Dual regulation of TERT activity through transcription and splicing by ΔNP63α
Abstract
P53 homolog p63 was shown to play a role in premature ageing phenotype found in mouse models through regulation of the replicative senescence. We previously showed that the forced ΔNp63α expression decreased the SIRT1 protein levels, and induced the replicative senescence of human keratinocytes, while the ectopic SIRT1 expression decreased the senescence. Using the ΔNp63α overexpressing and p63-/+ heterozygous mice, we found that ΔNp63α induced the mTERT promoter activation through the down regulation of the SIRT1 protein levels, inactivation of p53 deacetylation, decrease of the p53/Sp1 protein-protein interaction, and the overall induction of mTERT transcription regulation. In the same time, by a forming of protein-protein complexes with the ABBP1, ΔNp63α induced the mTERT RNA splicing leading to an increasing expression of spliced mTERT isoforms playing a role of dominant-negative inhibitors of mTERT activity and therefore decreasing the levels of TERT activity in mouse epidermal keratinocytes. The overall effect of the ΔNp63α overexpression resulted in decrease in telomerase activity and increase in replicative senescence observed in mouse keratinocytes. This dual molecular mechanism of telomerase regulation might underline the previously shown effect of ΔNp63α on premature ageing phenotype.
Introduction
Cell senescence and stress modulate the
proliferative potential of mammalian cells, suggesting that both are capable of
suppressing the formation of tumors [1-7]. Stresses and dysfunction of the
telomeric DNA/telomerase complex can trigger senescence. Impaired telomere
function activates the canonical DNA damage response pathway that engages p53
to initiate apoptosis or replicative senescence, while the inactivation of the
tumor suppressor genes (Rb and p53) allowing cells to escape
senescence [8-16]. The resulting cell immortalization is an essential component
of the tumorigenic phenotype of human cancer cells [8-12,17].
Skin
epidermis is one of the few regenerative tissues that express telomerase, the
ribonucleoprotein complex that can counteract telomere erosion, one of the
presently mostly favored potential mechanisms causing cellular ageing [18].
Altered functioning of both telomerase and telomere-interacting proteins is
present in some human premature ageing syndromes and in cancer, and recent
findings indicate that alterations that affect telomeres at the level of
chromatin structure might also have a role in human disease [18-24].
P53
transcriptional factors are involved in regulation of cellular senescence and
organismal ageing [14,16,25-30]. While p53 suppresses the onset of
malignancy and, thereby extends lifespan, it induces cellular senescence and
apoptosis upon DNA damage [8,9,14,16,25,26]. Transgenic mouse strains
(p53+/m) expressing the C-terminal p53 fragment along with the wild type p53
display an early onset of phenotypes associated with ageing [30]. The ΔN-isoform
of p53 recently reported [27,28] or ΔN-isoforms of p63 and p73
(all lacking the transactivation domain) might modulate an imbalance between
them and full-length p53 leading to an altered transcriptional function of p53
and in turn to an acceleration of the ageing process [30,31].
Sirtuins
possessing the histone deacetylase activity are implicated in the extension of
lifespan of eukaryotic cells [29,32-40]. Epigenetic alterations of the
expression of longevity genes by changing the level/pattern of histone
acetylation may be an important factor in determining the longevity of animals
[41,42]. SIRT1 encodes an NAD-dependent histone deacetylase that playing a
critical role in transcriptional silencing [39,40]. Studies have implicated
SIRT1 in binding to and deacetylating of the p53 protein (or Forkhead family
members), inhibiting p53-dependent apoptosis, preventing a premature cellular
senescence and leading to increase of organismal longevity [43,44].
We
previously showed an important role for p53 homolog p63 (ΔNp63α),
shown to be a key switch in skin renewal [25,45], in regulation of ageing
process in p53+/m and ΔNp63α transgenic mouse models [29,30]. P63 was also shown
to transcriptionally regulate many genes implicated in epithelial integrity,
differentiation, and ageing [31].
Results
ΔNp63α induces the SIRT1 degradation and the p53/SIRT1 protein interaction
Mice overexpressing a truncated mutant of
p53 (p53+/m, C-terminal part) or ΔNp63α were shown to exhibit a premature ageing of skin and
shortened life span suggesting that these mice share a common molecular
mechanism underlying these phenotypes [29,30]. The link between cellular
senescence/premature ageing and p53 family members was reported by several
groups [25,26,29,30]. P63 deficiency was found to induce cellular
senescence and to cause an accelerated ageing phenotype in adult mice showing
the conditional expression or depletion in stratified epithelia contributed to
ageing [29,30]. We have previously shown the expression of endogenous ΔNp63α in the p53+/m mice and overexpression of ΔNp63α in transgenic mice may play an important role in
premature ageing [29]. We also found that the formation of ΔNp63α/SIRT1 complexes led
to a decreased SIRT1 levels in both ΔNp63α transgenic and p53+/m mice [29]. We further
observed that the marked senescence in the ΔNp63α overexpressing cells that could be modulated by a
forced expression of SIRT1 [29].
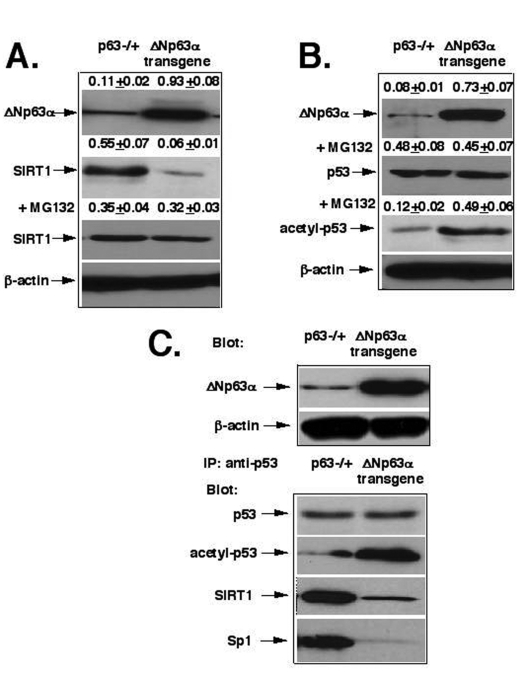
Figure 1. ΔNp63α mediates the SIRT1 degradation and p53 deacetylation. (A) The proteasome-dependent degradation
of SIRT1. (B) The deacetylation of p53. (C) The protein complex formation
between p53, SIRT1 and Sp1. Mice with heterozygous p63-/+ and ΔNp63αtransgenic
expression were sources for epidermal keratinocytes [29,45]. Total
lysates (2x105 cells) were used for immunoblotting with
indicated antibodies (dilutions: anti-ΔNp63, 1:500; anti-SIRT1, 1:300; anti-β-actin, 1:400;
anti-p53, 1:500; anti-acetyl-p53, 1:400; anti-Sp1, 1:300). Cells were also
treated with the proteasome inhibitor, MG-132 (20 μg/ml) for 24 h
before lysis. For immuno-precipitation (IP) experiments, we used total
lysates obtained from 1x106 cells/500 μl and anti-p53
antibodies (10 μg/500μl). Blots were quantitatively scanned using the
PhosphorImager and all
of the data (mean +SD) were from at least three independent experiments.
For
these studies, we used primary mouse epidermal keratinocytes obtained from mice
with p63-/+ heterozygous inactivation [45] and the ΔNp63α transgenic
mice [29], as previously described [46,47]. Using the primary mouse epidermal
cell culture, we found that the protein levels of SIRT1 were significantly
lower (by 9-fold) in cells obtained from the ΔNp63α transgenic mice
(0.06+0.01) than in the cells prepared from p63-/+ mice (0.55+0.07, Fig.
1A). We further found that the 26S proteasome inhibitor, MG-132, dramatically modulated
the SIRT1 protein degradation effect, which was likely to be induced by ΔNp63α dramatically
increasing the SIRT protein levels (Fig. 1A). We also showed that levels of
acetylated p53 were much greater (by 4- fold) in the ΔNp63α transgenic
mice (0.49+0.06) than in p63-/+ mice (0.12+0.02), while the p53 protein
levels were practically unaffected (Fig. 1B). Next, we observed that the
protein complex formation between p53, SIRT1 and Sp1 dramatically decreased in
the ΔNp63α transgenic
mice compared to p63-/+ mice (Fig. 1D).
ΔNp63α activates the transcription regulation of TERT core
promoter
The 3′-region of the core TERT
promoter contains a GC-box, which binds Sp1 and is essential for
transactivation and expression of the full-length telomerase [43,48-54].
Overexpression of Sp1 leads to a significant activation of transcription in a
cell type-specific manner, while an interaction with p53 could eliminate the
binding of Sp1, resulting in TERT repression [43]. To further examine this
phenomenon, we used the inhibitor/RNA silencing approach to investigate the
effect of the inhibition of SIRT1, p53 and Sp1 function on the transcriptional
regulation of mouse telomerase-reverse transcriptase (mTERT) promoter. The
epidermal cells form p63-/+ mice and the ΔNp63α transgenic
mice were transfected with shRNA for SIRT1, p53 and Sp1 or incubated with SIRT1
inhibitor, Sirtinol, as described elsewhere [36-38]. We, therefore, found that
the SIRT1 expression led to a decrease of acetylated p53, while both Sirtinol and
SIRT1 shRNA induced an increase of acetylated p53 (Fig. 2A). We further studied
the effect of these treatments on luciferase reporter activity driven by Sp1
binding element of the mTERT promoter [53,54]. Mouse keratinocytes
transfected with shRNA for SIRT1, p53 and Sp1 or treated with Sirtinol were
also co-transfected with the murine core TERT promoter-Luc reporter vector
(pGL3-347-Luc) containing the Sp1 binding site along with the Renilla
luciferase plasmid as described elsewhere (Methods). We showed that the
overexpression of ΔNp63α results in a significant increase in transcriptional
activity of the core mTERT promoter (Fig. 2B, samples 1 and 6). We also
observed that inhibition of SIRT1 expression or function, and p53 expression
led to an increase of luciferase reporter activity, while silencing of Sp1
induced the down regulation of luciferase reporter activity (Fig. 2B).
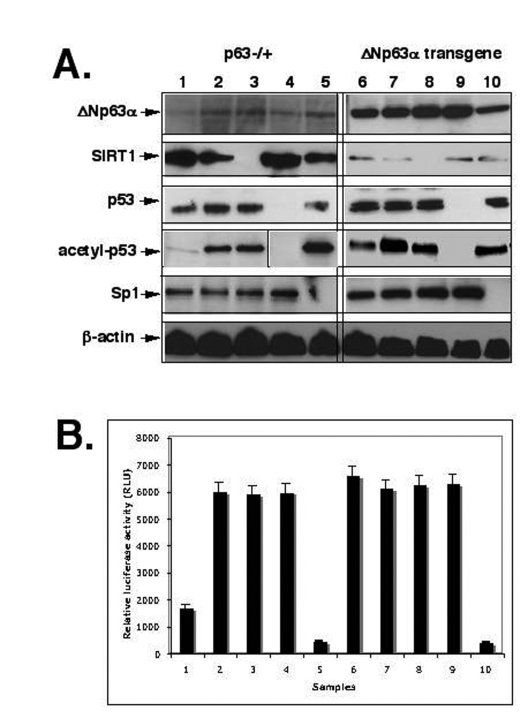
Figure 2. ShRNA silencing of ΔNp63-SIRT1-p53-Sp1 pathway. Mouse epidermal keratinocytes (2x105 cells) from p63-/+
(samples 1-5) or overexpressing ΔNp63α(samples 6-10) were treated with control
media (samples 1 and 6), SIRT1 inhibitor (Sirtinol, 100 μg/ml for 24 h;
samples 2 and 7), or transfected with the SIRT1 shRNA (samples 3 and 8), p53
shRNA (samples 4 and 9), and sh-Sp1 RNA (samples 5 and 10).
(A) Immunoblotting with indicated antibodies (dilutions: anti-ΔNp63, 1:500;
anti-SIRT1, 1:300; anti-Sp1, 1:300; anti-p53, 1:500; anti-acetyl-p53,
1:400; anti-β-actin, 1:400). The vertical lines separate data obtained from
independent protein gels.
(B) mTERT promoter luciferase reporter assay.
Mouse keratinocytes (1.0 x 105) were transfected with the pGL3-347-Luc
plasmid (0.5 μg) or the pGL3 control plasmid (0.5 μg) by using FuGENE6 transfection reagent
(Roche Diagnostics). 3 ng of the pRL-SV40 (Promega) was used as a
normalization control. Measurements were performed by using the Dual
Luciferase reporter assay system (Promega) and a BioOrbit 1251
luminometer. The activity of each TERT promoter fragment was expressed as
a relative value. All of the data (mean +SD) were from at least three
independent experiments.
We
then investigated whether the above-mentioned treatments affect endogenous
transcriptional regulation of mTERT promoter using the chromatin
immunoprecipitation (ChIP) approach using an antibody against Sp1 as described
elsewhere [53,54]. We thus found that ΔNp63α overexpression induced an interaction of Sp1
transcription factor with the core promoter of mTERT (Fig. 3). Similar effect
was found in cells transfected with shRNA inhibiting SIRT1 or p53 expression,
or in cells incubated with Sirtinol (100 μg/ml for 24 h). These
results suggested that both SIRT1 and p53 functions play a critical role in
transcription inhibition of the core mTERT promoter.
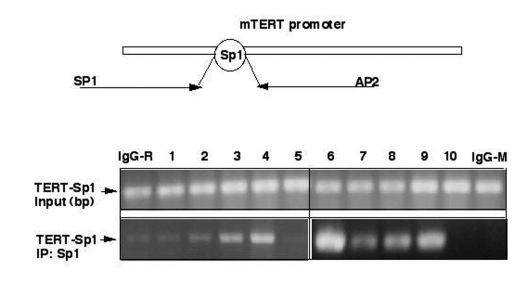
Figure 3. ΔNp63α modulates binding of Sp1 to Sp1 DNA-binding region by decreasing the SIRT1 protein levels and deacetylation of p53. Chromatin immunoprecipitation assay
(X-ChIP). Mouse epidermal keratinocytes (5x107 cells)
expressing heterozygous p63-/+ (samples 1-5) and overexpressing ΔNp63α(samples 6-10) were
treated with control media (samples 1 and 6), SIRT1 inhibitor (Sirtinol,
100 μg/ml for 24 h;
samples 2 and 7), SIRT1 shRNA (samples 3 and 8), p53 shRNA (samples 4 and
9), and shSp1 RNA (samples 5 and 10). The protein-DNA complexes were
precipitated with a primary antibody for Sp1. As negative controls, we used
immunoglobulins (IgG) from rabbit (IgG-R) or mouse (IgG-M) sera. The
mTERT-derived Sp1 promoter region using the following primers: sense (SP1),
5'-CTCACTGTCTGTGCAACCACAGCAGCTG-3'
(position-363), and antisense (AP2),
5'-AGAGCACCGCGGGGCAACGAGGAGCGCG-3' (position +143) giving raise to a
506 bp PCR product. The PCR products were run on
2% agarose gels and visualized by ethidium bromide staining.
ΔNp63α modulates the RNA splicing of mTERT
We have previously shown that ΔNp63α is implicated in
both transcriptional regulation and post-transcriptional processing/splicing
of downstream target genes [31,55]. We
previously reported that the ΔNp63α protein physically associated
with ABBP1, one of the key components of
RNA processing molecular machinery [55]. We found that the ΔNp63α ABBP1 protein
complexes contributed into the fibroblast growth factor receptor 2 receptor RNA
splicing leading to epithelial-mesenchymal transition [55]. Here we report
that these ΔNp63α ABBP1 protein
complexes were also involved in the post-transcriptional regulation of mTERT.
Telomerase
is a reverse transcriptase that adds telomeric repeats d(TTAGGG)n to
chromosomal ends [56]. In most normal somatic cells, telomerase is repressed
and telomeres progressively shorten, leading to limited proliferative lifespan
[2,56]. Telomerase reactivation is associated with cellular immortalization
and is a frequent event during tumorigenesis [2,11,13].
Structurally telomerase
is a ribonucleoprotein complex that consists of two essential components, TERT
and a template RNA, TR [56]. Telomerase ribonucleoprotein complex plays a
critical role in ageing, tumorigenesis, immortalization and "stemness"
phenotype [2,11,13,20,23,24,57]. A number of reports pointed-out that a
major control mechanism underlying the telomerase function lies at the level of
transcription and alternative splicing of TERT [18,42,48,49,51,53,59-62].
We
thus investigated whether the overexpression of the ΔNp63α protein would
affect the RNA splicing of mTERT and mTR in mouse epidermal cells. First, we
observed that the ΔNp63α overexpression led to an increasing level of the ΔNp63α ABBP1 protein
complexes (by 3-4-fold, Fig. 4A). Second, we found that the ΔNp63α overexpression
failed to affect expression of RNA component of telomerase complex (mTR) as
shown in Figure 4B (middle panel). And finally, we found that the ΔNp63α overexpression
dramatically induced the levels of α-splice isoform (by
5-6-fold) and β-splice isoform (by 1.5-2-fold) of mTERT (Fig. 4B,
upper panel and Fig. 4C), while levels of the full-length isoform of mTERT
remained unchanged in mouse epidermal keratinocytes from the ΔNp63α transgenic
mice compared to such levels found in cells from p63-/+ mice (Fig. 4B,
upper panel). As previously reported, these variants were not equal in their
ability to generate an active TERT complex [63-66]. Telomerase activity is
only provided by the full-length TERT [63-66]. The smaller splice variants (α and β) are inactive and may act as dominant-negative inhibitors
for telomerase activity [63-66]. The α-splice isoform
lacks a 12-residue region of the conserved reverse transcriptase motif A
(in-frame deletion), and the β-splice is missing a 182 bp-region resulting
in a non-sense mutation leading to premature stop codon, truncating the protein
before the conserved reverse transcriptase motifs B, C, and D [63-66].
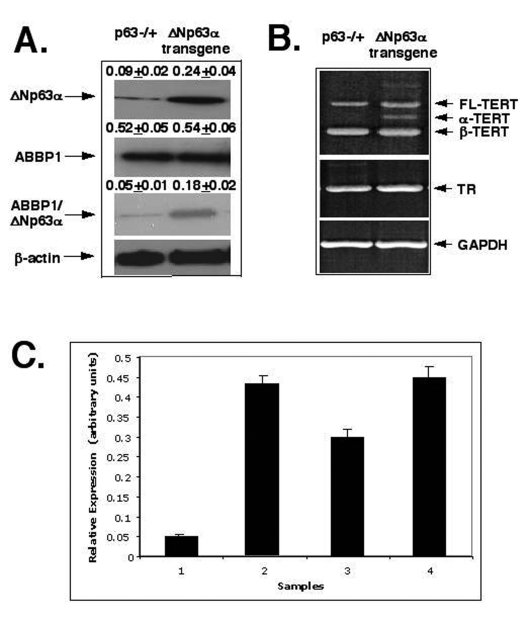
Figure 4. ΔNp63α increases levels of the mTERT-spliced isoforms via protein interaction with ABBP1. Mouse epidermal
keratinocytes (2x106 cells) expressing heterozygous p63-/+
and ΔNp63α transgene.
(A) Cells were tested for the levels of ΔNp63αand ABBP1 by immunoblotting and ABBP1ΔNp63αprotein complexes
using immunoprecipitation (IP) with an antibody to ABBP1 followed by
immunoblotting with an antibody to ΔNp63α. As a control, the protein level of β-actin was
monitored.
(B) Cells were examined for the expression of the mTERT and mTR
transcripts using RT-PCR. GAPDH was used in RT-PCR assay, as a control.
(C) The relative expression of TERT and TR was quantitatively analyzed and
plotted as bars using the Microsoft Excel software. All of the data (mean +SD) were
from at least three independent experiments. Samples: cells from p63-/+ mice, 1- TERT/GAPDH ratio; 2- TR/GAPDH ratio; cells from the ΔNp63αtransgenic mice, 3- TERT/GAPDH
ratio; 4- TR/GAPDH ratio. PCR experiments with
the 2164/ 2620 set of primers generated three products that represent the
full-length TERT transcript (457 bp), the α-splice transcript
(421 bp), and the β-splice transcript (275 bp). Sequence analysis revealed that
the longer transcripts were full-length one and the shorter transcripts
were α and β- spliced messages
of mTERT.
ΔNp63α modulates telomerase activity and increases cellular
senescence
To further examine the effect of the ΔNp63α overexpression on
the overall telomerase activity, we obtained the mouse epidermal keratinocytes
from the ΔNp63α transgenic
mice and p63-/- heterozygous mice. Cells were transfected with shRNA
against SIRT1, p53 or Sp1 for 72 h or treated with Sirtinol for 24 h. Resulting
cells were tested for telomerase activity using the TRAP assay, as described
elsewhere [63-66]. We first found that the level of telomerase activity in keratinocytes
from p63+/- mice is significantly greater than in cells from the ΔNp63α transgenic
mice (Fig. 5A, samples 1 and 6). Second, we observed that the treatment with
either SIRT1 inhibitor or shRNA against SIRT or p53 led to an increase in
telomerase activity in keratinocytes from p63+/- mice (Fig. 5A, samples
2-4), while no significant changes were seen in the ΔNp63α transgenic
mice (Fig. 5A, samples 7-9). Third, we showed that the Sp1 shRNA dramatically
decreased the telomerase activity in both mouse models (Fig. 5A, samples 5 and
10).
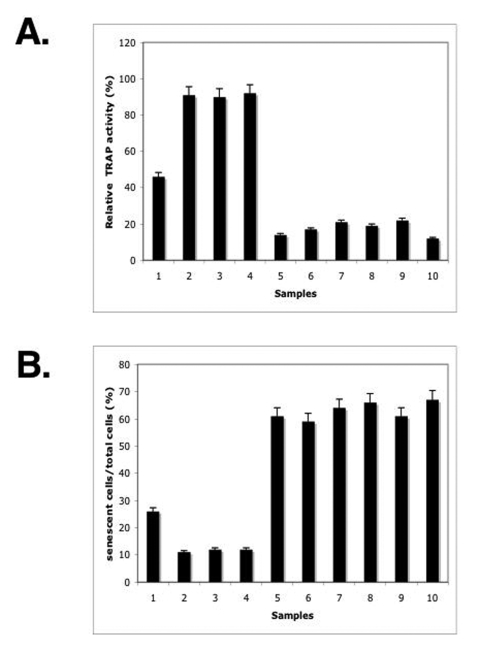
Figure 5. ΔNp63α overexpression modulated the overall telomerase activity and induced a S-β-gal activity. The mouse keratinocytes from the p63-/+ mice (samples 1, 3, 5, 7, 9) and ΔNp63αtransgenic mice (samples 2, 4, 6, 8, 10) were
treated with the control media (samples 1 and 2) or Sirtinol (100 μg/ml for 24h, samples 3 and 4)
or transfected for 72h with shRNA against SIRT1 (samples 5 and 6), p53
(samples 7 and 8) and Sp1 (samples 9 and 10).
(A) Telomerase activity. Telomerase activity was determined by the TRAP assay
using 1 μg of protein extract obtained from 2x105 cells.
Quantitative analysis was done using Molecular Dynamics densitometer and
ImageQuant software. The intensity of the positive control lane was taken
as 100%. The experiment was repeated three times, and error bars represent
mean ± S.D.
(B) S-β-gal
activity. The S-β-gal activitywas
measured using a senescence kit.
The
inhibition of endogenous telomerase activity resulting in telomere shortening
was shown to lead to a replicative senescence [11-22]. We previously showed
that the forced overexpression of ΔNp63α led to an increase in replicative senescence of human
squamous cell carcinoma cells that were distinguished by the presence of a
biomarker - senescence-associated β-galactosidase
(S-β-gal) as described [29].
Senescent cells show a series of morphological and physiological alterations including
a flat and enlarged morphology, an increase in acidic S-β-gal activity, chromatin condensation, and changes in
gene expression pattern. Here we observed that the level of the S-β-gal activity in keratinocytes from p63+/- mice
is significantly lower than in cells from the ΔNp63α transgenic mice (Fig.
5B, samples 1 and 6). Then, we showed that the treatment with either Sirtinol
or SIRT shRNA or p53 shRNA led to a decrease in the S-β-gal activity in keratinocytes from p63-/+ mice
(Fig. 5B, samples 2-4). Finally, we found that the Sp1 shRNA dramatically
increased the S-β-gal activity in p63-/+
mice (Fig. 5B, sample 5). In the same time, epidermal keratinocytes obtained
from the ΔNp63α transgenic
mice failed to display significant changes in the S-β-gal activity under above-mentioned experimental
conditions (Fig. 5B, samples 7-10).
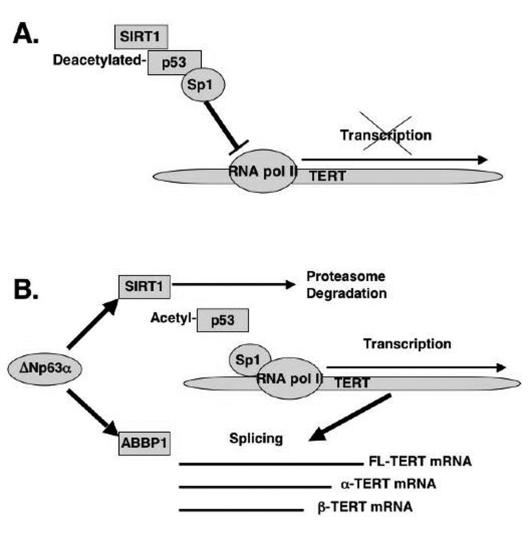
Figure 6. Schematic representation of regulation of TERT transcription and splicing by ΔNp63α. (A) mTERT transcription. (B) mTERT splicing.
Discussion
Normal
somatic cells undergo a limited number of divisions before entering an
irreversible growth-arrest state, a replicative cellular senescence, providing
a barrier against the unlimited proliferation and formation of cancer [1-7].
The molecular mechanism underlying the replicative senescence involves the
telomere shortening due to the inability to renew the telomere length by a
telomerase enzymatic complex [56]. Telomeres are specific DNA-protein complexes
present at the ends of linear chromosomes, which protect the latter from
degradation and fusion/recombination [56]. Telomeric DNA is synthesized by a
multisubunit enzymatic complex, telomerase, consisting of the telomerase
reverse transcriptase (TERT), an RNA component (TR) acting as a template, and
other associated proteins [56]. Replicative senescence can be overcome by
overexpression of the catalytic subunit of telomerase-reverse transcriptase
(TERT) as previously reported [59]. A growing number of reports showed that
various treatments could induce premature senescent phenotype through
regulation of TERT [3,5,8,10,15,25,52,57]. They include various types of
DNA damage, overexpression of oncogenes or mitogenic signals, and changes
affecting chromatin structure [3,5,8,10,15,25,52,57]. Replicative
senescence is likely to play a role in ageing of highly proliferative tissues
such as skin, endothelium and lymphoid tissues [18,60,62,64].
Telomerase
activity closely correlates with the expression of TERT, which could
potentially be regulated at the transcriptional (promoter) and
post-transcriptional (splicing) levels [2]. The TERT promoter activity is
usually regulated by a variety of transcription factors (AP-1, c-Myc, Sp1, Sp3,
NF-kB, Ets, and the estrogen receptor), and by chromatin remodeling and
epigenetic methylation mechanisms [3,5,8,10,15,25,48,52,57]. Several
variants of TERT are also generated by RNA splicing within the reverse
transcriptase region and the C-terminal part of the TERT gene and shown to
function as endogenous dominant-negative regulators/inhibitors of telomerase
activity [63-66]. Cells from skin cancers (melanomas) were shown to produce
the complete TERT mRNA along with one or more alternatively spliced transcripts
[62]. Depending of ratio between the full-length TERT and spliced TERT
isoforms, melanoma cells were characterized as positive and negative for telomerase
activity [48,62]. The high abundance of spliced TERT isoforms dramatically
inhibited the overall telomerase activity [62].
We
previously showed that the premature ageing in the p53+/m and ΔNp63α mice
was accompanied by increased ΔNp63α expression leading to induced cellular senescence
that was rescued by SIRT1 suggesting that ΔNp63α levels may affect ageing through regulation of SIRT1
[29]. Modulation of p63 function through genetic knockdown/RNA silencing [25,
26] or by dominant-negative inhibitor, ΔNp63α [29], could lead to a premature ageing phenotype,
however implicating SIRT1 regulation into the molecular mechanism underlying
the organismal ageing process [29,32-40].
In
the current report, we showed that from the first hand, ΔNp63α induced the mTERT
promoter activation through the down regulation of the SIRT1 protein levels,
inactivation of p53 deacetylation, decrease of the p53/Sp1 protein-protein
complexes, and the overall induction of mTERT transcription regulation (Fig.
6A). From the other hand, by a forming of protein-protein complexes with the
ABBP1-derived RNA processing/splicing complex (Fig. 6B), ΔNp63α induced the mTERT
RNA splicing leading to an increasing expression of spliced mTERT isoforms
playing a role of dominant-negative inhibitors of mTERT activity and therefore
decreasing the levels of TERT activity in mouse epidermal keratinocytes overexpressing
the ΔNp63α protein. The
overall effect of the ΔNp63α overexpression resulted in decrease in telomerase
activity and increase in replicative senescence observed in mouse
keratinocytes. This dual molecular mechanism of telomerase regulation might underline
the previously shown effect of p63 (ΔNp63α) on premature ageing phenotype observed in mice
overexpressing the ΔNp63α protein.
Methods
Antibodies and reagents.
We used a rabbit polyclonal antibody to ABBP1 (raised
against the C-terminal peptide SQRRGGHQNNYKPY by Affinity Bio-Reagents), a goat
polyclonal antibody to ΔNp63 (N-16, sc-8609), and a
mouse monoclonal antibody to p63 (4A4, sc-8431, both from Santa Cruz
Biotechnology), a mouse monoclonal antibody to SIRT1 (#07-131, Upstate Cell
Signaling Solutions), and a rabbit polyclonal antibody to mouse Sp1 (#S9809,
Sigma) or a rabbit polyclonal ChIP-grade antibody against Sp1 (Upstate
Biotechnology). We also used an agarose-conjugated p53 monoclonal antibody
(Ab-6; Oncogene Research Products), and anti-acetylated-Lys382 p53 antibody
(Cell Signaling Technology). We also used the SIRT1 inhibitor, Sirtinol
(#566320-5MG), and a 26S proteasome inhibitor, MG-132 (474791-1MG) that were
purchased from Calbiochem.
Preparation
of mouse keratinocytes.
P63-/+mice harboring the p63Brdm2 allele (obtained from Jackson Laboratories)
and ΔNp63α transgenic mice
(generated in our laboratory) were used [29,45] according to the regulations
of the Johns Hopkins University Animal Care and Use Committee (JHUACUC).
Primary keratinocytes were isolated from 3-4 day-old newborn pups by a
trypsinization [46,47]. Cells were plated at 3 x 106 cells
per 60-mm dish in chelex-treated low-calcium EMEM medium, BioWhittaker)
supplemented with 8% fetal bovine serum and 0.05 mM calcium) and grown at 37oC with 5% CO2. Total lysates were obtained
from cells flash-frozen in liquid N2 and transferred into a buffer A
as described [55]. The samples were homogenized on ice and centrifuged at
15,000 x g for 20 min at 4°C. The supernatants were separated on 12.5%
SDS-PAGE gels and probed with indicated antibodies. Immunoblotting and immuno-precipitation
was performed as described [55].
TERT
promoter luciferase assay.
The mouse
TERT promoter region encompassing Sp1 binding
element(-347
to +1) was kindly obtained from Drs. Charles Giardina and Rashimi R. Joshi,
University of Connecticut, Sparks, CT) as previously described [53,54]. For
the luciferase assay, mouse keratinocytes (1.0 x 105) were transfected with the
pGL3-347-Luc plasmid (0.5 μg) or the pGL3 control plasmid
(0.5 μg) by using FuGENE6 transfection reagent (Roche
Diagnostics). 3 ng of the pRL-SV40 (Promega) was used as a normalization
control. Cell lysates were obtained and measurements were performed by using
the Dual Luciferase reporter assay system (Promega) and a BioOrbit 1251
luminometer. The activity of each TERT promoter fragment was expressed as a
relative value. All of the data (mean +SD) were from at least three independent
experiments.
Small
hairpin RNA (shRNA), design and manipulation. ShRNA for mouse SIRT1
(#TR505485), Sp1 (#TR502115) and p53 (#TG500002) and scrambled shRNA were
purchased from Origene Technologies and used according to the manufacturer's
recommendations. Control and experimental shRNA (200 pmol/six-well plate) were
transiently introduced into mouse keratinocytes with aid of TurboFectin 8.0
(Origene Technologies), and 72 h later, total lysates were used for
immunoblotting.
Telomerase
activity assay.
For telomerase
activity detection, we used the PCR-mediated telomere repeat amplification
protocol (TRAP) as previously described [63-66]. As a negative control, cell
extract was substituted for lysis buffer. Two μl of cell lysate (protein
concentration 0.5 μg/μl)
were used per assay. The PCR products were run on a 10% polyacrylamide gel,
stained with SYBR Green (BioWhittaker Molecular Applications), and detected
using the Typhoon system (Molecular Dynamics). For quantitative analysis, the
ImageQuant version 5.2 software (Molecular Dynamics) was used. The area of
integration of all peaks was normalized to the signal from the internal
standard, then, after background subtraction, expressed relative to the
positive control signal (100 cell equivalent) that was run with each
experiment. The comparison of mean values between the different groups was
evaluated by ANOVA with Fisher's LSD test.
RT-PCR
assay.
Total RNA was isolated from
cells using Trizol reagent (Invitrogen). One μg
of total RNA was used to generate a cDNA from each sample using one-step RT-PCR
kit (Qiagen) and custom primers. mTR expression was monitored using the
following primers: sense (mTR, +1) 5'-CGTAATACGACTCAC TATAGGGT-3' and antisense
(mTR, +451), 5'-GCATGTGTGAGCCGAGTCCT-3' as described elsewhere [56]. The
mTERT spliced variants were detected with the following primers [56]: sense,
5'-GCCTGA GCTGTACTTTGTCAA-3', and antisense, 5'-CGCA AACAGCTTGTTCTCCATGTC-3'.
As a positive control we amplified the glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was amplified with the following primers: sense,
5'-ACCACAGTCCATGCCA TCAC-3' and antisense, 5'-TCCACCACCCTGTTGCT GTA-3'. PCR
products were separated by 2% agarose or in 4-20% gradient non-denatured PAG electrophoresis
and were visualized with ethidium bromide. All RT-PCR data was analyzed
digitally by Kodak 1D 3.5 software. The net intensity of RT-PCR bands for the
full-length mTERT and mTR were measured and normalized by net intensity of
GAPDH bands.
Chromatin immunoprecipitation (ChIP).
ChIP assays were performed using the antibody against
mouse Sp1 (Upstate Biotechnology) or rabbit immunoglobulins as negative
controls (Sigma) and ChIP assay kit (Upstate) on primary mouse keratinocytes as
previously described [53,54]. The proteins bound to DNA were cross-linked
using 1% formaldehyde for 10 min at 37°C
and the protein-DNA complexes were precipitated with a primary antibody against
Sp1. After reversal of the cross-links and DNA recovery, the latter was used as
a template to amplify the mTERT-derived Sp1 promoter region using the following
primers: sense, 5'-CTCA CTGTCTGTGCAACCACAGCAGCTG-3' (position-363), and
antisense, 5'-AGAGCACCGCGGGGCAA CGAGGAGCGCG-3' (position +143) producing a 506
bp PCR product. The PCR products were run on 2% agarose gels and visualized by
ethidium bromide.
Senescence-associated
β
-galactosidase (S-
β
-gal) activity.
Mouse keratinocytes were transfected with shRNA for
72 h or treated with Sirtinol for 24 h prior to assaying. The S-β
-gal activity was measured using a senescence kit
(Cell Signaling). Briefly, the cells were fixed with 3% formaldehyde solution
[29]. The cells were then washed and incubated with staining solution (1 mg/l,
5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside
(X-gal), 40 mM citric acid/sodium phosphate buffer, pH 6.0, 5 mM ferrocyanide,
5 mM ferricyanide, 150 mM NaCl, and 2 mM MgCl2) for 12-18 h to
visualize S-β-gal activity as
described [52]. Data were plotted as ratio of senescent cells over total cells
using Microsoft Excel software. All of the data (mean +SD) were from at least three
independent experiments.
Acknowledgments
We
thank Drs. Charles Giardino and Rashimi R. Joshi (University of Connecticut, Sparks, CT) for kindly providing us with pGL3-347 Luc plasmid.
Conflicts of Interest
The authors of this manuscript have no conflict of interests to declare.
References
-
1.
Adams
PD
Remodeling of chromatin structure in senescent cells and its potential impact on tumor suppression and ageing.
Gene.
2007;
397:
84
-93.
[PubMed]
.
-
2.
Blasco
MA
Telomeres and human disease: ageing, cancer and beyond.
Nat Rev Genet.
2005;
6:
611
-622.
[PubMed]
.
-
3.
Demidenko
ZN
and Blagosklonny
MV.
Growth stimulation leads to cellular senescence when the cell cycle is blocked.
Cell Cycle.
2008;
7:
3355
-3361.
[PubMed]
.
-
4.
Garcia
CK
, Wright
WE
and Shay
JW.
Human diseases of telomerase dysfunction: insights into tissue ageing.
Nucl Acids Res.
2007;
35:
7406
-7416.
[PubMed]
.
-
5.
Greer
EL
and Brunet
A.
Signaling networks in ageing.
J Cell Sci.
2008;
121:
407
-412.
[PubMed]
.
-
6.
Miura
T
, Mattson
MP
and Rao
MS.
Cellular lifespan and senescence signaling in embryonic stem cells.
Aging Cell.
2004;
3:
333
-343.
[PubMed]
.
-
7.
Smith
JR
and Pereira-Smith
OM.
Replicative Senescence: Implications for in Vivo Ageing and Tumor Suppression.
Science.
1996;
273:
63
-67.
[PubMed]
.
-
8.
Beliveau
A
, Bassett
E
, Lo
AT
, Garbe
J
, Rubio
MA
, Bissell
MJ
, Campisi
J
and Yaswen
P.
P53-dependent integration of telomere and growth factor deprivation signals.
Proc Natl Acad Sci U S A.
2007;
104:
4431
-4436.
[PubMed]
.
-
9.
Chen
JH
, Hales
CN
and Ozanne
SE.
DNA damage, cellular senescence and organismal ageing: causal or correlative.
Nucleic Acids Res.
2007;
35:
7417
-7428.
[PubMed]
.
-
10.
Courtois-Cox
S
, Genther-Williams
SM
, Reczek
EE
, Johnson
BW
, McGillicuddy
LT
, Johannessen
CM
, Hollstein
PE
, MacCollin
M
and Cichowski
K.
A negative feedback signaling network underlies oncogene-induced senescence.
Cancer Cell.
2006;
10:
459
-472.
[PubMed]
.
-
11.
Deng
Y
, Chan
SS
and Chang
S.
Telomere dysfunction and tumor suppression: the senescence connection.
Nat Rev Cancer.
2008;
8:
450
-458.
[PubMed]
.
-
12.
Epel
ES
, Blackburn
EH
, Lin
J
, Dhabhar
FS
, Adler
NE
, Morrow
JD
and Cawthon
RM.
Accelerated telomere shortening in response to life stress.
Proc Natl Acad Sci U S A.
2004;
101:
17312
-17315.
[PubMed]
.
-
13.
Feldser
DM
and Greider
CW.
Short telomeres limit tumor progression in vivo by inducing senescence.
Cancer Cell.
2007;
11:
461
-469.
[PubMed]
.
-
14.
Feng
Z
, Hu
W
, Rajagopal
G
and Levine
AJ.
The tumor suppressor p53: cancer and ageing.
Cell Cycle.
2008;
7:
842
-847.
[PubMed]
.
-
15.
Rudolph
KL
, Chang
S
, Lee
HW
, Blasco
M
, Gottlieb
GJ
, Greider
C
and DePinho
RA.
Longevity, stress response, and cancer in ageing telomerase-deficient mice.
Cell.
1999;
96:
701
-712.
[PubMed]
.
-
16.
Sekaric
P
, Shamanin
VA
, Luo
J
and Androphy
EJ.
hAda3 regulates p14ARF-induced p53 acetylation and senescence.
Oncogene.
2007;
26:
6261
-6268.
[PubMed]
.
-
17.
Shay
JW
and Wright
WE.
Senescence and immortalization: role of telomeres and telomerase.
Carcinogenesis.
2005;
26:
867
-874.
[PubMed]
.
-
18.
Boukamp
P
Skin ageing: a role for telomerase and telomere dynamics.
Curr Mol Med.
2005;
5:
171
-177.
[PubMed]
.
-
19.
Aisner
DL
, Wright
WE
and Shay
JW.
Telomerase regulation: not just flipping the switch.
Curr Opin Genet Dev.
2002;
12:
80
-85.
[PubMed]
.
-
20.
Armstrong
L
, Lako
M
, Lincoln
J
, Cairns
PM
and Hole
N.
mTert expression correlates with telomerase activity during the differentiation of murine embryonic stem cells.
Mech Dev.
2000;
97:
109
-116.
[PubMed]
.
-
21.
Kyo
S
and Inoue
M.
Complex regulatory mechanisms of telomerase activity in normal and cancer cells: how can we apply them for cancer therapy.
Oncogene.
2002;
21:
688
-697.
[PubMed]
.
-
22.
Minty
F
, Thurlow
JK
, Harrison
PR
and Parkinson
EK.
Telomere dysfunction in human keratinocytes elicits senescence and a novel transcription profile.
Exp Cell Res.
2008;
314:
2434
-2447.
[PubMed]
.
-
23.
Geserick
C
and Blasco
MA.
Novel roles for telomerase in ageing.
Mech Ageing Dev.
2006;
127:
579
-583.
[PubMed]
.
-
24.
Stewart
SA
and Weinberg
RA.
Telomeres: cancer to human ageing.
Annu Rev Cell Dev Biol.
2006;
22:
531
-557.
[PubMed]
.
-
25.
Guo
X
and Mills
AA.
P63, cellular senescence and tumor development.
Cell Cycle.
2007;
6:
305
-311.
[PubMed]
.
-
26.
Keyes
W
, Wu
Y
, Vogel
H
, Guo
X
, Lowe
S
and Mills
A.
P63 deficiency activates a program of cellular senescence and leads to accelerated ageing.
Genes Dev.
2005;
19:
1986
-1999.
[PubMed]
.
-
27.
Maier
B
, Gluba
W
, Bernier
B
, Turner
T
, Mohammad
K
, Guise
T
, Sutherland
A
, Thorner
M
and Scrable
H.
Modulation of mammalian life span by the short isoform of p53.
Genes Dev.
2004;
18:
306
-319.
[PubMed]
.
-
28.
Scrable
H
, Sasaki
T
and Maier
B.
DeltaNp53 or p44: priming the p53 pump.
Int J Biochem Cell Biol.
2005;
37:
913
-919.
[PubMed]
.
-
29.
Sommer
M
, Poliak
N
, Upadhyay
S
and Nelkin
B.
, Donehower L, Ratovitski E, Sidransky D. ΔNp63αoverexpression induces downregulation of SIRT1 and an accelerated ageing phenotype in the mouse.
Cell Cycle.
2006;
5:
2005
-2011.
[PubMed]
.
-
30.
Tyner
S
, Venkatachalam
S
, Choi
J
, Jones
S
, Ghebranious
N
, Igelmann
H
, Lu
X
, Soron
G
, Cooper
B
, Brayton
C
, Hee
Park S
, Thompson
T
, Karsenty
G
, Bradley
A
and Donehower
L.
P53 mutant mice that display early ageing-associated phenotypes.
Nature.
2002;
415:
45
-53.
[PubMed]
.
-
31.
Trink
B
, Osada
M
, Ratovitski
E
and Sidransky
D.
P63 transcriptional regulation of epithelial integrity and tumorigenesis.
Cell Cycle.
2007;
6:
240
-245.
[PubMed]
.
-
32.
Kim
EJ
, Kho
JH
, Kang
MR
and Um
SJ.
Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity.
Mol Cell.
2007;
28:
277
-290.
[PubMed]
.
-
33.
Longo
VD
and Kennedy
BK.
Sirtuins in ageing and age-related disease.
Cell.
2006;
126:
257
-268.
[PubMed]
.
-
34.
Motta
M
, Divecha
N
, Lemieux
M
, Kamel
C
, Chen
D
, Gu
W
, Bultsma
Y
, McBurney
M
and Guarente
L.
Mammalian SIRT1 represses forkhead transcription factors.
Cell.
2004;
116:
551
-563.
[PubMed]
.
-
35.
Narala
SR
, Allsopp
RC
, Wells
TB
, Zhang
G
, Prasad
P
, Coussens
MJ
, Rossi
DJ
, Weissman
IL
and Vaziri
H.
SIRT1 acts as a nutrient-sensitive growth suppressor and its loss is associated with increased AMPK and telomerase activity.
Mol Biol Cell.
2008;
19:
1210
-1219.
[PubMed]
.
-
36.
Ota
H
, Tokunaga
E
, Chang
K
, Hikasa
M
, Iijima
K
, Eto
M
, Kozaki
K
, Akishita
M
, Ouchi
Y
and Kaneki
M.
Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells.
Oncogene.
2006;
25:
176
-185.
[PubMed]
.
-
37.
Solomon
JM
, Pasupuleti
R
, Xu
L
, McDonagh
T
, Curtis
R
, DiStefano
PS
and Huber
LJ.
Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage.
Mol Cell Biol.
2006;
26:
28
-38.
[PubMed]
.
-
38.
Saunders
LR
and Verdin
E.
Sirtuins: critical regulators at the crossroads between cancer and ageing.
Oncogene.
2007;
26:
5489
-5504.
[PubMed]
.
-
39.
Vaziri
H
, Dessain
S
, Ng
Eaton E
, Imai
SI
, Frye
RA
, Pandita
T
, Guarente
L
and Weinberg
R.
hSIR2 (SIRT1) functions as an NAD-dependent p53 deacetylase.
Cell.
2001;
107, 149
-159.
[PubMed]
.
-
40.
Huang
J
, Gan
Q
, Han
L
, Li
J
, Zhang
H
, Sun
Y
, Zhang
Z
and Tong
T.
SIRT1 overexpression antagonizes cellular senescence with activated ERK/S6k1 signaling in human diploid fibroblasts.
PLoS ONE.
2008;
3:
e1710
[PubMed]
.
-
41.
Blasco
MA
The epigenetic regulation of mammalian telomeres.
Nat Rev Genet.
2007;
8:
299
-309.
[PubMed]
.
-
42.
Guilleret
I
and Benhattar
J.
Demethylation of the human telomerase catalytic subunit (hTERT) gene promoter reduced hTERT expression and telomerase activity and shortened telomeres.
Exp Cell Res.
2003;
289:
326
-334.
[PubMed]
.
-
43.
Koutsodontis
G
, Vasilaki
E
, Chou
WC
, Papakosta
P
and Kardassis
D.
Physical and functional interactions between members of the tumour suppressor p53 and the Sp families of transcription factors: importance for the regulation of genes involved in cell-cycle arrest and apoptosis.
Biochem J.
2005;
389:
443
-455.
[PubMed]
.
-
44.
You
H
and Mak
TW.
Crosstalk between p53 and FOXO transcription factors.
Cell Cycle.
2005;
4:
37
-38.
[PubMed]
.
-
45.
Mills
A
, Zheng
B
, Wang
X
, Vogel
H
, Roop
D
and Bradley
A.
P63 is a p53 homologue required for limb and epidermal morphogenesis.
Nature.
1999;
39:
708
-713.
[PubMed]
.
-
46.
Hager
B
, Bickenbach
JR
and Fleckman
P.
Long-term culture of murine epidermal keratinocytes.
J Invest Dermatol.
1999;
112:
971
-976.
[PubMed]
.
-
47.
Pirrone
A
, Hager
B
and Fleckman
P.
Primary mouse keratinocyte culture.
Methods Mol Biol.
2005;
289:
3
-14.
[PubMed]
.
-
48.
Cerezo
A
, Kalthoff
H
, Schuermann
M
, Schäfer
B
and Boukamp
P.
Dual regulation of telomerase activity through c-Myc-dependent inhibition and alternative splicing of hTERT.
J Cell Sci.
2002;
115:
1305
-1312.
[PubMed]
.
-
49.
Choi
J
, Southworth
LK
, Sarin
KY
, Venteicher
AS
, Ma
W
, Chang
W
, Cheung
P
, Jun
S
, Artandi
MK
, Shah
N
, Kim
SK
and Artandi
SE.
TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program.
PLoS Genet.
2008;
4:
e10
[PubMed]
.
-
50.
Pericuesta
E
, Ramírez
MA
, Villa-Diaz
A
, Relaño-Gines
A
, Torres
JM
, Nieto
M
, Pintado
B
and Gutiérrez-Adán
A.
The proximal promoter region of mTert is sufficient to regulate telomerase activity in ES cells and transgenic animals.
Reprod Biol Endocrinol.
2006;
4:
5
-9.
[PubMed]
.
-
51.
Takakura
M
, Kyo
S
, Inoue
M
, Wright
WE
and Shay
JW.
Function of AP-1 in transcription of the telomerase reverse transcriptase gene (TERT) in human and mouse cells.
Mol Cell Biol.
2005;
25:
8037
-8043.
[PubMed]
.
-
52.
Wu
KJ
, Grandori
C
, Amacker
M
, Simon-Vermot
N
, Polack
A
, Lingner
J
and Dalla-Favera
R.
Direct activation of TERT transcription by c-MYC.
Nat Genet.
1999;
21:
220
-224.
[PubMed]
.
-
53.
Yin
L
, Hubbard
AK
and Giardina
C.
NF-kappa B regulates transcription of the mouse telomerase catalytic subunit.
J Biol Chem.
2000;
275:
36671
-36675.
[PubMed]
.
-
54.
Zhang
Y
, Fan
S
, Meng
Q
, Ma
Y
, Katiyar
P
, Schlegel
R
and Rosen
EM.
BRCA1 interaction with human papillomavirus oncoproteins.
J Biol Chem.
2005;
280:
33165
-33177.
[PubMed]
.
-
55.
Fomenkov
A
, Huang
Y
, Topaloglu
O
, Brechman
A
, Osada
M
, Fomenkov
T
, Yuriditsky
E
, Trink
B
, Sidransky
D
and Ratovitski
E.
P63α mutations lead to aberrant splicing of keratinocyte growth factor receptor in the Hay-Wells syndrome.
J Biol Chem.
2003;
278:
23906
-23914.
[PubMed]
.
-
56.
Autexier
C
and Lue
NF.
The Structure and Function of Telomerase Reverse Transcriptase.
Annu Rev Biochem.
2006;
75:
493
-517.
[PubMed]
.
-
57.
Ye
X
, Zerlanko
B
, Kennedy
A
, Banumathy
G
, Zhang
R
and Adams
PD.
Downregulation of Wnt signaling is a trigger for formation of facultative heterochromatin and onset of cell senescence in primary human cells.
Mol Cell.
2007;
27:
183
-196.
[PubMed]
.
-
58.
Colgin
LM
, Wilkinson
C
, Englezou
A
, Kilian
A
, Robinson
MO
and Reddel
RR.
The hTERTα splice variant is a dominant negative inhibitor of telomerase activity.
Neoplasia.
2000;
2:
426
-432.
[PubMed]
.
-
59.
Counter
CM
, Meyerson
M
, Eaton
EN
, Ellisen
LW
, Caddle
SD
, Haber
DA
and Weinberg
RA.
Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), catalytic subunit of telomerase.
Oncogene.
1998;
16:
1217
-1222.
[PubMed]
.
-
60.
Fujiwara
M
, Kamma
H
, Wu
W
, Hamasaki
M
, Kaneko
S
, Horiguchi
H
, Matsui-Horiguchi
M
and Satoh
H.
Expression and alternative splicing pattern of human telomerase reverse transcriptase in human lung cancer cells.
Int J Oncol.
2004;
24:
925
-930.
[PubMed]
.
-
61.
Kilian
A
, Bowtell
DD
, Abud
HE
, Hime
GR
, Venter
DJ
, Keese
PK
, Duncan
L
, Reddel
RR
and Jefferson
RA.
Isolation of a candidate human telomerase catalytic subunit gene, which reveals complex splicing patterns in different cell types.
Hum Mol Genet.
1997;
6:
2011
-2019.
[PubMed]
.
-
62.
Villa
R
, Porta
CD
, Folini
M
, Daidone
MG
and Zaffaroni
N.
Possible regulation of telomerase activity by transcription and alternative splicing of telomerase reverse transcriptase in human melanoma.
J Invest Dermatol.
2001;
116:
867
-873.
[PubMed]
.
-
63.
Saldanha
SN
, Andrews
LG
and Tollefsbol
TO.
Analysis of telomerase activity and detection of its catalytic subunit, hTERT.
Anal Biochem.
2003;
315:
1
-21.
[PubMed]
.
-
64.
Ulaner
GA
, Hu
JF
, Vu
TH
, Giudice
LC
and Hoffman
AR.
Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts.
Cancer Res.
1998;
8:
4168
-4172.
[PubMed]
.
-
65.
Yi
X
, White
DM
, Aisner
DL
, Baur
JA
, Wright
WE
and Shay
JW.
An alternate splicing variant of the human telomerase catalytic subunit inhibits telomerase activity.
Neoplasia.
2000;
2:
433
-440.
[PubMed]
.
-
66.
Yi
X
, Shay
JW
and Wright
WE.
Quantitation of telomerase components and hTERT mRNA splicing patterns in immortal human cells.
Nucleic Acids Res.
2001;
29:
4818
-4825.
[PubMed]
.