Introduction
High NaCl induces DNA breaks and
oxidative damage to DNA and proteins [1-3], and also promotes cellular
senescence both in cell culture and in vivo [4]. Despite these changes, the
cells proliferate at a close to normal rate in culture and maintain their
function in renal inner medullas in vivo where NaCl is normally high.
Evidently, there must be mechanisms that promote survival and function of cells
despite the seemingly adverse high NaCl-induced changes. Ku86 is important in
this respect. It binds to the ends of DNA that is broken following ionizing
radiation (IR) and during V(D)J recombination, and it facilitates DNA repair by
aligning DNA ends for non-homologous end joining (NHEJ) [5]. We previously found
that Ku86 deficiency compromises
adaptation of cells to high NaCl [6]. This was most dramatic in the
radiosensitive xrs5 mutant cell line, derived from CHO-K1 cells by treating
them with ethyl methanesulphonate, resulting in Ku86 deficiency [7,8]. These
cells never recover from the initial cell cycle arrest induced by high NaCl.
They lose their epithelial appearance, become giant and multinucleated, and
disintegrate within 10 days after NaCl is raised to a level that normal cells
adapt to readily. Spontaneously immortalized mouse embryonic fibroblasts (mefs)
from Ku86-/- mice do proliferate despite high NaCl, but their growth
rate is greatly reduced compared to Ku86+/+ mefs. The number of
broken chromosomes is greater in Ku86-/- mefs exposed to high NaCl
than in Ku86+/+ mefs [6]. Since these high NaCl-induced changes that
occur in Ku86-/- cells resemble those known to be associated with
cellular senescence [9-11], we have in the present studies tested the hypothesis
that Ku86 deficiency might accelerate the cellular senescence induced by high
NaCl.
Results
xrs5
(Ku86 deficient) cells undergo rapid senescence when NaCl is elevated
In our previous studies we found that xrs5 cells undergo
dramatic morphological changes upon exposure to high NaCl. They change from
epithelial to fibroblast morphology, enlarge, flatten and become multinucleated
[6]. To test whether the cell have become senescent, we stained them for expression of senescence associated β-galactosidase (SA-β-gal). We confirm
that within 3 days of exposure to high NaCl the morphology of xrs5 cells
changes dramatically (Figure 1A) and now find that, in addition, they become
positive for SA-β-gal (Figure 1B), indicative of senescence. In contrast,
the appearance of the control CHO-K1 (wild type) cells does not change (Figure 1A) and they do not express SA-β-gal (Figure 1B). Also, we find diminished expression of
HSP70 in response to high NaCl, which is an additional indication of senescence
since, although high NaCl increases expression of HSP70 [12], senescence
reduces it [13,14].
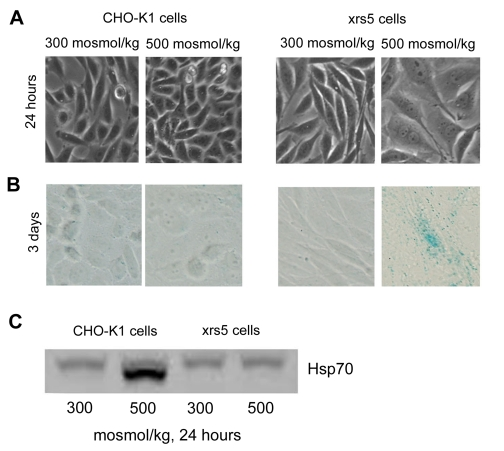
Figure 1. High NaCl induces rapid senescence of Ku86 deficient (xrs5) cells. Medium bathing CHO-K1 (wild
type) and xrs5 (ku86 mutant) cells grown at 300 mosmol/kg was acutely
changed to the same medium or to 500 mosmol/kg (NaCl added). (A)
Photographs after 24 hours. High NaCl rapidly induces cellular hypertrophy
in xrs5 cells. (B) Staining for senescence-associated β-galactosidise (SA-β-gal). Positive staining for SA-β-gal is detected 3 days after NaCl elevation. (C)
Western blot for Hsp70 expression. Hsp70 is not upregulated in xrs5 cells
exposed to high NaCl, consistent with senescence.
High NaCl increases expression of HSP70 in
CHO-K1 cells, but not in xrs5 cells (Figure 1C), providing an additional
indication that high NaCl induces senescence in Ku86 deficient cells.
Exposure
to high NaCl, starting in the larval stage, causes a greater reduction of the
life span of C. elegans that lack Ku86 activity than
of wild type
Previously,
we showed that exposure of C. elegans to high NaCl accelerates accumulation of
senescent cells and decreases their life span. In the present studies we tested
whether lack of Ku86 activity further diminishes longevity in the presence of
high NaCl. We compared the effect of high NaCl on wild type C. elegans to that
on cku80-/-C. elegans, which lack activity of the Ku86 homologue.
If NaCl is first elevated when the animals are adults (4 days old), life span
is little affected and does not differ between the mutants and the wild type
(Figure 2A). In contrast, if NaCl is first elevated while they are larvae (2
days old), life span decreases markedly and the life span of the mutants is
significantly less than the wild type (Figure 2B). Since somatic cells of C.
elegans do not proliferate once they reach adult stage [15], the difference may
lie in greater susceptibility to the effect of high NaCl of proliferating cells
in the larvae.
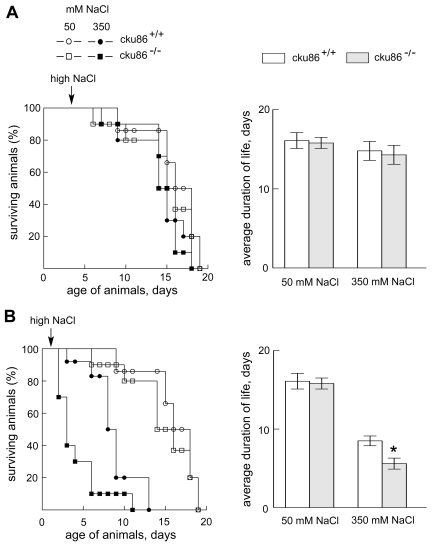
Figure 2. Absence of Ku86 reduces longevity of C. elegans in high NaCl, provided the exposure to high NaCl begins in the larval stage. C. elegans were placed on plates containing 50mM or
350 mM of NaCl beginning at the (A) adult stage (day 4 after
hatching) or (B) L2/L3 larva stage (day 2 after hatching). Every two
days worms were transferred to new plates to separate them from their
progeny. Left panels: % of animals surviving. Right panels: average
duration of life (mean ±SEM, * P < 0.05 relative to control (cku86+/+).
Knock
out of Ku86 accelerates cellular senescence in the mouse renal inner medulla in vivo
We next tested whether absence of Ku86
makes mouse renal cells more prone to high NaCl-induced cellular senescence in vivo, using expression of the cell
cycle regulator p16INK4 as an indicator [16,17]. NaCl normally is
always high in the renal inner medullary interstitium associated with its role
in the urinary concentrating mechanism, but it is not high in the renal cortex.
Using this assay, we previously found only low levels of cellular senescence in
both the renal cortex and medulla at 3 months of age. At 12 months expression
becomes high in the medulla, but not in the cortex [4]. In the present studies
we confirm that in Ku86+/+ mice p16INK4 is not elevated
in either renal inner medulla or cortex at 3 months (Figure 3). In contrast,
the renal medullas, but not cortex, of Ku86-/- mice already contain
numerous senescent cells at this age (Figure 3). Thus, absence of Ku86 greatly
accelerates accumulation of senescent cells in the renal inner medulla. As
previously noted [4], it is not renal medullary epithelial cells that become
prematurely senescent, but adjacent cells that surround the tubules.
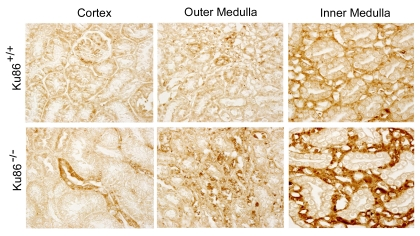
Figure 3. Immunocytochemical analysis of p16 INK4 in kidneys of 3 month old Ku86+/+ and Ku86-/- mice. Many senescent cells (brown stain) are present in
kidneys of Ku86-/- mice. p16INK4 is higher in the
renal medulla, where salt concentration normally is always high, than in
the cortex, where the salt concentration is similar to that in peripheral
blood. p16INK4 level and staining pattern in kidneys of 3 month
old Ku86-/- mice are similar to those observed previously in 12
month old wild type mice [4].
The
deficit in water conservation that occurs normally in old mice, occurs at an
earlier age in Ku86-/- mice
Antidiuresis,
which depends on intact function of the renal medulla, is important for water
conservation. Aged subjects are prone to dehydration [18,19]. The following
experiments were aimed at 1) finding if old mice have a deficit in water
conservation, 2) if so, whether it occurs prematurely in Ku86-/-mice, and 3) whether any deficit involves defective urinary concentrating
ability. We analyzed the response of Ku86+/+ versus Ku86-/-mice and of mice of various ages to mild water restriction. The mice were
maintained in individual metabolic cages. Their food was in the form of a gel,
maintaining a constant amount of dry food, but variable water content (Figure 4A). The experiment was divided into three periods: (I) gel food containing 43%
water, plus free access to drinking water; (II) and (III) no additional
drinking water; and (III) water content of the gel food reduced to 30% (Figure 4A). Body weight, food consumption, urine volume, urine osmolality, and
urinary vasopressin excretion rate were measured. Data are expressed relative
to period (I). Experiments were of two sorts: Ku86+/+ versus Ku86-/-mice at 3 month of age (Figure 4, left panels) and 2 month old versus 14-24
month old wild type mice (Figure 4, right panels). Changes in body weight (Figure 4B) are an index of fluid balance since consumption of dry food (Figure 4C)
either did not change significantly (Ku86-/- and 4-24 month old) or
varied slightly, uncorrelated with weight changes (Ku86+/+ and 2
month old). Three month old Ku86+/+ (Figure 4B, left panel)and
2 month old wild type mice (Figure 4B, right panel) do not lose weight during
the mild water restriction in periods (II) and (III). Evidently, they can
regulate their water balance to avoid net loss when water is restricted. In
contrast, the Ku86-/- (Figure 4B, left panel) and 14-24 month old
(Figure 4B, right panel) mice lose weight rapidly, indicating that ability to
maintain water balance decreases with age and that lack of Ku86 accelerates the
process. Greater excretion of antidiuretic hormone (ADH, Figure 4D) provides
additional evidence that Ku86-/- and 14-24 month old mice become
more dehydrated than Ku86+/+ and 2 month old mice following water
restriction.
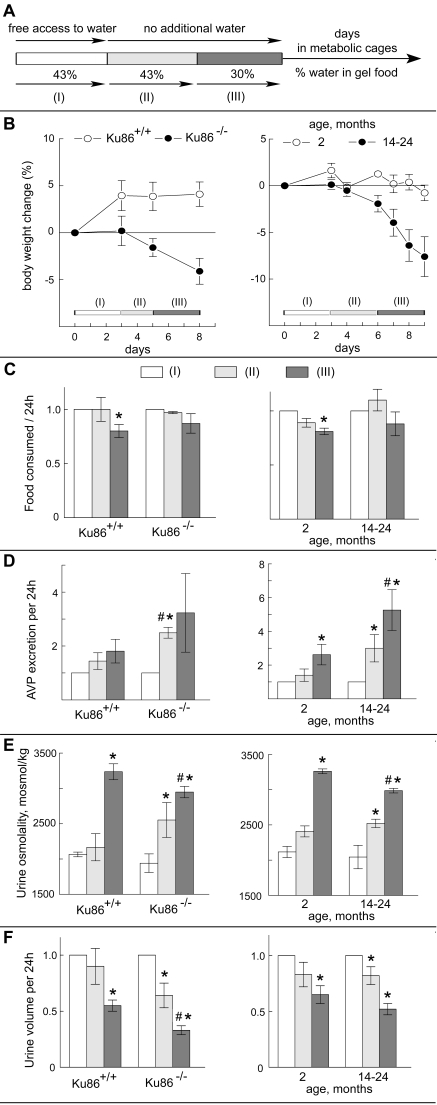
Figure 4. Effects of Ku86 deficiency and aging on water conservation. The experiment analyzes the
response to mild water restriction of 3 months old Ku86+/+ versus Ku86-/- mice (left panels) and of 2 months old versus
14-22 months old mice (right panels). (A) Experimental
design. Mice were subjected to 3 consecutive periods of
different water availability. During period I mice had free access to water
and the gel food containing 43% of water. Then, the supplemental drinking
water was removed and mice got water only from the gel food (period II).
During period III, the amount of water in the gel food was decreased to
30%. The periods lasted 3 days, except period (II) for Ku86+/+
and Ku86-/- mice which lasted 2 days. Body
weight (B), food consumption (C), Arginine Vasopressin (AVP)
excretion (D), urine osmolality (E) and urine volume (F)
were measured every 24h. Average values during each period were calculated and
normalized to period (I) for the same mouse. Data are
represented as mean
±SEM (n=3-5, * P < 0.05 relative to period I, # P<0.05 relative to
the same period in the parallel group).
Since
intake of water (limited to the gel food) is fixed during periods (II) and (III), the rapid weight loss of Ku86-/- mice and 14-24 month old mice must
be due to water loss. Given the evidence of senescence in the renal medullas of
these animals, our first thought was that the water loss might be due to
inability to concentrate their urine sufficiently. However, the Ku86-/-mice reduce water excretion in their urine even more than do the Ku86+/+ mice
(Figure 4F, left panel) and the 14-24 month old mice reduce their urine volume
at least as much as do the 2 month old mice Ku86+/+ (Figure 4F,
right panel), so excess loss of water in the urine is not the explanation.
Also, both the Ku86-/- and 14-24 month old mice make urine that is
highly concentrated (albeit slightly less than the Ku86+/+ 2 month
old mice) in response to water restriction (Figure 4E). Thus, poorly regulated
urinary loss does not account for the deficient water balance in these animals.
The alternative is poorly regulated extrarenal loss of water. However, our
present experiments do not identify the route of such loss.
We conclude that old mice do not conserve water as
well as young mice, apparently due to poorly regulated extrarenal loss, and
that the deficiency occurs at an earlier age in mice that lack Ku86.
Discussion
High
NaCl promotes cellular senescence
We
previously found that exposure to high NaCl promotes cellular senescence [4].
The evidence included that: 1) Chronic exposure to high NaCl induces senescence
in HeLa cells and accelerates senescence of primary mefs. 2) Elevated NaCl
reduces the life span of C. elegans, while increasing the number of senescent
cells. 3) Cells become senescent much faster in vivo in mouse renal inner
medullas, where they are normally exposed to elevated interstitial NaCl, than
in the renal cortex where they are not. High NaCl causes DNA damage and
oxidative stress [2,20], which are known precursors of cellular senescence
[9].
Ku86
deficiency accelerates high NaCl-induced cellular senescence in cultures
Cells
in culture adapt to high NaCl despite the presence of a continuously increased
number of DNA breaks. This evidently requires some mechanism for maintaining
chromatin integrity. We previously found that Ku heterodimers are important in
this respect, presumably because they bind to broken ends of DNA and align them
[6]. Thus, high NaCl fragments chromosomes more in Ku86-/- than in
Ku86+/+ mefs. In addition, high NaCl reduces the rate of
proliferation of Ku86-/- more than Ku86+/+ mefs, and a
senescent morphology appears, including cellular enlargement and flattening
[6]. The changes are even more striking in xrs5 cells, which were derived by
ethyl methane-sulphonate mutation of CHO-K1 cells, resulting in loss of Ku86
[7,8]. Upon exposure to high NaCl, these cells enlarge, flatten and become
multinucleated within 2 days, and their cell cycle becomes permanently arrested
[6]. Since these are morphological changes characteristic of cellular
senescence, we tested for that specifically in the present studies by using SA-β-gal, which is a marker of cellular senescence. We find
that by day 3 of exposure to high NaCl the cells do become positive for SA-β-gal (Figure 1), consistent with a role for Ku86 in
delaying high NaCl-induced senescence.
Ku86
deficiency accelerates high NaCl-induced cellular senescence in vivo
Since senescence pathways are modified in
immortalized cells [21,22] we conducted in vivo experiments to test whether
Ku86 protects normal cells from high NaCl-induced cellular senescence. In
previous studies we found that high NaCl accelerates accumulation of senescent
cells and decreases longevity of C. elegans [4]. Absence of cku86 further decreases
longevity of C. elegans exposed to high NaCl (Figure 2), consistent with a role
of Ku86 in delaying NaCl-induced senescence. It is of interest that the age at
which C. elegans are first exposed to high NaCl critically determines its
effect. NaCl reduces longevity of C. elegans only if they are first exposed to
it as larvae (2 days after hatching), not if they are first exposed to it as
adults (4 days after hatching) (Figure 2). A possible explanation is that
somatic cells of adult C. elegans, being postmitotic and unable to divide [15],
are not affected, while the dividing cells in the larvae are affected. The only
proliferating cells in adult C. elegans are contained in the gonads and embryos
in their reproductive tract, and those cells apparently are affected by
exposure to high NaCl. High NaCl decreases the number of progeny from wild type
C. elegans and the decrease is even greater in cku86 C. elegans [6].
We
also tested whether knockout of Ku86 might accelerate high NaCl-induced
cellular senescence in the kidney in vivo. NaCl is normally elevated in renal
inner medullary interstitial fluid, which powers the urinary concentrating and
diluting mechanisms. It is not elevated in the renal cortex. We previously
found that in 12 month old mice there are many more senescent cells in the
inner medulla than in the cortex [4]. In the present studies we tested younger
mice. We found that at 3 months of age there are already many more senescent
cells in the inner medullas of Ku86-/- mice than in Ku86+/+mice. We conclude that Ku86 delays the appearance of high NaCl-induced
senescence in mouse renal inner medullas in vivo.
Does
high NaCl-induced cellular senescence contribute to early aging of Ku86-/-mice?
Ku86-/-mice age prematurely [23,24]. They also have defective NHEJ DNA repair, severe
combined immunodeficiency (scid) [25,26], and chronic inflammation. These
other defects have been considered as possible causes of the accelerated aging.
However, immunodeficiency, alone, apparently is not the cause since mice
deleted for Rag-1, also suffer from scid and chronic inflammation, but do not
age prematurely [27]. Similarly, defective NHEJ, alone, apparently is not the
cause because defects in another NHEJ protein, DNA-PKcs, do not cause prominent
premature aging [28,29]. Having noted that Ku86-/- mice are
susceptible to dehydration from even a very limited restriction of water
(Figure 4), we were led to wonder whether they might be chronically dehydrated
enough to raise their blood NaCl sufficiently to contribute to premature
cellular senescence and aging. Old age is associated with dehydration [18,19,30]. The mechanisms implicated include decreased thirst, which leads to
insufficient water intake and impaired renal response to ADH, which leads to
excessive loss of water in the urine. (reviewed in [18,19]) We find that water
conservation is impaired in old mice and that Ku86 deficiency accelerates the
impairment (Figure 4), like it accelerates other aspects of aging.
Interestingly, water balance is impaired in Ku86-/- mice much
earlier than other aspects of aging, including kyphosis and premature closure
of growth plates. Thus, 3 month old Ku86-/- mice already have
impaired water conservation (Figure 4), whereas kyphosis does not occur until 6
months of age and premature growth plate closure until 5 months [23]. Thus,
impaired water conservation could be contributing to other aspects of premature
aging in Ku86-/- mice.
Methods
Cell culture.
Xrs5
(X-ray sensitive Chinese Hamster Ovary, no.CRL-2348, American Type Culture
Collection, Manassas, VA) is a mutant cell line which was derived from CHO-K1
cells (no.CCL-61, American Type
Culture Collection) by treating the cells with ethyl methanesulphonate.
These cells belong to X-ray complementation group 5 and are mutant in the p86
subunit of the Ku autoantigen [7,8]. We grew the cells in DMEM plus 10% fetal bovine serum (HyClone, Logan, UT). Osmolality of
control ("isotonic") medium was 300 mosmol/kg. High
NaCl medium was prepared by adding NaCl to the total osmolality indicated.
Staining of cells for SA-β-gal
activity.
The Senescence β-Galactosidase
Staining Kit (Cell Signaling, Beverly, MA) was used, as previously described
[4]. Senescent cells are indicated by blue color.
C. elegans
strains and culture
. Bristol N2 (Wild type) and cku80 (ok861) C.
elegans were provided by Caenorhabditis Genetic Center (CGC, Minneapolis, MN). The cku80(ok861) strain contains a homozygous 1,646-bp deletion,
including a large section of coding sequence, in the cku80 locus. The
deletion was confirmed by PCR in our previous publication [6]. The worms were
grown on Nematode Growth Medium agar plates spread with E. coli strain OP50
(obtained from CGC). Cultures were maintained at room temperature (about 20◦C).
Control Nematode Growth Medium contains 51mM NaCl, 1mM MgSO4, 1mM
CaCl2, 25mM KPO4, 5μg/ml cholesterol, 2.5g/l
peptone, and 17g/l agar [31]. We increased NaCl by adding 300 mM, as indicated.
To measure longevity we
transferred L2-L3 larvae or adult C. elegans to
control or high NaCl agar plates. Every other day the original worms were
transferred to new plates to separate them from their progeny. The number
surviving was counted every day. Worms were considered dead if they did not
respond to repeated prodding with a platinum wire.
Immunohistological
detection of p16Ink4 in kidney sections.
Mouse kidneys were fixed
overnight in 4% paraformaldehyde at 4°C, and then embedded in paraffin.
Sections were cut and mounted on silanized slides by American Histolabs (Gaithersburg, MD). Sections were stained with anti-p16 (sc-1207: Santa Cruz, Santa Cruz, CA) as previously described [4]. A Nikon E800 Widefield Microscope was used
for photography.
Measurement
of water balance.
The Ku86-/- mice used in this study were previously
described [25]. Wild type mice were purchased at age of 2-3 months from Taconic
(129S6, Model no.129SVE, Taconic Farms, Inc, Hudson, NY) and housed in the
NHLBI animal facility. All mouse studies were done under approved National
Heart, Lung, and Blood Institute and National Cancer Institute animal study
protocols and mice were housed in an Association for Assessment and
Accreditation of Laboratory Animal Care-accredited facilities. Mice were
maintained in mouse metabolic cages (Hatteras Instruments, Cary, NC) during the study under controlled temperature and light conditions (12-h light and dark
cycles).
The experiment
design is shown on Figure 4A. Initially, all mice received gelled food
containing 43% of water. The gelled food contained 3 ml of deionized water, 4 g
of balanced purified rodent diet (AIN-76A, Research Diets, New Brunswick, NJ),
and 70 mg of agar per 7 g of the food. Food in the metabolic cages wasprovided in excess so the mice could eat what they
wanted. Drinking water was provided ad libitum during this period. After 2 days
of adaptation, mice were subjected to 3 consecutive periods of differing water
availability (Figure 4A). During period I mice had free access to water and the
gel food containing 43% water. Then, supplemental drinking water was removed so
the only water was that contained in the gel food (period II). During period
III, the amount of water in gel food was decreased to 30% (1.7ml of water, 4g
of the rodent diet powder and 57 mg of agar). Body weight, urine volume, food
consumption, urine osmolality and urine Arginine Vasopressin (AVP)
concentration were measured every 24h. Urine was collected under mineral oil in
pre-weighed collection vials. Urine volume was measured gravimetrically, by
assuming a density of one. Gel food was supplied in preweighed plastic cups to
facilitate measurement of consumed food. Urine osmolality was measured using
Fiske Model 210 Freezing-Point Micro-Osmometer (Fiske Associates, Norwood, MA). AVP concentration in urine was measured using Vasopressin Enzyme Immunoassay
Kit (no. 900-017, Assay Designs, Ann Arbor, MI).
Statistics.
Average values during each period were normalized to
period (I). Data were evaluated by t-test, paired t-test comparison to period
(I), unpaired t-test for comparison between groups. A p-value less than 0.05
was considered significant.
We thank Joseph Handler for
suggestions on experimental design and Chris Combs and Daniela Malide of the
National Heart, Lung, and Blood Institute (NHLBI) Light Microscopy Core
Facility for help with microscopy. This research was supported by the
Intramural Research Program of the NIH, NHLBI and
NCI.
The
authors of this manuscript have no conflict of interests to declare.