Alterations in gene expression and sensitivity to genotoxic stress following HdmX or Hdm2 knockdown in human tumor cells harboring wild-type p53
Abstract
While half of all human tumors possess p53 mutations, inactivation of wild-type p53 can also occur through a variety of mechanisms that do not involve p53 gene mutation or deletion. Our laboratory has been interested in tumor cells possessing wild-type p53 protein and elevated levels of HdmX and/or Hdm2, two critical negative regulators of p53 function. In this study we utilized RNAi to knockdown HdmX or Hdm2 in MCF7 human breast cancer cells, which harbor wild-type p53 and elevated levels of HdmX and Hdm2 then examined gene expression changes and effects on cell growth. Cell cycle and growth assays confirmed that the loss of either HdmX or Hdm2 led to a significant growth inhibition and G1 cell cycle arrest. Although the removal of overexpressed HdmX/2 appears limited to an anti-proliferative effect in MCF7 cells, the loss of HdmX and/or Hdm2 enhanced cytotoxicity in these same cells exposed to DNA damage. Through the use of Affymetrix GeneChips and subsequent RT-qPCR validations, we uncovered a subset of anti-proliferative p53 target genes activated upon HdmX/2 knockdown. Interestingly, a second set of genes, normally transactivated by E2F1 as cells transverse the G1-S phase boundary, were found repressed in a p21-dependent manner following HdmX/2 knockdown. Taken together, these results provide novel insights into the reactivation of p53 in cells overexpressing HdmX and Hdm2.
Introduction
Only half of all human tumors contain
mutations in the p53 tumor suppressor gene [1], with the
other half retaining wild-type p53 but possessing defects in the expression of
p53 regulatory proteins and pathways. Under non-stress conditions, p53 protein
is maintained at a low basal level by constant ubiquitination and proteasomal
degradation [2]. Upon DNA
damage or various types of cellular stress, p53 is stabilized and functions as
a transcription factor to induce genes involved in cell cycle arrest,
apoptosis, and DNA repair [3]. The
stringent regulation of p53 involves a complex network
of proteins, and is critical for maintaining genomic stability and suppressing
tumor formation.
Hdm2
and its structural homologue HdmX represent two essential negative regulators
of p53 as demonstrated by their embryonic lethality in knockout mice and
subsequent rescue by concurrent elimination of p53 [4]. Hdm2
inactivates p53 function through direct association resulting in an inhibition
of transactivation [5] and, through
its E3 ligase activity targeting p53, by ubiquitin-mediated proteasome
degradation [6,7]. While
HdmX shows conservation in the Hdm2 E3 ligase ring finger domain through which
it can heterodimerize with Hdm2 [8,9], HdmX
lacks the ability to ubiquitinate p53 in vivo [10,11] and
thus can only antagonize p53 transactivation [12]. The
heterodimerization of Hdm2 and HdmX also plays a critical role in the response
to DNA damage enabling Hdm2 to promote the ubiquitination and rapid proteasomal
degradation of HdmX, thereby facilitating the tumor suppressor activity of p53 [13-15]. Thus,
the interactions between p53, Hdm2 and HdmX are critical for complete
regulation of p53 [4].
The
overexpression of either Hdm2 or HdmX can inhibit the activity of p53 and
directly contribute to tumor formation. It is not surprising that either one
or both proteins are found overexpressed in many human tumors and tumor cell
lines which harbor wild-type p53 [16]. Diverse
approaches to activate the wild-type p53 in these tumors include the use of
small molecule antagonists like Nutlin to inhibit the Hdm2-p53 interaction [17-19], and the
use of antisense oligonucleotides, antibodies, and small interfering RNAs
directed at Hdm2 or HdmX [20-23]. Recent
findings suggest that Hdm2 and HdmX are specific independent therapeutic
targets for activating wild-type p53 and that anti-cancer approaches that
target both Hdm2 and HdmX should be considered as a means of treatment for
tumors [16,18,24].
This
study undertook an examination of gene expression alterations and the
biological effects resulting from RNAi silencing of HdmX and Hdm2 in a breast
cancer cell line overexpressing both proteins. Unlike previous studies
examining only the biological effect of either HdmX or Hdm2 loss, this study focuses
on a cell line where both proteins are overexpressed and further compliments
those previous studies with a systematic examination of gene expression changes
following loss of HdmX or Hdm2. Interestingly, only p53 target genes primarily
associated with cell cycle arrest were induced. More striking was the
repression of a large group of E2F-regulated genes upon HdmX/2 knockdown.
Using siRNA approaches targeting p21, we were able to show that these
E2F-regulated genes were repressed through p53 activation of p21. Furthermore,
cell proliferation and colony formation assays confirmed that loss of HdmX or
Hdm2 inhibited tumor cell growth and could sensitize these cells to treatment
with doxorubicin. Taken together, these results suggest that in cells where
both Hdm2 and HdmX are overexpressed, removal of one leads to an
anti-proliferative effect in tumor cells harboring wild-type p53 and induction
of p53 cell cycle arrest genes that negatively feedback onto the E2F pathway.
Results
RNAi
knockdown of Hdm2 and HdmX in MCF7 cells
Given
that HdmX and Hdm2 are overexpressed in approximately 17% of human tumors [16] the
majority of which possess wild-type p53, this study set out to examine how loss
of Hdm2/X affected gene expression and tumor cell growth. MCF7, which possess
wild-type p53 [25] and
elevated levels of both HdmX and Hdm2 (Figure 1A) was the tumor cell line used
in these studies. To inactivate HdmX and Hdm2 we employed siRNA targeting each
gene as described in the materials and methods.
Before
performing the Affymetrix GeneChip experiments we developed a triple
transfection protocol that led to over 90% of the MCF7 cells taking up the
siRNA (data not shown). Next, the effectiveness of the knockdown was
assessed using RT-qPCR (data not shown) and Western blotting. Following the
triple transfection protocol HdmX and p53 protein levels were undetectable with
Hdm2 showing a greater than 80% reduction in protein expression (Figure 1B). As
expected, the loss of either HdmX or Hdm2 led to an increase in the levels of
p21. This p21 increase is p53-dependent since no increase in p21 protein levels
was detected upon concurrent knockdown of HdmX and p53. While it has been
suggested that Hdm2 controls the levels of p53 in non-stressed cells [26,27], in our
hands MCF7 cells showed only a slight increase in p53 protein levels following
the combined loss of HdmX and Hdm2. The inability of Hdm2 knockdown to result
in an increase in p53 protein could be the result of MCF7 cells harboring an
elevated level of HdmX. Consistent with this suggestion, the treatment of MCF7
cells with Nutlin leads to increased p53 protein levels through loss of Hdm2
binding to p53 and concurrent Hdm2 mediated degradation of HdmX [28].
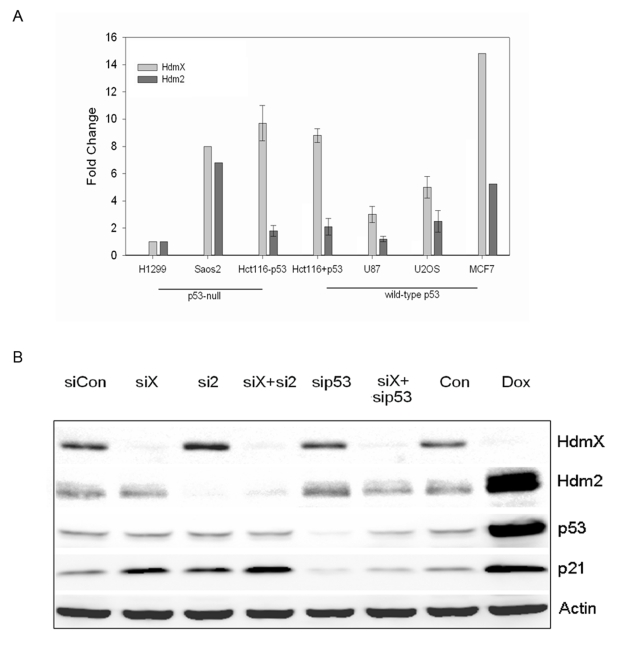
Figure 1. (A) RT-PCR analysis of hdmX and hdm2 gene expression in various human cell lines. The endogenous levels of hdmX and hdm2 were determined relative to H1299 cells. All samples were
normalized to GAPDH. (B) RNAi knockdown of HdmX or Hdm2 triggers
p53-dependent p21 induction. Western blot analysis of indicated proteins
from the various siRNA or doxorubicin (Dox) treated MCF7 cells. Knockdowns
of the indicated proteins were greater than 80%. Protein extracts were made
24 hours after the last siRNA transfection or treatment with 5 μg/ml doxorubicin.
Loss of Hdm2 and HdmX triggers inhibition of cell growth
Other
groups have reported that in cells where wild-type p53 is kept in check by
overexpression of HdmX or Hdm2, their inhibition can trigger alterations in
cell growth [29] and in some
conditions apoptosis [30]. To assess
the growth properties of RNAi knockdown of p53 regulators Hdm2 and HdmX,
siRNA-transfected MCF7 cells were plated at low density in 6 well plates and
allowed to grow for an additional 10 days. While transfection of siCon or
sip53 resulted in only minimal changes in
cell growth (Figure 2B), knockdown of either HdmX or Hdm2, alone or in combination led
to significantly fewer colonies (Figure 2A) and suppressed cell growth when
compared to siCon (Figure 2B). This decrease
in colony formation correlated with an increase in G1 arrest and not apoptosis
(i.e. sub-G1) as determined by flow cytometry (data not shown).
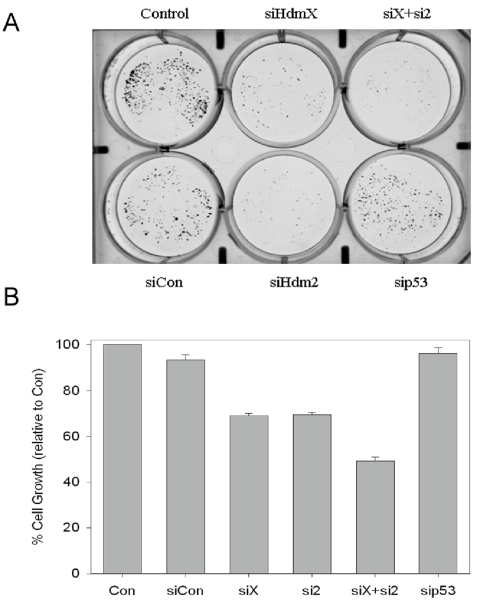
Figure 2. Loss of HdmX and/or Hdm2 inhibits MCF7 colony formation. (A) Following siRNA
transfections, MCF7 cells were seeded at 500 cells/well in 6-well plates.
The cells were allowed to grow for ten days then the colonies were stained
with crystal violet. Significantly fewer colonies were present following
knockdown of HdmX and/or Hdm2. The cells transfected with sip53 or a
non-targeting control (siCon) showed minimal effects on colony formation
relative to non-transfected control (Con/Control). (B) The percent
cell growth relative to untransfected control was determined by extracting
the stain in 10% acetic acid and quantifying the stain by reading
absorbance at 590 nm.
Loss
of HdmX or Hdm2 sensitizes MCF7 cells to DNA damage
Several recent studies using Nutlin and
various DNA damaging agents reported that blocking Mdm2:p53 association led to
increased chemosensitivity to DNA damaging agents [31,32]. To examine
whether knockdown of HdmX and Hdm2 can also elicit increased cytotoxicity to
DNA damage, MCF7 cells were transfected with the indicated siRNA leading to
alterations of gene expression (Figure 3B). Cells were then treated with
varying doses of doxorubicin and cell viability assessed. siRNAs targeting
HdmX or Hdm2 increased doxorubicin cytotoxicity, while removing both HdmX and
Hdm2 led to the greatest level of chemosensitivity (Figure 3A). Enhanced chemo-sensitivity
was also observed in cisplatin treatment of siHdmX or siHdm2 MCF7 cells (data
not shown).
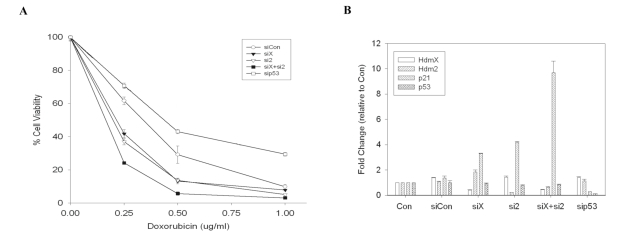
Figure 3. Knockdown of HdmX enhances doxorubicin-induced cytotoxicity. (A) Percent cell viability relative to untransfected untreated
control cells. MCF7 cells were treated with doxorubicin (0.25-1.0
μg/mL) for 48 hours and cell viability was determined by absorbance at
590 nm. The loss of HdmX and/or Hdm2 showed an enhanced cytotoxicity
relative to control cells. (B) RT-qPCR analysis of hdmX, hdm2, p21
and p53 gene expression in the indicated siRNA transfected MCF7 cells. The
hdmX, hdm2, and p53 transcripts were effectively knocked down by siRNA
prior to drug treatment.
Gene
expression profiles of MCF7 cells lacking HdmX or Hdm2
Having
established an effective knockdown approach with effects on cell growth and
increased sensitivity to DNA damage, we performed an Affymetrix GeneChip
experiment to assess how loss of HdmX or Hdm2 affected global gene expression
in MCF7 cells. Each RNAi transfection was performed in three separate bio-logical
replicates. The data analysis was carried out using GeneSpring GX software.
Given the similarity of biological function uncovered in the previous experi-ments
we focused our informatics on genes commonly altered following RNAi treatment
with siHdmX or siHdm2. In summary, cel files were normalized using GCRMA,
genes filtered by ANOVA and fold change, and genes significantly altered by
both siHdmX and siHdm2 but not siHdmX +
sip53 identified (see materials and methods for detailed approach). From this
approach we uncovered 394 gene alterations common to knockdown of both siHdmX
and siHdm2 (Supplementary Table 1).
p53
activation following loss of HdmX or Hdm2 triggers growth repressive genes
The
initial examination of the 394 genes focused on those genes (n=222) that were
increased following siHdmX or siHdm2 treatment relative to siCon. Thirteen
genes were identified that were known p53-regulated genes (Figure 4). As
expected these genes increased with siHdmX or siHdm2 treatment but had
expression levels comparable or lower than siCon when treated with siHdmX+sip53
or sip53. Interestingly, with the exception of Fas, this list of p53 target
genes consisted predominately of genes encoding proteins involved in cell cycle
arrest or DNA repair. Consistent with a model whereby p53 proapoptotic target
genes require p53 that is phosphorylated at serine 46 by HIPK2 [33-35], we
observed no detectable phosphorylation at serines 6, 15, 20, 46, or 392
following the RNAi transfection protocol employed in these studies (data not
shown).
To
confirm these results, we performed RT-qPCR using TaqMan primers targeting five
known p53 target genes, three of which were identified in our analysis. p21,
BTG2 and ACTA2 are p53 target genes that are associated with cell cycle arrest
or growth inhibition [36-38], while
Hdm2 is a negative regulator of p53 and Noxa a pro-apoptotic factor not
observed in our list of altered
genes [39]. MCF7
cells were either mock transfected (Mock), transfected with siRNA that does not
target any human gene (siCon) or transfected with siRNA to HdmX or Hdm2 either
alone or in combination. The results in Figure 5 demonstrate that relative to
siCon, knockdown of HdmX led to significant increases in hdm2, p21, BTG2 and
ACTA2 gene expression. No significant change in gene expression was observed
with Noxa, which is consistent with our GeneChip results. With the obvious
exception of hdm2, siRNA-targeting Hdm2 led to similar alterations in gene
expression (Figure 5). Finally, when both HdmX and Hdm2 were eliminated, the
levels of the cell cycle arrest genes p21, BTG2 and ACTA2 increased either
synergistically or additively while levels of Noxa remained unchanged. These
results validate our GeneChip data that p53-target genes were induced upon HdmX
or Hdm2 knockdown and that several of these genes encode proteins involved in
the cell cycle arrest.
p53
upregulation of p21 leads to global repression of E2F regulated genes
After searching for genes that were
directly upregulated by p53 we next evaluated those genes that were repressed
(N=172) following HdmX and Hdm2 knockdown (Figure 7). Within the list of downregulated
genes were a set of genes that encode proteins involved in G1-S phase
transition, the majority of which were known E2F1 regulated genes. It is known
that p21 can inhibit CDK/cyclins involved in Rb phosphorylation [40] and within
the literature we initially uncovered two reports where p53 activation led to
repression of TERT or Chk2, two known E2F-regulated genes [41,42]. To
determine whether repression of these genes was the result of an HdmX or
Hdm2-dependent p53 activation, MCF7 cells were treated with siHdm2 or siHdmX
alone or in combination with sip21. RNA was isolated and RT-qPCR performed to
monitor relative expression of cyclin A2 (CCNA2), p21 and E2F1. While E2F1 did
not make the 394 gene list, it possesses an E2F1 DNA binding site [43]. Relative
expression for each of the genes was normalized to GAPDH. As expected, loss of
HdmX or Hdm2 led to an increase in p21 and concomitant
decrease in both CCNA2 and E2F1 (Figure 7). In contrast, loss of Hdm2/X and
p21 completely abrogated CCNA2 and E2F1 repression consistent with p53
activation inactivating E2F1 transactivation via p21 induction.
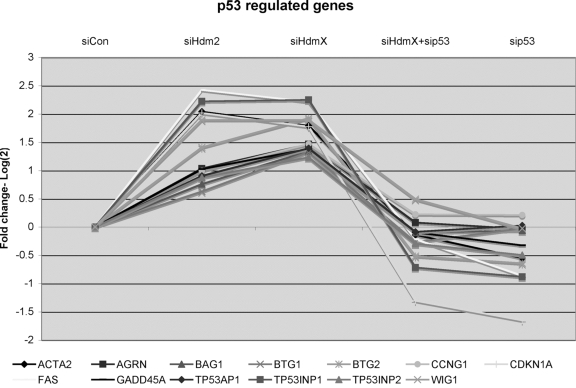
Figure 4. GeneChip expression of 13 known p53-regulated genes that were induced by knockdown of either siHdmX or siHdm2. Y-axis represents the average
fold change (log2) for each of the genes in the indicated siRNA
transfections relative to siCon (X-axis, conditions labeled at the top of
the chart).
Discussion
As
an essential tumor suppressor it is no surprise that human tumors demonstrate a
diverse array of genetic mechanisms to inactivate p53 function. Central to
this present study are tumors where one or both of the negative regulators of
p53, Hdm2 and HdmX, are overexpressed leading to loss of p53 activity. Previous
studies have focused on Hdm2 overexpression, where a small molecule inhibitor
Nutlin 3 has proven to activate wild-type p53 in cell lines with elevated Hdm2,
triggering apoptosis when combined with genotoxic agents that do not function
as anti-mitotics [44].
Unfortunately, Nutlins have not proven as effective in tumors where HdmX is
overexpressed [18,45-47],
suggesting the need for additional approaches aimed at blocking the HdmX:p53
association particularly given the recent observation of HdmX overexpression in
retinoblastoma [48].
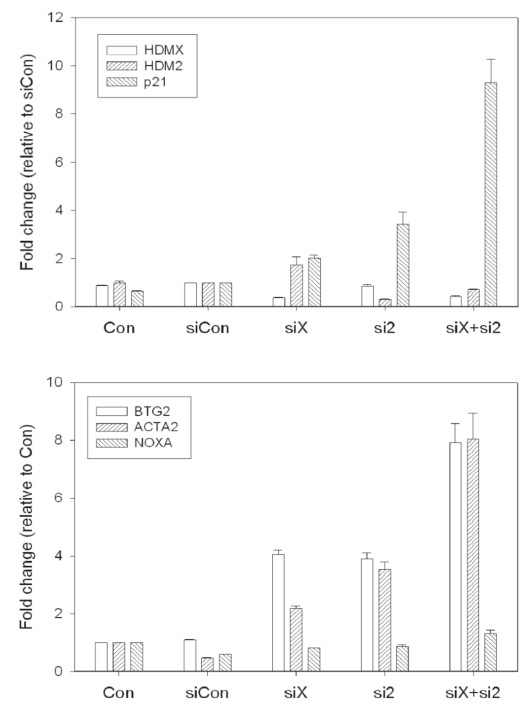
Figure 5. RT-qPCR validation of siRNA knockdown in MCF7 cells. (A). The hdmX, hdm2, and p21 mRNA
expression relative to siCon (non-targeting siRNA) is shown.
The p21 transcript is induced following loss of HdmX or Hdm2, and synergistically induced following
loss of both HdmX and Hdm2.
(B) BTG2, ACTA2, and NOXA mRNA expression relative to untransfected control (Con).
The p53 target genes, BTG2 and ACTA2, are induced by loss of HdmX and/or Hdm2, while the expression
of the proapoptotic gene, NOXA, is not altered.
Here
we have employed RNAi approaches and DNA microarrays to better understand the
activation of p53 in cells overexpressing Hdm2 and HdmX. In MCF7 cells a
growth arrest with no detectable apoptosis was observed following knockdown of
either Hdm2 or HdmX (Figure 2 and data not shown). While loss of either HdmX
or Hdm2 was sufficient to trigger an anti-proliferative effect, the combined
loss of both HdmX and Hdm2 resulted in a more significant growth inhibition.
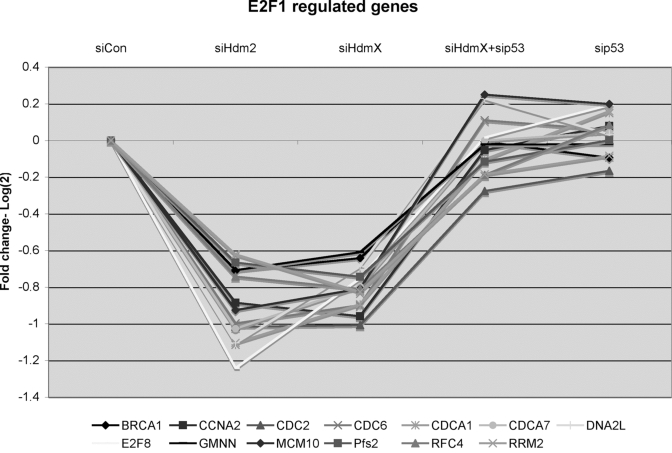
Figure 6. GeneChip expression of 13 reported E2F1-regulated genes that were repressed by knockdown of either siHdmX or siHdm2.
Y-axis represents the average fold change (log2) for each of the
genes in the indicated siRNA transfections relative to siCon (X-axis,
conditions labeled at the top of the chart).
Even
though this RNAi approach appears to activate p53 without triggering its
phosphorylation (data not shown), the loss of either HdmX or Hdm2 did
effectively sensitize the cells to doxorubicin with the loss of both Hdm2 and
HdmX being most sensitive to DNA damage (Figure 3). Surprisingly our results
showed only a modest elevation of endogenous p53 levels following loss of HdmX
and Hdm2 (Figure 1). This result maybe unique to MCF7 cells which harbor
elevated Hdm2 and HdmX, in contrast to most tumor cell lines with wild-type p53
that possessed only elevated Hdm2 (Figure 1A). Consistent with the need for
only one negative regulator to be elevated 65% of retinoblastoma tumors
overexpress HdmX and possess wild-type p53 [48]. Based on
our previous HdmX overexpression studies [10] we would
predict that the overexpression of HdmX might inhibit Hdm2 degradation of p53
in MCF7 cells and thus could explain why modulating Hdm2 levels in MCF7 cells
has no dramatic effect on p53 levels.
The
DNA microarray experiment directly tested whether HdmX or Hdm2 knockdown
triggered an increase in p53-regulated genes. While 394 genes were
significantly altered by either HdmX or Hdm2 knockdown (Supplementary Table 1), only a small
group was previously identified p53 targets (Figure 4). A few of the remaining
genes induced by HdmX or Hdm2 loss are likely novel p53 regulated genes (S.
Berberich, personal communication) but most probably represent downstream
effects of the cell cycle arrest induced by p53. Within the 13 identified p53
target genes it is noteworthy that only one apoptotic gene (Fas) was found
activated by loss of either HdmX or Hdm2. Upon careful examination of 16 known
p53 pro-apoptotic genes we found that several of them were repressed following
p53 knockdown, suggesting that their failure to be induced by loss of HdmX or
Hdm2 was not a cell-type specific phenotype. Rather, we propose that the
non-genotoxic release of p53 from Hdm2 of HdmX results in a preferential
activation of growth arrest target genes, like p21 (Figure 5). This model is
consistent with recent work suggesting that p53 promoter selection is dependent
on its phosphorylation [49].
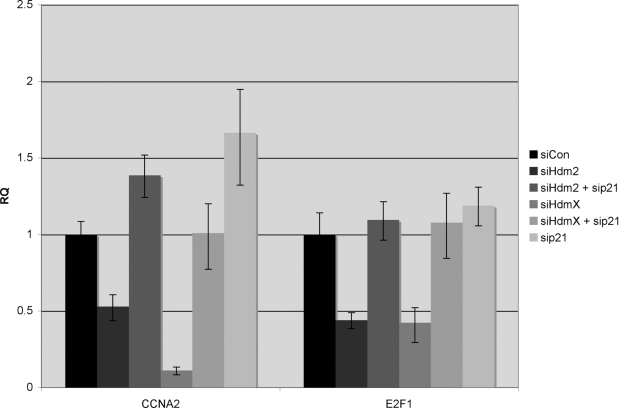
Figure 7. Repression of E2F1-regulated genes by Hdm2 or HdmX knockdown is blocked by concurrent knockdown of p21. MCF7 cells were transfected
with the indicated siRNA combinations. Twenty-four hours later, RNA was
isolated and subjected to RT-qPCR to quantify expression of CCNA2, p21 and
E2F1 after normalization to GAPDH. Expression levels (Y-axis) were
relative to siCon and reported as RQ values. Error bars represent the 95%
confidence interval of the relative expression.
Another interesting finding within the
microarray data was a subgroup of genes that were repressed upon HdmX and Hdm2
knockdown and could be classified as known E2F-regulated genes. Other groups
have noted that p53 activation of p21 could lead to the repression of TERT [42] or Chk2 [41], known
E2F-target genes, and another group recently reported similar findings using
microarray assays [50].
While
this report focused on genes commonly regulated by HdmX and Hdm2, it is worth
mentioning that within genes uniquely regulated by either HdmX or Hdm2 we did
not observe any additional p53 regulated genes (M. Markey, personal communication).
The common biological effects of HdmX or Hdm2-loss and significant overlap of
gene expression patterns are in contrast to recent in vivo studies where the
knockout of Mdm2 or MdmX in adult mouse tissues lead to non-overlapping roles
in regards to regulating p53 activity [51]. We
believe these findings point to either differences in cell culture verses
tissue studies or more likely represent a significant departure in the roles
that Hdm2 and HdmX play when expressed at physiological levels compared to the
elevated levels in tumor cells.
Finally
these studies demonstrate that non-genotoxic activation of p53 by knockdown of
its inhibitors Hdm2 and HdmX leads to the induction of genes involved in
cell-cycle arrest, as well as repression of genes along the E2F/Rb pathway that
promote cell cycle entry. These alterations in gene expression resulted in a decreased
population of proliferative cells without necessarily increasing apoptosis. A
non-genotoxic activation of p53 is one possible mechanism for the reduction in
cellular proliferation observed during aging. This further underscores the
critical importance of tumor suppressor activation in senescence and organismal
aging.
Materials
and methods
Cell
lines, antibodies, siRNA and chemotherapeutic agents.
The human
breast tumor cell line MCF7 was grown in Dulbecco's modified Eagle medium
(DMEM) supplemented with 10% bovine growth serum (BGS), and 10 μg/ml gentamicin
unless otherwise indicated. HdmX polyclonal antibody (Bethyl Laboratories,
Inc.), p21 polyclonal antibody C-19 (Santa Cruz Biotechnology, Inc.), p53
monoclonal antibody Ab-6 (Oncogene), Hdm2 monoclonal antibody SMP-14 (Santa
Cruz Biotechnology, Inc.) and beta-actin monoclonal antibody (Sigma, Inc.) were
used as indicated. A phosphorylation-specific p53 polyclonal antibody kit
(Cell Signaling Technology, Inc.) was utilized per manufacturer's protocol.
Horseradish peroxidase (HRP)-conjugated anti-mouse or anti-rabbit secondary
antibodies (Promega) were used with Super Signal substrate (Pierce) for
chemiluminescence detection of proteins. siGENOME duplex RNA targeting mRNA
from hdmX, hdm2, or p53, and a non-targeting control siRNA were obtained
from Dharmacon Research, Inc. and siRNA transfection was performed using
Oligofectamine or Lipofectamine 2000 (Invitrogen) as described below.
Doxorubicin hydro-chloride (Tocris Bioscience) was prepared as a 5 mg/ml stock
solution in water.
siRNA
transfection
. Cells were seeded at
200,000 cells per well in 6-well plates (for RNA isolation), or at 700,000
cells per 6-cm dish (for protein extraction) in antibiotic free DMEM containing
1% BGS in a small volume. Cells were reverse transfected with 100 nM siRNA
(Dharmacon Research, Inc.) at time of seeding using Lipofectamine 2000
(Invitrogen). After a five hour incubation, the media was removed and cells
were refed with DMEM containing 10% BGS. Twenty hours later, the cells were
transfected again with 100 nM siRNA in a small volume of serum free media using
Oligofectamine (Invitrogen). After a four-hour incubation, an equal volume of
DMEM containing 20% BGS was added to each well or dish without removing the
transfection mixture. Total RNA was isolated 24 hours post siRNA transfection
and protein was extracted at 48 hours post siRNA unless otherwise indicated.
Analysis
of Affymetrix GeneChips
. The
Affymetrix HG-U133 plus 2.0 GeneChips containing probe sets detecting over
54,000 transcripts were used in this study and each transfection condition was
performed in triplicate. GeneChip cel files were imported into GeneSpring GX
and preprocessed by GCRMA. Measurements less than 0.01 were then set to 0.01,
and each chip was normalized to the 50th percentile of the measurements taken
from that chip. Extra background correction was never applied. Each gene was
normalized to the median of the measurements for that gene, and then to the
median of that gene's expression in the siCon condition.
Initially
all genes were filtered in GeneSpring GX first by Welch ANOVA to find
expression changes based on siRNA treatment, using a p-value cut off of 0.05
and the Benjamini and Hochberg False Discovery Rate as a multiple testing
correction. The cross-gene error model was active and based on replicates.
From this list, genes were removed which varied between the mock and siCon
treatments by 1.5 fold with a p-value < 0.05. Next, lists of genes with
expression changes of 1.5 fold and a p-value < 0.05 were then made for
siHdm2 versus siCon and siHdmX versus siCon. We then eliminated all but the
union between these two lists. One gene that was repressed in the siHdm2
condition but upregulated in the siHdmX condition (encoding hypothetical
protein MGC5370) was manually removed. Finally, genes that were not changed
1.5 fold with a p-value of <0.05 between the siHdmX and siHdmX + sip53
conditions were removed leaving a total of 394 selected genes.
Quantitative
RT-pPCR
. Cells were lysed directly in
the culture dish and total RNA was isolated using the RNeasy kit (Qiagen)
according to manufacturer's protocol. The RNA was quantified by spectrophoto-meter
reading at 260 nm, and 1 μg RNA was reverse transcribed with random hexamers to
create cDNA using the TaqMan Reverse transcription kit (Applied Biosystems).
Quantitative PCR was performed in a 96-well micro titer plate format on an ABI
Prism 7900HT sequence detection system using 1 μl cDNA, TaqMan Universal PCR
master mix and Assay-on-Demand Gene Expression products (Applied Biosystems)
specific for genes of interest. Each cDNA sample was analyzed in triplicate
and fold change relative to control was calculated based on a PCR efficiency of
two and normalized to GAPDH (endogenous control) RNA levels. Average fold
change and standard deviation were obtained from 2-3 biological replicate
samples per treatment assayed in triplicate.
Western
blot analysis
. Frozen cells were
lysed in an aqueous extraction buffer composed of 120 mM NaCl, 50 mM Tris-HCl
(pH 8.0), 5 mM EGTA, 1 mM EDTA, 5 mM NaPPi, 10 mM NaF, 30 mM
para-nitrophenylphosphate, 1 mM Benzamidine, 0.1% NP-40 (Ipegal Ca-630), 0.2 mM
PMSF, and 1% protease inhibitor cocktail (Sigma), and soluble protein was
recovered by centrifugation. Protein concentration was determined using
Bradford reagent (Bio-Rad), and proteins were resolved on a sodium dodecyl
sulfate-10% polyacrylamide gel followed by transfer of proteins to a
polyvinylidene difluoride membrane (Millipore) using a Transblot system
(Bio-Rad). Immunoblotting was performed as previously described [52] using
appropriate primary antibodies at 1:1000-1:10,000 dilution and secondary
antibodies (goat anti-mouse or goat anti-rabbit HRP-conjugated, Promega) at
1:5000-1:10,000 dilution. Blots were exposed to chemiluminescent reagent
(Pierce) and protein was visualized on a FUJIFILM LAS-3000 image reader.
Colony
formation and cell viability assays
. Twenty-four
hours after the second siRNA transfection, the cells were trypsinized, counted
and seeded at 500 cells per well in 6-well plates for the colony formation
assay. The cells were allowed to grow for ten days, and then the colonies were
fixed and stained in 1% crystal violet in 70% methanol. The cell viability
assays were performed in 96-well plates using either CellQuanti-Blue™ Reagent
(BioAssay Systems) according to manufacturer's protocol or by staining the
cells with crystal violet, extracting the stain in 10% acetic acid, and then
reading absorbance at 590 nm. Again, cells were trypsinized after the second
siRNA transfection, counted and seeded at 20,000 cells per well. Cell
viability was determined at various time points post-seeding or following
treatment with chemotherapeutic agents for the times indicated.
Acknowledgments
This
work was funded by the National Cancer Institute (CA66430 to SJB). The
Biomedical Sciences Ph.D. program and NIH supported KAH. MM was supported by
NIH and the Center for Genomics Research. DNA microarray facilities and
bioinfomatic programs were provided by the Center for Genomics Research.
Conflicts of Interest
The authors of this manuscript have no conflict of interests to declare.
References
-
1.
Hollstein
M
, Rice
K
, Greenblatt
MS
, Soussi
T
, Fuchs
R
, Sorlie
T
, Hovig
E
, Smith-Sorensen
B
, Montesano
R
and Harris
CC.
Database of p53 gene somatic mutations in human tumors and cell lines.
Nucleic Acids Res.
1994;
22:
3551
-3555.
[PubMed]
.
-
2.
Kubbutat
MH
and Vousden
KH.
Keeping an old friend under control: regulation of p53 stability.
Mol Med Today.
1998;
4:
250
-256.
[PubMed]
.
-
3.
Vousden
KH
and X
Lu.
Live or let die: the cell's response to p53.
Nat Rev Cancer.
2002;
2:
594
-604.
[PubMed]
.
-
4.
Marine
JC
and Jochemsen
AG.
Mdmx and Mdm2: brothers in arms.
Cell Cycle.
2004;
3:
900
-904.
[PubMed]
.
-
5.
Oliner
JD
, Peitenol
JA
, Thiagalingam
S
, Gyuris
J
, Kinzler
KW
and Vogelstein
B.
Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53.
Nature.
1993;
362:
857
-860.
[PubMed]
.
-
6.
Haupt
Y
, Maya
R
and Oren
M.
Mdm2 promotes the rapid degradation of p53.
Nature.
1997;
387:
296
[PubMed]
.
-
7.
Kubbutat
MHG
, Jones
SN
and Vousden
KH.
Regulation of p53 stability by Mdm2.
Nature.
1997;
387:
299
-303.
[PubMed]
.
-
8.
Sharp
DA
, Kratowicz
SA
, Sank
MJ
and George
DL.
Stabilization of the MDM2 Oncoprotein by Interaction with the Structurally Related MDMX Protein.
J Biol Chem.
1999;
274:
38189
-38196.
[PubMed]
.
-
9.
Tanimura
S
, Ohtsuka
S
, Mitsui
K
, Shirouzu
K
, Yoshimura
A
and Ohtsubo
M.
MDM2 interacts with MDMX through their RING finger domains [In Process Citation].
FEBS Lett.
1999;
447:
5
-9.
[PubMed]
.
-
10.
Jackson
MW
and Berberich
SJ.
MdmX protects p53 from Mdm2-mediated degradation.
Mol Cell Biol.
2000;
20:
1001
-1007.
[PubMed]
.
-
11.
Stad
R
, Little
NA
, Xirodimas
DP
, Frenk
R
, van der Eb
AJ
, Lane
DP
, Saville
MK
and Jochemsen
AG.
Mdmx stabilizes p53 and Mdm2 via two distinct mechanisms.
EMBO Rep.
2001;
2:
1029
-1034.
[PubMed]
.
-
12.
Shvarts
A
, Steegenga
WT
, Riteco
N
, van
Laar T
, Dekker
P
, Bazuine
M
, van
Ham RC
, van der Houven
van Oordt W
, Hateboer
G
, van der Eb
AJ
and Jochemsen
AG.
MDMX: a novel p53-binding protein with some functional properties of MDM2.
The EMBO Journal.
1996;
15:
5349
-5357.
[PubMed]
.
-
13.
de Graaf
P
, Little
NA
, Ramos
YF
, Meulmeester
E
, Letteboer
SJ
and Jochemsen
AG.
Hdmx protein stability is regulated by the ubiquitin ligase activity of Mdm2.
J Biol Chem.
2003;
278:
38315
-38324.
[PubMed]
.
-
14.
Kawai
H
, Wiederschain
D
, Kitao
H
, Stuart
J
, Tsai
KK
and Yuan
ZM.
DNA damage-induced MDMX degradation is mediated by MDM2.
J Biol Chem.
2003;
278:
45946
-45953.
[PubMed]
.
-
15.
Pan
Y
and Chen
J.
MDM2 promotes ubiquitination and degradation of MDMX.
Mol Cell Biol.
2003;
23:
5113
-5121.
[PubMed]
.
-
16.
Toledo
F
and Wahl
GM.
Regulating the p53 pathway: in vitro hypotheses, in vivo veritas.
Nat Rev Cancer.
2006;
6:
909
-923.
[PubMed]
.
-
17.
Kojima
K
, Konopleva
M
, Samudio
IJ
, Shikami
M
, Cabreira-Hansen
M
, McQueen
T
, Ruvolo
V
, Tsao
T
, Zeng
Z
, Vassilev
LT
and Andreeff
M.
MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy.
Blood.
2005;
106:
3150
-3159.
[PubMed]
.
-
18.
Patton
JT
, Mayo
LD
, Singhi
AD
, Gudkov
AV
, Stark
GR
and Jackson
MW.
Levels of HdmX expression dictate the sensitivity of normal and transformed cells to Nutlin-3.
Cancer Res.
2006;
66:
3169
-3176.
[PubMed]
.
-
19.
Vassilev
LT
Small-Molecule Antagonists of p53-MDM2 Binding: Research Tools and Potential Therapeutics.
Cell Cycle.
2004;
3:
419
-421.
[PubMed]
.
-
20.
Chene
P
Inhibiting the p53-MDM2 interaction: an important target for cancer therapy.
Nat Rev Cancer.
2003;
3:
102
-109.
[PubMed]
.
-
21.
Linares
LK
and Scheffner
M.
The ubiquitin-protein ligase activity of Hdm2 is inhibited by nucleic acids.
FEBS Lett.
2003;
554:
73
-76.
[PubMed]
.
-
22.
Yu
Y
, Sun
P
, Sun
LC
, Liu
GY
, Chen
GH
, Shang
LH
, Wu
HB
, Hu
J
, Li
Y
, Mao
YL
, Sui
GJ
and Sun
XW.
Downregulation of MDM2 expression by RNAi inhibits LoVo human colorectal adenocarcinoma cells growth and the treatment of LoVo cells with mdm2siRNA3 enhances the sensitivity to cisplatin.
Biochem Biophys Res Commun.
2006;
339:
71
-78.
[PubMed]
.
-
23.
Zhang
R
, Wang
H
and Agrawal
S.
Novel antisense anti-MDM2 mixed-backbone oligonucleotides: proof of principle, in vitro and in vivo activities, and mechanisms.
Curr Cancer Drug Targets.
2005;
5:
43
-49.
[PubMed]
.
-
24.
Hu
B
, Gilkes
DM
and Chen
J.
Efficient p53 activation and apoptosis by simultaneous disruption of binding to MDM2 and MDMX.
Cancer Res.
2007;
67:
8810
-8817.
[PubMed]
.
-
25.
Ramos
YF
, Stad
R
, Attema
J
, Peltenburg
LT
, van der Eb
AJ
and Jochemsen
AG.
Aberrant expression of HDMX proteins in tumor cells correlates with wild-type p53.
Cancer Res.
2001;
61:
1839
-1842.
[PubMed]
.
-
26.
Fuchs
SY
, Adler
V
, Buschmann
T
, Wu
X
and Ronai
Z.
Mdm2 association with p53 targets its ubiquitination.
Oncogene.
1998;
17:
2543
-2547.
[PubMed]
.
-
27.
Little
NA
and Jochemsen
AG.
Hdmx and Mdm2 can repress transcription activation by p53 but not by p63.
Oncogene.
2001;
20:
4576
-4580.
[PubMed]
.
-
28.
Xia
M
, Knezevic
D
, Tovar
C
, Huang
B
, Heimbrook
DC
and Vassilev
LT.
Elevated MDM2 boosts the apoptotic activity of p53-MDM2 binding inhibitors by facilitating MDMX degradation.
Cell Cycle.
2008;
7:
1604
-1612.
[PubMed]
.
-
29.
Efeyan
A
, Ortega-Molina
A
, Velasco-Miguel
S
, Herranz
D
, Vassilev
LT
and Serrano
M.
Induction of p53-dependent senescence by the MDM2 antagonist nutlin-3a in mouse cells of fibroblast origin.
Cancer Res.
2007;
67:
7350
-7357.
[PubMed]
.
-
30.
Vassilev
LT
, Vu
BT
, Graves
B
, Carvajal
D
, Podlaski
F
, Filipovic
Z
, Kong
N
, Kammlott
U
, Lukacs
C
, Klein
C
, Fotouhi
N
and Liu
EA.
In vivo activation of the p53 pathway by small-molecule antagonists of MDM2.
Science.
2004;
303:
844
-848.
[PubMed]
.
-
31.
Barbieri
E
, Mehta
P
, Chen
Z
, Zhang
L
, Slack
A
, Berg
S
and Shohet
JM.
MDM2 inhibition sensitizes neuroblastoma to chemotherapy-induced apoptotic cell death.
Mol Cancer Ther.
2006;
5:
2358
-2365.
[PubMed]
.
-
32.
Coll-Mulet
L
, Iglesias-Serret
D
, Santidrian
AF
, Cosialls
AM
, de Frias
M
, Castano
E
, Campas
C
, Barragan
M
, de Sevilla
AF
, Domingo
A
, Vassilev
LT
, Pons
G
and Gil
J.
MDM2 antagonists activate p53 and synergize with genotoxic drugs in B-cell chronic lymphocytic leukemia cells.
Blood.
2006;
107:
4109
-4114.
[PubMed]
.
-
33.
D'Orazi
G
, Cecchinelli
B
, Bruno
T
, Manni
I
, Higashimoto
Y
, Saito
S
, Gostissa
M
, Coen
S
, Marchetti
A
, Del Sal
G
, Piaggio
G
, Fanciulli
M
, Appella
E
and Soddu
S.
Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis.
Nat Cell Biol.
2002;
4:
11
-19.
[PubMed]
.
-
34.
Hofmann
TG
, Moller
A
, Sirma
H
, Zentgraf
H
, Taya
Y
, Droge
W
, Will
H
and Schmitz
ML.
Regulation of p53 activity by its interaction with homeodomain-interacting protein kinase-2.
Nat Cell Biol.
2002;
4:
1
-10.
[PubMed]
.
-
35.
Oda
K
, Arakawa
H
, Tanaka
T
, Matsuda
K
, Tanikawa
C
, Mori
T
, Nishimori
H
, Tamai
K
, Tokino
T
, Nakamura
Y
and Taya
Y.
p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53.
Cell.
2000;
102:
849
-862.
[PubMed]
.
-
36.
el-Deiry
WS
Regulation of p53 downstream genes.
Semin Cancer Biol.
1998;
8:
345
-357.
[PubMed]
.
-
37.
Cui
XS
and Donehower
LA.
Differential gene expression in mouse mammary adenocarcinomas in the presence and absence of wild type p53.
Oncogene.
2000;
19:
5988
-5996.
[PubMed]
.
-
38.
Boiko
AD
, Porteous
S
, Razorenova
OV
, Krivokrysenko
VI
, Williams
BR
and Gudkov
AV.
A systematic search for downstream mediators of tumor suppressor function of p53 reveals a major role of BTG2 in suppression of Ras-induced transformation.
Genes Dev.
2006;
20:
236
-252.
[PubMed]
.
-
39.
Oda
E
, Ohki
R
, Murasawa
H
, Nemoto
J
, Shibue
T
, Yamashita
T
, Tokino
T
, Taniguchi
T
and Tanaka
N.
Noxa, a BH3-only member of the bcl-2 family and candidate mediator of p53-induced apoptosis.
Science.
2000;
288:
1053
-1058.
[PubMed]
.
-
40.
Boulaire
J
, Fotedar
A
and Fotedar
R.
The functions of the cdk-cyclin kinase inhibitor p21WAF1.
Pathol Biol (Paris).
2000;
48:
190
-202.
[PubMed]
.
-
41.
Gottifredi
V
, Karni-Schmidt
O
, Shieh
SS
and Prives
C.
p53 down-regulates CHK1 through p21 and the retinoblastoma protein.
Mol Cell Biol.
2001;
21:
1066
-1076.
[PubMed]
.
-
42.
Shats
I
, Milyavsky
M
, Tang
X
, Stambolsky
P
, Erez
N
, Brosh
R
, Kogan
I
, Braunstein
I
, Tzukerman
M
, Ginsberg
D
and Rotter
V.
p53-dependent down-regulation of telomerase is mediated by p21waf1.
J Biol Chem.
2004;
279:
50976
-50985.
[PubMed]
.
-
43.
Chen
KY
Transcription factors and the down-regulation of G1/S boundary genes in human diploid fibroblasts during senescence.
Front Biosci.
1997;
2:
d417
-426.
[PubMed]
.
-
44.
Carvajal
D
, Tovar
C
, Yang
H
, Vu
BT
, Heimbrook
DC
and Vassilev
LT.
Activation of p53 by MDM2 antagonists can protect proliferating cells from mitotic inhibitors.
Cancer Res.
2005;
65:
1918
-1924.
[PubMed]
.
-
45.
Hu
B
, Gilkes
DM
, Farooqi
B
, Sebti
SM
and Chen
J.
MDMX overexpression prevents P53 activation by the MDM2 inhibitor nutlin.
J Biol Chem.
2006;
281:
33030
-33035.
[PubMed]
.
-
46.
Kranz
D
and Dobbelstein
M.
Nongenotoxic p53 activation protects cells against S-phase-specific chemotherapy.
Cancer Res.
2006;
66:
10274
-10280.
[PubMed]
.
-
47.
Wade
M
, Wong
ET
, Tang
M
, Vassilev
LT
and Wahl
GM.
Hdmx modulates the outcome of p53 activation in human tumor cells.
J Biol Chem.
2006;
281:
33036
-33044.
[PubMed]
.
-
48.
Laurie
NA
, Donovan
SL
, Shih
CS
, Zhang
J
, Mills
N
, Fuller
C
, Teunisse
A
, Lam
S
, Ramos
Y
, Mohan
A
, Johnson
D
, Wilson
M
, Rodriguez-Galindo
C
, Quarto
M
, Francoz
S
, Mendrysa
SM
, Guy
RK
, Marine
JC
, Jochemsen
AG
and Dyer
MA.
Inactivation of the p53 pathway in retinoblastoma.
Nature.
2006;
444:
61
-66.
[PubMed]
.
-
49.
Mayo
LD
, Seo
YR
, Jackson
MW
, Smith
ML
, Guzman
JR
, Korgaonkar
CK
and Donner
DB.
Phosphorylation of human p53 at serine 46 determines promoter selection and whether apoptosis is attenuated or amplified.
J Biol Chem.
2005;
280:
25953
-25959.
[PubMed]
.
-
50.
Scian
MJ
, Carchman
EH
, Mohanraj
L
, Stagliano
KE
, Anderson
MA
, Deb
D
, Crane
BM
, Kiyono
T
, Windle
B
, Deb
SP
and Deb
S.
Wild-type p53 and p73 negatively regulate expression of proliferation related genes.
Oncogene.
2008;
27:
2583
-2593.
[PubMed]
.
-
51.
Francoz
S
, Froment
P
, Bogaerts
S
, De
Clercq S
, Maetens
M
, Doumont
G
, Bellefroid
E
and Marine
JC.
Mdm4 and Mdm2 cooperate to inhibit p53 activity in proliferating and quiescent cells in vivo.
Proc Natl Acad Sci U S A.
2006;
103:
3232
-3237.
[PubMed]
.
-
52.
Berberich
SJ
, Litteral
V
, Mayo
LD
, Tabesh
D
and Morris
D.
mdm-2 gene amplification in 3T3-L1 preadipocytes.
Differentiation.
1999;
64:
205
-212.
[PubMed]
.