 [PubMed]
-
34.
Meyer DH, Schumacher B. BiT age: A transcriptome-based aging clock near the theoretical limit of accuracy. Aging Cell. 2021; 20:e13320. https://doi.org/10.1111/acel.13320 [PubMed]
-
35.
Bell CG, Lowe R, Adams PD, Baccarelli AA, Beck S, Bell JT, Christensen BC, Gladyshev VN, Heijmans BT, Horvath S, Ideker T, Issa JJ, Kelsey KT, et al. DNA methylation aging clocks: challenges and recommendations. Genome Biol. 2019; 20:249. https://doi.org/10.1186/s13059-019-1824-y [PubMed]
-
36.
Alan T, Ariel F, Ritesh T, Rebeccah R, Hiroyuki M, Nicolas A, Herbert GK, Eric V. Development of a novel epigenetic clock resistant to changes in immune cell composition. bioRxiv. 2023: 2023.03.01.530561.
-
37.
Ying K, Liu H, Tarkhov AE, Sadler MC, Lu AT, Moqri M, Horvath S, Kutalik Z, Shen X, Gladyshev VN. Causality-enriched epigenetic age uncouples damage and adaptation. Nat Aging. 2024; 4:231–46. https://doi.org/10.1038/s43587-023-00557-0 [PubMed]
-
38.
Mamoshina P, Vieira A, Putin E, Zhavoronkov A. Applications of Deep Learning in Biomedicine. Mol Pharm. 2016; 13:1445–54. https://doi.org/10.1021/acs.molpharmaceut.5b00982 [PubMed]
-
39.
Martinez-Romero J, Mueller W, Fernandez M, Price N, Candia J, Bernier M, Camandola S, Vieira Ligo-Teixeira C, Palliyaguru D, Meirelles O, Hu Y-H, Li Z, Deighan A, et al. The blood has something to say: A hematology-based clock to measure aging in mice. Research Square. 2023. https://doi.org/10.21203/rs.3.rs-3017838/v1
-
40.
Chen Q, Dwaraka VB, Carreras-Gallo N, Mendez K, Chen Y, Begum S, Kachroo P, Prince N, Went H, Mendez T, Lin A, Turner L, Moqri M, et al. OMICmAge: An integrative multi-omics approach to quantify biological age with electronic medical records. bioRxiv. 2023; 2023.10.16.562114 https://doi.org/10.1101/2023.10.16.562114 [PubMed]
-
41.
Vijg J, Suh Y. Genome instability and aging. Annu Rev Physiol. 2013; 75:645–68. https://doi.org/10.1146/annurev-physiol-030212-183715 [PubMed]
-
42.
Yousefzadeh MJ, Zhao J, Bukata C, Wade EA, McGowan SJ, Angelini LA, Bank MP, Gurkar AU, McGuckian CA, Calubag MF, Kato JI, Burd CE, Robbins PD, Niedernhofer LJ. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell. 2020; 19:e13094. https://doi.org/10.1111/acel.13094 [PubMed]
-
43.
Bujarrabal-Dueso A, Sendtner G, Meyer DH, Chatzinikolaou G, Stratigi K, Garinis GA, Schumacher B. The DREAM complex functions as conserved master regulator of somatic DNA-repair capacities. Nat Struct Mol Biol. 2023; 30:475–88. https://doi.org/10.1038/s41594-023-00942-8 [PubMed]
-
44.
Yang JH, Petty CA, Dixon-McDougall T, Lopez MV, Tyshkovskiy A, Maybury-Lewis S, Tian X, Ibrahim N, Chen Z, Griffin PT, Arnold M, Li J, Martinez OA, et al. Chemically induced reprogramming to reverse cellular aging. Aging (Albany NY). 2023; 15:5966–89. https://doi.org/10.18632/aging.204896 [PubMed]
-
45.
Lu YR, Tian X, Sinclair DA. The Information Theory of Aging. Nat Aging. 2023; 3:1486–99. https://doi.org/10.1038/s43587-023-00527-6 [PubMed]
-
46.
Jing Y, Jiang X, Ji Q, Wu Z, Wang W, Liu Z, Guillen-Garcia P, Esteban CR, Reddy P, Horvath S, Li J, Geng L, Hu Q, et al. Genome-wide CRISPR activation screening in senescent cells reveals SOX5 as a driver and therapeutic target of rejuvenation. Cell Stem Cell. 2023; 30:1452–71.e10. https://doi.org/10.1016/j.stem.2023.09.007 [PubMed]
-
47.
Ocampo A, Reddy P, Martinez-Redondo P, Platero-Luengo A, Hatanaka F, Hishida T, Li M, Lam D, Kurita M, Beyret E, Araoka T, Vazquez-Ferrer E, Donoso D, et al. In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming. Cell. 2016; 167:1719–33.e12. https://doi.org/10.1016/j.cell.2016.11.052 [PubMed]
-
48.
Macip CC, Hasan R, Hoznek V, Kim J, Lu YR, Metzger LE 4th, Sethna S, Davidsohn N. Gene Therapy-Mediated Partial Reprogramming Extends Lifespan and Reverses Age-Related Changes in Aged Mice. Cell Reprogram. 2024; 26:24–32. https://doi.org/10.1089/cell.2023.0072 [PubMed]
-
49.
Stewart-Morgan KR, Petryk N, Groth A. Chromatin replication and epigenetic cell memory. Nat Cell Biol. 2020; 22:361–71. https://doi.org/10.1038/s41556-020-0487-y [PubMed]
-
50.
Wenger A, Biran A, Alcaraz N, Redó-Riveiro A, Sell AC, Krautz R, Flury V, Reverón-Gómez N, Solis-Mezarino V, Völker-Albert M, Imhof A, Andersson R, Brickman JM, Groth A. Symmetric inheritance of parental histones governs epigenome maintenance and embryonic stem cell identity. Nat Genet. 2023; 55:1567–78. https://doi.org/10.1038/s41588-023-01476-x [PubMed]
-
51.
Kang J, Benjamin DI, Kim S, Salvi JS, Dhaliwal G, Lam R, Goshayeshi A, Brett JO, Liu L, Rando TA. Depletion of SAM leading to loss of heterochromatin drives muscle stem cell ageing. Nat Metab. 2024; 6:153–68. https://doi.org/10.1038/s42255-023-00955-z [PubMed]
-
52.
Lu Y, Brommer B, Tian X, Krishnan A, Meer M, Wang C, Vera DL, Zeng Q, Yu D, Bonkowski MS, Yang JH, Zhou S, Hoffmann EM, et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature. 2020; 588:124–9. https://doi.org/10.1038/s41586-020-2975-4 [PubMed]
-
53.
Monteleon C, Menon S, Spice J, Mohammed A, Sebastiano V, Hsia E. LB1786 Transient epigenetic reprogramming by mRNA for skin rejuvenation. Journal of Investigative Dermatology. 2023; 143: B33. https://doi.org/10.1016/j.jid.2023.06.170
-
54.
Yücel AD, Gladyshev VN. The long and winding road of reprogramming-induced rejuvenation. Nat Commun. 2024; 15:1941. https://doi.org/10.1038/s41467-024-46020-5 [PubMed]
-
55.
Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014; 20:460–73. https://doi.org/10.1089/ars.2013.5371 [PubMed]
-
56.
Aman Y, Schmauck-Medina T, Hansen M, Morimoto RI, Simon AK, Bjedov I, Palikaras K, Simonsen A, Johansen T, Tavernarakis N, Rubinsztein DC, Partridge L, Kroemer G, et al. Autophagy in healthy aging and disease. Nat Aging. 2021; 1:634–50. https://doi.org/10.1038/s43587-021-00098-4 [PubMed]
-
57.
Juricic P, Lu YX, Leech T, Drews LF, Paulitz J, Lu J, Nespital T, Azami S, Regan JC, Funk E, Fröhlich J, Grönke S, Partridge L. Long-lasting geroprotection from brief rapamycin treatment in early adulthood by persistently increased intestinal autophagy. Nat Aging. 2022; 2:824–36. https://doi.org/10.1038/s43587-022-00278-w [PubMed]
-
58.
Wang H, Yan X, Zhang Y, Wang P, Li J, Zhang X. Mitophagy in Alzheimer’s Disease: A Bibliometric Analysis from 2007 to 2022. J Alzheimers Dis Rep. 2024; 8:101–28. https://doi.org/10.3233/ADR-230139 [PubMed]
-
59.
Hipp MS, Kasturi P, Hartl FU. The proteostasis network and its decline in ageing. Nat Rev Mol Cell Biol. 2019; 20:421–35. https://doi.org/10.1038/s41580-019-0101-y [PubMed]
-
60.
Rolland Y, Sierra F, Ferrucci L, Barzilai N, De Cabo R, Mannick J, Oliva A, Evans W, Angioni D, De Souto Barreto P, Raffin J, Vellas B, Kirkland JL, and G.C.T-TF group. Challenges in developing Geroscience trials. Nat Commun. 2023; 14:5038. https://doi.org/10.1038/s41467-023-39786-7 [PubMed]
-
61.
Nielsen JL, Bakula D, Scheibye-Knudsen M. Clinical Trials Targeting Aging. Front Aging. 2022; 3:820215. https://doi.org/10.3389/fragi.2022.820215 [PubMed]
-
62.
Guo J, Huang X, Dou L, Yan M, Shen T, Tang W, Li J. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct Target Ther. 2022; 7:391. https://doi.org/10.1038/s41392-022-01251-0 [PubMed]
-
63.
Green CL, Lamming DW, Fontana L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat Rev Mol Cell Biol. 2022; 23:56–73. https://doi.org/10.1038/s41580-021-00411-4 [PubMed]
-
64.
Partridge L, Fuentealba M, Kennedy BK. The quest to slow ageing through drug discovery. Nat Rev Drug Discov. 2020; 19:513–32. https://doi.org/10.1038/s41573-020-0067-7 [PubMed]
-
65.
Mannick JB, Lamming DW. Targeting the biology of aging with mTOR inhibitors. Nat Aging. 2023; 3:642–60. https://doi.org/10.1038/s43587-023-00416-y [PubMed]
-
66.
Ripa R, Ballhysa E, Steiner JD, Laboy R, Annibal A, Hochhard N, Latza C, Dolfi L, Calabrese C, Meyer AM, Polidori MC, Müller RU, Antebi A. Refeeding-associated AMPKγ1 complex activity is a hallmark of health and longevity. Nat Aging. 2023; 3:1544–60. https://doi.org/10.1038/s43587-023-00521-y [PubMed]
-
67.
Brandhorst S, Levine ME, Wei M, Shelehchi M, Morgan TE, Nayak KS, Dorff T, Hong K, Crimmins EM, Cohen P, Longo VD. Fasting-mimicking diet causes hepatic and blood markers changes indicating reduced biological age and disease risk. Nat Commun. 2024; 15:1309. https://doi.org/10.1038/s41467-024-45260-9 [PubMed]
-
68.
Acosta-Rodríguez V, Rijo-Ferreira F, Izumo M, Xu P, Wight-Carter M, Green CB, Takahashi JS. Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science. 2022; 376:1192–202. https://doi.org/10.1126/science.abk0297 [PubMed]
-
69.
Singh P, Gollapalli K, Mangiola S, Schranner D, Yusuf MA, Chamoli M, Shi SL, Lopes Bastos B, Nair T, Riermeier A, Vayndorf EM, Wu JZ, Nilakhe A, et al. Taurine deficiency as a driver of aging. Science. 2023; 380:eabn9257. https://doi.org/10.1126/science.abn9257 [PubMed]
-
70.
Luo S, Wong ICK, Chui CSL, Zheng J, Huang Y, Schooling CM, Yeung SLA. Effects of putative metformin targets on phenotypic age and leukocyte telomere length: a mendelian randomisation study using data from the UK Biobank. Lancet Healthy Longev. 2023; 4:e337–44. https://doi.org/10.1016/S2666-7568(23)00085-5 [PubMed]
-
71.
Reifsnyder PC, Flurkey K, Doty R, Calcutt NA, Koza RA, Harrison DE. Rapamycin/metformin co-treatment normalizes insulin sensitivity and reduces complications of metabolic syndrome in type 2 diabetic mice. Aging Cell. 2022; 21:e13666. https://doi.org/10.1111/acel.13666 [PubMed]
-
72.
Chieh C, Brett L, Min C, Wilson H, Jessie C, Laurent V, Reid O’Brien J, Ajay AV, Randall MC, Melissa MD, Gabriel S, Xudong F, Jenny CL, et al. The metabolite α-ketobutyrate increases health and life spans by activating AMPK. bioRxiv. 2022: 2022.10.10.511641.
-
73.
Asadi Shahmirzadi A, Edgar D, Liao CY, Hsu YM, Lucanic M, Asadi Shahmirzadi A, Wiley CD, Gan G, Kim DE, Kasler HG, Kuehnemann C, Kaplowitz B, Bhaumik D, et al. Alpha-Ketoglutarate, an Endogenous Metabolite, Extends Lifespan and Compresses Morbidity in Aging Mice. Cell Metab. 2020; 32:447–56.e6. https://doi.org/10.1016/j.cmet.2020.08.004 [PubMed]
-
74.
Regan JC, Lu YX, Ureña E, Meilenbrock RL, Catterson JH, Kißler D, Fröhlich J, Funk E, Partridge L. Sexual identity of enterocytes regulates autophagy to determine intestinal health, lifespan and responses to rapamycin. Nat Aging. 2022; 2:1145–58. https://doi.org/10.1038/s43587-022-00308-7 [PubMed]
-
75.
Lu YX, Regan JC, Eßer J, Drews LF, Weinseis T, Stinn J, Hahn O, Miller RA, Grönke S, Partridge L. A TORC1-histone axis regulates chromatin organisation and non-canonical induction of autophagy to ameliorate ageing. Elife. 2021; 10:e62233. https://doi.org/10.7554/eLife.62233 [PubMed]
-
76.
Amorim JA, Coppotelli G, Rolo AP, Palmeira CM, Ross JM, Sinclair DA. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat Rev Endocrinol. 2022; 18:243–58. https://doi.org/10.1038/s41574-021-00626-7 [PubMed]
-
77.
Wang CL, Ohkubo R, Mu WC, Chen W, Fan JL, Song Z, Maruichi A, Sudmant PH, Pisco AO, Dubal DB, Ji N, Chen D. The mitochondrial unfolded protein response regulates hippocampal neural stem cell aging. Cell Metab. 2023; 35:996–1008.e7. https://doi.org/10.1016/j.cmet.2023.04.012 [PubMed]
-
78.
Hogan KA, Chini CCS, Chini EN. The Multi-faceted Ecto-enzyme CD38: Roles in Immunomodulation, Cancer, Aging, and Metabolic Diseases. Front Immunol. 2019; 10:1187. https://doi.org/10.3389/fimmu.2019.01187 [PubMed]
-
79.
Membrez M, Migliavacca E, Christen S, Yaku K, Trieu J, Lee AK, Morandini F, Giner MP, Stiner J, Makarov MV, Garratt ES, Vasiloglou MF, Chanvillard L, et al. Trigonelline is an NAD+ precursor that improves muscle function during ageing and is reduced in human sarcopenia. Nat Metab. 2024; 6:433–47. https://doi.org/10.1038/s42255-024-00997-x [PubMed]
-
80.
Diessl J, Berndtsson J, Broeskamp F, Habernig L, Kohler V, Vazquez-Calvo C, Nandy A, Peselj C, Drobysheva S, Pelosi L, Vögtle FN, Pierrel F, Ott M, Büttner S. Manganese-driven CoQ deficiency. Nat Commun. 2022; 13:6061. https://doi.org/10.1038/s41467-022-33641-x [PubMed]
-
81.
Moiseeva V, Cisneros A, Sica V, Deryagin O, Lai Y, Jung S, Andrés E, An J, Segalés J, Ortet L, Lukesova V, Volpe G, Benguria A, et al. Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration. Nature. 2023; 613:169–78. https://doi.org/10.1038/s41586-022-05535-x [PubMed]
-
82.
Gurkar AU, Gerencser AA, Mora AL, Nelson AC, Zhang AR, Lagnado AB, Enninful A, Benz C, Furman D, Beaulieu D, Jurk D, Thompson EL, Wu F, et al. Spatial mapping of cellular senescence: emerging challenges and opportunities. Nat Aging. 2023; 3:776–90. https://doi.org/10.1038/s43587-023-00446-6 [PubMed]
-
83.
Chaib S, Tchkonia T, Kirkland JL. Cellular senescence and senolytics: the path to the clinic. Nat Med. 2022; 28:1556–68. https://doi.org/10.1038/s41591-022-01923-y [PubMed]
-
84.
Suda M, Shimizu I, Katsuumi G, Yoshida Y, Hayashi Y, Ikegami R, Matsumoto N, Yoshida Y, Mikawa R, Katayama A, Wada J, Seki M, Suzuki Y, et al. Senolytic vaccination improves normal and pathological age-related phenotypes and increases lifespan in progeroid mice. Nat Aging. 2021; 1:1117–26. https://doi.org/10.1038/s43587-021-00151-2 [PubMed]
-
85.
Amor C, Feucht J, Leibold J, Ho YJ, Zhu C, Alonso-Curbelo D, Mansilla-Soto J, Boyer JA, Li X, Giavridis T, Kulick A, Houlihan S, Peerschke E, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020; 583:127–32. https://doi.org/10.1038/s41586-020-2403-9 [PubMed]
-
86.
Kumar MA, Baba SK, Sadida HQ, Marzooqi SA, Jerobin J, Altemani FH, Algehainy N, Alanazi MA, Abou-Samra AB, Kumar R, Al-Shabeeb Akil AS, Macha MA, Mir R, Bhat AA. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct Target Ther. 2024; 9:27. https://doi.org/10.1038/s41392-024-01735-1 [PubMed]
-
87.
Paine PT, Nguyen A, Ocampo A. Partial cellular reprogramming: A deep dive into an emerging rejuvenation technology. Aging Cell. 2024; 23:e14039. https://doi.org/10.1111/acel.14039 [PubMed]
-
88.
Li X, Li C, Zhang W, Wang Y, Qian P, Huang H. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct Target Ther. 2023; 8:239. https://doi.org/10.1038/s41392-023-01502-8 [PubMed]
-
89.
Minhas PS, Latif-Hernandez A, McReynolds MR, Durairaj AS, Wang Q, Rubin A, Joshi AU, He JQ, Gauba E, Liu L, Wang C, Linde M, Sugiura Y, et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature. 2021; 590:122–8. https://doi.org/10.1038/s41586-020-03160-0 [PubMed]
-
90.
Wang LF, Gamage AM, Chan WOY, Hiller M, Teeling EC. Decoding bat immunity: the need for a coordinated research approach. Nat Rev Immunol. 2021; 21:269–71. https://doi.org/10.1038/s41577-021-00523-0 [PubMed]
-
91.
Huang Z, Whelan CV, Foley NM, Jebb D, Touzalin F, Petit EJ, Puechmaille SJ, Teeling EC. Longitudinal comparative transcriptomics reveals unique mechanisms underlying extended healthspan in bats. Nat Ecol Evol. 2019; 3:1110–20. https://doi.org/10.1038/s41559-019-0913-3 [PubMed]
-
92.
Arora S, Thompson PJ, Wang Y, Bhattacharyya A, Apostolopoulou H, Hatano R, Naikawadi RP, Shah A, Wolters PJ, Koliwad S, Bhattacharya M, Bhushan A. Invariant Natural Killer T cells coordinate removal of senescent cells. Med. 2021; 2:938–50. https://doi.org/10.1016/j.medj.2021.04.014 [PubMed]
-
93.
Sahoo D, Swanson L, Sayed IM, Katkar GD, Ibeawuchi SR, Mittal Y, Pranadinata RF, Tindle C, Fuller M, Stec DL, Chang JT, Sandborn WJ, Das S, Ghosh P. Artificial intelligence guided discovery of a barrier-protective therapy in inflammatory bowel disease. Nat Commun. 2021; 12:4246. https://doi.org/10.1038/s41467-021-24470-5 [PubMed]
-
94.
Ghosh TS, Shanahan F, O’Toole PW. The gut microbiome as a modulator of healthy ageing. Nat Rev Gastroenterol Hepatol. 2022; 19:565–84. https://doi.org/10.1038/s41575-022-00605-x [PubMed]
-
95.
Boehme M, Guzzetta KE, Bastiaanssen TFS, van de Wouw M, Moloney GM, Gual-Grau A, Spichak S, Olavarría-Ramírez L, Fitzgerald P, Morillas E, Ritz NL, Jaggar M, Cowan CSM, et al. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat Aging. 2021; 1:666–76. https://doi.org/10.1038/s43587-021-00093-9 [PubMed]
-
96.
Smith P, Willemsen D, Popkes M, Metge F, Gandiwa E, Reichard M, Valenzano DR. Regulation of life span by the gut microbiota in the short-lived African turquoise killifish. Elife. 2017; 6:e27014. https://doi.org/10.7554/eLife.27014 [PubMed]
-
97.
Verkade E, Shen W, Hovingh MV, Mulder NL, de Bruyn K, Koehorst M, de Vries HD, Bloks VW, Kuipers F, de Boer JF. Gut microbiota depletion aggravates bile acid-induced liver pathology in mice with a human-like bile acid composition. Clin Sci (Lond). 2023; 137:1637–50. https://doi.org/10.1042/CS20230812 [PubMed]
-
98.
Xu K, Guo Y, Wang Y, Ren Y, Low V, Cho S, Ping L, Peng K, Li X, Qiu Y, Liu Q, Li Z, Wang Z. Decreased Enterobacteriaceae translocation due to gut microbiota remodeling mediates the alleviation of premature aging by a high-fat diet. Aging Cell. 2023; 22:e13760. https://doi.org/10.1111/acel.13760 [PubMed]
-
99.
Statzer C, Park JYC, Ewald CY. Extracellular Matrix Dynamics as an Emerging yet Understudied Hallmark of Aging and Longevity. Aging Dis. 2023; 14:670–93. https://doi.org/10.14336/AD.2022.1116 [PubMed]
-
100.
Ewald CY. The Matrisome during Aging and Longevity: A Systems-Level Approach toward Defining Matreotypes Promoting Healthy Aging. Gerontology. 2020; 66:266–74. https://doi.org/10.1159/000504295 [PubMed]
-
101.
Brunet A, Goodell MA, Rando TA. Ageing and rejuvenation of tissue stem cells and their niches. Nat Rev Mol Cell Biol. 2023; 24:45–62. https://doi.org/10.1038/s41580-022-00510-w [PubMed]
-
102.
Easwaran S, Montell DJ. The molecular mechanisms of diapause and diapause-like reversible arrest. Biochem Soc Trans. 2023; 51:1847–56. https://doi.org/10.1042/BST20221431 [PubMed]
-
103.
Stevenson ME, Bieri G, Kaletsky R, St Ange J, Remesal L, Pratt KJB, Zhou S, Weng Y, Murphy CT, Villeda SA. Neuronal activation of Gαq EGL-30/GNAQ late in life rejuvenates cognition across species. Cell Rep. 2023; 42:113151. https://doi.org/10.1016/j.celrep.2023.113151 [PubMed]
-
104.
Sulaiman RS, Park B, Sheik Pran Babu SP, Si Y, Kharwadkar R, Mitter SK, Lee B, Sun W, Qi X, Boulton ME, Meroueh SO, Fei X, Seo SY, Corson TW. Chemical Proteomics Reveals Soluble Epoxide Hydrolase as a Therapeutic Target for Ocular Neovascularization. ACS Chem Biol. 2018; 13:45–52. https://doi.org/10.1021/acschembio.7b00854 [PubMed]
-
105.
Awan M, Buriak I, Fleck R, Fuller B, Goltsev A, Kerby J, Lowdell M, Mericka P, Petrenko A, Petrenko Y, Rogulska O, Stolzing A, Stacey GN. Dimethyl sulfoxide: a central player since the dawn of cryobiology, is efficacy balanced by toxicity? Regen Med. 2020; 15:1463–91. https://doi.org/10.2217/rme-2019-0145 [PubMed]
-
106.
Lee MB, Blue B, Muir M, Kaeberlein M. The million-molecule challenge: a moonshot project to rapidly advance longevity intervention discovery. Geroscience. 2023; 45:3103–13. https://doi.org/10.1007/s11357-023-00867-6 [PubMed]
-
107.
Seong KH, Matsumura T, Shimada-Niwa Y, Niwa R, Kang S. The Drosophila Individual Activity Monitoring and Detection System (DIAMonDS). Elife. 2020; 9:e58630. https://doi.org/10.7554/eLife.58630 [PubMed]
-
108.
Rahman M, Edwards H, Birze N, Gabrilska R, Rumbaugh KP, Blawzdziewicz J, Szewczyk NJ, Driscoll M, Vanapalli SA. NemaLife chip: a micropillar-based microfluidic culture device optimized for aging studies in crawling C. elegans. Sci Rep. 2020; 10:16190. https://doi.org/10.1038/s41598-020-73002-6 [PubMed]
-
109.
Wedell-Neergaard AS, Lang Lehrskov L, Christensen RH, Legaard GE, Dorph E, Larsen MK, Launbo N, Fagerlind SR, Seide SK, Nymand S, Ball M, Vinum N, Dahl CN, et al. Exercise-Induced Changes in Visceral Adipose Tissue Mass Are Regulated by IL-6 Signaling: A Randomized Controlled Trial. Cell Metab. 2019; 29:844–55.e3. https://doi.org/10.1016/j.cmet.2018.12.007 [PubMed]
-
110.
Cerqueira É, Marinho DA, Neiva HP, Lourenço O. Inflammatory Effects of High and Moderate Intensity Exercise-A Systematic Review. Front Physiol. 2020; 10:1550. https://doi.org/10.3389/fphys.2019.01550 [PubMed]
-
111.
Spadaro O, Youm Y, Shchukina I, Ryu S, Sidorov S, Ravussin A, Nguyen K, Aladyeva E, Predeus AN, Smith SR, Ravussin E, Galban C, Artyomov MN, Dixit VD. Caloric restriction in humans reveals immunometabolic regulators of health span. Science. 2022; 375:671–7. https://doi.org/10.1126/science.abg7292 [PubMed]
-
112.
D’Amico D, Andreux PA, Valdés P, Singh A, Rinsch C, Auwerx J. Impact of the Natural Compound Urolithin A on Health, Disease, and Aging. Trends Mol Med. 2021; 27:687–99. https://doi.org/10.1016/j.molmed.2021.04.009 [PubMed]
-
113.
Alsaleh G, Panse I, Swadling L, Zhang H, Richter FC, Meyer A, Lord J, Barnes E, Klenerman P, Green C, Simon AK. Autophagy in T cells from aged donors is maintained by spermidine and correlates with function and vaccine responses. Elife. 2020; 9:e57950. https://doi.org/10.7554/eLife.57950 [PubMed]
-
114.
Mannick JB, Morris M, Hockey HP, Roma G, Beibel M, Kulmatycki K, Watkins M, Shavlakadze T, Zhou W, Quinn D, Glass DJ, Klickstein LB. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci Transl Med. 2018; 10:eaaq1564. https://doi.org/10.1126/scitranslmed.aaq1564 [PubMed]
-
115.
Radenkovic D, Zhavoronkov A, Bischof E. AI in Longevity Medicine. In: Lidströmer N and Ashrafian H, eds. Artificial Intelligence in Medicine. (Cham: Springer International Publishing), 2020; pp. 1–13.
-
116.
Bischof E, Scheibye-Knudsen M, Siow R, Moskalev A. Longevity medicine: upskilling the physicians of tomorrow. Lancet Healthy Longev. 2021; 2:e187–88. https://doi.org/10.1016/S2666-7568(21)00024-6 [PubMed]
-
117.
Westerman K, Reaver A, Roy C, Ploch M, Sharoni E, Nogal B, Sinclair DA, Katz DL, Blumberg JB, Blander G. Longitudinal analysis of biomarker data from a personalized nutrition platform in healthy subjects. Sci Rep. 2018; 8:14685. https://doi.org/10.1038/s41598-018-33008-7 [PubMed]
-
118.
López-Otín C, Pietrocola F, Roiz-Valle D, Galluzzi L, Kroemer G. Meta-hallmarks of aging and cancer. Cell Metab. 2023; 35:12–35. https://doi.org/10.1016/j.cmet.2022.11.001 [PubMed]
-
119.
Pun FW, Leung GHD, Leung HW, Liu BHM, Long X, Ozerov IV, Wang J, Ren F, Aliper A, Izumchenko E, Moskalev A, de Magalhães JP, Zhavoronkov A. Hallmarks of aging-based dual-purpose disease and age-associated targets predicted using PandaOmics AI-powered discovery engine. Aging (Albany NY). 2022; 14:2475–506. https://doi.org/10.18632/aging.203960 [PubMed]
-
120.
Pun FW, Leung GHD, Leung HW, Rice J, Schmauck-Medina T, Lautrup S, Long X, Liu BHM, Wong CW, Ozerov IV, Aliper A, Ren F, Rosenberg AJ, et al. A comprehensive AI-driven analysis of large-scale omic datasets reveals novel dual-purpose targets for the treatment of cancer and aging. Aging Cell. 2023; 22:e14017. https://doi.org/10.1111/acel.14017 [PubMed]
-
121.
Moreno-Juste A, Gimeno-Miguel A, Poblador-Plou B, Calderón-Larrañaga A, Cano Del Pozo M, Forjaz MJ, Prados-Torres A, Gimeno-Feliú LA. Multimorbidity, social determinants and intersectionality in chronic patients. Results from the EpiChron Cohort. J Glob Health. 2023; 13:04014. https://doi.org/10.7189/13.04014 [PubMed]
-
122.
Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012; 380:37–43. https://doi.org/10.1016/S0140-6736(12)60240-2 [PubMed]
-
123.
Woods T, Palmarini N, Corner L, Barzilai N, Bethell LJ, Cox LS, Eyre H, Ferrucci L, Fried L, Furman D, Kennedy B, Roddam A, Scott A, Siow RC. Quantum Healthy Longevity for healthy people, planet, and growth. Lancet Healthy Longev. 2022; 3:e811–3. https://doi.org/10.1016/S2666-7568(22)00267-7 [PubMed]
-
124.
Scott AJ, Ellison M, Sinclair DA. The economic value of targeting aging. Nat Aging. 2021; 1:616–23. https://doi.org/10.1038/s43587-021-00080-0 [PubMed]
-
125.
Fantaccini S, Grassi L, Rampoldi A. The potential of DAOs for funding and collaborative development in the life sciences. Nat Biotechnol. 2024; 42:555–62. https://doi.org/10.1038/s41587-024-02189-0 [PubMed]
-
126.
DeFrancesco L, Klevecz A. Decentralized investor communities gain traction in biotech. Nat Biotechnol. 2022; 40:1310–5. https://doi.org/10.1038/s41587-022-01459-z [PubMed]