Abstract
Telomere length (TL) has been proposed as a marker of mitotic cell age and as a general index of human organismic aging. Short absolute leukocyte telomere length has been linked to cardiovascular-related morbidity and mortality. Our aim was to test whether the rate of change in leukocyte TL is related to mortality in a healthy elderly cohort. We examined a subsample of 236 randomly selected Caucasian participants from the MacArthur Health Aging Study (aged 70 to 79 years). DNA samples from baseline and 2.5 years later were assayed for mean TL of leukocytes. Percent change in TL was calculated as a measure of TL change (TLC). Associations between TL and TLC with 12-year overall and cardiovascular mortality were assessed. Over the 2.5 year period, 46% of the study participants showed maintenance of mean bulk TL, whereas 30% showed telomere shortening, and, unexpectedly, 24% showed telomere lengthening. For women, short baseline TL was related to greater mortality from cardiovascular disease (OR = 2.3; 95% CI: 1.0 - 5.3). For men, TLC (specifically shortening), but not baseline TL, was related to greater cardiovascular mortality, OR = 3.0 (95% CI: 1.1 - 8.2). This is the first demonstration that rate of telomere length change (TLC) predicts mortality and thus may be a useful prognostic factor for longevity.
Introduction
Understanding the aging
process is central to preventing age-related disease burden and premature
mortality. Many different measures have been suggested as having prognostic
value for mortality. Cellular aging may offer insights into organismic aging
relevant to diseases of aging such as CVD. Telomeres, the protective
nucleoprotein structures capping the ends of eukaryotic chromosomes, can serve
as markers of mitotic cell age and replicative potential. With every cell division,
a portion of the telomere cap is not replicated due to the "end
replication problem" - that is, DNA polymerase does not completely replicate
the end of a DNA strand [1]. Hence, cells in certain older organisms, including humans,
have shorter telomeres on average than cells in younger organisms.
Telomere length change (TLC) depends on many factors, prominent
among them the rate of cell divisions and level of telomerase, a cellular
ribonucleoprotein reverse transcriptase enzyme that replenishes telomeric DNA
and thus lengthens the telomere. In cells lacking sufficient levels of
telomerase, telomeres progressively shorten with successive cell divisions. If
the telomere shortening represents a clock ticking forward on cells' lifespans,
telomerase can slow or reverse this clock [2],
making the two an intricately interdependent dynamic system.
Indeed, in vitro studies show that telomeres can lengthen - activated B cell
telomere length increases as these cells multiply in germinal centers in
response to pathogenic challenge [3].
TLC in part reflects the balance between telomere elongation
by telomerase action versus telomere shortening processes.
Cellular senescence may underlie the progression of diseases
associated with organismic aging [4]. Mice bred without telomerase develop shorter telomeres, and
show premature aging, including hair graying, impaired wound healing, reduced
proliferation of lymphocytes, and, in later generations, early mortality and
infertility [5]. Humans with a rare genetic disorder (dyskeratosis congenita)
that leads to half the effective gene dosage of telomerase show early mortality
and increased incidences of fibrosis, cancer, progressive bone marrow failure
and other indications of premature aging, and other premature aging syndromes
are also often characterized by shortened telomeres [4,6-8].
Despite these lines of evidence, among the general population
of healthy humans without pathologic premature aging syndromes, little direct
data exist to link cellular aging with organismic aging.
The strongest evidence that
cellular aging, as reflected by shorter telomeres, might be associated with
organismic aging has until now been derived from cross sectional studies.
Shorter telomere length (TL) in leukocytes has been associated
cross-sectionally with CVD and its risk factors, including pulse pressure [9-11], obesity [12,13], vascular dementia [14], diabetes [13,15,16], CAD [17], and myocardial infarction [18] although not in all studies [19]. TL has also been shown to predict CVD events (MI and stroke)
in men under 73 years old [20]. Cawthon and colleagues found that TL predicted earlier
mortality, particularly from CVD and infectious disease, in a sample of 143
healthy men and women 60 years and older [21].
This suggested that poor telomere maintenance may serve as a
prognostic biomarker of risk of early mortality. Since then, additional
studies have found blood TL predicts mortality, in large twin studies [22,23],
and in Alzheimers [24], and stroke patients [25].
However, other reports, notably those with very elderly
cohorts, have failed to find an association between TL and mortality [26].
A single TL assessment, however, leaves open the possibility that
TL at birth, rather than rate of telomere attrition, accounts for this
association with mortality. One might have expected, given the low rate of
attrition throughout life, that TL at birth would be a strong predictor of TL
later in life. However, twin studies indicate non-genetic factors can have
significant effects on telomere length later in life; telomere length was
similarly related in identical compared to fraternal male twins over 70 years
old, suggesting a large non-genetic influence [27], and identical twins who exercised had longer leukocyte
telomeres than the identical twin who did not [28]. Further, twin studies show that telomere length predicts
mortality beyond genetic influences [22,23]. Hence, longitudinal studies that examine telomere changes
over time within individuals are needed to test the prognostic value of the
rate of telomere length change (TLC).
In one of the only published studies of TLC over time in humans, a
study of 70 adults found that a small percentage (10%) of subjects showed
leukocyte telomere length maintenance or lengthening over a ten year period [12].
No studies we are aware of in humans have systematically
examined TLC within a short period of only a few years, and how this may or may
not be linked to subsequent mortality. The current study examined TL and TLC
in a high functioning sample of 70-79 year olds. We aimed to: 1) Describe the
natural history of telomere length change over a 2.5 year period in a sample of
elderly men and women; and 2) Test TL and particularly TLC as predictors of
mortality. Lastly, we explored whether the combination of short TL and greater
TLC predicted greater risk of subsequent mortality than either one indicator
alone. We report here that TLC over the next 2.5 years did indeed predict
12-year mortality from cardiovascular disease in men. Hence we propose that the
rate of leukocyte telomere shortening is a potentially useful prognostic for
cardiovascular disease.
Results
Participants
The participants were aged 70 to 79 at baseline (1988), with an
average age of 73.7 years (SD = 2.87). Their ethnicity was Caucasian (100%).
The average BMI, blood pressure, alcohol intake, physical activity, and percent
of smokers and of those with diabetes are shown in Table 1. We also examined
sociodemo-graphic and health variables by short and long TL groups. As shown
in Table 1, there were no group differences in any of the sociodemographic or
health variables examined by long and short TL groups.
Table 1. Sociodemographic and health status for total sample and by short and long TL groups (% or Means and Standard Deviations).
*Short TL: defined as TL below the median/ Long TL: defined as TL above the median.
ap<0.01. There were no significant differences in these health and behavioral factors by TL group, above, or by sex and TL group (not shown).
|
Total sample
N = 235
|
Short TL*
N = 117
|
Long TL
N = 118
|
|
T/S ratio
|
1.1 (0.24)
|
0.94 (0.13) a
|
1.3 (0.16)
|
|
Mean Age
|
73.7 (2.9)
|
73.7 (2.9)
|
73.7 (2.9)
|
|
Mean Education (years)
|
10.5 (2.5)
|
10.6 (2.5)
|
10.4 (2.6)
|
|
Mean Diastolic BP
|
75.4 (10.1)
|
74.7 (10.0)
|
76.0 (10.2)
|
|
Mean Systolic BP
|
135.0 (17.1)
|
134.8 (16.5)
|
135.2 (17.7)
|
|
Hypertension (%)
|
49.4
|
47.9
|
50.9
|
|
Diabetes (%)
|
20.4
|
22.4
|
18.3
|
|
Mean Physical Activity
|
18.9 (25.5)
|
21.3 (27.3)
|
16.4 (23.4)
|
|
Current smokers (%)
|
18.7
|
16.2
|
21.2
|
|
Mean Alcohol intake (ounces/month)
|
4.5 (11.1)
|
4.5 (9.9)
|
4.5 (12.2)
|
Further, we examined these factors in TL groups by sex, and still
found no significant differences across the groups (men with long vs. short TL,
and women with long vs. short TL).
Natural history of Telomere Length Change (TLC) over 2.5 years
The average baseline TL was
1.1 t/s (4697 base pairs or bp), and ranged from 0.46 to 1.9 t/s. Consistent
with other studies, women had longer TL at baseline (mean t/s 1.17; SD = .233),
compared to men (mean t/s 1.09; SD = .233, p < .008). Hence, when TL was
divided into long and short, based on a median split for the entire sample of
men plus women, there tended to be more women in the long telomere group
(58.3% women), and more men in the short telomere length group (56.7% men). The
mean TL at the follow-up visit 2.5 years after baseline wassimilar, (mean t/s 1.1; with a range from
0.76 to 1.8). The raw t/s change score values ranged from -.75 to .60. This
corresponds to a range from a net loss of 1067 bp/year to a net gain of 925
bp/year, at the extremes. There was no significant gender difference in %TLC.
To quantify the extent of more substantial (and likely more
meaningful) decreases or increases in TL, we categorized people based on change
scores that were outside the 7% range of the variability expected for the
assay. To be conservative, we used differences of at least +/- 15% from the
baseline TL value as a cut point for indicating a reliable and large change
from baseline. Participants who showed less than a 15% change (increase or
decrease) from their baseline TL were categorized as TL Maintainers. TL maintainers
comprised 55% of the sample. For the purposes of this analysis, those who
showed a decrease in TL of greater than 15% are described as having significant
shortening, and comprised 30% of the sample. Those with greater than 15%
increase in TL are described as having significant lengthening, and were 24% of
the sample.
Predictors of %TLC
Spearman correlations with %TLC were performed for several
candidate sociodemographic and self reported health behaviors. There were no
consistent patterns and correlations were weak, as follows: Age (within the
narrow 70-79 year baseline age span for this cohort) was not related to TL or
%TLC for women, but was related to greater %TLC for men (r = -.27, p < .05),
in that older men showed greater rates of telomere attrition. BMI was related
to greater %TLC (greater decreases), in women (rho = -.25, p < .05), but not
significantly for men (rho = -.12, ns). Alcohol use was also related to
greater %TLC (greater decreases), again in women only (rho = -.31, p < .05).
TL and %TLC were not associated with education (rho = -.01, ns), pack years of
cigarettes (rho = -.04, ns), or physical activity (rho = .07), all ns.
Table 2. CVD Mortality rates (%) by gender for each Predictor.
*p < 0.05 difference within sex groups
| | Men | Women |
|
Baseline TL
|
Short
|
25.8%
| 29.4% * |
|
Long
|
24.0%
| 13.2% |
|
TL Change
|
Shortened
| 46.7% * |
16.7%
|
|
Maintained
| 17.7% |
18.4%
|
TL and %TLC predict mortality?
By 2000 (12 years from the beginning of
the study), 102 (43.4%) participants were known to have died, according to
death certificates (42 women, 60 men). There were no associations of TL or %TLC
on overall 12 year mortality. We then examined mortality from different causes
in relation to leukocyte TL or telomere length change. There were not enough
deaths due to infectious disease (n = 6) to examine independently. More than
half of deaths (53) were from cardiovascular disease (24 women, 29 men), the
main outcome in this study. CVD Mortality rates by gender for baseline TL and
%TLC are listed in Table 2. For the sample as a whole (men and women
combined), baseline TL weakly predicted CVD mortality, a relationship which
achieved only marginal statistical significance, p < .10. This trend is
consistent with Cawthon's previous study (2003). However, when we examined the
sample by gender, women with shorter baseline TL were 2.3 times more likely to
die from CVD over the next 12 years compared with those with longer baseline TL
(95% CI = 1.0 - 5.3, p < 0.05, Table 3, Figure 1). Specifically, 20% of
women had died from CVD, and of these, the majority (62.5%) were in the short
TL group. This effect held only for women, with no association of TL with CVD
mortality for men (p = .60). Although not significant, Cawthon et al (2003)
also found a marginally stronger effect of TL on mortality for women as
compared with men.
We next examined rate of telomere
shortening (%TLC), categorically, in relation to subsequent mortality,
comparing those in the lowest quartile of %TLC (representing those with the
greatest shortening) to the rest of the sample. For women, there was no
association of 12 year CVD mortality with the rate of TL shortening during the
2.5 years monitored at the beginning of the 12 year period (p = .98). Strikingly,
TL shortening rate in the men was linked to greater CVD mortality (hazard ratio
= 3.0, 95% CI = 1.1 - 8.2, p < .04, Table 3, Figure 2).
A final set of secondary
analyses examined the combined impact of having both shorter baseline TL and
experiencing a decline in TL over time on CVD mortality and overall mortality.
Given the strong relation between baseline TL and change in TL, with greater
shortening seen in those with longer rather than shorter baseline TL, there
were insufficient numbers of participants who had both short TL and shortening
over time when examining CVD mortality. When examining overall mortality, there
were nine participants in the both short baseline and shortening over time
category. A Chi Square testing baseline TL (long vs. short) by change
(shortening vs. no shortening) by mortality was significant, X2 (3) = 8.70, p
< .03. The men with short baseline TL and maintenance or lengthening over
time were more likely to be alive 12 years later (20 of 29, 69%), compared to those
with short baseline TL and telomere shortening (1 of 8, 13%). In contrast,
for men with relatively long baseline telomeres, there was no apparent effect
of rate of shortening on mortality: those with telomere lengthening over time
tended to be equally likely to be alive 12 years later (40%), compared to those
with telomere shortening (58%).
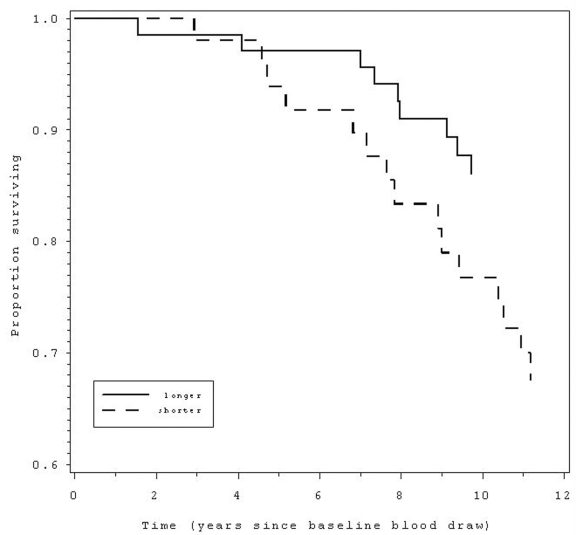
Figure 1.
Those with shorter (below median) telomere length at baseline (dashed line) had 2.3 times greater
likelihood of mortality over the following 12 years compared to those with
longer telomeres (solid line).
Discussion
Telomere maintenance has emerged as a significant
determinant of the ability of mitotic cells to continue proliferating [29].
Cawthon et al. (2003) have previously shown in a cross
sectional study that telomere length in late human adulthood can predict
longevity. Here we extend and replicate these findings: we found that in
older women, short telomere length (below average baseline TL) was associated
with almost three times the risk of 12-year mortality from cardiovascular
disease compared to women with longer baseline TL. Further, we report a novel
association of failure to maintain telomere length with cardiovascular disease
mortality; we found that men who showed leukocyte telomere shortening over the
short period of 2.5 years were subsequently three times more likely to die from
heart disease than those who maintained leukocyte telomere length. Exploratory
analyses also found that among men, those with both shorter baseline TL and
shortening over the 2.5 year interval (though only a small subset of the total
sample) had extremely high 12-year mortality (87%), as compared to men who also
had short baseline telomeres, but who showed stable maintenance or lengthening
of their telomeres during this period (31%). Given the small sample size this
secondary analysis must be replicated.
What factors might lead to faster telomere
shortening? Telomere shortening, especially in the face of already short
telomeres, is indicative of insufficient telomerase, as cells with short
telomeres, but with adequate telomerase, can maintain proliferation and
longevity [5,30]. Thus, it is in part the co-occurrence of short telomeres and
low telomerase activity that appears to increase the risk of cell death in
vitro [31]. More specifically, in relation to CVD, telomerase is crucial
for healthy cardiovascular cell functioning [32], and has been linked to cardiovascular disease risk factors in
vivo [33]. We speculate that low telomerase, as indicated by the rate of
telomere shortening, may also have contributed to the more rapid decline in
cardiovascular health and subsequent earlier mortality observed in men.
Another interesting finding
was that in general, people in this study with short leukocyte telomeres tended
to have a slower rate of telomere shortening over time, compared to those with
longer telomeres. This relation between telomere length and change in telomere
length was strong (r = -.71). Such a finding is consistent with the available
information about telomerase action and the consequences of telomere shortening
in cells, in which telomerase preferentially elongates shorter rather than the
longer telomeres [31,34],
and cells with critically short telomeres become
underrepresented in the cell population because they cease to proliferate.
This inverse relationship also underscores the potential importance of
adjusting for baseline telomere length when examining rate of change, since we found
the two are strongly inversely related. One untested possibility for this
inverse relationship is that people with short telomeres may have upregulated
telomerase, which would lead to less attrition per replication, and thus
prevent loss of telomere length over years. However, there is likely to be
strong selection for those cells in vivo that have maintained telomeres above
critically short lengths, and thus there may be an in vivo selection for cells
with short telomeres that also have higher levels of telomerase. These are all
salient questions for future research.
It is notable that cross-sectional TL did predict mortality in
this sample of women, given their older age (70 to 79 years old). This is
consistent with two population-based twin studies examining cross-sectional
telomere length in people of this age range or older [22,23] but discrepant with four studies, which have found weaker [21] or no [26,35,36] effects for mortality in participants over 70 years old.
The present study used a subset of participants from the MacArthur Study of
Successful Aging, which only enrolled participants with good cognitive and
physical functioning. In this respect, it is an atypical sample of elderly
people, who are possibly biologically younger than the unselected elderly
samples typically studied. This may explain why TL served as a predictor in
this elderly sample but not in other elderly samples. Further, selection bias
for healthy elderly men in the present study may in part account for why
cross-sectional TL in men did not predict mortality in men, as it did in
women. Men who are very healthy at 70 to 80 years old (as in this study) are
likely even more highly selected than the women given the higher mortality
rates for men [37]. Thus they may be more selected for having some underlying
resiliency toward age-related diseases than would be true for women.
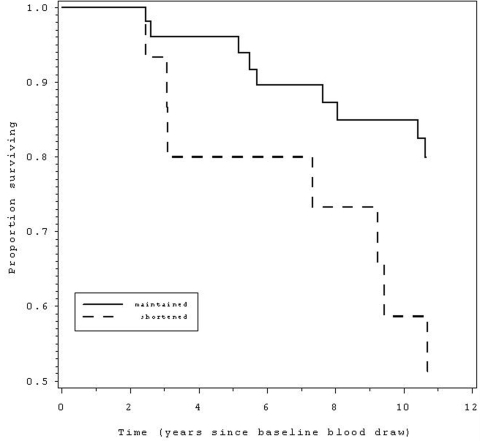
Figure 2.
Those with telomere shortening over a 2.5 year period (dashed line) had 3.0 times
greater likelihood ofmortality over the 12 years since the baseline blood draw,
compared to those without telomere shortening (solid line).
The mechanism by which peripheral blood leukocyte telomere
shortening may be linked to mortality is not yet understood. Leukocyte
telomere length may serve as a proxy for whole body aging or cardiovascular
aging, because leukocyte telomere length has been correlated with telomere
length in other tissues [14,38]. Leukocyte telomere length may also serve as a proxy for
biochemical stress, such as oxidative stress and inflammation, known to both
shorten telomeres and contribute to CVD [39]. Both alcohol excess and obesity are thought to create a milieu
of oxidative stress [40,41]. Indeed, among women in the current study, greater obesity and
alcohol use and obesity at baseline were related to greater telomere attrition
rate. The finding with obesity is consistent with other studies which have
found concurrent cross-sectional relationships between BMI and TL [13]. Alcohol use has not been previously linked to telomere length.
Lastly, leukocyte telomere shortening has important functional
consequences that may contribute directly to the pathogenesis of CVD. Short
telomeres lead to cessation of cell division and can elicit cell death and, in
the absence of fully functional cellular damage checkpoints, can lead to
genomic instability via end-to-end chromosome fusions [5,6,31]. When telomeres become critically shortened in leukocytes, these
cells become senescent and secrete pro-inflammatory cytokines [42,43]. Thus, a senescent immune system can contribute to a
pro-inflammatory milieu, and senescent macrophages can contribute directly to
atherosclerotic plaques [32].
While such mechanistic pathways are yet to be elucidated, our
findings suggest that telomere rate of change may be an important predictor of
human longevity. Rate of attrition is informative in that it may reflect
genetic, biological, and lifestyle (behavioral) factors. This study suggests
the possibility that telomere length changes over the short term might be a
clinically useful measure of health status and risk. However, this study is
limited in that we cannot infer causality, and the findings need replication
from larger samples before TLC is considered a validated predictor of
mortality.
Table 3. Hazards Ratios and 95% CI for telomere predictors for CVD mortality, adjusted for age.
*p < .05 difference within sex groups
| | MEN | WOMEN |
| Baseline TL |
Short
|
1.2 (0.6-2.6)
| 2.3
(1.0-5.3)* |
|
Long
|
Reference
|
Reference
|
| TL Change |
Shortened
| 3.0 (1.1-8.2)* |
1.0 (0.3-3.7)
|
|
Maintained/+
|
Reference
|
Reference
|
Materials and Methods
Participants and procedures.
Participants were men and women, from a population-based
sample in East Boston (one site of the three-site MacArthur Study of Successful
Aging). Subjects were selected from within a larger population-based
NIA-funded cohort study by a score in the upper tertile on six markers of
mental and physical health, as described in detail in Berkman, Seeman et al, 1992, and all provided written informed
consent. This study received approval from an institutional review board at
each site and was conducted in accordance with the 1964 Declaration of Helsinki (see The World Medical Association: The
Declaration of Helsinki www.wma.net/e/policy/b3ht) and International
Conference on Harmonization/Good Clinical Practice guidelines. Written informed
consent was obtained from each patient before participation.
Though they had good cognitive and physical function, they could
have chronic disease. Response rates were over 90%. Men (N=116) and women
(n=120) were randomly sampled from among the approximately 800 with available
baseline (1988) and follow-up (1991) DNA. Blood was drawn at various times of
day on the baseline visit, and on a follow-up visit for 134 participants, 2.5
years later (1991), including 66 men and 68 women. Buffy coat was stored at
-800C. DNA was later extracted at the UCLA General Clinical Research Center (Los Angeles), frozen, and shipped to Dr. Cawthon's laboratory (University of Utah) for TL assays (see below).
Statistical analyses.
We first examined the relation between TL and TLC variables to
see which measure of TLC was most independent of baseline TL. Spearman
correlation was performed between TL and %TLC (rho = -.71, p < .0001). The
correlation between TL and raw TLC (rather than % change) was similarly large (rho
= -.66, p < .0001): Those with longer TL at baseline showed
greater rate of change (shortening), and conversely, those with shorter TL at
baseline showed slower rate of change (less shortening or more lengthening). In
light of this, we chose percent change (vs. a raw change score) as the measure
of change independent of baseline length, because it adjusts for baseline TL,
and hence percent of telomere length change (%TLC) was used in all analyses.
TL and %TLC were used as continuous variables. To examine
correlates of TL and %TLC, Spearman rank order correlations were used. To
examine whether TL and %TLC were predictors of mortality, Cox Proportional
Hazards analyses were used, using survival time calculated in days.
TL and %TLC
were also exa-mined as categorical variables - baseline TL (short
or long, based on a median split) and shortening over time (yes or no, with
shortening reflecting the lowest (most negative) 25%ile of change. Lastly,
given known gender differences in TL and mortality, all analyses were done
across the group and also by gender.
Telomere length assay.
Blood was drawn in subject's home and processed soon after.
Buffy coat was stored at -800C. DNA was extracted by the UCLA GCRC,
with Gentra system kits. Telomere length was measured from DNA using a
PCR-based assay as follows: Two quantitative PCR reactions are performed in
separate reactions, consisting of an amplification of telomere sequence (T),
and of a specific single copy gene in the genome (S). The ratio of T/S
corresponds to telomere length, when multiplied by a standardization factor. The
conversion factor from t/s to base pairs (bp) for this study is 4270. All
assays are performed in duplicate, have high reliability and validity [44], and have been used in multiple prior studies [21,24].
Telomere length, as measured by T/S ratio, was normally
distributed, with a skew of 0.33 and kurtosis 0.14. TLC was calculated as both
raw change (TL2 minus TL1), and a percent change ((TL2-TL1)*100)/TL1, and
categorically into TL change groups, as described below. We excluded one
subject with a change in telomere length greater than four standard deviations
from the mean, making the final sample size 235.
Mortality.
Twelve-year
mortality data (from 1988 to 2000) were obtained from a National Death Index
search that provided date and cause of death information based on death
certificate data. Data on overall mortality and CVD mortality was used, as
described in Results below.
Health behaviors and indices.
Smoking was
measured by pack-years (average reported packs/day * years smoked). Alcohol
consumption was measured based on participants' reports of how much beer, wine
and hard liquor they usually consume per month, and then converted to an
average monthly quantity of ethyl alcohol consumed. Physical activity was
measured as the sum of reported participation in strenuous work activities and
strenuous recreational activities. The detailed measures are described in
Seeman et al, 1995 [45]. Height and weight were based on self report. BMI was
calculated as weight/height2 in kg/m2. Blood pressure
was measured as the average of two resting, seated assessments. Presence of
diabetes was determined by self report.
Acknowledgments
The study was supported by the
USC/UCLA Center on Biodemography and Population Health (CBPH), NIH grant
5P30AG017265-099002, and the John D. and Catherine T.
MacArthur Foundation Network on Socioeconomic Status and Health.
Conflicts of Interest
No authors had financial and personal relationships that could bias this work.
References
-
1.
Blackburn
EH
Telomeres and telomerase: their mechanisms of action and the effects of altering their functions.
FEBS Lett.
2005;
579:
859
-862.
[PubMed]
.
-
2.
Chan
SW
and Blackburn
EH.
Telomerase and ATM/Tel1p protect telomeres from nonhomologous end joining.
Mol Cell.
2003;
11:
1379
-1387.
[PubMed]
.
-
3.
Weng
NP
, Granger
L
and Hodes
RJ.
Telomere lengthening and telomerase activation during human B cell differentiation.
Proc Natl Acad Sci U S A.
1997;
94:
10827
-10832.
[PubMed]
.
-
4.
Erusalimsky
JD
and Kurz
DJ.
Cellular senescence in vivo: its relevance in ageing and cardiovascular disease.
Exp Gerontol.
2005;
40:
634
-642.
[PubMed]
.
-
5.
Edo
MD
and Andres
V.
Aging, telomeres, and atherosclerosis.
Cardiovasc Res.
2005;
213
-221.
[PubMed]
.
-
6.
Allsopp
RC
, Vaziri
H
and Patterson
C.
Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A.
Nov 1.
1992;
89:
10114
-10118.
.
-
7.
Blasco
MA
Telomeres and human disease: ageing, cancer and beyond.
Nat Rev Genet.
2005;
6:
611
-622.
[PubMed]
.
-
8.
Chang
S
, Multani
AS
, Cabrera
NG
, Naylor
M
, Laud
P
, Lombard
D
, Pathak
S
, Guarente
L
and DePinho
R.
Essential role of limiting telomeres in the pathogenesis of Werner syndrome.
Nat Genet.
2004;
36:
877
-882.
[PubMed]
.
-
9.
Jeanclos
E
, Schork
NJ
, Kyvik
KO
, Kimura
M
, Skurnick
JH
and Aviv
A.
Telomere length inversely correlates with pulse pressure and is highly familial.
Hypertension.
2000;
36:
195
-200.
[PubMed]
.
-
10.
Nawrot
TS
, Staessen
JA
, Gardner
JP
and Aviv
A.
Telomere length and possible link to X chromosome.
Lancet.
2004;
363:
507
-510.
[PubMed]
.
-
11.
Benetos
A
, Okuda
K
, Lajemi
M
, Kimura
M
, Thomas
F
, Skurnick
J
, Labat
C
, Bean
K
and Aviv
A.
Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity.
Hypertension.
2001;
37:
381
-385.
.
-
12.
Gardner
JP
, Li
S
, Srinivasan
S
, Chen
W
, Kimura
M
, Lu
X
, Berenson
G
and Aviv
A.
Rise in insulin resistance is associated with escalated telomere attrition.
Circulation.
2005;
111:
2171
-2177.
[PubMed]
.
-
13.
Valdes
AM
, Andrew
T
, Gardner
J
, Kimura
M
, Oelsner
E
, Cherkas
L
, Aviv
A
and Spector
T.
Obesity, cigarette smoking, and telomere length in women.
Lancet.
2005;
366:
662
-664.
[PubMed]
.
-
14.
von
Zglinicki T
, Serra
V
, Lorenz
M
, Saretzki
G
, Lenzen-Grossimlighaus
R
, Gessner
R
, Risch
A
and Steinhagen-Thiessen
E.
Short telomeres in patients with vascular dementia: an indicator of low antioxidative capacity and a possible risk factor.
Lab Invest.
2000;
80:
1739
-1747.
[PubMed]
.
-
15.
Aviv
A
, Valdes
A
, Gardner
JP
, Swaminathan
R
, Kimura
M
and Spector
TD.
Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation.
J Clin Endocrinol Metab.
2006;
91:
635
-640.
[PubMed]
.
-
16.
Sampson
MJ
, Winterbone
MS
, Hughes
JC
, Dozio
N
and Hughes
DA.
Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes.
Diabetes Care.
2006;
29:
283
-289.
[PubMed]
.
-
17.
Samani
N
, Boultby
R
, Butler
R
, Thompson
J
and Goodall
AH.
Telomere shortening in atherosclerosis.
The Lancet.
2001;
358:
472
-473.
.
-
18.
Brouilette
S
, Singh
RK
, Thompson
JR
, Goodall
AH
and Samani
NJ.
White cell telomere length and risk of premature myocardial infarction.
Arterioscler Thromb Vasc Biol.
2003;
23:
842
-846.
[PubMed]
.
-
19.
Kurz
DJ
, Kloeckener-Gruissem
B
, Akhmedov
A
, Akhmedov
A
, Eberli
F
, Buhler
I
, Berger
W
, Bertel
O
and Luscher
T.
Degenerative Aortic Valve Stenosis, but not Coronary Disease, Is Associated With Shorter Telomere Length in the Elderly.
Arterioscler Thromb Vasc Biol.
2006;
26:
e114
[PubMed]
.
-
20.
Fitzpatrick
AL
, Kronmal
RA
, Gardner
JP
, Psaty
BM
, Jenny
NS
, Tracy
RP
, Walston
J
, Kimura
M
and Aviv
A.
Leukocyte telomere length and cardiovascular disease in the cardiovascular health study.
Am J Epidemiol.
2006;
165:
14
-21.
[PubMed]
.
-
21.
Cawthon
R
, Smith
K
, O'Brien
E
, Sivatchenko
A
and Kerber
R.
Association between telomere length in blood and mortality in people aged 60 years or older.
Lancet.
2003;
361:
393
-395.
[PubMed]
.
-
22.
Bakaysa
SL
, Mucci
LA
, Slagboom
PE
, Boomsma
DI
, McClearn
GE
, Johansson
B
and Pedersen
NL.
Telomere length predicts survival independent of genetic influences.
Aging Cell.
2007;
6:
769
-774.
[PubMed]
.
-
23.
Kimura
M
, Hjelmborg
JV
, Gardner
JP
, Bathum
L
, Brimacombe
M
, Lu
X
, Christiansen
L
, Vaupel
JW
, Aviv
A
and Christensen
K.
Telomere length and mortality: a study of leukocytes in elderly Danish twins.
Am J Epidemiol.
2008;
167:
799
-806.
[PubMed]
.
-
24.
Honig
LS
, Schupf
N
, Lee
JH
, Tang
MX
and Mayeux
R.
Shorter telomeres are associated with mortality in those with APOE epsilon4 and dementia.
Ann Neurol.
2006;
60:
181
-187.
[PubMed]
.
-
25.
Martin-Ruiz
C
, Dickinson
HO
, Keys
B
, Rowan
E
, Kenny
RA
and Von
Zglinicki T.
Telomere length predicts poststroke mortality, dementia, and cognitive decline.
Ann Neurol.
2006;
60:
174
-180.
[PubMed]
.
-
26.
Bischoff
C
, Petersen
HC
, Graakjaer
J
, Andersen-Ranberg
K
, Vaupel
JW
, Bohr
VA
, Kolvraa
S
and Christensen
K.
No association between telomere length and survival among the elderly and oldest old.
Epidemiology.
2006;
17:
190
-194.
[PubMed]
.
-
27.
Huda
N
, Tanaka
H
, Herbert
BS
, Reed
T
and Gilley
D.
Shared environmental factors associated with telomere length maintenance in elderly male twins.
Aging Cell.
2007;
6:
709
-713.
[PubMed]
.
-
28.
Cherkas
LF
, Hunkin
JL
, Kato
BS
, Richards
J
, Gardner
JP
, Surdulescu
GL
, Kimura
M
, Lu
X
, Spector
TD
and Aviv
A.
The association between physical activity in leasure time and leukocyte telomere length.
Archives of Internal Medicine.
2008;
168:
154
-158.
[PubMed]
.
-
29.
Yu
GL
, Bradley
JD
, Attardi
LD
and Blackburn
EH.
In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs.
Nature.
1990;
344:
126
-132.
[PubMed]
.
-
30.
Blackburn
EH
Structure and function of telomeres.
Nature.
1991;
350:
569
-573.
[PubMed]
.
-
31.
Blackburn
EH
Telomere states and cell fates.
Nature.
2000;
408:
53
-56.
[PubMed]
.
-
32.
Serrano
AL
and Andres
V.
Telomeres and cardiovascular disease: does size matter.
Circ Res.
2004;
94:
575
-584.
[PubMed]
.
-
33.
Epel
ES
, Lin
J
, Wilhelm
FH
, Wolkowitz
OM
, Cawthon
R
, Adler
NE
, Dolbier
C
, Mendes
WB
and Blackburn
EH.
Cell aging in relation to stress arousal and cardiovascular disease risk factors.
Psychoneuroendocrinology.
2006;
31:
277
-287.
[PubMed]
.
-
34.
Chan
SW
, Blackburn
EH
, Chan
S
, Blackburn
EH
, Chan
S
, Chang
J
, Fulton
TB
, Krauskopf
A
, McEachern
M
, Prescott
J
, Roy
J
, Smith
C
and Wang
H.
Telomerase and ATM/Tel1p protect telomeres from nonhomologous end joining: Molecular manifestations and molecular determinants of telomere capping.
Mol Cell.
2003;
11:
1379
-1387.
[PubMed]
.
-
35.
Harris
SE
, Deary
IJ
, MacIntyre
A
, Deary
I
, MacIntyre
A
, Lamb
K
, Radhakrishnan
K
, Starr
JM
, Whalley
LJ
and Shiels
PG.
The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people.
Neurosci Lett.
2006;
406:
260
-264.
[PubMed]
.
-
36.
Martin-Ruiz
CM
, Gussekloo
J
, van
Heemst D
, von
Zglinicki T
and Westendorp
RG.
Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study.
Aging Cell.
2005;
4:
287
-290.
[PubMed]
.
-
37.
Barford
A
, Dorling
D
, Davey
Smith G
and Shaw
M.
Life expectancy: women now on top everywhere.
British Medical Journal.
2006;
332:
808
[PubMed]
.
-
38.
Friedrich
U
, Griese
E
, Schwab
M
, Fritz
P
, Thon
K
and Klotz
U.
Telomere length in different tissues of elderly patients.
Mech Ageing Dev.
2000;
119:
89
-99.
[PubMed]
.
-
39.
Aviv
A
Telomeres, sex, reactive oxygen species, and human cardiovascular aging.
J Mol Med.
2002;
80:
689
-695.
[PubMed]
.
-
40.
Albano
E
Alcohol, oxidative stress and free radical damage.
Proc Nutr Soc.
2006;
65:
278
-290.
[PubMed]
.
-
41.
de Ferranti
S
and Mozaffarian
D.
The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences.
Clin Chem.
2008;
54:
945
-955.
[PubMed]
.
-
42.
Effros
RB
, Dagarag
M
, Spaulding
C
and Man
J.
The role of CD8+ T-cell replicative senescence in human aging.
Immunol Rev.
2005;
205:
147
-157.
[PubMed]
.
-
43.
Effros
RB
T cell replicative senescence: pleiotropic effects on human aging.
Ann N Y Acad Sci.
2004;
1019:
123
-126.
[PubMed]
.
-
44.
Cawthon
RM
Telomere measurement by quantitative PCR.
Nucleic Acids Res.
2002;
30:
e47
[PubMed]
.
-
45.
Seeman
TE
, Berkman
LF
, Charpentier
PA
, Blazer
DG
, Albert
MS
and Tinetti
ME.
Behavioral and psychosocial predictors of physical performance: MacArthur studies of successful aging.
J Gerontol A Biol Sci Med Sci.
1995;
50:
M177
-183.
[PubMed]
.