Validation of anti-aging drugs by treating age-related diseases
Abstract
Humans die from age-related diseases, which are deadly manifestations of the aging process. In order to extend life span, an anti-aging drug must delay age-related diseases. All together age-related diseases are the best biomarker of aging. Once a drug is used for treatment of any one chronic disease, its effect against other diseases (atherosclerosis, cancer, prostate enlargement, osteoporosis, insulin resistance, Alzheimer's and Parkinson's diseases, age-related macular degeneration) may be evaluated in the same group of patients. If the group is large, then the anti-aging effect could be validated in a couple of years. Startlingly, retrospective analysis of clinical and preclinical data reveals four potential anti-aging modalities.
Problem
The discovery of anti-aging drugs is no longer a fantasy.
Numerous genes for aging and longevity have been identified in diverse
organisms, revealing potential targets for potential anti-aging drugs. But how
could potential anti-aging drug be introduced to humans? There are two
problems. First, the effect of anti-aging agents on human aging may require
almost a lifetime to determine [1]. Second, it is seemingly desirable to test
anti-aging drugs in healthy individuals. However, all drugs have side effects.
And, in healthy individuals, side effects would preclude clinical trials. How
might these problems be solved? How could we validate anti-aging drugs in
humans without life-long trials in healthy individuals?
Solution
The solution includes two steps. First,
we must find an indication for a drug to treat at least one chronic disease.
Then this drug could be tested in humans, not as an anti-aging drug, but as
therapy for a particular disease. In fact this approach has been suggested for
introduction of activators of sirtuins to the clinic [2,3].
Second,
we must find a biomarker of aging that absolutely predicts longevity. Then
using this biomarker, the anti-aging effect could be evaluated in the same
patients.
Aging and age-related diseases
Aging
can be defined as an increase in the probability of death. This is how the rate
of aging can be measured. Humans die not from ‘healthy' aging but from
age-related diseases. Healthy aging (a late onset of disease) is associated
with longevity. For example, centenarians show significant delay in the onset
of age-related diseases, including cardiovascular disease, type 2 diabetes,
cancer and Alzheimer's disease. In other words, those who live longer are
healthier and vice versa [4,5]. Since, by definition, all age-dependent
diseases are connected with aging, these diseases are connected to each other.
In fact, aging humans often suffer from many diseases simultaneously: diabetes,
atherosclerosis, hypertension, macular degeneration, prostate enlargement and
prostate cancer (in men) or breast cancer (in women), Alzheimer's disease and
osteoarthritis. This is why elimination of one disease (e.g., cancer) will not
radically extend maximal human lifespan. And as calculated, "the complete
resolution of Alzheimer's disease would add about 19 days onto average life
expectancy" [6]. But if a drug delays or stops all diseases, a person must live
longer. Otherwise what would be the cause of death, if all causes were delayed?
Since human longevity is limited by death from age-related diseases, a true
anti-aging drug must delay age-related diseases. In other words, unless a drug
delays age-related diseases, it will not extend lifespan. And vice versa, if a
drug prevents age-related diseases, it must extend life span.
Biomarker of organismal aging
Given
that (a) an increase in the death rate is a measure of aging and (b) the death
rate is determined diseases taken together, then we can conclude that the sum
of all age-related diseases is the best biomarker of aging. Any one age-related
disease is not a biomarker of aging because, in addition to aging, numerous
factors contribute to the incidence of a particular disease. For example,
smoking increases the risk for lung cancer but not for Parkinson's disease.
Yet, aging is a risk factor for both diseases. And, even for lung cancer, aging
is a bigger risk factor than is smoking. Aging is the biggest risk factor for
all age-related diseases. Whether aging and disease have a common mechanism or
whether aging simply increases vulnerability to diseases, in any case, the
inhibition of aging will delay diseases, thus extending life span.
Disease-specific drugs versus anti-aging agents
Slowing
aging would delay all age-related diseases. If a drug is effective against one
particular disease only, such a drug is not anti-aging. And current drugs are
not anti-aging. For example, insulin compensates diabetes. Yet, insulin does
not treat cancer. And vice versa chemotherapy may treat cancer but does not
treat diabetes. So neither chemotherapy nor insulin is an anti-aging modality.
Furthermore, both insulin and chemotherapy may accelerate aging.
Metformin
The underlying cause of age-related type
II diabetes is insulin resistance. Insulin treatment does not ‘treat' the
cause, it just compensates for resistance. Unlike insulin, metformin, an oral
anti-diabetic drug, restores insulin sensitivity in type diabetes type II.
Remarkably, metformin decreases the incidence of breast cancer [7,8]. Also,
metformin is considered for cancer treatment [9] and inhibits atherosclerosis
in diabetic mice [10]. Metformin is used to induce ovulation in patients with
polycystic ovary syndrome (PCOS). Six months of 1700 mg/d metformin treatment
improved fertility in anovulatory PCOS women [11,12]. Given such effects on
infertility, type II diabetes, cancer and atherosclerosis, it is plausible that
metformin slows aging. In fact, it extends life span in rodents [13-15].
Calorie restriction
Calorie restriction (CR) extends life span from yeast and worms to rodents and perhaps
humans [16-18]. If we did not already know that CR slows aging, how might we
figure that out based solely on clinical data? Unrestricted food consumption
leads to obesity associated with diabetes, atherosclerosis, thrombosis,
hypertension, cancer (especially breast, prostate and colon cancer), coronary
heart disease, stroke, osteoporosis and Alzheimer's disease [19-25]. In other
words, unrestricted eating in humans (ad libitum in rodents) accelerates most,
if not all, diseases of aging. So we can conclude that CR delays all diseases
of aging. This suggests that CR is an anti-aging modality. And it is known that
CR extends life span in almost all organisms from yeast to mammals.
From metformin and calorie restriction to rapamycin
Numerous factors including insulin, glucose and amino acids activate the
nutrient-sensing TOR (target of rapamycin) pathway. When the TOR pathway is
activated, it acts via S6K to deplete the insulin-receptor-substrate (IRS1/2),
causing insulin resistance (Figure 1). As shown in Figure 1, metformin
indirectly (by activating AMPK) inhibits TOR and thereby restores insulin
sensitivity [26].
CR decreases levels of nutrients and insulin and thus de-activates TOR (Figure 1).
It is possible that the anti-aging effects of CR and metformin are due to
inhibition of the TOR pathway. Like CR, rapamycin decreases size of fat cells
and animal weight. When rats (15 weeks old) were either treated 1 mg/kg
rapamycin 3 times per week for 12 weeks, rapamycin decreased their weight. Mean
adipocyte diameter was decreased from 36 μm to 25 μm. At the end of the study,
mean body weight in the rapamycin-treated rats was 356 g instead of 507 g, in
spite of comparable food intake [27]. So rapamycin imitated CR. CR may also
extend life span by activating sirtuins. Probably, sirtuins, AMPK and mTOR are
linked in the common network [28].
Genetic
inhibition of the TOR pathway slows down aging in diverse organisms, including
yeast, worms, flies and mice [29-33]. If
genetic inhibition of the TOR pathway slows aging, then rapamycin, a drug that inhibits
TOR, must slow aging too. Once used for any indication, even unrelated to
age-related diseases (such as renal transplantation, for instance), an
anti-aging drug should slow down age-related diseases such as cancer,
osteoporosis and atherosclerosis. Rapamycin is already used in renal transplant
patients.
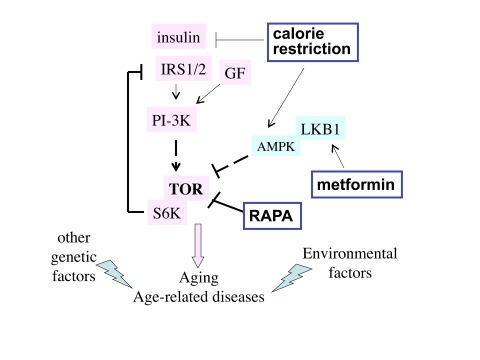
Figure 1. The TOR intracellular signaling pathway. Nutrients, GF (growth factors)
and insulin activate the TOR pathway, which is involved in aging and
age-related diseases. Other genetic factors and environmental factors
(e.g., smoking) contribute to specific age-related diseases. Three
potential anti-aging modalities (metformin, calorie restriction and
rapamycin) all inhibit the TOR pathway.
Retrospective analysis of the clinical use of rapamycin
Rapamycin has been used in renal-transplant patients for several years. Since rapamycin
was viewed as an immunossupressive drug (not as an anti-aging drug) it was
expected that it would cause cancer.
Unexpectedly, it turned out that rapamycin prevented cancer, and even cured pre-existing cancer and Kaposi's
sarcoma in renal transplant patients [34-44]. Furthermore, temsirolimus, an
analog of rapamycin, has recently been approved for cancer therapy [45]. Also,
everolimus, a TOR inhibitor, markedly delayed tumor development in transgenic
mice that spontaneously develop ovarian carcinomas [46]. Would TOR inhibitors
extend life span in transgenic mice? Since rapamycin delays cancer, it must
prolong the life span of cancer-prone mice, who would otherwise die from
cancer. Of course, humans die from a variety of age-related diseases, not from
just one disease. To prolong life
span dramatically, rapamycin must delay most of them.
In
renal transplant patients, rapamycin increases blood lipoproteins [47]. This is
considered to be a negative side effect. Yet, this results from mobilization of
fat from the fat tissue (lipolysis) [48,49]. This is exactly what happens
during starvation or calorie restriction (CR). And CR extends life span. Furthermore,
rapamycin reduces the accumulation of cholesterol within the arterial wall [50,51]. Thus, lipolysis of fat tissue and decreased uptake of cholesterol by
tissues both contribute to high levels of lipids in blood (Figure 2). Despite
hypercholesterolemia, rapamycin prevents atherosclerosis in animals [52]. In
animal models, systemic administration of rapamycin reduces neointimal
thickening and slows the progression of atherosclerosis in apoE-deficient mice
with elevated levels of cholesterol [53-55]. In patients with coronary
atherosclerosis, oral rapamycin prevents re-stenosis after implantation of
metal stents [56]. As a case report, it has been described that conversion to
everolimus (an analog of rapamycin) resulted in decrease in blood pressure
[57]. In kidney transplant patients, 2 years after transplantation, body-mass
index was significantly lower in the rapamycin-based treatment arm compared to
cyclosporine [27].
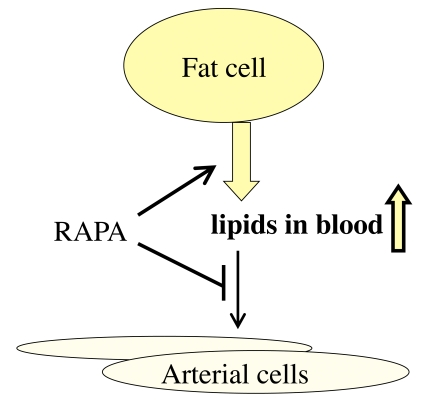
Figure 2. Re-interpretation of the hyperlipidemic side effect of rapamycin. Rapamycin
activates adipose tissue lipase, thus mobilizing lipids from the fat tissue
(lipolysis). This effect imitates starvation. Also, rapamycin inhibits
lipoprotein lipase thus preventing utilization of lipids by the fat tissue
and blocking lipid uptake by the arterial wall. This results in increase
in blood lipids.
Multiple indications for a single drug
If
a drug is indicated to treat most age-related diseases, then this drug could be
defined as an anti-aging drug. The probability that a non-anti-aging drug would
have independent activities against all diseases is exceedingly low.
Rapamycin
analogs are approved to treat certain cancers [45]. Based on preclinical data,
rapamycin has been considered in such pathologies as obesity [58],
atherosclerosis [53-55], cardiac hypertrophy [59-64], aortic aneurysm [65],
osteoporosis [66-68], organ fibrosis (liver, renal, cardiac fibrosis) [64,69,70-75], neurodegeneration [76,77], Alzheimer's disease [78,79], Parkinson's
disease [80-82], psoriasis [80], skin scars and keloids [83], multiple
sclerosis [84], arthritis [85,86], and renal hypertrophy in diabetes [87].
May rapamycin increase human life span?
In principle, life-extending effect of
anti-aging drug might be limited by side effects. Although chronic
administration of rapamycin is associated with some undesirable effects in
transplant patients (see for references [88]), they might be avoided by
administrating rapamycin in pulses (for example, once a week). For example,
chronic administration of rapamycin impairs wound healing. In theory, a pulse
treatment might rejuvenate wound-healing cells [88]. A single dose of rapamycin
reverses insulin resistance, whereas chronic administration of rapamycin may
precipitate diabetes in certain conditions. Clinical trials will be needed to
determine benefits of pulse treatment with rapamycin. Alternatively, rapamycin
can be combined with ‘complementary' drugs. Thus, hyperlipidemia caused by
rapamycin may deteriorate insulin-resistance. Yet, hyperlipidemia caused by
rapamycin can be controlled by lipid-lowering drugs. A combination of rapamycin
with resveratrol may be especially intriguing.
Resveratrol
Resveratrol, an activator of SIRT1 in mammals, extends life span in diverse species
[89,90].
Resveratrol was shown to prevent cancer, atherosclerosis, neuro-degeneration
and insulin-resistance (diabetes type II) [10,91-100]. Resveratrol also
indirectly inhibits PI-3K/mTOR/S6K pathway [101-105]. SIRT1 and mTOR could be
members of the same sirtuin/TOR network. The link between TOR and sirtuins has
been suggested [28]. It is likely that TOR (pro-aging pathway) and sirtuins (anti-aging
pathway) antagonize each other [106]. However, inhibition of the TOR pathway by
resveratrol occurs at near-toxic concentrations [107].
The ability of resveratrol to extend life span may be
limited by its toxicity at high doses due to off-target effects. Therefore,
more selective activators of SIRT1 undergo clinical trials [3]. Importantly,
these drugs will be developed to treat age-related diseases such as type 2
diabetes [3]. This is the only possible strategy for a drug to enter the
clinic. But here is an additional aspect: this is the only practical way of how
anti-aging effect can be evaluated too. Once used for treatment of diabetes,
sirtuin activators might delay heart diseases, cancer, neurodegeneration and
other age-related diseases in the same patients. And delaying of all diseases
must extend life span, thus validating a drug as anti-aging.
Conclusion
It was previously assumed that anti-aging drugs should be tested in healthy
individuals. Ironically, the best biomarker of aging is the occurrence of
age-related diseases. And this is how anti-aging drugs can be validated in the
clinic (by showing that a putative anti-aging drug can prevent or delay the
onset of all age-related diseases). Then such drugs could be approved for
prevention of any particular age-related disease in healthy individuals. Thus,
potential anti-aging drugs should be introduced to the clinical trials for
therapy of a particular disease but be ultimately approved for prevention of all
age-related diseases in healthy individuals. And this is synonymous to the
approval of a drug as anti-aging.
Acknowledgments
This work was not funded by any
sources. The author is a founder of Oncotarget but is not employed by the
company and declares no conflicts of interests.
References
-
1.
Hadley
EC
, lakatta
EG
, Morrison-Bogorad
M
, Warner
HR
and Hodes
RJ.
The future of aging therapies.
Cell.
2005;
120:
557
-567.
[PubMed]
.
-
2.
Sinclair
DA
and Guarente
L.
Unlocking the secrets of longevity genes.
Sci Am.
2006;
294:
48
-51.
[PubMed]
.
-
3.
Milne
JC
, Lambert
PD
, Schenk
S
, Carney
DP
, Smith
JJ
, Gagne
DJ
, Jin
L
, Boss
O
, Perni
RB
, Vu
CB
, Bemis
JE
, Xie
R
, Disch
JS
, Ng
PY
, Nunes
JJ
, Lynch
AV
, Yang
H
, Galonek
H
, Israelian
K
, Choy
W
, Iffland
A
, Lavu
S
, Medvedik
O
, Sinclair
DA
, Olefsky
JM
, Jirousek
MR
, Elliott
PJ
and Westphal
CH.
Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes.
Nature.
2007;
450:
712
-716.
[PubMed]
.
-
4.
Perls
T
, Kunkel
L
and Puca
A.
The genetics of aging.
Curr Opin Genet Dev.
2002;
12:
362
-369.
[PubMed]
.
-
5.
Curtis
R
, Geesaman
BJ
and DiStefano
PS.
Ageing and metabolism: drug discovery opportunities.
Nat Rev Drug Discov.
2005;
4:
569
-580.
[PubMed]
.
-
6.
Hayflick
L
The future of ageing.
Nature.
2000;
408:
267
-269.
[PubMed]
.
-
7.
Evans
JM
Metformin and reduced risk of cancer in diabetic patients.
BMJ.
2005;
330:
1304
-1305.
[PubMed]
.
-
8.
Johnson
JA
, Majumdar
SR
, Simpson
SH
and Toth
EL.
Decreasedmortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes.
Diabetes Care.
2002;
25:
2244
-2248.
[PubMed]
.
-
9.
Buzzai
M
, Jones
RG
, Amaravadi
RK
, Lum
JJ
, DeBerardinis
RJ
, Zhao
F
, Viollet
B
and Thompson
CB.
Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth.
Cancer Res.
2007;
67:
6745
-6752.
[PubMed]
.
-
10.
Zang
M
, Xu
S
, Maitland-Toolan
KA
, Zuccollo
A
, Hou
X
, Jiang
B
, Wierzbicki
M
, Verbeuren
TJ
and Cohen
RA.
Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice.
Diabetes.
2006;
55:
2180
-2191.
[PubMed]
.
-
11.
Cheang
KI
, Sharma
ST
and Nestler
JE.
Is metformin a primary ovulatory agent in patients with polycystic ovary syndrome.
Gynecol Endocrinol.
2006;
22:
595
-604.
[PubMed]
.
-
12.
Palomba
S
, Orio
FJ
, Falbo
A
, Russo
T
, Tolino
A
and Zullo
F.
Clomiphene citrate versus metformin as first-line approach for the treatment of anovulation in infertile patients with polycystic ovary syndrome.
J Clin Endocrinol Metab.
2007;
92:
3498
-3503.
[PubMed]
.
-
13.
Dilman
VM
and Anisimov
VN.
Effect of treatment with phenformin, diphenylhydantoin or L-dopa on life span and tumour incidence in C3H/Sn mice.
Gerontology.
1980;
26:
241
-246.
[PubMed]
.
-
14.
Anisimov
VN
, Egormin
PA
, Bershtein
LM
, Zabezhinskii
MA
, Piskunova
TS
, Popovich
IG
and Semenchenko
AV.
Metformin decelerates aging and development of mammary tumors in HER-2/neu transgenic mice.
Bull Exp Biol Med.
2005;
139:
721
-723.
[PubMed]
.
-
15.
Anisimov
VN
, Berstein
LM
, Egormin
PA
, Piskunova
TS
, Popovich
IG
, Zabezhinski
MA
, Tyndyk
ML
, Yurova
MV
, Kovalenko
IG
, Poroshina
TE
and Semenchenko
AV.
Metformin slows down aging and extends life span of female SHR mice.
Cell Cycle.
2008;
7:
2769
-2773.
[PubMed]
.
-
16.
Heilbronn
LK
and Ravussin
E.
Calorie restriction and aging: review of the literature and implications for studies in humans.
Am J Clin Nutr.
2003;
78:
361
-369.
[PubMed]
.
-
17.
Kennedy
BK
, Steffen
KK
and Kaeberlein
M.
Ruminations on dietary restriction and aging.
Cell Mol Life Sci.
2007;
64:
1323
-1328.
[PubMed]
.
-
18.
Everitt
AV
and Le
Couteur DG.
Life extension by calorie restriction in humans.
Ann N Y Acad Sci.
2007;
1114:
428
-433.
[PubMed]
.
-
19.
Anisimov
VN
Premature ageing prevention: limitations and perspectives of pharmacological intervention.
Curr Drug Targets.
2006;
7:
1485
-1504.
[PubMed]
.
-
20.
Ingram
DK
, Zhu
M
, Mamczarz
J
, Zou
S
, Lane
MA
, Roth
GS
and deCabo
R.
Calorie restriction mimetics: an emerging research field.
Aging Cell.
2006;
5:
97
-108.
[PubMed]
.
-
21.
Holloszy
JO
and Fontana
L.
Caloric restriction in humans.
Exp Gerontol.
2007;
42:
709
-12.
[PubMed]
.
-
22.
Wilson
PW
and Kannel
WB.
Obesity, diabetes, and risk of cardiovascular disease in the elderly.
Am J Geriatr Cardiol.
2002;
11:
119
-123.
[PubMed]
.
-
23.
Zamboni
M
, Mazzali
G
, Zoico
E
, Harris
TB
, Meigs
JB
, Di Francesco
V
, Fantin
F
, Bissoli
L
and Bosello
O.
Health consequences of obesity in the elderly: a review of four unresolved questions.
Int J Obes (Lond).
2005;
29:
1011
-1029.
[PubMed]
.
-
24.
Fontana
L
, Meyer
TE
, Klein
S
and Holloszy
JO.
Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans.
Proc Natl Acad Sci U S A.
2004;
101:
6659
-6663.
[PubMed]
.
-
25.
Bray
GA
Medical consequences of obesity.
J Clin Endocrinol Metab.
2004;
89:
2583
-2594.
[PubMed]
.
-
26.
Shaw
RJ
, Lamia
KA
, Vasquez
D
, Koo
SH
, Bardeesy
N
, Depinho
RA
, Montminy
M
and Cantley
LC.
The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin.
Science.
2005;
310:
1642
-1646.
[PubMed]
.
-
27.
Rovira
J
, Marcelo
Arellano E
, Burke
JT
, Brault
Y
, Moya-Rull
D
, Bañón-Maneus
E
, Ramírez-Bajo
MJ
, Gutiérrez-Dalmau
A
, Revuelta
I
, Quintana
LF
, Campistol
JM
and Diekmann
F.
Effect of mTOR inhibitor on body weight: from an experimental rat model to human transplant patients.
Transpl Int.
2008;
21:
992
-998.
[PubMed]
.
-
28.
Medvedik
O
, Lamming
DW
, Kim
KD
and Sinclair
DA.
MSN2 and MSN4 Link Calorie Restriction and TOR to Sirtuin-Mediated Lifespan Extension in Saccharomyces cerevisiae.
PLoS Biol.
2007;
5:
e261
[PubMed]
.
-
29.
Vellai
T
, Takacs-Vellai
K
, Zhang
Y
, Kovacs
AL
, Orosz
L
and Muller
F.
Genetics: influence of TOR kinase on lifespan in C. elegans.
Nature.
2003;
426:
620
[PubMed]
.
-
30.
Jia
K
, Chen
D
and Riddle
DL.
The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span.
Development.
2004;
131:
3897
-3906.
[PubMed]
.
-
31.
Tatar
M
, Kopelman
A
, Epstein
D
, Tu
MP
, Yin
CM
and Garofalo
RS.
A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function.
Science.
2001;
292:
107
-110.
[PubMed]
.
-
32.
Kapahi
P
, Zid
BM
, Harper
T
, Koslover
D
, Sapin
V
and Benzer
S.
Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway.
Curr Biol.
2004;
14:
885
-890.
[PubMed]
.
-
33.
Bartke
A
Long-lived Klotho mice: new insights into the roles of IGF-1 and insulin in aging.
Trends Endocrinol Metab.
2006;
17:
33
-35.
[PubMed]
.
-
34.
Law
BK
Rapamycin: an anti-cancer immunosuppressant.
Crit Rev Oncol Hematol.
2005;
56:
47
-60.
[PubMed]
.
-
35.
Nungaray
N
, Arriola
M
, Gutierrez
MJ
, Oliva
E
, Hernandez
E
, Gonzalez
E
, Andres
A
and Morales
JM.
Rapamycin at six years can exhibit normal renal function without proteinuria or neoplasia after renal transplantation. A single-center experience.
Transplant Proc.
2005;
37:
3727
-3728.
[PubMed]
.
-
36.
Kauffman
HM
, Cherikh
WS
, Cheng
Y
, Hanto
DW
and Kahan
BD.
Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies.
Transplantation.
2005;
80:
883
-889.
[PubMed]
.
-
37.
Zmonarski
SC
, Boratynska
M
, Rabczynski
J
, Kazimierczak
K
and Klinger
M.
Regression of Kaposi's sarcoma in renal graft recipients after conversion to sirolimus treatment.
Transplant Proc.
2005;
37:
964
-966.
[PubMed]
.
-
38.
Rizell
M
, Cahlin
C
, Friman
S
, Hafstrom
L
, Lonn
L
, Olausson
M
and Lindner
P.
Impressive regression of primary liver cancer after treatment with sirolimus.
Acta Oncol.
2005;
44:
496
[PubMed]
.
-
39.
Stallone
G
, Schena
A
, Infante
B
, Di Paolo
S
, Loverre
A
, Maggio
G
, Ranieri
E
, Gesualdo
L
, Schena
FP
and Grandaliano
G.
Sirolimus for Kaposi's sarcoma in renal-transplant recipients.
N Engl J Med.
2005;
352:
1317
-1323.
[PubMed]
.
-
40.
Yakupoglu
YK
, Buell
JF
, Woodle
S
and Kahan
BD.
Individualization of Immunosuppressive Therapy. III. Sirolimus Associated With a Reduced Incidence of Malignancy.
Transplant Proc.
2006;
38:
358
-361.
[PubMed]
.
-
41.
Campistol
JM
, Eris
J
, Oberbauer
R
, Friend
P
, Hutchison
B
, Morales
JM
, Claesson
K
, Stallone
G
, Russ
G
, Rostaing
L
, Kreis
H
, Burke
JT
, Brault
Y
, Scarola
JA
and Neylan
JF.
Sirolimus Therapy after Early Cyclosporine Withdrawal Reduces the Risk for Cancer in Adult Renal Transplantation.
J Am Soc Nephrol.
2006;
17:
581
-589.
[PubMed]
.
-
42.
Mathew
T
, Kreis
H
and Friend
P.
Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies.
Clin Transplant.
2004;
18:
446
-449.
[PubMed]
.
-
43.
Mohsin
N
, Budruddin
M
, Pakkyara
A
, Darweesh
A
, Nayyer
M
, Amitabh
J
and Daar
AS.
Complete regression of visceral Kaposi's sarcoma after conversion to sirolimus.
Exp Clin Transplant.
2005;
3:
366
-369.
[PubMed]
.
-
44.
Cullis
B
, D'Souza
R
, McCullagh
P
, Harries
S
, Nicholls
A
, Lee
R
and Bingham
C.
Sirolimus-induced remission of posttransplantation lymphoproliferative disorder.
Am J Kidney Dis.
2006;
47:
e67
-72.
[PubMed]
.
-
45.
Hudes
G
Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma.
N Engl J Med.
2007;
356:
2271
-81.
[PubMed]
.
-
46.
Mabuchi
S
, Altomare
DA
, Connolly
DC
, Klein-Szanto
A
, Litwin
S
, Hoelzle
MK
, Hensley
HH
, Hamilton
TC
and Testa
JR.
RAD001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer.
Cancer Res.
2007;
67:
2408
-2413.
[PubMed]
.
-
47.
Legendre
C
, Campistol
JM
, Squifflet
JP
and Burke
JT.
Cardiovascular risk factors of sirolimus compared with cyclosporine: early experience from two randomized trials in renal transplantation.
Transplant Proc.
2003;
35:
151S
-153S.
[PubMed]
.
-
48.
Morrisett
JD
, Abdel-Fattah
G
, Hoogeveen
R
, Mitchell
E
, Ballantyne
CM
, Pownall
HJ
, Opekun
AR
, Jaffe
JS
, Oppermann
S
and Kahan
BD.
Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients.
J Lipid Res.
2002;
43:
1170
-1180.
[PubMed]
.
-
49.
Morrisett
JD
, Abdel-Fattah
G
and Kahan
BD.
Sirolimus changes lipid concentrations and lipoprotein metabolism in kidney transplant recipients.
Transplant Proc.
2003;
35:
143S
-150S.
[PubMed]
.
-
50.
Basso
MD
, Nambi
P
and Adelman
SJ.
Effect of sirolimus on the cholesterol content in ApoE knockout mice.
Transplant Proc.
2003;
35:
3136
-3138.
[PubMed]
.
-
51.
Ma
KL
, Ruan
XZ
, Powis
SH
, Moorhead
JF
and Varghese
Z.
Anti-atherosclerotic effects of sirolimus on human vascular smooth muscle cells.
Am J Physiol Heart Circ Physiol.
2007;
292:
H2721
-2728.
[PubMed]
.
-
52.
Mueller
MA
, Beutner
F
, Teupser
d
, Ceglarek
U
and Thiery
J.
Prevention of atherosclerosis by the mTOR inhibitor everolimis in LDLR-/- mice despite hypercholesterolemia.
Atherosclerosis.
2008;
198:
39
-48.
[PubMed]
.
-
53.
Pakala
R
, Stabile
E
, Jang
GJ
, Clavijo
L
and Waksman
R.
Rapamycin attenuates atherosclerotic plaque progression in apolipoprotein E knockout mice: inhibitory effect on monocyte chemotaxis.
J Cardiovasc Pharmacol.
2005;
46:
481
-486.
[PubMed]
.
-
54.
Elloso
MM
, Azrolan
N
, Sehgal
SN
, Hsu
PL
, Phiel
KL
, Kopec
CA
, Basso
MD
and Adelman
SJ.
Protective effect of the immuno-suppressant sirolimus against aortic atherosclerosis in apo E-deficient mice.
Am J Transplant.
2003;
3:
562
-569.
[PubMed]
.
-
55.
Waksman
R
, Pakala
R
and Burnett
MS.
Oral rapamycin inhibits growth of atherosclerotic plaque in apoE knock-out mice.
Cardiovasc Radiat Med.
2003;
4:
34
-38.
[PubMed]
.
-
56.
Rodriguez
AE
, Granada
JF
, Rodriguez-Alemparte
M
, Vigo
CF
, Delgado
J
, Fernandez-Pereira
C
, Pocovi
A
, Rodriguez-Granillo
AM
, Schulz
D
, Raizner
AE
, Palacios
I
, O'neill
W
, Kaluza
GL
, Stone
G
and Investigators
OI.
Oral Rapamycin After Coronary Bare-Metal Stent Implantation to Prevent Restenosis The Prospective, Randomized Oral Rapamycin in Argentina (ORAR II) Study.
J Am Coll Cardiol.
2006;
47:
1522
-1529.
[PubMed]
.
-
57.
Pascual
J
, Fernández
AM
, Marcén
R
and Ortuño
J.
Conversion to everolimus in a patient with arterial hypertension and recurrent cutaneous neoplasia - a case report.
Nephrol Dial Transplant.
2006;
21 Suppl 3:
iii38
-iii41.
[PubMed]
.
-
58.
Um
SH
, Frigerio
F
, Watanabe
M
, Picard
F
, Joaquin
M
, Sticker
M
, Fumagalli
S
, Allegrini
PR
, Kozma
SC
, Auwerx
J
and Thomas
G.
Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity.
Nature.
2004;
431:
200
-205.
[PubMed]
.
-
59.
Oudit
GY
, Sun
H
, Kerfant
BG
, Crackower
MA
, Penninger
JM
and Backx
PH.
The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease.
J Mol Cell Cardiol.
2004;
37:
449
-471.
[PubMed]
.
-
60.
Tu
VC
, Bahl
JJ
and Chen
QM.
Signals of oxidant-induced cardiomyocyte hypertrophy: key activation of p70 S6 kinase-1 and phosphoinositide 3-kinase.
J Pharmacol Exp Ther.
2002;
300:
1101
-1110.
[PubMed]
.
-
61.
Sadoshima
J
and Izumo
S.
Rapamycin selectively inhibits angiotensin II-induced increase in protein synthesis in cardiac myocytes in vitro. Potential role of 70-kD S6 kinase in angiotensin II-induced cardiac hypertrophy.
Circ Res.
1995;
77:
1040
-1052.
[PubMed]
.
-
62.
Shioi
T
, McMullen
JR
, Kang
PM
, Douglas
PS
, Obata
T
, Franke
TF
, Cantley
LC
and Izumo
S.
Akt/protein kinase B promotes organ growth in transgenic mice.
Mol Cell Biol.
2002;
22:
2799
-2809.
[PubMed]
.
-
63.
Ha
T
, Li
Y
, Gao
X
, McMullen
JR ST
, Izumo
S
, Kelley
JL
, Zhao
A
, Haddad
GE
, Williams
DL
, Browder
IW
, Kao
RL
and Li
C.
Attenuation of cardiac hypertrophy by inhibiting both mTOR and NFkappaB activation in vivo.
Free Radic Biol Med.
2005;
39:
1570
-1580.
[PubMed]
.
-
64.
McMullen
JR
, Sherwood
MC
, Tarnavski
O
, Zhang
L
, Dorfman
AL
, Shioi
T
and Izumo
S.
Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload.
Circulation.
2004;
109:
3050
-3055.
[PubMed]
.
-
65.
Lawrence
DM
, Singh
RS
, Franklin
DP
, Carey
DJ
and Elmore
JR.
Rapamycin suppresses experimental aortic aneurysm growth.
J Vasc Surg.
2004;
40:
334
-338.
[PubMed]
.
-
66.
Kneissel
M
, Luong-Nguyen
NH
, Baptist
M
, Cortesi
R
, Zumstein-Mecker
S
, Kossida
S
, O'Reilly
T
, Lane
H
and Susa
M.
Everolimus suppresses cancellous bone loss, bone resorption, and cathepsin K expression by osteoclasts.
Bone.
2004;
35:
1144
-1156.
[PubMed]
.
-
67.
Glantschnig
H
, Fisher
JE
, Wesolowski
G
, Rodan
GA
and Reszka
AA.
M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase.
Cell Death Differ.
2003;
10:
1165
-1177.
[PubMed]
.
-
68.
Sugatani
T
and Hruska
KA.
Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors.
J Biol Chem.
2005;
280:
3583
-3589.
[PubMed]
.
-
69.
Shegogue
D
and Trojanowska
M.
Mammalian target of rapamycin positively regulates collagen type I production via a phosphatidylinositol 3-kinase-independent pathway.
J Biol Chem.
2004;
279:
23166
-23175.
[PubMed]
.
-
70.
Gabele
E
, Reif
S
, Tsukada
S
, Bataller
R
, Yata
Y
, Morris
T
, Schrum
LW
, Brenner
DA
and Rippe
RA.
The role of p70S6K in hepatic stellate cell collagen gene expression and cell proliferation.
J Biol Chem.
2005;
280:
13374
-13382.
[PubMed]
.
-
71.
Bonegio
RG
, Fuhro
R
, Wang
Z
, Valeri
CR
, Andry
C
, Salant
DJ
and Lieberthal
W.
Rapamycin ameliorates proteinuria-associated tubulointerstitial inflammation and fibrosis in experimental membranous nephropathy.
J Am Soc Nephrol.
2005;
16:
2063
-2072.
[PubMed]
.
-
72.
Poulalhon
N
, Farge
D
, Roos
N
, Tacheau
C
, Neuzillet
C
, Michel
L
, Mauviel
A
and Verrecchia
F.
Modulation of collagen and MMP-1 gene expression in fibroblasts by the immunosuppressive drug rapamycin: A direct role as an anti-fibrotic agent.
J Biol Chem.
2006;
281:
33045
-52.
[PubMed]
.
-
73.
Gao
XM
, Wong
G
, Wang
B
, Kiriazis
H
, Moore
XL
, Su
YD
, Dart
A
and Du
XJ.
Inhibition of mTOR reduces chronic pressure-overload cardiac hypertrophy and fibrosis.
J Hypertens.
2006;
24:
1663
-1670.
[PubMed]
.
-
74.
Shioi
T
, McMullen
JR
, Tarnavski
O
, Converso
K
, Sherwood
MC
, Manning
WJ
and Izumo
S.
Rapamycin attenuates load-induced cardiac hypertrophy in mice.
Circulation.
2003;
107:
1664
-1670.
[PubMed]
.
-
75.
Wu
MJ
, Wen
MC
, Chiu
YT
, Chiou
YY
, Shu
KH
and Tang
MJ.
Rapamycin attenuates unilateral ureteral obstruction-induced renal fibrosis.
Kidney Int.
2006;
69:
2029
-2036.
[PubMed]
.
-
76.
Khurana
V
, Lu
Y
, Steinhilb
ML
, Oldham
S
, Shulman
JM
and Feany
MB.
TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model.
Curr Biol.
2006;
16:
230
-241.
[PubMed]
.
-
77.
Berger
Z
, Ravikumar
B
, Menzies
FM
, Oroz
LG
, Underwood
BR
, Pangalos
MN
, Schmitt
I
, Wullner
U
, Evert
BO
, O'Kane
CJ
and Rubinsztein
DC.
Rapamycin alleviates toxicity of different aggregate-prone proteins.
Hum Mol Genet.
2006;
15:
433
-442.
[PubMed]
.
-
78.
Li
X
, Alafuzoff
I
, Soininen
H
, Winblad
B
and Pei
JJ.
Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer's disease brain.
FEBS J.
2005;
272:
4211
-4220.
[PubMed]
.
-
79.
An
WL
, Cowburn
RF
, Li
L
, Braak
H
, Alafuzoff
I
, Iqbal
K
, Iqbal
IG
, Winblad
B
and Pei
JJ.
Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer's disease.
Am J Pathol.
2003;
163:
591
-607.
[PubMed]
.
-
80.
Marsland
AM
and Griffiths
CE.
The macrolide immunosuppressants in dermatology: mechanisms of action.
Eur J Dermatol.
2002;
12:
618
-622.
[PubMed]
.
-
81.
Wullschleger
S
, Loewith
R
and Hall
MN.
TOR signaling in growth and metabolism.
Cell.
2006;
124:
471
-484.
[PubMed]
.
-
82.
Pannu
J
and Trojanowska
M.
Recent advances in fibroblast signaling and biology in scleroderma.
Curr Opin Rheumatol.
2004;
16:
739
-745.
[PubMed]
.
-
83.
Ong
CT
, Khoo
YT
, Mukhopadhyay
A
, Do
DV
, Lim
IJ
, Aalami
O
and Phan
TT.
mTOR as a potential therapeutic target for treatment of keloids and excessive scars.
Exp Dermatol.
2007;
16:
394
-404.
[PubMed]
.
-
84.
Farrell
R
, Heaney
D
and Giovannoni
G.
Emerging therapies in multiple sclerosis.
Expert Opin Emerg Drugs.
2005;
10:
797
-816.
[PubMed]
.
-
85.
Carlson
RP
, Hartman
DA
, Tomchek
LA
, Walter
TL
, Lugay
JR
, Calhoun
W
, Sehgal
SN
and Chang
JY.
Rapamycin, a potential disease-modifying antiarthritic drug.
J Pharmacol Exp Ther.
1993;
266:
1125
-1138.
[PubMed]
.
-
86.
Foroncewicz
B
, Mucha
K
, Paczek
L
, Chmura
A
and Rowinski
W.
Efficacy of rapamycin in patient with juvenile rheumatoid arthritis.
Transpl Int.
2005;
18:
366
-368.
[PubMed]
.
-
87.
Sakaguchi
M
, Isono
M
, Isshiki
K
, Sugimoto
T
, Koya
D
and Kashiwagi
A.
Inhibition of mTOR signaling with rapamycin attenuates renal hypertrophy in the early diabetic mice.
Biochem Biophys Res Commun.
2006;
340:
296
-301.
[PubMed]
.
-
88.
Blagosklonny
MV
Aging, stem cells, and mammalian target of rapamycin: a prospect of pharmacologic rejuvenation of aging stem cells.
Rejuvenation Res.
2008;
11:
801
-808.
[PubMed]
.
-
89.
Howitz
KT
, Bitterman
KJ
, Cohen
HY
, Lamming
DW
, Lavu
S
, Wood
JG
, Zipkin
RE
, Chung
P
, Kisielewski
A
, Zhang
LL
, Scherer
B
and Sinclair
DA.
Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan.
Nature.
2003;
425:
191
-196.
[PubMed]
.
-
90.
Bauer
JH
, Goupil
S
, Garber
GB
and Helfand
SL.
An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster.
Proc Natl Acad Sci U S A.
2004;
101:
12980
-12985.
[PubMed]
.
-
91.
Jang
M
, Cai
L
, Udeani
GO
, Slowing
KV
, Thomas
CF
, Beecher
CW
, Fong
HH
, Farnsworth
NR
, Kinghorn
AD
, Mehta
RG
, Moon
RC
and Pezzuto
JM.
Cancer chemopreventive activity of resveratrol, a natural product derived from grapes.
Science.
1997;
275:
218
-220.
[PubMed]
.
-
92.
Baur
JA
and Sinclair
DA.
Therapeutic potential of resveratrol: the in vivo evidence.
Nat Rev Drug Discov.
2006;
5:
493
-506.
[PubMed]
.
-
93.
Westphal
CH
, Dipp
MA
and Guarente
L.
A therapeutic role for sirtuins in diseases of aging.
Trends Biochem Sci.
2007;
555-60:
555
-560.
[PubMed]
.
-
94.
Lavu
S
, Boss
O
, Elliott
PJ
and Lambert
PD.
Sirtuins--novel therapeutic targets to treat age-associated diseases.
Nat Rev Drug Discov.
2008;
10:
841
-853.
[PubMed]
.
-
95.
Wang
RH
, Sengupta
K
, Li
C
, Kim
HS
, Cao
L
, Xiao
C
, Kim
S
, Xu
X
, Zheng
Y
, Chilton
B
, Jia
R
, Zheng
ZM
, Appella
E
, Wang
XW
, Ried
T
and Deng
CX.
Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice.
Cancer Cell.
2008;
14:
312
-323.
[PubMed]
.
-
96.
Guarente
L
Sirtuins in aging and disease.
Cold Spring Harb Symp Quant Biol.
2007;
72:
483
-488.
[PubMed]
.
-
97.
Kim
D
, Nguyen
MD
, Dobbin
MM
, Fischer
A
, Sananbenesi
F
, Rodgers
JT
, Delalle
I
, Baur
JA
, Sui
G
, Armour
SM
, Puigserver
P
, Sinclair
DA
and Tsai
LH.
SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis.
EMBO J.
2007;
26:
3169
-3179.
[PubMed]
.
-
98.
Harikumar
KB
and Aggarwal
BB.
Resveratrol: a multitargeted agent for age-associated chronic diseases.
Cell Cycle.
2008;
7:
1020
-1035.
[PubMed]
.
-
99.
Lagouge
M
, Argmann
C
, Gerhart-Hines
Z
, Meziane
H
, Lerin
C
, Daussin
F
, Messadeq
N
, Milne
J
, Lambert
P
, Elliott
P
, Geny
B
, Laakso
M
, Puigserver
P
and Auwerx
J.
Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1alpha.
Cell.
2006;
127:
1109
-1122.
[PubMed]
.
-
100.
Baur
JA
, Pearson
KJ
, Price
NL
, Jamieson
HA
, Lerin
C
, Kalra
A
, Prabhu
VV
, Allard
JS
, Lopez-Lluch
G
, Lewis
K
, Pistell
PJ
, Poosala
S
, Becker
KG
, Boss
O
, Gwinn
D
, Wang
M
, Ramaswamy
S
, Fishbein
KW
, Spencer
RG
, Lakatta
EG
, Le
Couteur D
, Shaw
RJ
, Navas
P
, Puigserver
P
, Ingram
DK
, de Cabo
R
and Sinclair
DA.
Resveratrol improves health and survival of mice on a high-calorie diet.
Nature.
2006;
444:
337
-342.
[PubMed]
.
-
101.
Chan
AY
, Dolinsky
VW
, Soltys
CL
, Viollet
B
, Baksh
S
, Light
PE
and Dyck
JR.
Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt.
J Biol Chem.
2008;
283:
24194
-24201.
[PubMed]
.
-
102.
Cao
Z
, Fang
J
, Xia
C
, Shi
X
and Jiang
BH.
trans-3,4,5'-Trihydroxystibene inhibits hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in human ovarian cancer cells.
Clin Cancer Res.
2004;
10:
5253
-5263.
[PubMed]
.
-
103.
Brito
PM
, Devillard
R
, Nègre-Salvayre
A
, Almeida
LM
, Dinis
TC
, Salvayre
R
and Augé
N.
Resveratrol inhibits the mTOR mitogenic signaling evoked by oxidized LDL in smooth muscle cells.
Atherosclerosis.
2008;
In press
.
-
104.
Fröjdö
S
, Cozzone
D
, Vidal
H
and Pirola
L.
Resveratrol is a class IA phosphoinositide 3-kinase inhibitor.
Biochem J.
2007;
406:
511
-518.
[PubMed]
.
-
105.
Haider
UG
, Sorescu
D
, Griendling
KK
, Vollmar
AM
and Dirsch
VM.
Resveratrol suppresses angiotensin II-induced Akt/protein kinase B and p70 S6 kinase phosphorylation and subsequent hypertrophy in rat aortic smooth muscle cells.
Mol Pharmacol.
2002;
62:
772
-777.
[PubMed]
.
-
106.
Blagosklonny
MV
An anti-aging drug today: from senescence-promoting genes to anti-aging pill.
Drug Discov Today.
2007;
12:
218
-224.
[PubMed]
.
-
107.
Demidenko
ZN
and Blagosklonny
MV.
At concentrations that inhibit mTOR, resveratrol suppresses cellular senescence.
Cell Cycle.
2009;
8:
In press
.