Cyclooxygenase-1 null mice show reduced neuroinflammation in response to β-amyloid
Abstract
Several independent epidemiological studies indicate that chronic use of non-steroidal anti-inflammatory drugs can reduce the risk of developing Alzheimer's disease (AD), supporting the inflammatory cascade hypothesis. Although the first clinical trial with indomethacin, a preferential cyclooxygenase (COX)-1 inhibitor, showed beneficial effects, subsequent large clinical trials, mostly using COX-2 inhibitors, failed to show any beneficial effect in AD patients with mild to severe cognitive impairment. These combined data suggest that either an early treatment is crucial to stop the mechanisms underlying the disease before the onset of the symptoms, or that preferential COX-1 inhibition, rather than COX-2, is beneficial. Therefore, a full understanding of the physiological, pathological, and/or neuroprotective role of COX isoforms may help to develop better therapeutic strategies for the prevention or treatment of AD. In this study, we examined the effect of COX-1 genetic deletion on the inflammatory response and neurodegeneration induced by β-amyloid. β-amyloid (Aβ1-42) was centrally injected in the lateral ventricle of COX-1-deficient (COX-1-/-) and their respective wild-type (WT) mice. In COX-1-/- mice, Aβ1-42-induced inflammatory response and neuronal damage were attenuated compared to WT mice, as shown by Fluoro-Jade B and nitrotyrosine staining. These results indicate that inhibition of COX-1 activity may be valid therapeutic strategy to reduce brain inflammatory response and neurodegeneration.
Introduction
Alzheimer's
disease (AD) is an aging-related progressive neurodegenerative disease,
characterized by massive neuronal and synaptic loss, accompanied by
neuropathological changes, such as neurofibrillary tangles and senile plaques,
in the hippocampus, neocortex, and subcortical structures [1]. The senile
plaques are primarily composed of amyloid beta peptide (Aβ), which is a
40-42 amino acid peptide fragment of the amyloid protein precursor. However,
the mechanism by which Aβ causes neuronal injury
and cognitive impairment is unclear. AD is also thought to have a local,
non-immune mediated neuroinflammatory component with clusters of activated microglia,
increased inflammatory
proteins (complement
factors, acute-phase protein, pro-inflammatory cytokines) [2-4], and
increased COX-1-expressing microglia surrounding amyloid plaques [2]. Changes in
COX-2 expression in AD are discrepant and seem to depend on the stage of the
disease, with an upregulation of COX-2 in early AD, and a downregulation in
advanced AD stages, which also correlate with PGE2 levels in the
CSF, which are increased in probable AD patients and decrease with the
progression of the disease [5,6]. Several
independent epidemiological studies have shown that early use of non steroidal
anti-inflammatory drugs (NSAIDs), which inhibit COX activity, significantly
reduces the risk of developing AD later in life suggesting that inflammation is
critical for the progression of the disease [7-13]. However,
although a 6-month, double-blinded, placebo-controlled study with indomethacin,
a preferential COX-1 inhibitor, appeared to protect AD patients from cognitive
decline [14], subsequent
large-scale randomized clinical trials, mostly with selective COX-2 inhibitors,
did not show any beneficial effects in AD patients with mild to severe symptoms
[15-18]. Supporting these clinical data, indomethacin, but not the COX-2
selective nimesulide, significantly reduced levels of Aβ in the
hippocampus and cortex of transgenic mouse models of AD [19]. While the
clinical data seem to rule out a protective effect of selective COX-2
inhibition in AD, it is still unclear whether COX-2 inhibitors can improve the
pathology in animal models of AD. For instance, COX-2 inhibition blocks Aβ-mediated
suppression of long-term potentiation and memory function, independently of
reductions in Aβ1-42 or in inflammation [20]. However,
the selective COX-2 inhibitor celecoxib has been shown to increase Aβ levels
[21,22], and in a model of acute inflammation, both genetic deletion and
pharmacological inhibition of COX-2 worsen the neuroinflammatory response to
lipopolysaccharide (LPS) [23]. These
combined data suggest that either NSAIDs have rather a preventive than a
therapeutic effect or that preferential COX-1 inhibition is a better therapeutic
approach than selective targeting COX-2, or that the beneficial effects are due
to COX-independent effects of NSAIDs. In particular, ibuprofen, flurbiprofen,
and diclofenac have been shown to reduce serum Aβ1-42 levels, a
major component of senile plaques in AD [24-28].
However, a recent report from a pooled dataset from six prospective studies
indicated that NSAIDs use reduced the risk of AD without any apparent advantage
for the subset of NSAIDs shown to selectively lowering Aβ1-42[29]. While COX-1
and COX-2 are both differentially expressed in different stages of AD
pathology, their specific roles in the pathogenesis of AD is unclear.
Therefore, a full understanding of the physiological, pathological, and/or
neuroprotective role of COX isoforms may help to develop better therapeutic
strategies for the prevention or treatment of AD.
Partial reproduction of AD neuropathology
and cognitive deficits has been achieved with pharma-cological and genetic
approaches. Most injection models use synthetic peptide Aβ1-40
or Aβ1-42, which are analogous to peptides found in neuritic
plaques in AD patients [30]. Mice with a null mutation of COX gene
have been a useful tool for investigating the role of each COX isoform in both
physiological and pathological conditions in the CNS by overcoming the
complexity of dosing paradigm, duration of treatment, and possible unspecific
inhibition of both COX isoform [31]. In this
study, we assessed the effect of intracerebroventricular (i.c.v.) injection of
Aβ1-42 on acute neuroinflammatory response in COX-1-deficient
(COX-1-/-) mice and their respective wild-type mice (WT)
controls. We showed that COX-1-/- mice are more resistant
than WT mice to Aβ1-42-induced neuronal death and exhibit a
marked reduction in the inflammatory response.
Results
The
inflammatory response is reduced in COX-1-/- mice after
Aβ1-42 injection
Aβ1-42
or the control reverse peptide Aβ42-1 was unilaterally injected
into the lateral ventricle, as reported [32-35]. Seven
days later, brains were removed and coronal sections were processed for
immunohistochemistry. We assessed microglial activation in the brain using
IBA-1 as a microglial marker. Aβ1-42 administration caused a
robust inflammatory response within the CA1 and CA3 areas of the hippocampus of
WT mice characterized primarily by the presence of activated microglia (Figure 1A, D, J). Intense IBA-1-immunoreactive microglia with enhanced staining
intensity, retracted processes, perikaryal hypertrophy, and amoeboid appearance
were observed in the CA3 area of hippocampus of WT mice (Figure 1G). In COX-1-/-
mice, IBA-1-immunreactive microglia retained a resting morphology with
specifically small cell bodies, thin, and ramified processes (Figure 1B, E, H,
J). In reverse peptide Aβ42-1-injected mice, only a few faintly
IBA-1-immunoreactive microglia were observed in the hippocampus (Figure 1C, F,
I, J). Staining with CD11b, another marker for microglia gave results similar
to that of IBA-1 (data not shown).
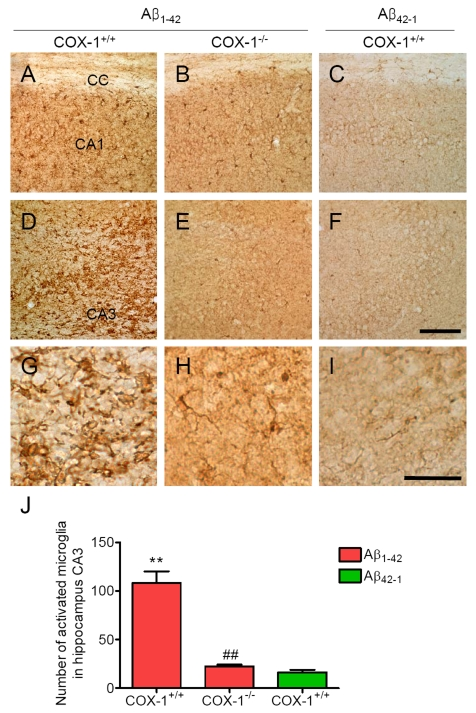
Figure 1. Increased microglial activation in the hippocampus 7 d after Aβ 1-42 administration. Representative
photomicrographs of the CA1 and CA3 of the hippocampus from WT mice (A,
D) injected with Aβ1-42
that shows numerous activated microglia with short, less-ramified
processes, perikaryal hypertrophy, and amoeboid appearance (G). CA1 and CA3 areas of the hippocampus from Aβ1-42-injected
COX-1-/- mice (B, E) show many resting microglia
with ramified morphology (H). Scale bar: A-F, 100
μm; G-I, 50 μm. (J) Comparison of the number of activated
microglia from the CA3 area. Mean ± SEM (n = 3-4 per group); **P
< 0.01 compared with the Aβ42-1-injected WT mice; ##P
< 0.01 compared with the Aβ1-42-injected WT mice.
We
then assessed astrocytes immunoreactivity by staining the brain of WT and COX-1-/-
mice with the astrocytic marker glial fibrillary acidic protein (GFAP).
GFAP-immunoreactive astrocytes in response to Aβ1-42 injection
were markedly attenuated in the brain of COX-1-/- mice (Figure 2B, E, H) compared to WT mice (Figure 2A, D, G). These results indicate that
Aβ1-42 administration induced less severe glial cell activation
in COX-1-/- mice compared to WT mice.
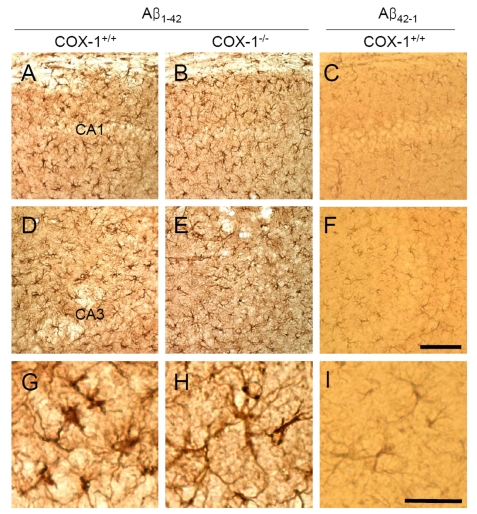
Figure 2. Increased astrocytic activation in the hippocampus 7 d after Aβ 1-42 administration. Representative
photomicrographs of the CA1 and CA3 of the hippocampus from WT mice (A,
D, G) injected with Aβ1-42 that shows numerous robustly
GFAP-immunoreactive astrocytes compared with Aβ1-42-injected
COX-1-/- mice (B, E, H). Scale
bar: A-F, 100 μm; G-I, 50 μm.
COX-1
deficiency leads to reduced neuronal damage following Aβ1-42
injection
We
next assessed neuronal damage in the brain using the fluorescent marker
Fluoro-Jade B (FJB), which selectively labels injured neurons [36,37]. Aβ1-42
administration caused a significant neuronal damage, characterized by the
presence of FJB-positive neurons within the CA3 areas of hippocampus of WT
mice (Figure 3A, J). In contrast, Aβ1-42-injected COX-1-/-
mice showed few scattered FJB-positive neurons in the CA3 of hippocampus (Figure 3B, J). In same sections stained with DAPI or adjacent sections stained with
cresyl violet, a similar distribution of neuronal loss and gliosis was found in
the CA3 areas of hippocampus in Aβ1-42-injected
WT mice (Figure 3D, G). FJB and Nissl staining showed that hippocampal CA3
neurons in COX-1-/- mice were better preserved than in WT
mice (Figure 3E, H). These results indicate that Aβ1-42
administration induced less severe neuronal damage in COX-1-/-
mice compared to WT mice.
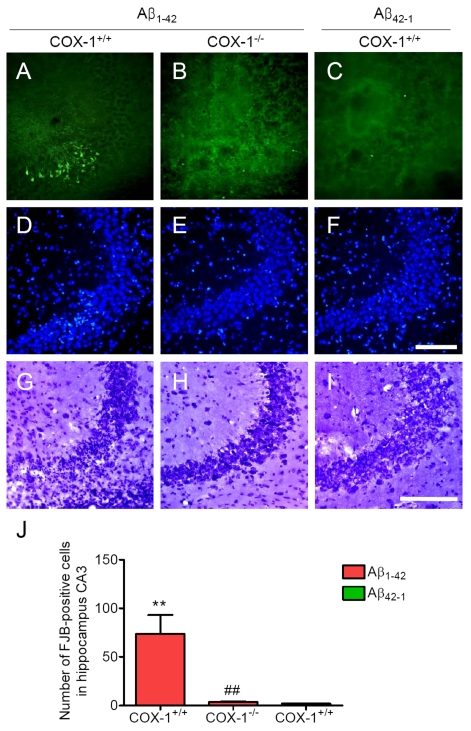
Figure 3. Increased degenerating neurons in the hippocampus 7 d after. Aβ1-42
administration. (A-C)
Representative photomicrographs of the CA3 of the hippocampus from WT mice
(A) injected with Aβ1-42 that shows numerous FJB-positive cells
compared with Aβ1-42-injected COX-1-/-
mice (B). Representative photomicro-graphs of DAPI (D-F) and
Nissl staining (G-I) in the CA3 of hippocampus from Aβ1-42-injected WT (D, G) and COX-1-/-mice (E, H).
Scale bar: A-I, 100 μm. (J) Comparison of the number of
FJB-positive cells from the CA3 area. Mean ± SEM (n = 3-4 per
group); **P < 0.01 compared with the Aβ42-1-injected
WT mice; ##P < 0.01 compared with the Aβ1-42-injected
WT mice.
COX-1-/-
mice exhibit reduced oxidative damage following Aβ1-42
administration
An
important component of Aβ1-42-induced neurotoxic process is
mediated by oxidative damage [38], which can
be evaluated by assessing protein carbonyls and nitrotyrosine levels [39]. To
determine whether oxidative damage is involved in the process of Aβ1-42-induced
neurotoxic process, we investigated oxidized amino acid, nitrotyrosine levels
using sections adjacent to those used for FJB staining. We found an increase in
nitrotyrosine-immunoreactive cells in the brain of WT mice (Figure 4A, D),
which was markedly attenuated in the
brain of COX-1-/- mice (Figure 4B, E). These results indicate
that Aβ1-42 administration induced less severe oxidative damage
in COX-1-/- mice compared to WT mice.
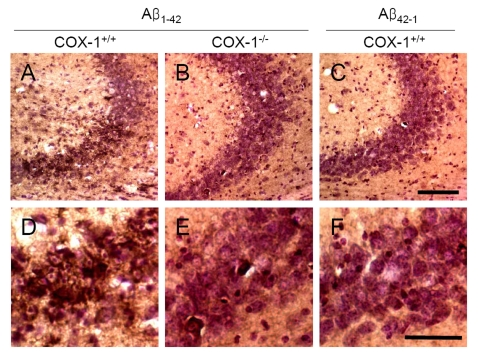
Figure 4. Increased oxidative damage in the hippocampus 7 d after Aβ 1-42 administration. Representative
photomicrographs of the CA1 and CA3 of the hippocampus from WT mice (A,
D) injected with Aβ1-42
that show numerous robustly nitrotyrosine-immunoreactive cells compared
with Aβ1-42-injected COX-1-/- mice (B,
E). Scale bar: A-C, 100 μm; D-F, 50 μm.
PG
generation is reduced in Aβ1-42-injected COX-1-/-
mice
To determine the contribution of COX-1 to
PG production after Aβ1-42 injection, we measured the levels of
PGE2, PGF2α, and TXB2 24 h after Aβ1-42
administration. We observed significant reduction in levels of PGE2 (Figure 5A), PGF2α (Figure 5B), and TXB2 (Figure 5C) inAβ1-42-injected COX-1-/- mice.
These
results suggest that the reduced levels of PGE2, PGF2α,
and TXB2 in COX-1-/- mice could contribute, in
part, to the observed differences in glial and neuronal response to Aβ1-42
administration.
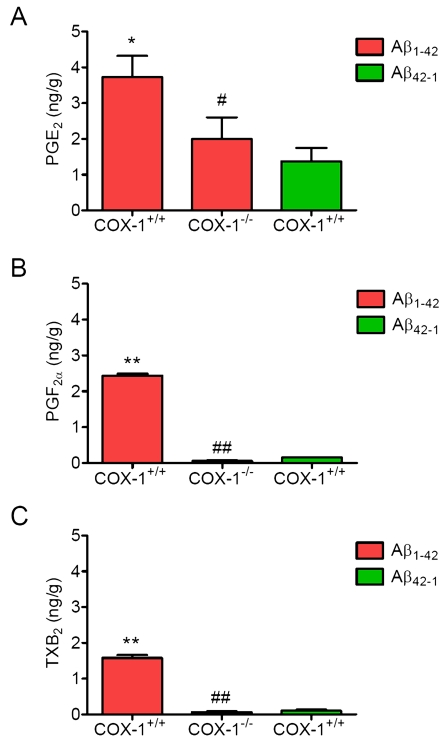
Figure 5. Effects of COX-1 deficiency on PG production 24 h after Aβ 1-42 administration. Aβ1-42-injected
WT mice show significantly more PGE2
(A), PGF2α (B), and TXB2 levels (C)
than COX-1-/- mice. Mean ± SEM (n = 3-4 per
group); *P < 0.05, **P < 0.01
compared with the Aβ42-1-injected WT mice; #P
< 0.05, ##P < 0.01 compared with the Aβ1-42-injected
WT mice.
Discussion
In
this study, genetic deletion of COX-1 led to a decrease in the
inflammatory response and neuronal damage in response to Aβ1-42, and
this effect was associated with alteration of PG production. We show that Aβ1-42-induced
oxidative damage and degenerating neurons, as well as glial activation, were
less severe in COX-1-/- mice compared to WT mice. These data
suggest that COX-1 facilitates activation of glial cells and supports
inflammatory processes and oxidative stress that evolve in neuronal damage, and
support previous data from our lab showing that COX-1-/- mice
have a decreased inflammatory response, oxidative stress and neuronal damage
after central injection of LPS [37].
Glial
cell activation, in turn, results in enhanced production of a variety of
proinflammatory and oxidative mediators, including cytokines, chemokines, and
reactive oxygen/nitrogen species [40-42].
Oxidative stress has been recognized to play an important role in the pathogenesis of AD and linked to the presence
of Aβ by the
finding of several characteristics, such as enhanced protein, DNA oxidation,
and lipid peroxidation in specific regions of the postmortem brain [43-48]. A previous study suggested that
oxidative DNA damage reduces the expression of highly vulnerable genes involved
in neuronal survival and learning memory, initiating a program of brain aging
that starts early in adult life [49]. In addition, lipid peroxidation
leads to a reduction in membrane fluidity, alteration of membrane-bounded
protein, receptors, and ion channels, and generation of Aβ that induces
more oxidative stress and calcium influx that induces glutamate excitotoxicity
and cell death [50,51]. The abundant polyunsaturated
lipid content, high oxygen consumption, high metal ion concentration, and low
regenerative capacity, as well as relatively low antioxidant levels compared with other
tissues make brain highly susceptible to oxidative damage [49,52]. In addition, oxidative stress
differentially affects brain regions, as levels of peroxidizable unsaturated
lipids and antioxidant enzymes, and membrane-bound protein differ between brain
regions. In this regard, continuous icv infusion of Aβ1-42 results in a significant
reduction of endogenous antioxidant systems, including Mn-superoxide dismutase
(Mn-SOD), glutathione, glutathione peroxidase, and
glutathione-S-transferase-π in the hippocampus, cortex, substantia nigra,
and thalamus [53]. Importantly, these alterations
of each antioxidant enzyme were not uniform, but rather specific in a brain
region-dependent manner (e.g. Mn-SOD in CA3), indicating a heterogenous
susceptibility to the Aβ1-42-induced
oxidative stress.
Our
results show that a single injection of Aβ1-42 resulted in a similar spatial distribution of
reactive glial cells, nitrotyrosine, and degenerating neurons in the CA3 of
hippocampus, suggesting the possibility that glial cell-derived reactive
oxygen/nitrogen species may be involved in the impaired neuronal function,
which has been described in this model [32,33,54,55].
Indeed, several studies have shown that pretreatment with antioxidants or
minocycline, a tetracycline derivative with anti-inflammatory and
neuroprotective properties, tend to ameliorate the Aβ1-42-induced
oxidative damage and behavioral deficits [32,33,56].
Although, variable in terms of the injected Aβ peptide sequences, injection methods, and employed behavioral
tests, previous studies have consistently shown the occurrence of behavioral
deficits related to memory impairment after intracerebral injection of Aβ peptide [32,33,57-59].
Therefore, Aβ injection is a useful in vivo model for Aβ toxicity, which is an important component in the progression of AD.
Gene deletion of COX-1 decreased glial cell activation
and attenuated nitrotyrosine induction. The decreased oxidative damage in COX-1-/- mice
suggests that COX-1 deletion can reduce the activity of free-radical
generating enzymes such as inducible nitric oxide (iNOS), NADPH oxidase, and
myeloperoxidase (MPO). These data are consistent with recent observations that
genetic deletion of COX-1 significantly reduces LPS-induced expression
of both superoxide (O2-) and NO-forming enzymes and thus
subsequently attenuates the levels of nitrotyrosine and protein carbonyls,
which are considered as biomarkers of oxidative stress [37]. Although, the precise
mechanism(s) by which COX-1 regulates free radical-generating enzymes in
inflammatory cascade have not been clearly established, it is possible that
because of its predominant localization in microglia, COX-1 can modulate the
induction of O2-, as well as NO, from NADPH oxidase and
iNOS, which, in turn, can enhance the production of more potent free radicals
such as peroxynitrite (ONOO-). In addition, O2-
and NO act as potent cell signaling molecules and amplify production of
TNF-α and PGE2 by upregulation of COX-2 [60]. These initial effects combined
with the activation of seconddary signaling cascades activate a robust immune
response that consequently causes neuronal damage and death.
The results from epidemiological data
indicating that NSAIDs are effective in preventing or delaying the onset of AD
combined with the failure of COX-2 selective inhibitors in clinical trials in
AD patients with moderate to severe AD suggest that either an early treatment
is crucial to stop the mechanisms underlying the disease before the onset of
the symptoms or that COX-2 selective inhibitors are not effective in delaying
the progression of AD. In this regard, an intriguing hypothesis is that the
protective effects of NSAIDs may be related to COX-1 rather than COX-2
inhibition. Supporting this hypothesis, COX-1 selective inhibitors (SC-560 and
valeryl salicylate), but not COX-2 selective inhibitors (SC-236 and DuP-697),
reduce Aβ1-42-induced PGs production and neurotoxicity in
postmortem human microglia and in murine cortical neurons [61,62].
Furthermore, a small double blind, placebo-controlled study with indomethacin,
a preferential COX-1 inhibitor [63], appeared
to protect mild to moderately impaired AD patients from cognitive decline [14].
Interestingly, COX-1 is prominently expressed by microglia in rodent and human
brain [2,4] and
appears to be increased in AD brain [2]. Double
immunostaining for Aβ and COX-1 indicates clustering of COX-1 positive
microglia with classicaland neuritic plaques, although there is no indication
that COX-1 is upregulated in activated microglia [64]. However,
LPS-induced PGE2 secretion can be reduced by COX-1 genetic deletion
and by COX-1 selective inhibitors [37,61,65],
suggesting that it is dependent on the constitutive COX-1 activity. In
contrast, COX-2 has not been detected in microglia and astrocytes in AD [66]. These
combined data suggest that COX-2 may not be the exclusive COX isoform
responsible for patho-physiological consequences in neurodegenerative diseases,
especially in AD, but that COX-1 also plays a critical role in the process of
neuroinflammation and neurodegeneration.
In
summary, we show that COX-1 facilitates activation of glial cells and supports
inflammatory processes and that genetic deletion of COX-1 significantly
attenuates the oxidative stress and neuronal damage in response to Aβ1-42. This
effect may be due to the predominant localization of COX-1 in microglial cells,
where, through its prostaglandin products contributes to the neuroinflammatory
cascade of events that ultimately lead to neuronal damage or death. Therefore,
COX-1 may represent a viable therapeutic target to treat neuroinflammation and
neurodegeneration.
Materials
and methods
Animals and stereotaxic A
β
1-42
administration.
Three-month-old male
homozygous COX-1-/- and their WT mice (COX-1+/+)
on a C57BL/6-129/Ola genetic background were used [67]. Mice were received at our animal facility at 6 weeks of age
from a NIEHS colony maintained by Taconic Farms (Germantown, NY) with heterozygous by heterozygous breedings for greater than 35
generations. In order to prevent the inclusion of strain or genetic background
confounders between COX null and wild type mice, all of the mice used in
this study were progeny derived from heterozygous by heterozygous mating and
therefore all contained the same strain and genetic background [67,68]. The mice were housed at
25°C in our animal facility with a 12 h light/dark cyclewith free access to food and water. All animal procedures were approved
by the National Institutes of Health (NIH) Animal Care and Use Committee in accordance with NIH guidelines on the
care and use of laboratory animals. Aβ1-42 and reverse peptide Aβ42-1 (American Peptide, Sunnyvale,
CA) were reconstituted in phosphate-buffered saline (pH 7.4) and aggregated by
incubation at 37°C for 4 days before use as described previously [69]. Aβ1-42 and Aβ42-1 (400 pmol per mouse) were administered
intracerebroventricularly (i.c.v) into the lateral ventricle using a 10 μl
syringe with a fine needle (World Precision Instruments, Sarasota, FL) and a
syringe pump (Stoelting, Wood Dale, IL) at a rate of 1 μl/min. The dose of Aβ1-42 and Aβ42-1 was selected based on previous
studies [32-35]. The coordinates for
the stereotaxic injections were -2.3mm dorsal/ventral, -1.0 mm
lateral, and -0.5 mmanterior/posterior from the bregma [70].
Tissue
preparation and histology.
Mice were transcardially perfused withsaline
followed by 4% paraformaldehyde. Brains were postfixedovernight in
the same medium and placed in 30% sucrose, before sectioning (30 μm).
Immunohistochemistry and double immunofluorescence were performed as described
previously [71]. Rabbit
anti-IBA-1 (1:500; Wako), mouse anti-GFAP (1:200; Sigma-Aldrich), and mouse
anti-nitrotyrosine (1:100; Chemicon, Temecula, CA) were used as primary
antibodies. The slides were visualized by brightfield microscopy (Olympus) and digitally photographed. FJB, a fluorochrome for the sensitive histochemicallocalization of neuronal degeneration, was used to identifydegenerating
neurons [72]. Brainsections were mounted on gelatin-coated slides and completelydried.
Then sections were rehydrated through graded concentrationsof
alcohol (100, 70, and 50%; 1 min each), and rinsed for 1 min in
distillated water. The slides were incubated in a solution of 0.06% potassium
permanganate for 20 min, rinsedin distilled water for 1 min, and
transferred to FJB (Histochem, Jefferson, AR) stainingsolution
(0.001% FJB/0.1% acetic acid) for 20 min. The slides were thereafter rinsed
three times in distilled water and air dried then immersedin xylene
and coverslipped with mounting media. The slides were visualized by fluorescent
microscopy (Olympus) and digitally photographed. Because the FJB staining was
obvious on digital imaging, the number of FJB-positive cells per section was
quantified as described previously [73]. The number
of microglia per section was quantified by counting the number of IBA-1-stained
cell bodies within 0.3 mm2 area of the CA3. For each measurement,
two blinded independent investigators counted 3-4 brains per group, 3 sections
per brain.
Measurement of prostanoids.
Prostanoids
were purified from the lipid extract as previously described [74] and levels
were determined using specific enzyme immunoassay (EIA) kits, PGE2,
PGF2α, and TXB2, (Oxford Biomedical, Oxford, MI).
Statistics.
All data are expressed as mean ± SEM. Statistical
significance was assessed with one-way analysis of variance (ANOVA) followed by
Bonferroni's post hoc test using GraphPad Prism version 4.00 (GraphPad
Software, San Diego, CA). Significance was taken at P < 0.05.
Acknowledgments
This
work was supported by the Intramural Research Program of the National Institute
on Aging, National Institutes of Health. We thank Dr. Robert Langenbach for
providing COX-1-/- and WT mice. We also thank Drs. Saba Aid,
Sara Palumbo, and Christopher D. Toscano for experimental suggestions and
critical comments.
Conflicts of Interest
The
authors in this manuscript have no conflict of interests to declare.
References
-
1.
Mattson
MP
Pathways towards and away from Alzheimer's disease.
Nature.
2004;
430:
631
-639.
[PubMed]
.
-
2.
Yermakova
AV
, Rollins
J
, Callahan
LM
, Rogers
J
and O'Banion
MK.
Cyclooxygenase-1 in human Alzheimer and control brain: quantitative analysis of expression by microglia and CA3 hippocampal neurons.
J Neuropathol Exp Neurol.
1999;
58:
1135
-1146.
[PubMed]
.
-
3.
Ho
L
, Luterman
JD
, Aisen
PS
, Pasinetti
GM
, Montine
TJ
and Morrow
JD.
Elevated CSF prostaglandin E2 levels in patients with probable AD.
Neurology.
2000;
55:
323
[PubMed]
.
-
4.
Hoozemans
JJ
, Rozemuller
AJ
, Janssen
I
, De
Groot CJ
, Veerhuis
R
and Eikelenboom
P.
Cyclooxygenase expression in microglia and neurons in Alzheimer's disease and control brain.
Acta Neuropathol (Berl).
2001;
101:
2
-8.
[PubMed]
.
-
5.
Minghetti
L
Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases.
J Neuropathol Exp Neurol.
2004;
63:
901
-910.
[PubMed]
.
-
6.
Combrinck
M
, Williams
J
, De
Berardinis MA
, Warden
D
, Puopolo
M
, Smith
AD
and Minghetti
L.
Levels of CSF prostaglandin E2, cognitive decline, and survival in Alzheimer's disease.
J Neurol Neurosurg Psychiatry.
2006;
77:
85
-88.
[PubMed]
.
-
7.
Andersen
K
, Launer
LJ
, Ott
A
, Hoes
AW
, Breteler
MM
and Hofman
A.
Do nonsteroidal anti-inflammatory drugs decrease the risk for Alzheimer's disease? The Rotterdam Study.
Neurology.
1995;
45:
1441
-1445.
[PubMed]
.
-
8.
McGeer
PL
, Schulzer
M
and McGeer
EG.
Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies.
Neurology.
1996;
47:
425
-432.
[PubMed]
.
-
9.
Stewart
WF
, Kawas
C
, Corrada
M
and Metter
EJ.
Risk of Alzheimer's disease and duration of NSAID use.
Neurology.
1997;
48:
626
-632.
[PubMed]
.
-
10.
Anthony
JC
, Breitner
JC
, Zandi
PP
, Meyer
MR
, Jurasova
I
, Norton
MC
and Stone
SV.
Reduced prevalence of AD in users of NSAIDs and H2 receptor antagonists: the Cache County study.
Neurology.
2000;
54:
2066
-2071.
[PubMed]
.
-
11.
in
t' Veld BA
, Ruitenberg
A
, Hofman
A
, Launer
LJ
, van
Duijn CM
, Stijnen
T
, Breteler
MM
and Stricker
BH.
Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease.
N Engl J Med.
2001;
345:
1515
-1521.
[PubMed]
.
-
12.
McGeer
PL
and McGeer
EG.
NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies.
Neurobiol Aging.
2007;
28:
639
-647.
[PubMed]
.
-
13.
Vlad
SC
, Miller
DR
, Kowall
NW
and Felson
DT.
Protective effects of NSAIDs on the development of Alzheimer disease.
Neurology.
2008;
70:
1672
-1677.
[PubMed]
.
-
14.
Rogers
J
, Kirby
LC
, Hempelman
SR
, Berry
DL
, McGeer
PL
, Kaszniak
AW
, Zalinski
J
, Cofield
M
, Mansukhani
L
and Willson
P.
Clinical trial of indomethacin in Alzheimer's disease.
Neurology.
1993;
43:
1609
-1611.
[PubMed]
.
-
15.
Scharf
S
, Mander
A
, Ugoni
A
, Vajda
F
and Christophidis
N.
A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer's disease.
Neurology.
1999;
53:
197
-201.
[PubMed]
.
-
16.
Aisen
PS
, Schafer
KA
, Grundman
M
, Pfeiffer
E
, Sano
M
, Davis
KL
, Farlow
MR
, Jin
S
, Thomas
RG
and Thal
LJ.
Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial.
Jama.
2003;
289:
2819
-2826.
[PubMed]
.
-
17.
Reines
SA
, Block
GA
, Morris
JC
, Liu
G
, Nessly
ML
, Lines
CR
, Norman
BA
and Baranak
CC.
Rofecoxib: no effect on Alzheimer's disease in a 1-year, randomized, blinded, controlled study.
Neurology.
2004;
62:
66
-71.
[PubMed]
.
-
18.
Thal
LJ
, Ferris
SH
, Kirby
L
, Block
GA
, Lines
CR
, Yuen
E
, Assaid
C
, Nessly
ML
, Norman
BA
, Baranak
CC
and Reines
SA.
A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment.
Neuropsychopharmacology.
2005;
30:
1204
-1215.
[PubMed]
.
-
19.
Sung
S
, Yang
H
, Uryu
K
, Lee
EB
, Zhao
L
, Shineman
D
, Trojanowski
JQ
, Lee
VM
and Pratico
D.
Modulation of nuclear factor-kappa B activity by indomethacin influences A beta levels but not A beta precursor protein metabolism in a model of Alzheimer's disease.
Am J Pathol.
2004;
165:
2197
-2206.
[PubMed]
.
-
20.
Kotilinek
LA
, Westerman
MA
, Wang
Q
, Panizzon
K
, Lim
GP
, Simonyi
A
, Lesne
S
, Falinska
A
, Younkin
LH
, Younkin
SG
, Rowan
M
, Cleary
J
, Wallis
RA
, Sun
GY
, Cole
G
, Frautschy
S
, Anwyl
R
and Ashe
KH.
Cyclooxygenase-2 inhibition improves amyloid-beta-mediated suppression of memory and synaptic plasticity.
Brain.
2008;
131:
651
-664.
[PubMed]
.
-
21.
Jantzen
PT
, Connor
KE
, DiCarlo
G
, Wenk
GL
, Wallace
JL
, Rojiani
AM
, Coppola
D
, Morgan
D
and Gordon
MN.
Microglial activation and beta -amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice.
J Neurosci.
2002;
22:
2246
-2254.
[PubMed]
.
-
22.
Kukar
T
, Murphy
MP
, Eriksen
JL
, Sagi
SA
, Weggen
S
, Smith
TE
, Ladd
T
, Khan
MA
, Kache
R
, Beard
J
, Dodson
M
, Merit
S
, Ozols
VV
, Anastasiadis
PZ
, Das
P
, Fauq
A
, Koo
EH
and Golde
TE.
Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Abeta42 production.
Nat Med.
2005;
11:
545
-550.
[PubMed]
.
-
23.
Aid
S
, Langenbach
R
and Bosetti
F.
Neuroinflammatory response to lipopolysaccharide is exacerbated in mice genetically deficient in cyclooxygenase-2.
J Neuroinflammation.
2008;
5:
17
[PubMed]
.
-
24.
Eriksen
JL
, Sagi
SA
, Smith
TE
, Weggen
S
, Das
P
, McLendon
DC
, Ozols
VV
, Jessing
KW
, Zavitz
KH
, Koo
EH
and Golde
TE.
NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo.
J Clin Invest.
2003;
112:
440
-449.
[PubMed]
.
-
25.
Gasparini
L
, Rusconi
L
, Xu
H
, del
Soldato P
and Ongini
E.
Modulation of beta-amyloid metabolism by non-steroidal anti-inflammatory drugs in neuronal cell cultures.
J Neurochem.
2004;
88:
337
-348.
[PubMed]
.
-
26.
Morihara
T
, Teter
B
, Yang
F
, Lim
GP
, Boudinot
S
, Boudinot
FD
, Frautschy
SA
and Cole
GM.
Ibuprofen suppresses interleukin-1beta induction of pro-amyloidogenic alpha1-antichymotrypsin to ameliorate beta-amyloid (Abeta) pathology in Alzheimer's models.
Neuropsychopharmacology.
2005;
30:
1111
-1120.
[PubMed]
.
-
27.
Peretto
I
, Radaelli
S
, Parini
C
, Zandi
M
, Raveglia
LF
, Dondio
G
, Fontanella
L
, Misiano
P
, Bigogno
C
, Rizzi
A
, Riccardi
B
, Biscaioli
M
, Marchetti
S
, Puccini
P
, Catinella
S
, Rondelli
I
, Cenacchi
V
, Bolzoni
PT
, Caruso
P
, Villetti
G
, Facchinetti
F
, Del Giudice
E
, Moretto
N
and Imbimbo
BP.
Synthesis and biological activity of flurbiprofen analogues as selective inhibitors of beta-amyloid(1)(-)(42) secretion.
J Med Chem.
2005;
48:
5705
-5720.
[PubMed]
.
-
28.
Kukar
TL
, Ladd
TB
, Bann
MA
, Fraering
PC
, Narlawar
R
, Maharvi
GM
, Healy
B
, Chapman
R
, Welzel
AT
, Price
RW
, Moore
B
, Rangachari
V
, Cusack
B
, Eriksen
J
, Jansen-West
K
, Verbeeck
C
, Yager
D
, Eckman
C
, Ye
W
, Sagi
S
, Cottrell
BA
, Torpey
J
, Rosenberry
TL
, Fauq
A
, Wolfe
MS
, Schmidt
B
, Walsh
DM
, Koo
EH
and Golde
TE.
Substrate-targeting gamma-secretase modulators.
Nature.
2008;
453:
925
-929.
[PubMed]
.
-
29.
Szekely
CA
, Green
RC
, Breitner
JC
, Ostbye
T
, Beiser
AS
, Corrada
MM
, Dodge
HH
, Ganguli
M
, Kawas
CH
, Kuller
LH
, Psaty
BM
, Resnick
SM
, Wolf
PA
, Zonderman
AB
, Welsh-Bohmer
KA
and Zandi
PP.
No advantage of A beta 42-lowering NSAIDs for prevention of Alzheimer dementia in six pooled cohort studies.
Neurology.
2008;
70:
2291
-2298.
[PubMed]
.
-
30.
Yamada
K
and Nabeshima
T.
Animal models of Alzheimer's disease and evaluation of anti-dementia drugs.
Pharmacol Ther.
2000;
88:
93
-113.
[PubMed]
.
-
31.
Bosetti
F
Arachidonic acid metabolism in brain physiology and pathology: lessons from genetically altered mouse models.
J Neurochem.
2007;
102:
577
-586.
[PubMed]
.
-
32.
Yan
JJ
, Cho
JY
, Kim
HS
, Kim
KL
, Jung
JS
, Huh
SO
, Suh
HW
, Kim
YH
and Song
DK.
Protection against beta-amyloid peptide toxicity in vivo with long-term administration of ferulic acid.
Br J Pharmacol.
2001;
133:
89
-96.
[PubMed]
.
-
33.
Jhoo
JH
, Kim
HC
, Nabeshima
T
, Yamada
K
, Shin
EJ
, Jhoo
WK
, Kim
W
, Kang
KS
, Jo
SA
and Woo
JI.
Beta-amyloid (1-42)-induced learning and memory deficits in mice: involvement of oxidative burdens in the hippocampus and cerebral cortex.
Behav Brain Res.
2004;
155:
185
-196.
[PubMed]
.
-
34.
Prediger
RD
, Franco
JL
, Pandolfo
P
, Medeiros
R
, Duarte
FS
, Di Giunta
G
, Figueiredo
CP
, Farina
M
, Calixto
JB
, Takahashi
RN
and Dafre
AL.
Differential susceptibility following beta-amyloid peptide-(1-40) administration in C57BL/6 and Swiss albino mice: Evidence for a dissociation between cognitive deficits and the glutathione system response.
Behav Brain Res.
2007;
177:
205
-213.
[PubMed]
.
-
35.
Medeiros
R
, Prediger
RD
, Passos
GF
, Pandolfo
P
, Duarte
FS
, Franco
JL
, Dafre
AL
, Di Giunta
G
, Figueiredo
CP
, Takahashi
RN
, Campos
MM
and Calixto
JB.
Connecting TNF-alpha signaling pathways to iNOS expression in a mouse model of Alzheimer's disease: relevance for the behavioral and synaptic deficits induced by amyloid beta protein.
J Neurosci.
2007;
27:
5394
-5404.
[PubMed]
.
-
36.
Schmued
LC
, Albertson
C
and Slikker
W Jr.
Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration.
Brain Res.
1997;
751:
37
-46.
[PubMed]
.
-
37.
Choi
SH
, Langenbach
R
and Bosetti
F.
Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury.
Faseb J.
2008;
22:
1491
-1501.
[PubMed]
.
-
38.
Yan
SD
, Chen
X
, Fu
J
, Chen
M
, Zhu
H
, Roher
A
, Slattery
T
, Zhao
L
, Nagashima
M
, Morser
J
, Migheli
A
, Nawroth
P
, Stern
D
and Schmidt
AM.
RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease.
Nature.
1996;
382:
685
-691.
[PubMed]
.
-
39.
Liberatore
GT
, Jackson-Lewis
V
, Vukosavic
S
, Mandir
AS
, Vila
M
, McAuliffe
WG
, Dawson
VL
, Dawson
TM
and Przedborski
S.
Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease.
Nat Med.
1999;
5:
1403
-1409.
[PubMed]
.
-
40.
Block
ML
, Zecca
L
and Hong
JS.
Microglia-mediated neurotoxicity: uncovering the molecular mechanisms.
Nat Rev Neurosci.
2007;
8:
57
-69.
[PubMed]
.
-
41.
McGeer
PL
, Itagaki
S
, Tago
H
and McGeer
EG.
Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR.
Neurosci Lett.
1987;
79:
195
-200.
[PubMed]
.
-
42.
Nelson
PT
, Soma
LA
and Lavi
E.
Microglia in diseases of the central nervous system.
Ann Med.
2002;
34:
491
-500.
[PubMed]
.
-
43.
Butterfield
DA
and Lauderback
CM.
Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress.
Free Radic Biol Med.
2002;
32:
1050
-1060.
[PubMed]
.
-
44.
Castegna
A
, Thongboonkerd
V
, Klein
JB
, Lynn
B
, Markesbery
WR
and Butterfield
DA.
Proteomic identification of nitrated proteins in Alzheimer's disease brain.
J Neurochem.
2003;
85:
1394
-1401.
[PubMed]
.
-
45.
Hensley
K
, Maidt
ML
, Yu
Z
, Sang
H
, Markesbery
WR
and Floyd
RA.
Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer brain indicates region-specific accumulation.
J Neurosci.
1998;
18:
8126
-8132.
[PubMed]
.
-
46.
Markesbery
WR
and Carney
JM.
Oxidative alterations in Alzheimer's disease.
Brain Pathol.
1999;
9:
133
-146.
[PubMed]
.
-
47.
Smith
MA
, Richey
Harris PL
, Sayre
LM
, Beckman
JS
and Perry
G.
Widespread peroxynitrite-mediated damage in Alzheimer's disease.
J Neurosci.
1997;
17:
2653
-2657.
[PubMed]
.
-
48.
Sultana
R
, Perluigi
M
and Butterfield
DA.
Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer's disease: insights into mechanism of neurodegeneration from redox proteomics.
Antioxid Redox Signal.
2006;
8:
2021
-2037.
[PubMed]
.
-
49.
Lu
T
, Pan
Y
, Kao
SY
, Li
C
, Kohane
I
, Chan
J
and Yankner
BA.
Gene regulation and DNA damage in the ageing human brain.
Nature.
2004;
429:
883
-891.
[PubMed]
.
-
50.
Lauritzen
L
, Hansen
HS
, Jorgensen
MH
and Michaelsen
KF.
The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina.
Prog Lipid Res.
2001;
40:
1
-94.
[PubMed]
.
-
51.
Yehuda
S
, Rabinovitz
S
and Mostofsky
DI.
Essential fatty acids are mediators of brain biochemistry and cognitive functions.
J Neurosci Res.
1999;
56:
565
-570.
[PubMed]
.
-
52.
Lane
RM
and Farlow
MR.
Lipid homeostasis and apolipoprotein E in the development and progression of Alzheimer's disease.
J Lipid Res.
2005;
46:
949
-968.
[PubMed]
.
-
53.
Kim
HC
, Yamada
K
, Nitta
A
, Olariu
A
, Tran
MH
, Mizuno
M
, Nakajima
A
, Nagai
T
, Kamei
H
, Jhoo
WK
, Im
DH
, Shin
EJ
, Hjelle
OP
, Ottersen
OP
, Park
SC
, Kato
K
, Mirault
ME
and Nabeshima
T.
Immunocytochemical evidence that amyloid beta (1-42) impairs endogenous antioxidant systems in vivo.
Neuroscience.
2003;
119:
399
-419.
[PubMed]
.
-
54.
Weldon
DT
, Rogers
SD
, Ghilardi
JR
, Finke
MP
, Cleary
JP
, O'Hare
E
, Esler
WP
, Maggio
JE
and Mantyh
PW.
Fibrillar beta-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo.
J Neurosci.
1998;
18:
2161
-2173.
[PubMed]
.
-
55.
Klein
AM
, Kowall
NW
and Ferrante
RJ.
Neurotoxicity and oxidative damage of beta amyloid 1-42 versus beta amyloid 1-40 in the mouse cerebral cortex.
Ann N Y Acad Sci.
1999;
893:
314
-320.
[PubMed]
.
-
56.
Ryu
JK
and McLarnon
JG.
Minocycline or iNOS inhibition block 3-nitrotyrosine increases and blood-brain barrier leakiness in amyloid beta-peptide-injected rat hippocampus.
Exp Neurol.
2006;
198:
552
-557.
[PubMed]
.
-
57.
O'Hare
E
, Weldon
DT
, Mantyh
PW
, Ghilardi
JR
, Finke
MP
, Kuskowski
MA
, Maggio
JE
, Shephard
RA
and Cleary
J.
Delayed behavioral effects following intrahippocampal injection of aggregated A beta (1-42).
Brain Res.
1999;
815:
1
-10.
[PubMed]
.
-
58.
Nakamura
S
, Murayama
N
, Noshita
T
, Annoura
H
and Ohno
T.
Progressive brain dysfunction following intracerebroventricular infusion of beta(1-42)-amyloid peptide.
Brain Res.
2001;
912:
128
-136.
[PubMed]
.
-
59.
Christensen
R
, Marcussen
AB
, Wortwein
G
, Knudsen
GM
and Aznar
S.
Abeta(1-42) injection causes memory impairment, lowered cortical and serum BDNF levels, and decreased hippocampal 5-HT(2A) levels.
Exp Neurol.
2008;
210:
164
-171.
[PubMed]
.
-
60.
Qin
L
, Liu
Y
, Wang
T
, Wei
SJ
, Block
ML
, Wilson
B
, Liu
B
and Hong
JS.
NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia.
J Biol Chem.
2004;
279:
1415
-1421.
[PubMed]
.
-
61.
Hoozemans
JJ
, Veerhuis
R
, Janssen
I
, van
Elk EJ
, Rozemuller
AJ
and Eikelenboom
P.
The role of cyclo-oxygenase 1 and 2 activity in prostaglandin E(2) secretion by cultured human adult microglia: implications for Alzheimer's disease.
Brain Res.
2002;
951:
218
-226.
[PubMed]
.
-
62.
Bate
C
, Veerhuis
R
, Eikelenboom
P
and Williams
A.
Neurones treated with cyclo-oxygenase-1 inhibitors are resistant to amyloid-beta1-42.
Neuroreport.
2003;
14:
2099
-2103.
[PubMed]
.
-
63.
Barnett
J
, Chow
J
, Ives
D
, Chiou
M
, Mackenzie
R
, Osen
E
, Nguyen
B
, Tsing
S
, Bach
C
and Freire
J.
Purification, characterization and selective inhibition of human prostaglandin G/H synthase 1 and 2 expressed in the baculovirus system.
Biochim Biophys Acta.
1994;
1209:
130
-139.
[PubMed]
.
-
64.
Hoozemans
JJ
, Rozemuller
JM
, van
Haastert ES
, Veerhuis
R
and Eikelenboom
P.
Cyclooxygenase-1 and -2 in the different stages of Alzheimer's disease pathology.
Curr Pharm Des.
2008;
14:
1419
-1427.
[PubMed]
.
-
65.
Candelario-Jalil
E
, Taheri
S
, Yang
Y
, Sood
R
, Grossetete
M
, Estrada
EY
, Fiebich
BL
and Rosenberg
GA.
Cyclooxygenase inhibition limits blood-brain barrier disruption following intracerebral injection of tumor necrosis factor-alpha in the rat.
J Pharmacol Exp Ther.
2007;
323:
488
-498.
[PubMed]
.
-
66.
Hoozemans
JJ
, Veerhuis
R
, Rozemuller
AJ
and Eikelenboom
P.
Non-steroidal anti-inflammatory drugs and cyclooxygenase in Alzheimer's disease.
Curr Drug Targets.
2003;
4:
461
-468.
[PubMed]
.
-
67.
Langenbach
R
, Morham
SG
, Tiano
HF
, Loftin
CD
, Ghanayem
BI
, Chulada
PC
, Mahler
JF
, Lee
CA
, Goulding
EH
, Kluckman
KD
, Kim
HS
and Smithies
O.
Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration.
Cell.
1995;
83:
483
-492.
[PubMed]
.
-
68.
Toscano
CD
, Prabhu
VV
, Langenbach
R
, Becker
KG
and Bosetti
F.
Differential gene expression patterns in cyclooxygenase-1 and cyclooxygenase-2 deficient mouse brain.
Genome Biol.
2007;
8:
R14
[PubMed]
.
-
69.
Maurice
T
, Lockhart
BP
and Privat
A.
Amnesia induced in mice by centrally administered beta-amyloid peptides involves cholinergic dysfunction.
Brain Res.
1996;
706:
181
-193.
[PubMed]
.
-
70.
Paxinos
G
and Franklin
KBJ.
San Diego, Calif, London
Academic
The mouse brain in stereotaxic coordinates. edn 2nd.
2001;
.
-
71.
Choi
SH
, Lee
da Y
, Kim
SU
and Jin
BK.
Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: role of microglial NADPH oxidase.
J Neurosci.
2005;
25:
4082
-4090.
[PubMed]
.
-
72.
Schmued
LC
and Hopkins
KJ.
Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration.
Brain Res.
2000;
874:
123
-130.
[PubMed]
.
-
73.
El
Khoury J
, Toft
M
, Hickman
SE
, Means
TK
, Terada
K
, Geula
C
and Luster
AD.
Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease.
Nat Med.
2007;
13:
432
-438.
[PubMed]
.
-
74.
Powell
WS
Reversed-phase high-pressure liquid chromatography of arachidonic acid metabolites formed by cyclooxygenase and lipoxygenases.
Anal Biochem.
1985;
148:
59
-69.
[PubMed]
.