dSir2 mediates the increased spontaneous physical activity in flies on calorie restriction
Abstract
Calorie restriction (CR) is the most effective way to increase life span and delay the onset of age-related symptoms in animals. We have previously reported that CR affects a variety of physiological phenotypes in flies and results in dramatic behavioral, physical and demographic changes. Here we show effects of low and high calorie levels on the spontaneous physical activity of flies. Wild type flies maintained on a low calorie diet exhibit higher spontaneous activity compared to flies on higher calorie diets. This increase is dependent on the presence of Sir2 since a low calorie diet does not increase the activity of dSir2 null flies. Similarly, increasing dSir2 activity by feeding flies resveratrol, a CR mimetic, increases spontaneous physical activity of flies on high caloric food. InDrosophila, spontaneous physical activity therefore closely mimics life span in its dependence on Sir2.
Introduction
Aging
in humans, and a wide variety of other animals, is characterized by the decline
of physiological activity and function. Learning, sensory function,
reproduction, cardiac function and locomotor activity all senesce [1]. However,
the restriction of dietary calories substantially slows the aging process and
extends the lifespan of organisms including yeast, nematodes, fruit flies and
rodents [2-6]. Beneficial effects of limited CR in primates and humans have
been reported but the effects on maximal longevity are unknown [7,8].
Several lines of evidence suggest that
the extension of life span due to CR in yeast, nematodes, and fruit flies is mediated
by an increase in the activity of the silent information
regulator 2 (Sir2) gene [9-13]. Neither yeast, worms nor flies with reduced or
absent Sir2 activity exhibit longer life span on reduced calorie media [12,14,15]. Similarly, survival of SirT1-null mice was decreased after exposure to CR
[16]. Overexpression of Sir2 genetically, or increase in Sir2 activity
mediated by the drug resveratrol, increases life span without calorie
restriction in yeast, nematodes, fruit flies and fish [13,17,18]. Consistent
with this is the finding that overexpression of SIRT1 in transgenic mice
confers many of the physiological changes associated with CR [19]. In addition,
as would be expected if the increase in life span due to CR is mediated by an
increase in dSir2 activity, CR does not increase the life span of dSir2
overexpressing flies [12,13]. However, there are several reports suggesting
that in worms and yeast the Sir2 gene is not necessary for CR life span
extension and that addition of resveratrol does not increase the longevity of
yeast, worm or flies [20-26].
In
addition to increased longevity, mice respond to CR with a general increase in
physical activity that is also mediated by an increase in Sir2 activity [27].
Increases in five different measurements of physical activity such as walking,
jumping and distance traveled, usually observed in CR mice, were not observed
in mice lacking functional Sirt1, the mouse ortholog of yeast Sir2 [16,27]. In
addition, transgenic mice over-expression SIRT1 have increased rotarod
performance [19]. Resveratrol given to mice on a standard diet mimics the
effects of CR, reduces age-related pathology and improves performance on the
rotarod with age [28]. Resveratrol given to mice on high calorie food also
promotes numerous beneficial effects: increased insulin sensitivity, decreased
organ pathology, increased activity of peroxisome proliferator-activated
receptor-γ cooactivator 1α (PGC-1α) and AMP-activated protein kinase (MAMPK) and increased mitochondrial
number [29,30]. In addition, this intervention also improves mouse
neuromuscular function, and affects balance and motor coordination (improved
rotarod performance, increased running time and consumption of oxygen in muscle
fibers) [29,30].
Drosophila are typical of other animals, both in their pattern
of senescence and in their response to CR. Flies decline with age by a variety
of measures including learning, walking, flying, resting, phototaxis, jumping
and general locomotor activity [31-33]. There are a number of reports studying
locomotor activity of flies and its change with age. The reported studies used
a variety of techniques, such as locomotion, geotaxis, fast phototaxis, RING
assays, or Trikinetics activity monitors. Such techniques record the
performance of the flies in particular events or continuously monitor
spontaneous physical activity during 24 hours [33-39]. Several new
sophisticated techniques for tracking of the 3D movements of flies have been
described recently, designed for special uses such as behavioral analysis,
free-flight response to motion and recording fly movements and gene expression
[40,41]. Using Trikinetics activity monitors we found higher spontaneous
physical activity of mixed population of flies under CR [42]. We used computer
controlled "population activity monitors" to record spontaneous physical
activity of each gender under several life extending conditions. Here, we
report that CR is associated with increased spontaneous physical activity in
Drosophila, similar to mammalian studies in mice, and that this increase is
mediated by the fly Sir2 ortholog [16,27]. In addition, feeding the flies
resveratrol, a CR mimetic, increased their spontaneous physical activity on a
high calorie diet, further confirming the role of dSir2 in mediating increased
spontaneous physical activity in CR flies.
Results
Daily
spontaneous physical activity is affected by caloric intake
We
previously reported that the 24-hour activity of flies (mixed genders) depends
on the calorie content of the food, with increased activity associated with a
low calorie diet [47]. In order to determine gender-specific effects of diet on
spontaneous physical activity, male and female Canton-S wild type flies were
aged together on food with low (0.5X) and high (1.5X) calorie contents
post-eclosion. The caloric content of 0.5X food is 50% that of the 1.0X food,
and flies kept on 0.5X food have extended lifespan [42,43]. From 3 days of
age, we recorded spontaneous activity of flies separated by gender in 3 groups
of 10 male or female flies. Each cohort was transferred into the population
monitors with 0.5X or 1.5X food and placed in a temperature controlled
incubator set at 25°C, with a 12 hour light-dark cycle. Using computer
controlled population activity monitors, we were able to monitor spontaneous
physical activity of the flies throughout most of their first 10 days of life.
The days when the flies were passed to new vials were not used for
calculations. There is a significant increase in the 24 hour spontaneous
physical activity (total) observed in male flies kept at 0.5X food compared to
flies kept on 1.5 food level at age 4 days [t(1, 58) = 7.21; η2= 0.47], Figure 1A, Table 1. A similar statistical difference in the
total spontaneous activity of male flies kept on 0.5X and 1.5X was found at age
9, [t(1, 58) = 6.59; η2= 0.43], suggesting that the difference in activity
is not only associated with very young age of 4 days. Female flies show a
significant increases in spontaneous physical activity associated with low
calorie food at age 4, [t(1, 58) = 9.15; η2= 0.59], but not at
age 9, Figure 1B, Table 1. However, this result may be due to the high standard
errors of the means observed in recorded mobility for female flies on 0.5X and
1.5X food levels at age 9. We also examined if there is a gender specific
difference in the levels of physical activity of the flies in response to 0.5X
vs. 1.5X food levels. There is no significant difference in the activity of the
male and female flies on 0.5X food levels at both ages, nor on 1.5X food level
at age 9. However, there is a statistically significant increase in the
spontaneous physical activity of the female flies on 1.5X food levels at age 4
[t(1, 47.2) = -4.575; p<0.001; η2= 0.265]. Interestingly,
the activity of the female flies is higher than males at both food levels at
age 4, but lower at age 9 days, Figure 1C. These data further confirm that,
like mammals,
male and female flies respond to a low calorie diet with increased spontaneous
physical activity.
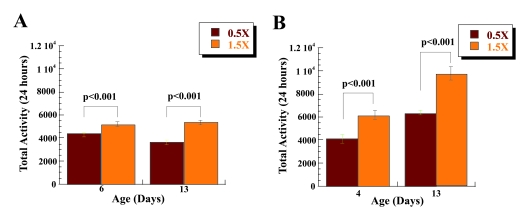
Figure 1. Low calorie diet is associated with increased spontaneous physical activity of Drosophila. Sum of 24-hour
spontaneous physical activity of Canton-S male (A) and female
(B) flies on a low (0.5X) and a high (1.5X) calorie food based on
collected data for days 4 and 9. Both male and female flies on 0.5X food
have increased spontaneous physical activity compared to the flies on l.5X
food. The mobility was based on the mean mobility of 3 vials with 10 male
or 10 female flies each, and expressed as mean total activity per vial
during 24 hours +/- SEM. (C) Mean total 24 hours spontaneous
activity of male and female CS flies on 0.5X and 1.5X food at age 4
and 9 expressed per vial. Statistical significance was determined by using
two-tailed Student's t-test for independent samples.
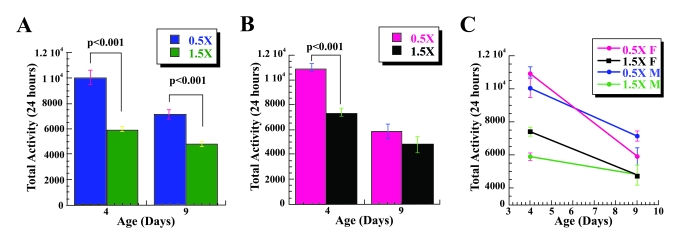
Figure 2. Increase in activity of male flies on low calorie food is mediated by dSir2. dSir4.5/dSir4.5
(A) and dSir5.26/dSir5.26 (B)
homozygous dSir2 null male flies have significantly lower 24-hour
spontaneous physical activity on 0.5X (brown) & 1.5X (orange) calorie
diet at two different ages. The mobility was based on the mean mobility of
3 vials with 10 male flies each, and expressed as mean total activity per
vial during 24 hours +/- SEM. Statistical significance was determined by
using two-tailed Student's t-test with unequal variances.
Table 1. Increased activity of flies on low calorie diet is mediated by dSir2.
|
Gender
|
Genotype
|
Food levels
|
Age
|
Mean Activity
|
SE
|
η2 |
| | | | | | |
|
M
| CS |
0.5
|
4
|
10006.00
|
547.12
|
0.47*
|
|
M
| CS |
1.5
|
4
|
5882.66
|
165.63
| |
|
M
| CS |
0.5
|
9
|
7112.33
|
287.12
|
0.43*
|
|
M
| CS |
1.5
|
9
|
4776.00
|
208.02
| |
| | | | | | |
|
F
| CS |
0.5
|
4
|
10893.66
|
266.94
|
0.59*
|
|
F
| CS |
1.5
|
4
|
7365.33
|
278.55
| |
|
F
| CS |
0.5
|
9
|
5876.33
|
551.43
|
0.17
|
|
F
| CS |
1.5
|
9
|
4759.33
|
594.62
| |
| | | | | | |
|
M
| dSir24.5/dSir24.5 |
0.5
|
6
|
4279.33
|
77.64
|
0.502*
|
|
M
| dSir24.5/dSir24.5 |
1.5
|
6
|
5195.00
|
91.22
| |
|
M
| dSir24.5/dSir24. |
0.5
|
13
|
3630.33
|
141.57
|
0.556*
|
|
M
| dSir24.5/dSir246 |
1.5
|
13
|
5372.67
|
147.47
| |
| | | | | | |
|
M
| dSir25.26/dSir25.26 |
0.5
|
4
|
4415.66
|
359.69
|
0.211*
|
|
M
| dSir25.26/dSir25.26 |
1.5
|
4
|
6475.00
|
378.45
| |
|
M
| dSir25.26/dSir25.26 |
0.5
|
13
|
6723.67
|
228.39
|
0.298*
|
|
M
| dSir25.26/dSir25.26 |
1.5
|
13
|
10249.33
|
673.63
| |
| | | | | | |
|
M
| CS |
0.5 50Res
|
9
|
3224.67
|
41.65
| 0.222* |
|
M
| CS |
0.5 100Res
|
9
|
3775.67
|
309.48
| |
|
M
| CS |
0.5 200Res
|
9
|
2358.33
|
156.99
| |
|
M
| CS |
0.5 EtOH
|
9
|
4645.50
|
229.85
| |
|
M
| CS |
1.5 50Res
|
9
|
5364.00
|
261.26
| |
|
M
| CS |
1.5 100Res
|
9
|
4663.67
|
170.89
| |
|
M
| CS |
1.5 200Res
|
9
|
5046.67
|
715.19
| |
|
M
| CS |
1.5 EtOH
|
9
|
3331.83
|
134.98
| |
| | | | | | |
|
M
| CS |
0.5 200Res
|
6
|
4192.67
|
136.62
| 0.23* |
|
M
| CS |
0.5 EtOH
|
6
|
5116.33
|
248.51
| |
|
M
| CS |
1.5 200Res
|
6
|
7932.00
|
883.47
| |
|
M
| CS |
1.5 EtOH
|
6
|
4805.33
|
271.69
| |
| | | | | | |
|
M
| yw |
0.5 200Res
|
3
|
8880.67
|
611.66
|
0.248
|
|
M
| yw |
0.5 EtOH
|
3
|
7847.00
|
641.64
| |
|
M
| yw |
1.5 200Res
|
4
|
12721.67
|
259.56
|
0.724*
|
|
M
| yw |
1.5 EtOH
|
4
|
7028.00
|
381.95
| |
Increased
activity in flies on low calorie food is mediated by dSir2
Our
life span studies reveal that the beneficial effects of CR on fly survivorship
accrue and are mediated by dSir2 [12]. In order to examine if increased
activity under CR conditions is mediated by dSir2, we determined the
activity of the dSir4.5/dSir4.5 and dSir25.26/dSir25.26,dSir2 - homozygous null mutants on 0.5X and 1.5X food levels. Both dSir4.5/dSir4.5
and dSir25.26/dSir25.26 flies have lower
spontaneous physical activity associated with CR, Figure 2A and B, Table 1. In
contrast to wild type flies that have increased spontaneous physical activity
on low calorie diet, we found that two different dSir2 null homozygous
flies have significantly lower spontaneous physical activity on 0.5X food
compared to the 1.5X, Figure 2A and B, Table 1. Decreased activity on 0.5X food was observed at two different ages, 6 and 13
for dSir4.5/dSir4.5 flies (age 6 [t(1, 58) = 7.64;
p< 0.001; η2= 0.502], age 13 [t (1, 58) = 8.523; p< 0.001; η2= 0.556] and 4 and 13 for dSir5.26/dSir5.26
flies (age 4 [t (1, 58) = 3.944; p< 0.001; η2= 0.211, age 13; [t (1, 35.6 = 4.957; p<
0.001; η2= 0.298]).
While activity of dSir4.5/dSir4.5 is similar in
flies at ages 6 and 13, there is a significant increase in the spontaneous
activity of dSir5.26/dSir5.26 flies at age 13
compared to age 4 on 0.5X food [t (1, 49.1) = 5.417; p< 0.001; η2= 0.336] and 1.5X food [t (1,
45.648) = 4.885; p< 0.001; η2= 0.291]. Decreased physical activity observed in dSir2 mutant
flies on 0.5X food in comparison to 1.5 X food suggests that the presence of
dSir2 is not only necessary for increased spontaneous physical activity of the
flies on low calorie diet, but importantly, that dSir2 deficiency has negative
effects on activity under CR conditions.
Resveratrol
restores normal physical activity to flies on a high calorie diet
The drug resveratrol, a polyphenolic
STAC, increases the life span of yeast, worms, fruit flies and fish by
activating Sir2 [13,17,18]. Moreover, this chemical activation of Sir2
increases the running time of mice fed a high fat diet [29,30]. We wanted to
determine if dietary administration of resveratrol to flies maintained on a
high calorie diet could boast the low spontaneous physical activity seen under
these conditions. In order to evaluate the effects of different concentrations
of resveratrol on fly spontaneous activity we recorded the spontaneous activity
of male CS flies on 0.5X and 1.5X food with 50 μM, 100 μM and
200 μM of resveratrol or ethanol controls, Figure 3A.
Statistical differences between means were found using a one-way analysis of
variance (ANOVA) [F(7, 292)= 11.927, p<0.001, η2=0.222],
Table 1. We found that addition of 50 μM, 100 μM or 200 μM of
resveratrol increases the spontaneous physical activity of male flies on a high
calorie diet to the levels of activity observed in control flies on 0.5X EtOH
food, Figure 3A, Table 1. A similar increase in activity of flies on 1.5X Res
food was observed at all three levels of resveratrol, suggesting that once the
increase in physical activity reaches a certain threshold, additional increases
in dSir2 activity do not further raise the physical activity of the flies.
Addition of any of the three concentrations of resveratrol to the low calorie
diet decreases the activity of flies compared to 0.5X EtOH controls, suggesting
a negative effect of resveratrol on the spontaneous activity of CR flies,
Figure 3A, Supplementary Table 1A and Supplementary Table 1B. However, the biggest negative impact on
activity at 0.5X was observed with 200 μM of
resveratrol. Statistical analysis is in Supplementary Table 1A and Supplementary Table 1B.
We
also examined if addition of resveratrol increases the physical activity of
younger flies fed a high calorie diet. We found that of 200 μM of resveratrol boasts the low spontaneous activity
of male CS flies on 1.5X food to levels higher than 0.5X at age 6,
Figure 3B, Table 1, Supplementary Table 1A and Supplementary Table 1B. An analysis of variance was
performed to test mean differences of mobility between high calorie diet with
addition of ethanol (1.5X EtOH) or resveratrol (1.5X Res), and low calorie diet
with resveratrol (0.5X Res) or with ethanol (0.5X EtOH). A statistically
significant difference was found between food groups for males at age 6 [F(3, 116)
= 11.77; η2= 0.23). At age 6, spontaneous physical activity of
flies on 1.5X Res was significantly increased compared to flies on all food
conditions determined by Tukey's HSD post-hoc analysis, Supplementary Table 1A and Supplementary Table 1B.
The flies on 0.5X with addition of 200 μM of resveratrol
have the lowest activity. This suggests that the levels of dSir2 activity may
directly determine fly activity- excess, as in case of addition of 200 μM resveratrol, or none as in case of dSir2
mutant flies, decreases fly activity. Another
explanation for low activity of CS flies on 0.5X Res food could be that
concentration of 200 μM of resveratrol is too high and may have some negative
effects on flies that are already under stress caused by CR.
In
order to confirm that the addition of resveratrol increases the low spontaneous
activity of flies on 1.5X food, we also determined the activity of yw,
another wild type genetic background. As can be seen from the Figure 3C,
addition of 200 μM resveratrol (1.5X 200Res)
to high calorie diet significantly increases fly spontaneous physical activity
compared all other food regimens: 1.5X with diluent ethanol (1.5X EtOH), or
flies on 0.5X with resveratrol (0.5X 200Res) or diluent (0.5X EtOH). However, yw
flies on 0.5X with resveratrol did not have the lowest activity as CS
flies did. The different response of yw flies to addition of resveratrol
to 0.5X food may be explained by different genetic backgrounds and slightly
younger age. yw flies were 3 and 4 days old, while CS were 6 and
9 days old.
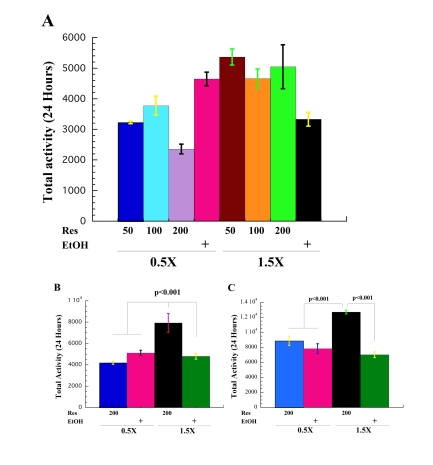
Figure 3. Resveratrol rescues low activity of the flies on high-calorie diet. (A)
Effect of 50 μM,
100 μM and 200 μM of resveratrol
in 0.5X (0.5X 50Res, 0.5X 100Res, 0.5 200Res) and 1.5X (1.5 50Res, 1.5
100Res, 1.5X 200Res) food on CS male spontaneous physical activity
compared to activity of flies on 0.5X and 1.5X food that contain ethanol
(0.5 EtOH and 1.5 EtOH) used as resveratrol solvent. (B) Total
daily spontaneous physical activity of male CS flies on 0.5X with
200 μM resveratrol
(0.5 200Res) and 1.5X with μM
200 resveratrol (1.5 200Res) compared to the male flies on 0.5X and 1.5X
food that contain ethanol (0.5 EtOH and 1.5 EtOH). The data are mean total
24s spontaneous activities collected independently for 3 vials with 10
flies each collected at age 9 (A) and 6 (B) days, except in A
where there were 6 vials of 0.5X EtOH and 1.5X EtOH. A Tukey HSD post-hoc
test was conducted on the food means to determine which means are pairwise
statistical different from one another. Results of statistical analysis are
in Supplementary Table 1A and Supplementary Table 1B. (C) Male yw wild type flies on 1.5X
food with addition of 200 μM
of resveratrol (1.5 200Res) have the highest activity compared to the flies
on 1.5X EtOH, 0.5X 200Res and 0.5X EtOH. Flies were 3 (0.5X 200Res and 0.5X
EtOH) and 4 (1.5XRes and 1.5EtOH) days old. Statistical significance was
determined by using two-tailed Student's t-test with unequal variances.
Discussion
Calorie
restriction increases spontaneous physical activity in Drosophila
Caloric
uptake affects many physiological functions [6,44]; this is especially true of
spontaneous physical activity [45,46]. Within a range of caloric intake above
starvation for flies, greater calorie consumption leads to higher body weight,
and a higher rate of reproduction but a shorter life span [42]. We have
previously reported that low calorie diet increases spontaneous physical
activity of flies [42]. By using computer-controlled activity monitors, we were
able to monitor spontaneous physical activity of flies longitudinally during
the first 10 days of life. Comparisons of total spontaneous physical activity
on days 4 and 9 of life reveal that total activity decreases with age but a
CR-mediated increase in the activity of male flies is maintained. Females have
increased activity associated with low calorie diet at age 4 but not at age 9.
Sir2
mediates increased spontaneous physical activity of flies on low calorie food
Our studies on the molecular mechanisms
underlying life span extension by CR suggest that dSir2 mediates the CR
response [11,12]. When Sir2 is overexpressed or activated by the drug
resveratrol in yeast, worms, flies, or mice, there is an increase in life span
on a rich diet that mimics the response to CR, even though nutrient supplies
are superabundant [12,13,17,29,30,47]. Overexpression of SIRT1 in transgenic
mice confers many of the same phenotypes as CR in mice, including increased
performance in a rotarod assay [19]. Consistent with Sir2-mediating the
response to low calories, no further increases are obtained when Sir2
overexpression is combined with CR [12,13]. When Sir2 is reduced or absent, CR
no longer induces longer life span in yeast, worms or flies [12,14,15]. Chen
et al. (2005) reported that CR increases five different measurements of
physical activity such as walking, jumping and distance traveled, but such
increases were not observed in mice lacking the mouse orthologue of Sir2 [27].
Similarly, another group reported that SirT1-null mice don't increase their
activity on CR and have lower total 24-hour activity on regular food [16]. Thus,
we were prompted to examine whether increased spontaneous physical activity in Drosophila on low calorie diet is mediated by dSir2. We now report that
increased spontaneous physical activity of Drosophila on low calorie
food is mediated by dSir2, as is the case for mice. Furthermore, we found that
the spontaneous physical activity of flies lacking Sir2 is lower on low calorie
food compared to high. While Sir2 is necessary for increased mobility on low
calorie, its absence actually has a negative effect on mobility when flies are
raised on low calories. Several potential molecular mechanisms that could
contribute to lower spontaneous physical activity of calorie restricted dSir2
null flies come to mind. First, Sir2 has been implicated in energy metabolism and
Sir2 deficiency, such as in SirT1-null mice, results in inefficient metabolism
characterized by lower food utilization, altered mitochondria and metabolic
rate and lower activity [16]. A role of Sir2 in regulating the amplitude of the
circadian rhythm has also been described [48,49].
Resveratrol
restores spontaneous physical activity of flies on a high calorie diet
The
drug resveratrol, a polyphenolic STAC, increases the life span of yeast, worms,
fruit flies and fish by activating Sir2 [13,17,18]. Administration of
resveratrol to wild type mice on either a regular diet or a high-calorie diet
mimics effects of CR, postpones age-related pathology and has other benefits
[28-30]. An effect of resveratrol on the physical activity in mice is also
observed; it increases rotarod performance and endurance running, but decreases
total spontaneous physical activity at high doses [29,30]. Similarly, addition
of resveratrol postponed age-related decreases in locomotor activity of
short-lived vertebrate fish, N. furzeri [18]. Consistently, SIRT1
transgenic mice overexpressing SirT1 have better performance on the
rotarod [19]. We found that the administration of resveratrol to control flies
on a high calorie diet increases their physical activity in two different control
strains, CS and yw. Similar non-dose dependent restoration of
activity on high calorie diet was observed when CS flies were subjected
to three different doses of resveratrol suggesting the presence of a threshold
for the increase in spontaneous activity of flies on high calorie diet that can
be reached at certain levels of resveratrol, so that additional increases in
resveratrol concentration and subsequent increases in dSir2 activity does not
further increase spontaneous activity of the flies. Interestingly, addition of
resveratrol to a low calorie diet decreases the spontaneous physical activity
of CS flies but not yw flies. The decrease in spontaneous
physical activity of calorie restricted CS flies was most pronounced in
flies on the highest level of resveratrol of 200 μM.
High levels of resveratrol may have some toxic effects, for instance negative
effects were reported when rats and mice were exposed extremely high doses of
resveratrol [28,50].
Similarly
significant decrease in ambulatory locomotor activity and numbers of rears was
observed in mice on high calorie diet with high doses of resveratrol, and in
mice on high calorie diet after treatment with high doses of SRT1720, a potent
SirT1 activator [51]. The effects of high levels of resveratrol may be through
activation of Ser/Thr kinase AMPK, a known metabolic regulator that is also
activated by CR or other targets and pathways known to be activated by
resveratrol treatment [29]. The different response of CS and yw
flies to the addition of resveratrol under CR conditions can be explained by
different genetic backgrounds, which has been shown to effect survivorship,
age-dependent changes in locomotor activity of male and female flies and
response to CR [36,52].
Our
results further indicate that the Sir2 orthologue, dSir2, mediates the
CR-induced increase in spontaneous physical activity observed in flies.
Consistent with this conclusion, the activation of Sir2 by resveratrol leads to
an increase in activity on high calorie food. Interestingly, we found that the
activity of flies on a low calorie diet is sensitive to the levels of dSir2
activity, too much or none results in lowered activities. Illustrating this is
the fact that dSir2 null mutant flies on a low calorie diet have lower activity
compared to the mutants on high calorie diet. Furthermore, the addition of
resveratrol, a dSir2 activator, to 0.5X food similarly decreases CS
flies activity. In this study, we used a computer assisted measure of
spontaneous physical activity to extend our earlier findings on mobility in
flies, and show that Sir2-mediated increases in spontaneous physical activity
occur under CR and addition of resveratrol, conditions known to lead to
Sir2-mediated increases in life span.
Materials and Methods
Fly
stocks, food preparation and maintenance
were described previously [12,45].
The Canton-S strain is the standard wild-type background line obtained
from the Bloomington Stock Center. dSir24.5 and dSir25.26
mutant flies are null for dSir2 gene (S. Smolik). Flies were maintained
in a humidified temperature-controlled environmental chamber at 25°C (Percival
Scientific) on a 12-hour light: dark cycle with light on at 6:AM.
Dietary
calorie content of Drosophila food.
Standard laboratory corn media as
well as food marked as 0.5X and 1.5X were used. The two food levels are
standardized as 1.0X being the food that has 100 g/L of sucrose (MP
Biomedicals, Inc), 100 g/L of brewer's yeast (MP Biomedicals, Inc) and 20 g/L
of agar [42,43].
Resveratrol (Sigma) dissolved in EtOH was
added to the food during its preparation in final concentration of 50 μM, 100 μM and
200 μM. For control experiments the
same volume of EtOH was added to the food. Food was prepared as previously
described [13].
Spontaneous
physical activity monitors.
20 male and 20 female flies were aged together on
appropriate food since the day of their eclosion. On day 3 flies were separated
by gender and three subgroups of 10 males or female flies were placed in
population monitors and their physical activity was recorded every 10 minutes
for the first 10 days of their life (Drosophila population monitor by
Trikinetics Inc., Waltham, MA, USA). Reading chambers have circular rings of
infrared beams at three different levels, which allow recording every time when
fly crosses the rings. Activity monitors were kept in temperature control
incubators set at 25°C on a 12-h light-dark cycle. The daylight period began at
6:00AM. Flies were replaced with a new set of flies of the same ages every two
to three days. Recorded activities for the days when flies were replaced were
not used for calculations.
Statistical
analysis.
A two-tailed Student's t-test was used for the analysis of the
effects of 0.5X and 1.5X food levels on the mobility of wild type CS anddSir2 null flies. Similar analysis was performed to analyze the effects
of addition of 200 μM of resveratrol or EtOH to
0.5X and 1.5X food on the mobility of wild type yw male flies. A one-way
analysis of variance (ANOVA) was performed to assess whether there are
differences between mean activity of male CS flies on low (0.5X) and
high (1.5X) calorie diet with addition of different doses of resveratrol or
ethanol. A Tukey HSD post-hoc test was conducted on the mean mobility of wild
type CS male flies to analyze the effects of addition of different doses
of resveratrol or EtOH to 0.5X and 1.5X food on the mobility and to determine
which means are pairwise statistically significantly different from one
another.
Supplementary Materials
*The mean difference is significant at the 0.05 levels.
* = p < .05
** = p < .01
*** = p < .001
A Tukey HSD post-hoc test was conducted on the mean 24 hour
spontaneous physical activity of male wild type CS flies kept
on low food with 50 μM, 100?μM and 200 μM resveratrol
(0.5 50Res, 0.5 100Res, 0.5 200Res), 0.5 low calorie food
with ethanol, (0.5 EtOH) or high calorie food with 50 μM,
100 μM and 200 μM resveratrol (1.5 50Res, 1.5 100Res,
1.5 200Res) or ethanol (1.5 EtOH) to determine which means
are paiwise statistically significantly different from one
another. Flies were kept at 25°C during recording of the
spontaneous physical activity. Flies were 9 days old.
A Tukey HSD post-hoc test was conducted on the mean 24 hour
spontaneous physical activity of male wild type CS flies kept
on low food with 200 μM resveratrol (0.5 200Res), 0.5 low
calorie food with ethanol, (0.5 EtOH) or high calorie food with
200 μM resveratrol (1.5 200Res) or ethanol (1.5 EtOH) to
determine which means are paiwise statistically significantly
different from one another. Flies were kept at 25°C C during
recording of the spontaneous physical activity. Flies were and
6 days old.
Acknowledgments
We
thank Suzanne Kowalski for technical help and Drs. Stewart Frankel, Joseph Jack
and Robert A. Reenan for critical reading of the manuscript and Drs. Stephen L.
Helfand and Stormy Chamberlain for helpful discussion. We are grateful to Dr.
Daniel J. Denis for expert statistical analysis. This work was supported by
grant from the National Institutes of Health (AG23088 to B.R.).
Conflicts of Interest
The
author of this manuscript has no conflict of interests to declare.
References
-
1.
Finch
CE
Chicago
University of Chicago Press
Longevity, senescence, and the genome.
1990;
.
-
2.
Lin
SJ
, Kaeberlein
M
, Andalis
AA
, Sturtz
LA
, Defossez
PA
, Culotta
VC
, Fink
GR
and Guarente
L.
Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration.
Nature.
2002;
418:
344
-348.
[PubMed]
.
-
3.
Comfort
A
Effect of delayed and resumed growth on the longevity of a fish (Lebistes reticulates, Peters) in captivity.
Gerontologia.
1963;
49:
150
-155.
[PubMed]
.
-
4.
McCay
CM
, Crowell
MF
and Maynard
LA.
The effect of retarded growth upon the lenght of life span and upon the ultimate body size.
J Nutr.
1935;
10:
63
-79.
.
-
5.
Sinclair
DA
Toward a unified theory of caloric restriction and longevity regulation.
Mech Ageing Dev.
2005;
126:
987
-1002.
[PubMed]
.
-
6.
Bishop
NA
and Guarente
L.
Genetic links between diet and lifespan: shared mechanisms from yeast to humans.
Nat Rev Genet.
2007;
8:
835
-844.
[PubMed]
.
-
7.
Mattison
JA
, Roth
GS
, Lane
MA
and Ingram
DK.
Dietary restriction in aging nonhuman primates.
Interdiscip Top Gerontol.
2007;
35:
137
-158.
[PubMed]
.
-
8.
Fontana
L
and Klein
S.
Aging, Adiposity, and Calorie Restriction.
JAMA.
2008;
297:
986
-994.
[PubMed]
.
-
9.
Kaeberlein
M
, McVey
M
and Guarente
L.
The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms.
Genes Dev.
1999;
13:
2570
-2580.
[PubMed]
.
-
10.
Tissenbaum
HA
and Guarente
L.
Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans.
Nature.
2001;
410:
227
-230.
[PubMed]
.
-
11.
Rogina
B
, Helfand
SL
and Frankel
S.
Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction.
Science.
2002;
298:
1745
[PubMed]
.
-
12.
Rogina
B
and Helfand
SL.
Sir2 mediates longevity in the fly through a pathway related to calorie restriction.
Proc Natl Acad Sci U S A.
2004;
101:
15998
-6003.
[PubMed]
.
-
13.
Wood
JG
, Rogina
B
, Lavu
S
, Howitz
K
, Helfand
SL
, Tatar
M
and Sinclair
DA.
Sirtuin activators mimic caloric restriction and delay ageing in metazoans.
Nature.
2004;
430:
686
-689.
[PubMed]
.
-
14.
Lin
SJ
, Defossez
PA
and Guarente
L.
Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae.
Science.
2000;
289:
2126
-2128.
[PubMed]
.
-
15.
Wang
Y
and Tissenbaum
HA.
Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO.
Mech Ageing Dev.
2006;
127:
48
-56.
[PubMed]
.
-
16.
Boily
G
, Seifert
EL
, Bevilacqua
L
, He
XH
, Sabourin
G
, Estey
C
, Moffat
C
, Crawford
S
, Saliba
S
, Jardine
K
, Xuan
J
, Evans
M
, Harper
ME
and McBurney
MW.
SirT1 regulates energy metabolism and response to caloric restriction in mice.
PLoS ONE.
2008;
3:
e1759
[PubMed]
.
-
17.
Howitz
KT
, Bitterman
KJ
, Cohen
HY
, Lamming
DW
, Lavu
S
, Wood
JG
, Zipkin
RE
, Chung
P
, Kisielewski
A
, Zhang
LL
, Scherer
B
and Sinclair
DA.
Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan.
Nature.
2003;
425:
191
-196.
[PubMed]
.
-
18.
Valenzano
DR
, Terzibasi
E
, Genade
T
, Cattaneo
A
, Domenici
L
and Cellerino
A.
Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate.
Curr Biol.
2006;
16:
296
-300.
[PubMed]
.
-
19.
Bordone
L
, Cohen
D
, Robinson
A
, Motta
MC
, van
Veen E
, Czopik
A
, Steele
AD
, Crowe
H
, Marmor
S
, Luo
J
, Gu
W
and Guarente
L.
SIRT1 transgenic mice show phenotypes resembling calorie restriction.
Aging Cell.
2007;
6:
759
-767.
[PubMed]
.
-
20.
Lamming
DW
, Latorre-Esteves
M
, Medvedik
O
, Wong
SN
, Tsang
FA
, Wang
C
, Lin
SJ
and Sinclair
DA.
HST2 mediates SIR2-independent life-span extension by calorie restriction.
Science.
2005;
309:
1861
-1864.
[PubMed]
.
-
21.
Hansen
M
, Taubert
S
, Crawford
D
, Libina
N
, Lee
SJ
and Kenyon
C.
Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans.
Aging Cell.
2007;
6:
95
-110.
[PubMed]
.
-
22.
Lee
GD
, Wilson
MA
, Zhu
M
, Wolkow
CA
, de Cabo
R
, Ingram
DK
and Zou
S.
Dietary deprivation extends lifespan in Caenorhabditis elegans.
Aging Cell.
2006;
5:
515
-524.
[PubMed]
.
-
23.
Kaeberlein
M
, Kirkland
KT
, Fields
S
and Kennedy
BK.
Sir2-independent life span extension by calorie restriction in yeast.
PLoS Biol.
2004;
2:
E296
[PubMed]
.
-
24.
Kaeberlein
M
, Powers
RW 3rd
, Steffen
KK
, Westman
EA
, Hu
D
, Dang
N
, Kerr
EO
, Kirkland
KT
, Fields
S
and Kennedy
BK.
Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients.
Science.
2005;
310:
1193
-1196.
[PubMed]
.
-
25.
Tsuchiya
M
, Dang
N
, Kerr
EO
, Hu
D
, Steffen
KK
, Oakes
JA
, Kennedy
BK
and Kaeberlein
M.
Sirtuin-independent effects of nicotinamide on lifespan extension from calorie restriction in yeast.
Aging Cell.
2006;
5:
505
-514.
[PubMed]
.
-
26.
Bass
TM
, Weinkove
D
, Houthoofd
K
, Gems
D
and Partridge
L.
Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans.
Mech Ageing Dev.
2007;
128:
546
-552.
[PubMed]
.
-
27.
Chen
D
, Steele
AD
, Lindquist
S
and Guarente
L.
Increase in activity during calorie restriction requires Sirt1.
Science.
2005;
310:
1641
[PubMed]
.
-
28.
Pearson
KJ
, Baur
JA
, Lewis
KN
, Peshkin
L
, Price
NL
, Labinskyy
N
, Swindell
WR
, Kamara
D
, Minor
RK
, Perez
E
, Jamieson
HA
, Zhang
Y
and Dunn
SR.
Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span.
Cell Metab.
2008;
8:
157
-168.
[PubMed]
.
-
29.
Baur
JA
, Pearson
KJ
, Price
NL
, Jamieson
HA
, Lerin
C
, Kalra
A
, Prabhu
VV
, Allard
JS
, Lopez-Lluch
G
, Lewis
K
, Pistell
PJ
, Poosala
S
and Becker
KG.
, Resveratrol improves health and survival of mice on a high-calorie diet.
Nature.
2006;
444:
337
-342.
[PubMed]
.
-
30.
Lagouge
M
, Argmann
C
, Gerhart-Hines
Z
, Meziane
H
, Lerin
C
, Daussin
F
, Messadeq
N
, Milne
J
, Lambert
P
, Elliott
P
, Geny
B
, Laakso
M
and Puigserver
P.
Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha.
Cell.
2006;
127:
1109
-1122.
[PubMed]
.
-
31.
Koh
K
, Evans
JM
, Hendricks
JC
and Sehgal
A.
A Drosophila model for age-associated changes in sleep:wake cycles.
Proc Natl Acad Sci U S A.
2006;
103:
13843
-13847.
[PubMed]
.
-
32.
Carey
JR
, Papadopoulos
N
, Kouloussis
N
, Katsoyannos
B
, Müller
HG
, Wang
JL
and Tseng
YK.
Age-specific and lifetime behavior patterns in Drosophila melanogaster and the Mediterranean fruit fly, Ceratitis capitata.
Exp Gerontol.
2006;
41:
93
-97.
[PubMed]
.
-
33.
Simon
AF
, Liang
DT
and Krantz
DE.
Differential decline in behavioral performance of Drosophila melanogaster with age.
Mech Ageing Dev.
2006;
127:
647
-651.
[PubMed]
.
-
34.
Miquel
J
, Lundgren
PR
, Bensch
KG
and Atlan
H.
Effects of temperature of the life span, vitality and fine structure of Drosophila melanogaster.
Mech Ageing Dev.
1976;
5:
347
-370.
[PubMed]
.
-
35.
Le
Bourg E
and Lints
FA.
A longitudinal study of the effects of age on spontaneous locomotor activity in Drosophila melanogaster.
Gerontology.
1984;
30:
79
-86.
[PubMed]
.
-
36.
Fernandez
JR
Differences in locomotor activity across the lifespan of Drosophila melanogaster.
Experimental Gerontology.
1999;
34:
621
-631.
[PubMed]
.
-
37.
Marden
JH
, Rogina
B
, Montooth
KL
and Helfand
SL.
Conditional tradeoffs between aging and organismal performance of Indy long-lived mutant flies.
Proc Natl Acad Sci U S A.
2003;
100:
3369
-3373.
[PubMed]
.
-
38.
Grotewiel
MS
, Martin
I
, Bhandari
P
and Cook-Wiens
E.
Functional senescence in Drosophila melanogaster.
Ageing Res Rev.
2005;
4:
372
-397.
[PubMed]
.
-
39.
Rhodenizer
D
, Martin
I
, Bhandari
P
, Pletcher
SD
and Grotewiel
M.
Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed.
Exp Gerontol.
2008;
43:
739
-748.
[PubMed]
.
-
40.
Fry
SN
TrackFly: virtual reality for a behavioral system analysis in free-flying fruit flies.
Journal of Neuroscience Methods.
2008;
171:
110
-117.
[PubMed]
.
-
41.
Grover
D
, Yang
J
, Tavaré
S
and Tower
J.
Simultaneous tracking of fly movement and gene expression using GFP.
BMC Biotechnol.
2008;
8:
93
[PubMed]
.
-
42.
Bross
TG
, Rogina
B
and Helfand
SL.
Behavioral, physical, and demographic changes in Drosophila populations through dietary restriction.
Aging Cell.
2005;
4:
309
-317.
[PubMed]
.
-
43.
Chapman
T
and Partridge
L.
Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males.
Proc Biol Sci.
1996;
263:
755
-759.
[PubMed]
.
-
44.
Mair
W
Dillin A. Aging and survival: the genetics of life span extension by dietary restriction.
Annu Rev Biochem.
2008;
77:
727
-754.
[PubMed]
.
-
45.
Duffy
PH
, Feuers
RJ
, Leakey
JA
, Nakamura
K
, Turturro
A
and Hart
RW.
Effect of chronic caloric restriction on physiological variables related to energy metabolism in the male Fischer 344 rat.
Mech Ageing Dev.
1989;
48:
117
-133.
[PubMed]
.
-
46.
Weed
JL
, Lane
MA
, Roth
GS
, Speer
DL
and Ingram
DK.
Activity measures in rhesus monkeys on long-term calorie restriction.
Physiol Behav.
1997;
62:
97
-103.
[PubMed]
.
-
47.
Guarente
L
and Picard
F.
Calorie restriction--the SIR2 connection.
Cell.
2005;
120:
473
-482.
[PubMed]
.
-
48.
Nakahata
Y
, Kaluzova
M
, Grimaldi
B
, Sahar
S
, Hirayama
J
, Chen
D
, Guarente
LP
and Sassone-Corsi
P.
The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control.
Cell.
2008;
134:
329
-340.
[PubMed]
.
-
49.
Asher
G
, Gatfield
D
, Stratmann
M
, Reinke
H
, Dibner
C
, Kreppel
F
, Mostoslavsky
R
, Alt
FW
and Schibler
U.
SIRT1 regulates circadian clock gene expression through PER2 deacetylation.
Cell.
2008;
134:
317
-328.
[PubMed]
.
-
50.
Crowell
JA
, Korytko
PJ
, Morrissey
RL
, Booth
TD
and Levine
BS.
Resveratrol-associated renal toxicity.
Toxicol Sci.
2004;
82:
614
-619.
[PubMed]
.
-
51.
Feige
JN
, Lagouge
M
, Canto
C
, Strehle
A
, Houten
SM
, Milne
JC
, Lambert
PD
, Mataki
C
, Elliott
PJ
and Auwerx
J.
Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation.
Cell Metab.
2008;
8:
347
-358.
[PubMed]
.
-
52.
Grandison
RC
, Wong
R
, Bass
TM
, Partridge
L
and Piper
MD.
Effect of a standardised dietary restriction protocol on multiple laboratory strains of Drosophila melanogaster.
PLoS ONE.
2009;
4:
e4067
[PubMed]
.